Abstract
Cerebrospinal fluid (CSF) circulation and turnover provides a sink for the elimination of solutes from the brain interstitium, serving an important homeostatic role for the function of the central nervous system. Disruption of normal CSF circulation and turnover is believed to contribute to the development of many diseases, including neurodegenerative conditions such as Alzheimer’s disease, ischemic and traumatic brain injury, and neuroinflammatory conditions such as multiple sclerosis. Recent insights into CSF biology suggesting that CSF and interstitial fluid exchange along a brain-wide network of perivascular spaces termed the ‘glymphatic’ system suggest that CSF circulation may interact intimately with glial and vascular function to regulate basic aspects of brain function. Dysfunction within this glial vascular network, which is a feature of the aging and injured brain, is a potentially critical link between brain injury, neuroinflammation and the development of chronic neurodegeneration. Ongoing research within this field may provide a powerful new framework for understanding the common links between neurodegenerative, neurovascular and neuroinflammatory disease, in addition to providing potentially novel therapeutic targets for these conditions.
Introduction
Cerebrospinal fluid (CSF), a clear, secreted fluid filling the cerebral ventricles and surrounding the brain and spinal cord within the subarachnoid space (SAS), serves several functions in the central nervous system (CNS). CSF provides buoyancy to support the weight of the brain and acts as a protective layer to cushion it from injury. CSF also serves regulatory functions, including distribution of neurotrophic factors and stabilization of brain pH and chemical gradients, in addition to supplying an excretory pathway out of CNS for solutes that cannot readily cross the blood brain barrier (BBB). Contributions to CNS development and repair mechanisms have also been noted [1].
Throughout the course of the 20th Century, painstaking surgical, physiological and biophysical experiments led to the development of a classical description of CSF secretion, circulation and reabsorption that remains the principal model to this day. Within this model, CSF is secreted actively by the choroid plexuses (CPs) and secretory epithelia in the lateral, third and fourth cerebral ventricles [2]. CSF moves by bulk flow, driven by arterial pulsation and respiration, through the ventricular system, exiting into the SAS via the foramina of Luschka and Magendie [3]. From the SAS, CSF is believed to be reabsorbed into the blood stream either through arachnoid villi, valve-like structures within the walls of dural sinuses, or by traveling along cranial or spinal nerve sheaths to reach the peripheral lymphatic drainage. The excretory function of CSF did not come to prominence until the second half of the 20th century with the work of Davson, who suggested that CSF and the brain interstitial fluid (ISF) interacted, and the CSF serves as a “sink” for solutes from the brain parenchyma [4].
Recent research, aided by advances in imaging technology, suggests that CSF circulation may not be as linear as the classical model suggests, and that CSF and brain ISF exchange dynamically along organized anatomical pathways. The movement of CSF through and the clearance of ISF and its associated solutes from the brain parenchyma along perivascular pathways has important implications for current understanding of basic physiological processes such as CNS waste clearance, distribution of trophic factors, nutrients, and neuroactive compounds, and peripheral immune surveillance of the CNS. Derangement of the functions of CSF circulation may play a key role in the development of a wide range of CNS pathologies, including neurodegenerative diseases such as Alzheimer’s disease (AD), neuroinflammatory conditions such as multiple sclerosis (MS) and neurovascular conditions such as cerebral ischemia, traumatic brain injury (TBI) and subarachnoid hemorrhage [5–10]. Alterations in CSF secretion, circulation and reabsorption are also directly involved in the pathogenesis of different CNS pathologies, including hydrocephalus, pseudotumor cerebri, and neoplasms of the choroid plexus [11–13].
Basic aspects of CSF circulation and CP biology have been the subject of several excellent recent reviews [2, 14–16]. Thus in the present review, we will focus on the implications that recent insights into the interactions between CSF circulation and the brain ISF may have for current understanding of AD, MS and neurovascular diseases.
Central Nervous System Fluid Dynamics
Choroid Plexus and CSF Secretion
CSF is produced primarily by the CPs which are found in the lateral, third and fourth ventricles (Fig. 1C, D) [17]. The CPs were identified as the primary source of CSF by Dandy in 1919, when he observed that hydrocephalus induced by the blockage of the foramen of Monro could be prevented by removal of the CP [18]. Further work has identified extrachoroidal sources, including ependymal cells, limited trans-capillary fluid flux, and metabolic water production that contribute to CSF production [19–21]. It is widely agreed, however, that approximately 80% of total CSF is secreted by the CP [3].
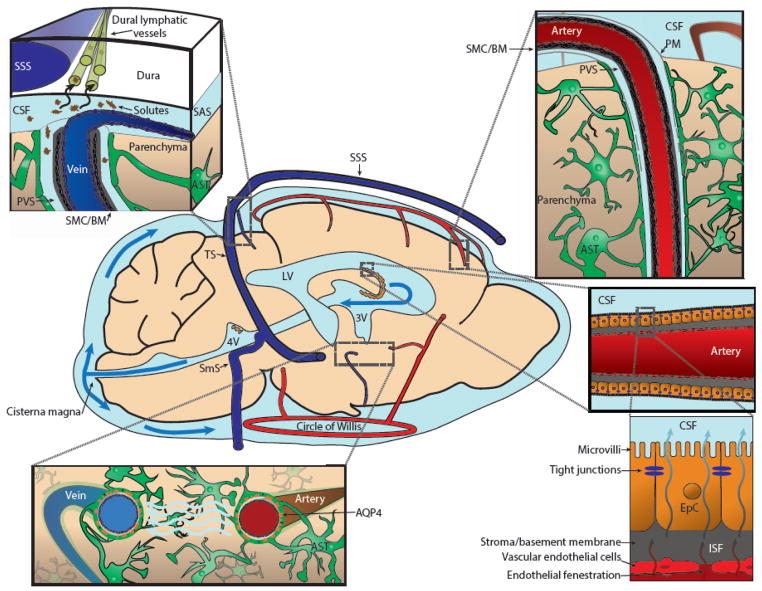
Figure 1. Schematic of CSF production and circulation in the brain.
Cerebrospinal fluid (CSF) is formed in the choroid plexuses in the 3rd (3V), 4th (4V) and lateral ventricles (LV, Bottom Right), then exits into the subarachnoid space (SAS) at the cisterna magna. Classically, CSF is cleared by bulk reabsorption into the bloodstream via arachnoid villi or by clearance to peripheral lymphatic vessels accessed along cranial nerve sheathes. More recent research suggests that from the SAS, a portion of the CSF circulates into the parenchyma of the brain along perivascular spaces (PVS) surrounding penetrating arteries, exchanging with surrounding brain interstitial fluid (ISF, Top Right). ISF and its associated solutes and wastes are in turn cleared along PVSs surrounding large caliber draining veins. This processes is supported by astroglial water transport through the aquaporin-4 (AQP4) water channel, which is localized to the perivascular astrocytic endfeet that surround the cerebral vasculature (Bottom Left). Interstitial solutes are cleared along peri-venous spaces into cisternal CSF spaces, where they have access to sinus-associated lymphatic vessels (Top Left). Abbreviations: Astrocyte (AST), basement membrane (BM), epithelial cells (EpC), pia mater (PM), smooth muscle cell (VSM), sigmoid sinus (SmS), superior saggital sinus (SSS), transverse sinus (TS). Adapted from [129].
In 1960, De Rougemont et al. demonstrated that the electrolyte content of the CSF extracted at the CP was distinct from that of blood plasma, physiologically linking the CPs to CSF production [22]. The secretion and composition of the CSF is tightly regulated by the CPs, which are complex structures comprised of a plexus of fenestrated capillaries surrounded by a layer of cuboidal epithelial cells, with an intervening stromal space between these two components (Fig. 1D). The epithelial cells are polarized, with the apical CSF-facing side possessing microvilli and tight junctions that constitute the blood-CSF barrier (BCSFB), which is in many respects analogous in function to the BBB of the cerebrovascular endothelium [23].
Under physiological conditions, CSF is actively secreted, largely independently of choroidal blood flow, through the concerted activity of numerous membrane proteins. These include apical Na+/K+ ATPase, the aquaporin-1 (AQP1) water channel, and numerous secondary ion transporters and channels localized specifically to basolateral and/or apical membranes that facilitate ionic flux comprised largely of Na+, HCO3− and Cl− into the ventricular lumen [24–26]. The respective contributions of these transporters and channels to CSF secretion are complex and recently have been comprehensively reviewed [15]. The net result of this secretory process is that CSF is produced with stable ionic composition that is distinct from that of plasma.
The CP also utilizes systems of transporter proteins to coordinate both the entry and efflux of specific solutes essential to normal function of neuronal and glial cell types into and from the CNS [27]. One such example of nutrient delivery is folate transport. Folate receptors (FRα) are localized to the membrane of the CP and package reduced folates (MeTHF) from the blood into endosomes where they are acidified. The MeTHF is then ferried out of the endosome by a proton-coupled folate transporter (SLC46A1), and is ultimately delivered into the CSF via facilitated diffusion mediated by the reduced folate carrier (RFC) [28, 29].
In contrast, CP can also mediate solute clearance from the CSF, as occurs with the removal of L-Glutamate, an excitatory neurotransmitter associated with neuroexcitatory conditions such as epilepsy. Excitatory amino acid transporter 3 (EAAT3) is localized to the apical (CSF) side of the CP and facilitates transport into the CP [30].
CSF Circulation and Reabsorption
Due to the nature of CSF circulation, which occurs dynamically across large anatomical distances, the majority of contemporary research on the topic utilizes phase-contrast magnetic resonance imaging (PC-MRI). These approaches, however, are limited by the scales of CSF movement that occur. CSF flow is comparatively slow and generally not observed across many voxels during any given measurement. Additionally, the space that CSF travels through is typically very small in any given plane and therefore total available voxels for detection are limited. Despite these limitations, PC-MRI has provided key insights into the basic principles of CSF flow. Earlier approaches evaluating rates of CSF production, flow and reabsorption including tracer infusion and CP lesion studies also often produced conflicting or controversial results [3]. Here we will attempt to summarize the current leading perspectives in the field.
As detailed above, CSF is largely generated by the CPs. The exact rate of CSF formation varies with neuroendocrine and hormonal modulation and also across species. In humans, the production rate lies between 0.3–0.6 ml/min, turning over a total CSF volume of 150 ml 4 times per day. In mice, CSF is produced at a rate of ~350 nl/min, turning over a total CSF volume of 40 μl 12–13 times per day [16, 31].
PC-MRI studies indicate that through the course of each cardiac or respiratory cycle, bidirectional CSF flow through narrow segments of the ventricular system such as the cerebral aqueduct may occur. Net flow of CSF however, originates in the ventricles (primarily the third and lateral ventricles) and travels through the cerebral aqueduct, to the 4th ventricle where it exits into the SAS through the foramina of Luschka and Magendie (Fig. 1C) [32]. Conventional understanding dictates that a portion of the subarachoid CSF will continue through the SAS along the spinal cord, exiting along spinal nerve roots, while the remainder will exit the cranium via arachnoid villi and the cranial nerves [15]. As detailed below, the recently described lymphatic vessels associated with the dural sinuses may also be involved in CSF reabsorption (Fig. 1A) [33, 34].
Reabsorption of the CP-derived CSF circulating through the ventricles and SASs into the peripheral lymphatic system or blood stream is critical to the clearance of metabolites and waste products. CSF bulk flow and reabsorption are driven by pulsations within the cranium, but are supported by CSF pressure maintained by equalization of the rates of CSF secretion and reabsorption. Due to enclosure within the cranium, changes in CSF pressure can occur rapidly when the rates of CSF secretion and reabsorption are not balanced, leading to conditions such as hydrocephalus [15, 16]. The pulsations driving CSF from its origin at the CPs to the efflux pathways from the cranium are generated by both the respiratory and cardiac cycles. Utilizing PC-MRI Feinberg and Mark illustrated that CSF velocity at the cerebral aqueduct varies across the cardiac cycle and has peak velocities phase-locked to cardiac systole [35]. Recent studies using PC-MRI have also implicated respiration in the bulk flow of CSF as changes in CSF movement through the ventricular system varied with the respiratory cycle and could be manipulated by alterations in the respiratory patterns of study participants [32, 36]. Interestingly, the magnitude of respiration-associated changes in CSF velocity were substantially greater than those occurring in phase with the cardiac cycle. In addition, other long term factors may modulate pulsatile CSF flow in the cranium, which will be discussed in subsequent sections.
CSF Communication with Brain Extracellular Fluid
Classical CSF studies demonstrate that the CSF serves as a sink to interstitial solutes, as tracers injected into different brain regions drain to different CSF compartments [37]. Two modes of movement, diffusion and bulk flow (or convection), could potentially account for the movement of solutes within the brain extracellular space and their clearance either to internal or external CSF compartments. Diffusion results from the thermal motion of a molecule down its concentration gradient. The speed of diffusion is strongly influenced by the mass of the solute, with large macromolecules moving only slowly through the brain extracellular space [38]. Bulk flow occurs on a larger scale and refers to movement of fluid in which all solutes move at the same rate, regardless of size [20, 39].
A series of seminal studies by Cserr and colleagues demonstrated that interstitial solutes are cleared from the brain by bulk flow and that ISF and CSF exchange along perivascular spaces surrounding cerebral blood vessels. In a study carried out in the rat caudate nucleus, Cserr et al. reported that inert extracellular tracers with masses spanning 2 orders of magnitude (900 Da – 69,000 Da) were eliminated from the brain at the same rate, suggesting that clearance occurred by bulk flow rather than diffusion [40]. These findings were confirmed in a later pharmacokinetic study by Groothuis et al. [41], and more recently in a radio-tracer study in mice using mannitol (182 Da) and dextran (10 kD) [7]. Cserr and colleagues estimated the rate of brain ISF flow to be 0.11–0.29 μl*g−1*min−1, a value in line with the rate of lymph flow throughout the peripheral organs [37].
Bulk flow within the brain parenchyma has repeatedly been attributed to specific anatomical domains, as opposed to being a feature of the wider extracellular compartment. Rosenberg et al. [42] reported that bulk flow within the brain extracellular space occurred primarily along white matter tracks, however no difference was observed by Cserr and colleagues when comparing efflux kinetics between the caudate nucleus and internal capsule [37]. In this and subsequent studies, these investigators observed that much of the tracer injected into the brain parenchyma could be detected in perivascular spaces surrounding cerebral blood vessels [37, 43], leading them to propose ‘the subarachnoid perivascular space is part of a large, interconnecting network of intracranial extracellular channels’, facilitating efflux of interstitial solutes and exchange with the surrounding CSF compartments [43]. The ensheathment of leptomeningeal blood vessels perivascular ‘sleeves’ comprised of the pia mater described by Weller and colleagues [44] is believed to provide the anatomical basis for the free movement of fluid and solutes along these cerebral blood vessels. In their extensive pharmacokinetic study, Groothuis et al. reported that for molecules exhibiting uptake across the blood brain barrier, efflux from the parenchyma was dependent upon local diffusion, while other solutes appeared to be cleared from the brain interstitium by a combination of bulk flow and diffusion [41]. This is in line with the notion that efflux of interstitial solutes results from the combined influence of diffusion within the dimensions of the brain extracellular space coupled to efflux via bulk flow along ‘privileged’ long-distance pathways such as white matter tracks and perivascular spaces [3].
A related and widely accepted hypothesis articulated by Weller, Carare and colleagues proposes that ISF and solutes are cleared along these perivascular pathways, moving first along the capillary basal lamina, then vascular smooth muscle basement membrane, then traveling through perivascular spaces to reach the cervical lymphatics along the internal carotid arteries [45]. This hypothesis is supported by parenchymal tracer injection experiments in which signal was detected along capillaries and within the walls of cortical arteries. This group has proposed that in cerebral amyloid angiopathy (CAA), amyloid β acts as a sort of endogenous tracer and that the deposition of amyloid β within the walls of cerebral arteries in this condition reflects aggregation within the brain’s clearance pathway for interstitial solutes. [46]. However, whether the patterns of tracer accumulation observed in these experimental studies, and whether patterns of vascular amyloid β aggregation observed in human CAA cases reflect pathways of endogenous interstitial solute clearance remains to be determined.
Rapid Perivascular Transport and the Glymphatic System
A series of studies from Grady and colleagues reported that CSF from the subarachnoid space rapidly entered the brain along perivascular spaces surrounding penetrating arteries [47, 48]. In these studies, horseradish peroxidase (a 44 kD protein) injected into the cat and dog ventricular and subarachnoid CSF compartments moved rapidly (within minutes) into the cortex along perivascular spaces surrounding penetrating arteries. These findings are in conflict with subsequent studies by Cserr and colleagues that found little evidence for bulk flow of CSF tracers into the brain under control conditions after injection into the subarachnoid space or into perivascular spaces of leptomeningeal arteries [43, 49]. While Cserr and colleagues proposed that ISF exchanged slowly with CSF by bulk flow through the perivascular network, Grady and colleagues proposed that CSF-ISF exchange was rapid and polarized along the vasculature, with CSF entering the brain along perivascular spaces surrounding arteries and ISF exiting the brain along perivascular spaces surrounding veins.
A series of recent studies, utilizing novel in vivo approaches that permit the dynamic imaging of CSF tracers, have brought new perspectives to these questions. Studies employing dynamic in vivo 2-photon imaging after injection of fluorescent CSF tracers in mice indicates that subarachnoid CSF from the cisternal compartments enters and travels along perivascular spaces surrounding cerebral arteries, gaining access to the brain parenchyma and exchanging with the surrounding ISF (Fig. 1B, E) [6, 7, 50, 51]. This finding has been confirmed in rats and by visualizing brain-wide CSF contrast movement along perivascular pathways by dynamic contrast-enhanced MRI (DCE-MRI) [9, 52–54]. Further studies demonstrate that macromolecules introduced across the nasal mucosa circulate along these perivascular pathways and gain access to the brain parenchyma [55]. Using these dynamic and depth-resolved imaging techniques, many of the questions surrounding timing, rates and direction of perivascular flow that were left unresolved by earlier investigations in fixed or frozen tissue can now be defined.
Using intra-parenchymal injections of inert tracer molecules such as fluorescent dextrans, or proteins including amyloid β or tau, it was observed that ISF and interstitial solutes are cleared from the brain along distinct anatomical pathways, following subcortical white matter tracks and perivascular spaces surrounding large-caliber draining veins and emerging into cisternal CSF compartments associated with dural sinuses, including the quadrigeminal and basal cisterns (Fig. 1A) [5, 7].
The anatomical pathway permitting communication of cisternal CSF with CSF flowing along perivascular spaces is not clear. One possibility is that subarachnoid CSF enters perivascular spaces proximally, through fenestrations of the ensheathing pia mater within the cisterns containing the circle of Willis. CSF could then be conducted distally through these perivascular spaces to enter the brain parenchyma along penetrating arteries. As of yet, no ultrastructural description of the leptomeningeal investment of the communicating arteries comprising the Circle of Willis or the proximal segments of the conduit arteries that branch off of them has been conducted, making it difficult to definitively identify the mode of exchange between subarachnoid CSF of the cisterns and CSF within perivascular spaces surrounding cerebral arteries. As discussed below, interstitial solute clearance along perivascular spaces surrounding large caliber draining veins appears to provide a route for the delivery of interstitial solutes from within the brain parenchyma to the putative lymphatic vessels that have been recently described in association with dural sinuses (Fig. 1A) [33, 34]. Thus, although the precise anatomical bases have not yet been fully defined, it appears that perivascular spaces surrounding both cerebral arteries and large caliber draining veins provide pathways for the bidirectional exchange of CSF from the cisternal compartments with the ISF throughout the brain parenchyma.
Although the findings reported most recently by Nedergaard and colleagues are in many ways simply an elaboration of earlier tracer studies defining exchange of CSF and ISF along perivascular pathways [7, 37, 43, 47, 48, 50, 52], these studies have provided additional details concerning the role that glial cells including astrocytes play in perivascular CSF-ISF exchange. Moreover, they provide strong evidence for the physiological regulation of the dynamics of perivascular fluid exchange that may offer key insight into the interrelationship between CSF-ISF dynamics, physiological brain function, and the development of neurological disease.
One key finding from these studies was the observation that the astroglial water channel aquaporin-4 (AQP4), which is localized primarily to perivascular astrocytic endfeet ensheathing the cerebral vasculature, supports both perivascular influx of CSF into and through the brain interstitium as well as the clearance of interstitial solutes from the brain parenchyma [7]. Owing the dependence of perivascular CSF-ISF exchange upon glial water transport and its assumption of the conventional peripheral lymphatic function of interstitial solute clearance, Nedergaard and colleagues termed this brain-wide perivascular network the ‘glymphatic’ system [56].
In a follow-up study, Xie et al. reported that glymphatic pathway function was a feature of the sleeping, rather than the waking brain [51]. Compared to both naturally sleeping and anesthetized mice, the rate of perivascular CSF tracer influx into the waking cortex after infusion into the cisterna magna was reduced by ~95%. In parallel experiments, the authors reported that both the clearance of the inert radio-tracer 14C-inulin and radio-labeled 125I-amyloid β were reduced by approximately one half in the waking compared to the sleeping or anesthetized brain. These data are in agreement with the findings of Groothuis et al. who reported that the clearance of the inert tracer 14C-Sucrose from the brain interstitium was markedly slowed in awake behaving rats compared to those under ketamine-xylazine anesthesia [41]. This study may shed light on the discrepancies between experimental work carried out by Cserr and colleagues and more recent work focusing on the so-called ‘glymphatic’ system. In their study, Groothuis reported similar efflux kinetics under pentobarbital anesthesia to those observed in the studies by Cserr and colleagues. However compared to pentobarbital anesthesia, tracer efflux was more than 5–10 times more rapid under other anesthesia regimes or in the awake-behaving brain [41]. Here it is important to note that in the recent studies focusing on the glymphatic system, animals under ketamine-xylazine or isofluorane anesthesia exhibited perivascular CSF movement and ISF solute clearance that were nearly identical to those observed in the naturally sleeping brain [51]. While this has not yet been evaluated experimentally, methodological difference including the influence of anesthesia may underlie observed differences in the direction and rate of perivascular fluid movement in the CNS.
Underlying these changes in perivascular CSF-ISF exchange in interstitial solute clearance appears to be sleep-wake changes in the dimensions of the brain extracellular spaces. From in vivo electrophysiological recordings collected in mouse cortex using the gold-standard tetramethylammonium (TMA) micro-iontophoresis method [38], Xie et al. reported that during sleep, the brain extracellular space increases in volume by ~60% [51]. This effect, as well as the effect of arousal on perivascular CSF-ISF exchange and interstitial solute clearance, appeared to be in part regulated by cortical noradrenergic tone, as local pharmacological blockade of noradrenergic receptors restored waking extracellular volume fraction, perivascular CSF influx and interstitial solute clearance to levels observed during natural sleep or anesthesia. These findings suggest that the processes of CSF circulation, including its exchange with the interstitial compartment along perivascular pathways, may be under dynamic physiological regulation. In agreeance with this hypothesis, a recent pharmacokinetic study reported that in the rat, CSF turnover is more rapid during resting compared to the active portion of the diurnal cycle [57].
AQP4 and Other Transporters
The most well-known family of water transporters are the aquaporins (AQPs). Originally discovered in red blood cells, at least 10 different AQPs are involved in water transport in tissues including the renal ducts (AQP2 & AQP6), the lens of the eye (AQP1), and the CPs (AQP1) [25, 58–60]. Identified in 1994, AQP4 is exclusively expressed within the brain in ependymal cells and astrocytes [61, 62]. Astroglial AQP4 in the perivascular endfeet abutting the cerebral vasculature is organized into dense crystalline plaques that are visible as square arrays on freeze-fracture transmission electron microscopy that occupy as much as 50% of surface area facing the cerebral microvasculature [63, 64]. AQP4 is anchored within these endfeet domains by its association with extracellular proteins of the subendothelial basal lamina [65]. Although many studies have investigated the role of AQP4 in the movement of water into and out of the CNS under pathological conditions such as cerebral edema, little has been understood regarding the physiological significance of AQP4’s perivascular localization [66].
With the observation that CSF and ISF exchange along perivascular pathways that are bounded by astroglial endfeet exhibiting high levels of AQP4 localization, Iliff and colleagues proposed that perivascular AQP4 organizes water transport through the brain along the axes of the cerebral vasculature, permitting the rapid transport of water into and out of these perivascular pathways [7]. Because approximately one-tenth of astroglial AQP4 is localized to the vast network of peri-synaptic processes, water that transits AQP4 at perivascular endfeet is readily distributed to the wider interstitium, just as water from the bulk interstitium can readily enter the perivascular spaces across these endfeet [67]. Based on this concept, Iliff, Nedergaard and colleagues have proposed that the movement of interstitial solutes through the brain parenchyma, including those that are not taken up by astrocytes, is facilitated through this astrocyte-mediated communication of perivascular and bulk interstitial water compartments [7].
One corollary to this proposal is that the loss of perivascular AQP4 localization should have a similar effect to that of the loss of global AQP4 expression. Loss of perivascular AQP4 localization is a phenotype that has previously been identified in several neurological disorders including epilepsy [68], AD [69], and TBI [5, 70]. As will be detailed below, loss of perivascular AQP4 localization in both the aging mouse brain and the young mouse brain after TBI were both associated with impairment of perivascular CSF-ISF exchange and the clearance of interstitial solutes [5, 6].
The mechanism underlying AQP4 mis-localization in the aging and diseased brain is currently an area of active research, but is not yet well understood. In most mammals two isoforms of APQ4, the products of distinct AQP4 transcripts, are primarily expressed. The longer isoform M1, contains an extended N-terminus tail and is unable to form the arrays found at the perivascular astrocyte endfeet, and instead flows freely though the plasma membrane [71]. The shorter M23 isoform forms the square arrays found at the endfeet and is largely immobile within the cell membrane [72–74]. It is possible that glial injury results in a change in AQP4 expression, from the M23 to the M1 isoform, leading to changes in perivascular AQP4 localization. Alternatively, BBB disruption associated with brain injury may disrupt interactions between AQP4 and protein components of the basal lamina, such as agrin, disrupting the associations that anchor AQP4 at the perivascular endfoot [67]. Neither of these hypotheses, however, have yet been directly evaluated.
In addition to AQP4, other solute transporters and channels are known to mediate significant water fluxes in the brain. For example, a number of co-transporters move a large number of water molecules with each catalytic cycle. Transporters of this type that are found in astrocytes include EAAT, NKCC1, KCC4, and GLUT1 [60]. In addition to playing key roles in the secretion of CSF at the CP, these transporters may also participate in the movement of water between glial cells and the surrounding interstitial and perivascular compartment [15].
A significant criticism of the glymphatic hypothesis centers around the proposed role of AQP4 in perivascular fluid movement and interstitial solute clearance. These were articulated by by Verkman and colleagues in reply to a perspective piece by Thrane et al. on the potential role of the glymphatic system in the formation and resolution of cerebral edema [75–77]. In their letter, Verkman and colleagues question how hydrostatic pressure can drive water movement through AQP4 channels in perivascular endfeet given the small amplitude of arterial wall pulsations within the perivascular spaces. They further question how, given the permeability of AQP4 for water, but not solutes, uptake of water across the perivascular astocytic endfeet can facilitate the movement of solutes into or out of the perivascular spaces [76].
As Thrane and colleagues point out in their reply, hydrostatic pressure originating from the pulsation of the arterial wall is likely only one of many factors contributing to perivascular fluid movement [77]. Indeed, when cerebral arterial pulsation was reduced surgically by internal carotid artery ligation, perivascular fluid movement was reduced only a small amount [50], compared to the reduction observed in the Aqp4 null mouse [7] or in wild type mice in the waking state [51]. In addition, it is also important to consider the fact that sources of hydrostatic pressure other than local perivascular dilation that would be influenced by arterial ligation may be present. During each cardiac cycle, a pulse wave propagates through the brain vasculature. As systolic and diastolic elements are conducted through the different cisternal compartments, pressure gradients within discrete CSF compartments are produced. Some of these are transient, appearing and dissipating with each cardiac cycle, while others comprise standing hydrostatic pressure gradients between CSF compartments. For example, phase-contrast MRI shows that with each cardiac cycle, a pressure gradient originates in the cisterna magna, propagating to the prepontine, interpeduncular and suprasellar cisterns. This pressure gradient diminishes toward the detection limit over the cerebral convexity, but is maintained along the middle cerebral artery [78]. These global pressure gradients clearly drive bulk CSF flow through larger CSF spaces, and may drive CSF flow through communicating distal perivascular spaces. Thus, one limitation of evaluating the biophysics of perivascular fluid movement and AQP4 only on the microscopic scale is that global drivers of fluid dynamics are not accounted for. A similar point is made by Thrane and colleagues, where they cite global factors such as the direct current (DC) between CSF and ISF and the effect of posture on tissue fluid dynamics [54, 77].
Verkman and colleagues also raise the issue of the ‘salt accumulation problem’, in which water flow into the astrocyte endfoot would result in increased osmolyte concentration in the perivascular space and corresponding dilution of osmolytes in the cell. Here it is important to note that while AQP4 is enriched 10:1 in perivascular endfoot domains compared to other processes, the surface area of these other fine processes are many times greater than that of the perivascular domains [67, 79]. Thus, water entering astrocytes at perivascular endfeet is free to diffuse out of astrocytes into the wider extracellular space through this large exchange surface, preventing the accumulation of osmotic gradients within the astrocytes.
Relationship of CSF Dynamics to Neurological Disease
Alzheimer’s Disease
AD, the most common cause of dementia, is a neurodegenerative disease characterized histopathologically by the formation of extracellular senile plaques composed primarily of amyloid β and intracellular neurofibrillary tangles (NFTs) comprised of hyperphosphorylated tau. According to the dominant ‘Amyloid Cascade Hypothesis of AD’, increasing levels of interstitial amyloid β species impair synaptic function, promoting tau aggregation and NFT formation and resulting in neurodegeneration [80, 81]. AD pathology is associated with pronounced neuroinflammation, including reactive astrogliosis and microgliosis [82]. CSF dynamics have also been found to change in the aging brain [83, 84]. While both AD-associated neuroinflammation and changes in CSF dynamics have been regarded as distinct contributors to the development of AD within the aging brain, recent studies suggest that these events may interact with one another during the onset of AD.
Amyloid β is formed from the amyloid protein precursor (APP) following successive cleavages by β- and γ-secretases to form the pro-fibrillary amyloid β1–42 that is a key component of senile plaques and the more soluble amyloid β1–40 that deposits in the walls of leptomeningeal and cerebral arteries in the condition cerebral amyloid angiopathy (CAA) [85]. Amyloid β is produced in the brain during synaptic activity, and in the healthy young brain is rapidly eliminated by cellular uptake into microglia and astrocytes, efflux across the BBB via solute transporters including P-glycoprotein (PGP) and low density lipoprotein receptor-related protein 1 (LRP-1), and clearance along the perivascular glymphatic pathway [6, 7, 51, 86–88]. During waking hours, when glymphatic pathway function and amyloid β clearance are markedly reduced, interstitial and CSF levels of amyloid β increase in mice and humans respectively [51, 89]. Then with the onset of sleep, when perivascular CSF-ISF exchange is active and interstitial amyloid β clearance is more rapid, interstitial amyloid β levels decline. These findings indicate that perivascular CSF-ISF exchange along the glymphatic pathway is a key contributor to the physiological clearance of amyloid β from the brain.
In the aging brain, BBB amyloid β efflux pathways are impaired, including the down-regulation of PGP and LRP-1, resulting in slowed interstitial amyloid β clearance that may contribute to amyloid β aggregation and subsequent neurodegeneration [90]. Amyloid β efflux transporter expression within the CP, however, increases in the aging brain, suggesting a potential compensatory role for the CP and BCSFB in the elimination of amyloid β from the ventricular CSF and associated ISF [91]. CSF secretion by the CPs slows with age and with the onset of AD and the deposition of amyloid β within the CP [92, 93], reducing the rate of CSF turnover and the efficacy of amyloid β clearance with the bulk reabsorption of CSF via arachnoid villi and along cranial nerve sheaths.
Impairment of glymphatic pathway function, including perivascular CSF-ISF exchange and the clearance of interstitial amyloid β, is similarly a feature of the aging brain [6]. Whether slowing of perivascular CSF flux through the aging brain is in part the result of reduced CSF secretion by the CP is unclear. When CSF secretion was experimentally impaired by the administration of the carbonic anhydrase inhibitor acetazolamide, glymphatic pathway function was slowed, suggesting that reduced CSF secretion at the CP may contribute to impaired glymphatic pathway function in the aging brain [94]. However, perivascular AQP4 localization was also markedly reduced in the aging brain and was significantly associated with slowing of perivascular CSF influx into and through the brain parenchyma [6]. Whether loss of perivascular AQP4 localization promotes amyloid β aggregation in the aging brain has not yet been formally evaluated. Yet the ability of amyloid β deposits, either in the form of senile plaques or in the form of CAA, to cause mis-localization of AQP4 suggests the presence of a feed-forward pathogenic cycle, with reactive astrogliosis impairing glymphatic pathway function and amyloid β clearance, promoting amyloid β deposition, which in turn causes further neuroinflammation and glymphatic pathway dysfunction.
NFTs are a second histopathological feature associated with AD. Tau is a microtubule associated protein that in healthy cells is involved in stabilization of microtubules and is localized to neuronal axons. Under physiological conditions, phosphorylation is important for modulation of tau binding to microtubules and tau is the target of several kinases, including cdk5, PKA, and CamKII among others [95]. NFTs occur when tau becomes abnormally phosphorylated, dissociates from microtubules, relocates to the somato-dendritic compartments of the cell, and forms tangles. Whether aging or disease-related changes in CSF circulation or glymphatic pathway function promote NFT formation in AD remains speculative. Recent studies utilizing in vivo microdialysis in mice suggests that tau is released into the interstitium of the healthy young brain during synaptic activity [96]. Other recent studies suggest that tau aggregates can pass from cell to cell through the extracellular compartment, seeding aggregate formation in neighboring cells in a ‘prion-like’ manner [97]. These findings provide a powerful mechanistic explanation for the classical neuroanatomical spread of neurodegenerative diseases characterized by the mis-accumulation of protein aggregates, including AD. Moreover, they suggest the intriguing possibility that age- and disease-associated changes in processes that determine the dynamics of macromolecular movement within and clearance from the brain interstitium, including CSF circulation and glymphatic pathway function, may be intimately involved in the spread of protein aggregates along neuroanatomical pathways.
Another clinical condition frequently associated with AD that may be affected by changes in CSF circulation is CAA. As described above, the widely accepted model of CAA pathogenesis proposed by Weller and Carare posits that under physiological conditions amyloid β1–40 is cleared along perivascular spaces surrounding penetrating and leptomeningeal arteries in a direction opposite to that of blood flow, while the impairment of amyloid β1–40 clearance along these routes leads to the deposition of amyloid β1–40 within the walls of cerebral arteries in CAA [45, 46, 98, 99]. In this model, the direction of flow in the perivascular space is in opposition to the perivascular CSF influx detected with dynamic imaging approaches by Nedergaard and colleagues, and others [5–7, 9, 50–52, 54, 55, 100]. One possible explanation for these discrepancies is that fluid and solutes move both outward and inward along different glial-vascular elements within the perivascular space, with amyloid β efflux occurring along the cerebral arterial basement membrane and subarachnoid CSF influx occurring through a space immediately bounded by astrocyte endfeet (Figure 1B). A second possibility is that fluid and solute movement can occur in both directions depending on physiological or pathophysiological context, and that these differences are revealed under the different experimental conditions in these studies. Currently, the basis of these discrepancies remains unclear.
The recent observations of Iliff, Nedergaard and colleagues showing CSF tracer circulation into the brain along penetrating cerebral arteries suggest that amyloid β-laden CSF from the subarachnoid space may in fact be re-circulating into and through the brain along perivascular pathways. Under physiological conditions, with intact BBB amyloid β efflux mechanisms [101], this recirculating amyloid β would be readily eliminated. However, in the aging or injured brain in which endogenous mechanisms of BBB amyloid β efflux are impaired, recirculation along perivascular spaces may promote amyloid β deposition within the arterial wall. This model may explain the pattern of amyloid β deposition seen in post mortem studies, including the finding that amyloid β deposition is greatest in pial arteries and decreases in deeper layers of the cortex.
Neurovascular Disease: Stroke, Traumatic Brain Injury and Subarachnoid Hemorrhage
Brain injury, including ischemic and traumatic injury (TBI), are two key contributors to human death and disability and are associated with substantial disruption of CSF circulation, which in turn, may promote secondary injury and impede recovery of surviving tissue. The CP may be directly damaged by either ischemic or traumatic injury, leading to the breakdown of the BCSFB and the accumulation of fluid within the cranium. Compounding these effects, debris mobilized within the CSF from the ischemic or traumatic lesion may interrupt CSF flow and block CSF reabsorption sites, leading to a dangerous increase in intracranial pressure that can result in further tissue ischemia and ultimately brain herniation [102, 103]. Breakdown of the BCSFB following ischemic or traumatic brain injury may also increase its permeability to leukocyte trafficking, promoting immune cell infiltration into the injured brain [104].
Beyond the acute and subacute effects of ischemic or traumatic brain injury, these conditions are also associated with the development of dementia, including vascular dementia and Alzheimer’s disease, in the years following injury [105, 106]. In addition to regional ischemic infarcts, small ischemic lesions, including microinfarcts and lacunar infarcts, are key contributors to the development of vascular dementia [107]. Similarly, although moderate-to-severe TBI is most widely reported to be associated with an increase in AD risk, exposure to even a single episode of mild TBI (or concussion) may be sufficient to confer vulnerability to the development of early onset dementia [108–110]. Recently published studies suggest that the association between TBI, including mild TBI, and ischemic injury, including relatively small microvascular lesions, may exert widespread and long-lasting effects upon glymphatic pathway function, rendering the injured brain vulnerable to protein mis-aggregation and neurodegeneration.
In a mouse model of moderate-to-severe TBI, perivascular AQP4 localization was lost in reactive astrocytes throughout the cortex for at least 28 days post-injury [70]. In the same model, a subsequent study reported that impairment of perivascular AQP4 localization after TBI is associated with persistently impaired glymphatic function, slowing perivascular CSF influx into the brain and the clearance of ISF solutes from the brain [5]. Importantly, when glymphatic pathway function was reduced by genetic deletion of the Aqp4 gene, levels of phosphorylated tau in the post-traumatic cortex increased and neurocognitive function worsened 28 days post-injury, suggesting that chronic impairment of glymphatic function after TBI may promote protein mis-aggregation and neurodegeneration in the post-traumatic brain. Although glymphatic function has not yet been evaluated in an experimental model of mild TBI, perivascular AQP4 localization was lost to a similar extent after mild TBI as for moderate-to-severe TBI, suggesting that similar processes may be at work [70].
Similar results are observed in experimental models of cerebral ischemia. In a mouse model of focal cerebral ischemia, the influx of intracisternally-injected MRI contrast agent was evaluated. During the acute ischemic period, glymphatic pathway function was impaired in the ipsilateral cortex, while 24 hours after ischemia after re-canalization of the occluded artery had occurred, CSF tracer influx returned to baseline values [9]. In another mouse model of transient cerebral ischemia, Badaut and colleagues reported that AQP4 expression was increased while perivascular AQP4 localization was reduced 48 hours after ischemic injury [111, 112]. In a third model of diffuse cerebral microinfarcts, Wang et al. reported that microscopic vascular lesions were associated with wide fields of reactive astrogliosis, with persistently reactive astrocytes exhibiting reduced perivascular AQP4 localization for up to 28 days after injury [113]. These findings suggest that in the chronic phase after ischemic injury, persistent loss of perivascular AQP4 localization in reactive astrocytes may impair glymphatic pathway function and the associated clearance of harmful interstitial solutes such as amyloid β, promoting neurodegeneration in the post-ischemic brain.
Traumatic and ischemic brain injury share many common pathological features, such as BBB disruption, cerebral edema, and the development of neuroinflammation including the induction of reactive astrogliosis. As observed in the aging and AD brain, loss of perivascular AQP4 localization appears to be a general feature of reactive astrocytes in the post-traumatic and post-ischemic brain [70, 114, 115]. Based upon these findings, Iliff, Nedergaard and colleagues have proposed that persistent reactive astrogliosis in the injured brain chronically impairs glymphatic pathway function, promoting protein mis-aggregation and neurodegeneration, and perhaps providing the basis for the association between ischemic and traumatic brain injury and the development of dementia later in life [116].
In the setting of subarachnoid hemorrhage (SAH), blood entering the subarachnoid CSF compartment causes a direct increase in ICP. Changes in CSF circulation, including the blockage of CSF efflux routes such as the arachnoid villi by fibrin clots and other blood components contribute to the occurrence of hydrocephalus after SAH [117]. Studies in experimental animal models of SAH in monkeys, cats, and mice suggest that intrathecal anticoagulation or fibrinolysis can restore CSF outflow resistance to normal levels and restore CSF flow through the subarachnoid space [10, 118, 119]. In two studies carried out in mice, perivascular CSF influx into the brain was reduced after induction of experimental subarachnoid hemorrhage. In both cases, glymphatic function was at least partially restored with the intrathecal administration of the fibrinolytic tissue-type plasminogen activator (tPA) [9, 10]. Siler et al. reported that improvement of perivascular CSF circulation with intrathecal tPA was associated with both reduced ICP and improved cortical blood flow 24 hours after hemorrhage, suggesting that impairment of glymphatic function after SAH may contribute to secondary injury progression in this neurovascular condition.
Multiple Sclerosis
In addition to neurodegenerative diseases and brain injury, another class of neurological disorders afflicting the CNS closely tied to CSF function are neuroinflammatory disorders. The most common and extensively studied of these is multiple sclerosis (MS). MS is characterized by loss of oligodendrocyte myelination of CNS neurons thought to result from the autoimmune destruction of these cells.
A key determinants to CNS autoimmune disease progression is access of peripheral immune cells to their CNS targets. Within the cerebral vasculature, infiltration of peripheral immune cells is restricted by the BBB. Interestingly, in the rodent experimental autoimmune encephalomyelitis (EAE) model of MS, clinical indicators of disease progression are only observed when immune cells leave perivascular spaces surrounding post-capillary venules to reach the wider brain parenchyma, suggesting that both the glial limitans and the perivascular astroglial ensheathment of the cerebral vasculature function to restrict immune access to the CNS [120, 121]. In addition, CNS endothelial cells constitutively express CXCL12 which binds CXCR4 expressing T-cells, and prevents their exit from perivascular and leptomeningeal locations during immune surveillance [122, 123].
In the healthy brain, the BCSFB at the CP serves as an entry point of peripheral immune cells into the CSF. From here, peripheral immune cells appear to circulate through perivascular, leptomeningeal and ventricular CSF compartments, but do not penetrate into the brain parenchyma. From these locations, it is widely believed that these peripheral immune cells conduct immune surveillance of the brain [8]. In MS, the CP may provide the route utilized by auto-aggressive T-cells to gain access to the brain parenchyma. This process includes the breach of the BCSFB by T-Cells, permitting access to the ventricular CSF compartment. The mechanisms governing this process are still under investigation, however some evidence suggests that CCR6 T-helper cells mediate the crossing of the BCSFB via the CCL20 ligand, which is expressed by CP epithelial cells [124].
CSF biomarkers are important for the diagnosis of subsets of autoimmune disorders, a role that will likely increase with further research. The CNS demyelinating autoimmune disorder neuromyelitis optica (NMO), which was for a long time considered a variant of MS, is characterized by transverse myelitis and optic neuritis which are also seen in other autoimmune disorders, creating difficulty in distinguishing between this and other diseases. Recently it was discovered that patients with NMO test positive for AQP4 auto-antibodies while MS patients do not [125–127]. This distinction permits differentiation of these conditions from one another, and has turned attention towards the CSF for identification of other distinguishing biomarkers.
Discovery of AQP4-targeting auto-antibodies in NMO poses an interesting question regarding the role of CSF circulation in autoimmune disorders. If auto-antibodies target and inhibit AQP4 in astrocytes, then glymphatic flow through the parenchyma may also be disrupted. In addition, in an EAE model of MS, while antibodies targeting AQP4 have not been identified, a loss of perivascular AQP4 localization is observed [128]. Thus, impairment of AQP4 function, either by targeting by autoantibodies in NMO or by its mis-localization in MS, may lead to further consequences including accumulation of neurotoxic solutes in the parenchyma and progression of neurodegeneration.
Sinus-Associated Lymphatic Vessels
As detailed above, the brain has long been thought to lack a classical lymphatic vasculature, resulting in successive models, including most recently the ‘glymphatic’ system, to account for the exchange of ISF and CSF to facilitate the efflux of interstitial solutes from the brain. Two studies published recently may shed new light on these issues. Independent research groups recently reported in mice the presence of a network of putative lymphatic vessels associated with the dural sinuses throughout the cranium [33, 34]. Both studies observed that small vessels expressing lymphatic endothelial cell markers (including LYVE-1, VEGF3, and others) were found associated both with dural sinuses and certain dural arteries, exiting the cranium along large veins and arteries in the base of the skull. In the study conducted by Louveau et al., tracers infused in the ventricular CSF compartment were taken up into these sinus-associated vessels and cleared to the deep cervical lymph nodes. The study by Aspelund et al. reported that tracers injected into the brain interstitium were similarly cleared to these sinus-associated vessels, and thence to the deep cervical lymphatics. Although neither study quantified the proportion of CSF or ISF that is cleared along these apparently conventional lymphatic pathways, if these results are validated in other species, then these findings will suggest that at least a portion of ISF and CSF are cleared from the cranium along dural lymphatic vessels in addition to other previously identified clearance pathways including the arachnoid villi and peri-neural sheaths.
In their description of the glymphatic system, Iliff, Nedergaard and colleagues reported that while CSF enters the brain along perivascular spaces surrounding penetrating arteries, ISF was cleared from the brain along perivascular spaces surrounding large caliber draining veins that emptied into sinus-associated cisternal compartments, including the quadrigeminal and basal cisterns [7]. The recent description of sinus-associated lymphatic vessels appears to mesh well with these observations. Large caliber draining veins form the origin of the dural sinus structures; for example the internal cerebral veins merge to form the Great Vein of Galen, which in turn joins the inferior sagittal sinus to form the straight sinus. The drainage of interstitial solutes along these peri-venous routes conceivably provides either direct access to the most distal segments of these sinus-associated lymphatic structures or to the cisternal CSF compartments immediately associated with them (Fig. 1A). In this way, the recently described glymphatic pathway may represent the anatomical route for the movement of CSF into the interstitium and for the ISF to these lymphatic vessels. Dural lymphatic vessels in turn provide the anatomical route for these solutes out of the cranium.
Assuming that these vessels are found to be present in primates as well, the clinical implications of these recent findings may be great indeed. Sinus-associated lymphatic vessels, if involved in the clearance of interstitial solutes, may be disrupted in the setting of neurodegenerative, neurovascular or neuroinflammatory diseases. An intriguing possibility in the setting of MS is that dural lymphatic vessels may serve as an entry point for malfunctioning immune cells into the CSF. Both studies noted the presence of peripheral immune cells in the sinus-associated vessels, which may indicate a role for these vessels in immune-surveillance of the CNS or immune cell migration. Because it lies outside the BBB, sinus-associated vessels may prove to be an important therapeutic target for the treatment of these conditions. Clearly, further evaluation of these structures and their functional significance is warranted.
Conclusion
Diseases of the CNS, including neurodegenerative, neurovascular and neuroinflammatory conditions, have long been known to be associated with changes in the CSF circulation. Classically, these associations have tended to center around pathological changes in CSF secretion, caused by aging, amyloid β loading into the CP, or direct injury to the CP, or around impairment of CSF reabsorption such as occurs after ischemic or traumatic injury or in SAH. In the case of AD, slowing of CSF turnover is proposed to impair the clearance of amyloid β from the brain, promoting amyloid β plaque deposition. In the setting of brain injury, these effects are believed to promote secondary injury by promoting cerebral edema and elevated ICP. Recent studies describing the glymphatic pathway for the perivascular exchange of CSF and ISF, suggest that CSF circulation, and glial and vascular function are intimately associated and together facilitate the appropriate clearance of interstitial solutes from the brain parenchyma. Aging, brain injury and neuroinflammation, are each associated with persistent reactive gliosis, which may impair glymphatic pathway function, slowing the clearance of toxic metabolites from the brain interstitium and setting the stage for the development of neurodegeneration in the chronic phases of these conditions.
Highlights.
Current understanding of CSF production and circulation is described.
A recently proposed mechanism for CSF-mediated brain waste clearance is described.
The potential involvement of the glymphatic pathway in neurological disorders is examined.
A newly discovered cranial lymphatic system and its implications are discussed.
Acknowledgments
Work in the authors’ lab is supported by grants from the American Heart Association, the National Institutes of Health and the Paul G. Allen Family Foundation. The authors thank Douglas Zeppenfeld for helpful discussion of this review article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, Ye P, D’Ercole AJ, Wong ET, LaMantia AS, Walsh CA. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davson H. Physiology of the cerebrospinal fluid. Churchill; London: 1967. [Google Scholar]
- 5.Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, Plog BA, Ding F, Deane R, Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engelhardt B, Ransohoff RM. Capture, crawl, cross: the T cell code to breach the blood-brain barriers. Trends Immunol. 2012;33:579–589. doi: 10.1016/j.it.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Gaberel T, Gakuba C, Goulay R, Martinez De Lizarrondo S, Hanouz JL, Emery E, Touze E, Vivien D, Gauberti M. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke. 2014;45:3092–3096. doi: 10.1161/STROKEAHA.114.006617. [DOI] [PubMed] [Google Scholar]
- 10.Siler DA, Gonzalez JA, Wang RK, Cetas JS, Alkayed NJ. Intracisternal administration of tissue plasminogen activator improves cerebrospinal fluid flow and cortical perfusion after subarachnoid hemorrhage in mice. Transl Stroke Res. 2014;5:227–237. doi: 10.1007/s12975-014-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oi S. Classification of hydrocephalus: critical analysis of classification categories and advantages of “Multi-categorical Hydrocephalus Classification” (Mc HC) Childs Nerv Syst. 2011;27:1523–1533. doi: 10.1007/s00381-011-1542-6. [DOI] [PubMed] [Google Scholar]
- 12.Sheldon CA, Kwon YJ, Liu GT, McCormack SE. An integrated mechanism of pediatric pseudotumor cerebri syndrome: evidence of bioenergetic and hormonal regulation of cerebrospinal fluid dynamics. Pediatr Res. 2015;77:282–289. doi: 10.1038/pr.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyers SP, Khademian ZP, Chuang SH, Pollack IF, Korones DN, Zimmerman RA. Choroid plexus carcinomas in children: MRI features and patient outcomes. Neuroradiology. 2004;46:770–780. doi: 10.1007/s00234-004-1238-7. [DOI] [PubMed] [Google Scholar]
- 14.Wolburg H, Paulus W. Choroid plexus: biology and pathology. Acta Neuropathol. 2010;119:75–88. doi: 10.1007/s00401-009-0627-8. [DOI] [PubMed] [Google Scholar]
- 15.Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev. 2013;93:1847–1892. doi: 10.1152/physrev.00004.2013. [DOI] [PubMed] [Google Scholar]
- 16.Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davson H, Segal MB. Physiology of the CSF and blood-brain barriers. CRC Press; Boca Raton: 1996. [Google Scholar]
- 18.Dandy WE. Extirpation of the Choroid Plexus of the Lateral Ventricles in Communicating Hydrocephalus. Ann Surg. 1918;68:569–579. doi: 10.1097/00000658-191812000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollay M, Curl F. Secretion of cerebrospinal fluid by the ventricular ependyma of the rabbit. Am J Physiol. 1967;213:1031–1038. doi: 10.1152/ajplegacy.1967.213.4.1031. [DOI] [PubMed] [Google Scholar]
- 20.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Cserr HF. Role of secretion and bulk flow of brain interstitial fluid in brain volume regulation. Ann N Y Acad Sci. 1988;529:9–20. doi: 10.1111/j.1749-6632.1988.tb51415.x. [DOI] [PubMed] [Google Scholar]
- 22.de R, Ames A, 3rd, Nesbett FB, Hofmann HF. Fluid formed by choroid plexus; a technique for its collection and a comparison of its electrolyte composition with serum and cisternal fluids. J Neurophysiol. 1960;23:485–495. doi: 10.1152/jn.1960.23.5.485. [DOI] [PubMed] [Google Scholar]
- 23.Wolburg H, Wolburg-Buchholz K, Liebner S, Engelhardt B. Claudin-1, claudin-2 and claudin-11 are present in tight junctions of choroid plexus epithelium of the mouse. Neurosci Lett. 2001;307:77–80. doi: 10.1016/s0304-3940(01)01927-9. [DOI] [PubMed] [Google Scholar]
- 24.Millar ID, Brown PD. NBCe2 exhibits a 3 HCO3(−):1 Na+ stoichiometry in mouse choroid plexus epithelial cells. Biochem Biophys Res Commun. 2008;373:550–554. doi: 10.1016/j.bbrc.2008.06.053. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen S, Smith BL, Christensen EI, Agre P. Distribution of the aquaporin CHIP in secretory and resorptive epithelia and capillary endothelia. Proc Natl Acad Sci U S A. 1993;90:7275–7279. doi: 10.1073/pnas.90.15.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kao L, Kurtz LM, Shao X, Papadopoulos MC, Liu L, Bok D, Nusinowitz S, Chen B, Stella SL, Andre M, Weinreb J, Luong SS, Piri N, Kwong JM, Newman D, Kurtz I. Severe neurologic impairment in mice with targeted disruption of the electrogenic sodium bicarbonate cotransporter NBCe2 (Slc4a5 gene) J Biol Chem. 2011;286:32563–32574. doi: 10.1074/jbc.M111.249961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spector R. Micronutrient homeostasis in mammalian brain and cerebrospinal fluid. J Neurochem. 1989;53:1667–1674. doi: 10.1111/j.1471-4159.1989.tb09229.x. [DOI] [PubMed] [Google Scholar]
- 28.Spector R, Johanson C. Micronutrient and urate transport in choroid plexus and kidney: implications for drug therapy. Pharm Res. 2006;23:2515–2524. doi: 10.1007/s11095-006-9091-5. [DOI] [PubMed] [Google Scholar]
- 29.Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem. 2009;284:4267–4274. doi: 10.1074/jbc.M807665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akanuma S, Sakurai T, Tachikawa M, Kubo Y, Hosoya K. Transporter-mediated L-glutamate elimination from cerebrospinal fluid: possible involvement of excitatory amino acid transporters expressed in ependymal cells and choroid plexus epithelial cells. Fluids Barriers CNS. 2015;12:11. doi: 10.1186/s12987-015-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005;19:76–78. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- 32.Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gartner J, Frahm J. Inspiration is the major regulator of human CSF flow. J Neurosci. 2015;35:2485–2491. doi: 10.1523/JNEUROSCI.3246-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feinberg DA, Mark AS. Human brain motion and cerebrospinal fluid circulation demonstrated with MR velocity imaging. Radiology. 1987;163:793–799. doi: 10.1148/radiology.163.3.3575734. [DOI] [PubMed] [Google Scholar]
- 36.Yamada S, Miyazaki M, Yamashita Y, Ouyang C, Yui M, Nakahashi M, Shimizu S, Aoki I, Morohoshi Y, McComb JG. Influence of respiration on cerebrospinal fluid movement using magnetic resonance spin labeling. Fluids Barriers CNS. 2013;10:36. doi: 10.1186/2045-8118-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szentistvanyi I, Patlak CS, Ellis RA, Cserr HF. Drainage of interstitial fluid from different regions of rat brain. Am J Physiol. 1984;246:F835–844. doi: 10.1152/ajprenal.1984.246.6.F835. [DOI] [PubMed] [Google Scholar]
- 38.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiol Rev. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cserr HF. Physiology of the choroid plexus. Physiol Rev. 1971;51:273–311. doi: 10.1152/physrev.1971.51.2.273. [DOI] [PubMed] [Google Scholar]
- 40.Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol. 1981;240:F319–328. doi: 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- 41.Groothuis DR, Vavra MW, Schlageter KE, Kang EW, Itskovich AC, Hertzler S, Allen CV, Lipton HL. Efflux of drugs and solutes from brain: the interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J Cereb Blood Flow Metab. 2007;27:43–56. doi: 10.1038/sj.jcbfm.9600315. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg GA, Kyner WT, Estrada E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am J Physiol. 1980;238:F42–49. doi: 10.1152/ajprenal.1980.238.1.F42. [DOI] [PubMed] [Google Scholar]
- 43.Ichimura T, Fraser PA, Cserr HF. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain Res. 1991;545:103–113. doi: 10.1016/0006-8993(91)91275-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990;170:111–123. [PMC free article] [PubMed] [Google Scholar]
- 45.Weller RO, Djuanda E, Yow HY, Carare RO. Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol. 2009;117:1–14. doi: 10.1007/s00401-008-0457-0. [DOI] [PubMed] [Google Scholar]
- 46.Hawkes CA, Hartig W, Kacza J, Schliebs R, Weller RO, Nicoll JA, Carare RO. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011;121:431–443. doi: 10.1007/s00401-011-0801-7. [DOI] [PubMed] [Google Scholar]
- 47.Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv Neurol. 1990;52:431–439. [PubMed] [Google Scholar]
- 48.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 49.Pullen RG, DePasquale M, Cserr HF. Bulk flow of cerebrospinal fluid into brain in response to acute hyperosmolality. Am J Physiol. 1987;253:F538–545. doi: 10.1152/ajprenal.1987.253.3.F538. [DOI] [PubMed] [Google Scholar]
- 50.Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, Benveniste H, Iliff JJ, Nedergaard M. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med. 2013;11:107. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H. The Effect of Body Posture on Brain Glymphatic Transport. J Neurosci. 2015;35:11034–11044. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 2015;35:371–381. doi: 10.1038/jcbfm.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013;340:1529–1530. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kervezee L, Hartman R, van den Berg DJ, Shimizu S, Emoto-Yamamoto Y, Meijer JH, de Lange EC. Diurnal variation in P-glycoprotein-mediated transport and cerebrospinal fluid turnover in the brain. AAPS J. 2014;16:1029–1037. doi: 10.1208/s12248-014-9625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohshiro K, Yaoita E, Yoshida Y, Fujinaka H, Matsuki A, Kamiie J, Kovalenko P, Yamamoto T. Expression and immunolocalization of AQP6 in intercalated cells of the rat kidney collecting duct. Arch Histol Cytol. 2001;64:329–338. doi: 10.1679/aohc.64.329. [DOI] [PubMed] [Google Scholar]
- 59.Zeidel ML, Ambudkar SV, Smith BL, Agre P. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry. 1992;31:7436–7440. doi: 10.1021/bi00148a002. [DOI] [PubMed] [Google Scholar]
- 60.MacAulay N, Zeuthen T. Water transport between CNS compartments: contributions of aquaporins and cotransporters. Neuroscience. 2010;168:941–956. doi: 10.1016/j.neuroscience.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 61.Hasegawa H, Ma T, Skach W, Matthay MA, Verkman AS. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem. 1994;269:5497–5500. [PubMed] [Google Scholar]
- 62.Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci U S A. 1994;91:13052–13056. doi: 10.1073/pnas.91.26.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verbavatz JM, Ma T, Gobin R, Verkman AS. Absence of orthogonal arrays in kidney, brain and muscle from transgenic knockout mice lacking water channel aquaporin-4. J Cell Sci. 1997;110(Pt 22):2855–2860. doi: 10.1242/jcs.110.22.2855. [DOI] [PubMed] [Google Scholar]
- 64.Yang B, Brown D, Verkman AS. The mercurial insensitive water channel (AQP-4) forms orthogonal arrays in stably transfected Chinese hamster ovary cells. J Biol Chem. 1996;271:4577–4580. [PubMed] [Google Scholar]
- 65.Camassa LM, Lunde LK, Hoddevik EH, Stensland M, Boldt HB, De Souza GA, Ottersen OP, Amiry-Moghaddam M. Mechanisms underlying AQP4 accumulation in astrocyte endfeet. Glia. 2015 doi: 10.1002/glia.22878. [DOI] [PubMed] [Google Scholar]
- 66.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93:1543–1562. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eid T, Lee TS, Thomas MJ, Amiry-Moghaddam M, Bjornsen LP, Spencer DD, Agre P, Ottersen OP, de Lanerolle NC. Loss of perivascular aquaporin 4 may underlie deficient water and K+ homeostasis in the human epileptogenic hippocampus. Proc Natl Acad Sci U S A. 2005;102:1193–1198. doi: 10.1073/pnas.0409308102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J, Lunde LK, Nuntagij P, Oguchi T, Camassa LM, Nilsson LN, Lannfelt L, Xu Y, Amiry-Moghaddam M, Ottersen OP, Torp R. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;27:711–722. doi: 10.3233/JAD-2011-110725. [DOI] [PubMed] [Google Scholar]
- 70.Ren Z, Iliff JJ, Yang L, Yang J, Chen X, Chen MJ, Giese RN, Wang B, Shi X, Nedergaard M. ‘Hit & Run’ model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J Cereb Blood Flow Metab. 2013;33:834–845. doi: 10.1038/jcbfm.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crane JM, Tajima M, Verkman AS. Live-cell imaging of aquaporin-4 diffusion and interactions in orthogonal arrays of particles. Neuroscience. 2010;168:892–902. doi: 10.1016/j.neuroscience.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fenton RA, Moeller HB, Zelenina M, Snaebjornsson MT, Holen T, MacAulay N. Differential water permeability and regulation of three aquaporin 4 isoforms. Cell Mol Life Sci. 2010;67:829–840. doi: 10.1007/s00018-009-0218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Furman CS, Gorelick-Feldman DA, Davidson KG, Yasumura T, Neely JD, Agre P, Rash JE. Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms. Proc Natl Acad Sci U S A. 2003;100:13609–13614. doi: 10.1073/pnas.2235843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silberstein C, Bouley R, Huang Y, Fang P, Pastor-Soler N, Brown D, Van Hoek AN. Membrane organization and function of M1 and M23 isoforms of aquaporin-4 in epithelial cells. Am J Physiol Renal Physiol. 2004;287:F501–511. doi: 10.1152/ajprenal.00439.2003. [DOI] [PubMed] [Google Scholar]
- 75.Thrane AS, Rangroo Thrane V, Nedergaard M. Drowning stars: reassessing the role of astrocytes in brain edema. Trends Neurosci. 2014;37:620–628. doi: 10.1016/j.tins.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith AJ, Jin BJ, Verkman AS. Muddying the water in brain edema? Trends Neurosci. 2015;38:331–332. doi: 10.1016/j.tins.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thrane AS, Rangroo Thrane V, Plog BA, Nedergaard M. Filtering the muddied waters of brain edema. Trends Neurosci. 2015;38:333–335. doi: 10.1016/j.tins.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 78.Matsumae M, Hirayama A, Atsumi H, Yatsushiro S, Kuroda K. Velocity and pressure gradients of cerebrospinal fluid assessed with magnetic resonance imaging. Journal of neurosurgery. 2014;120:218–227. doi: 10.3171/2013.7.JNS121859. [DOI] [PubMed] [Google Scholar]
- 79.Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Muller cells and fibrous astrocytes. J Neurosci. 1998;18:2506–2519. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 81.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 82.Rogers J, Strohmeyer R, Kovelowski CJ, Li R. Microglia and inflammatory mechanisms in the clearance of amyloid beta peptide. Glia. 2002;40:260–269. doi: 10.1002/glia.10153. [DOI] [PubMed] [Google Scholar]
- 83.Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- 84.Albeck MJ, Skak C, Nielsen PR, Olsen KS, Borgesen SE, Gjerris F. Age dependency of resistance to cerebrospinal fluid outflow. J Neurosurg. 1998;89:275–278. doi: 10.3171/jns.1998.89.2.0275. [DOI] [PubMed] [Google Scholar]
- 85.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2011;37:75–93. doi: 10.1111/j.1365-2990.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 86.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 87.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 88.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 89.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pascale CL, Miller MC, Chiu C, Boylan M, Caralopoulos IN, Gonzalez L, Johanson CE, Silverberg GD. Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS. 2011;8:21. doi: 10.1186/2045-8118-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Masseguin C, LePanse S, Corman B, Verbavatz JM, Gabrion J. Aging affects choroidal proteins involved in CSF production in Sprague-Dawley rats. Neurobiol Aging. 2005;26:917–927. doi: 10.1016/j.neurobiolaging.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 93.Silverberg GD, Heit G, Huhn S, Jaffe RA, Chang SD, Bronte-Stewart H, Rubenstein E, Possin K, Saul TA. The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology. 2001;57:1763–1766. doi: 10.1212/wnl.57.10.1763. [DOI] [PubMed] [Google Scholar]
- 94.Plog BA, Dashnaw ML, Hitomi E, Peng W, Liao Y, Lou N, Deane R, Nedergaard M. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35:518–526. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Billingsley ML, Kincaid RL. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J. 1997;323(Pt 3):577–591. doi: 10.1042/bj3230577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, Cirrito JR, Patel TK, Hochgrafe K, Mandelkow EM, Holtzman DM. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–393. doi: 10.1084/jem.20131685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holmes BB, Diamond MI. Prion-like properties of Tau protein: the importance of extracellular Tau as a therapeutic target. J Biol Chem. 2014;289:19855–19861. doi: 10.1074/jbc.R114.549295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hawkes CA, Sullivan PM, Hands S, Weller RO, Nicoll JA, Carare RO. Disruption of arterial perivascular drainage of amyloid-beta from the brains of mice expressing the human APOE epsilon4 allele. PLoS One. 2012;7:e41636. doi: 10.1371/journal.pone.0041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weller RO, Subash M, Preston SD, Mazanti I, Carare RO. Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol. 2008;18:253–266. doi: 10.1111/j.1750-3639.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rangroo Thrane V, Thrane AS, Plog BA, Thiyagarajan M, Iliff JJ, Deane R, Nagelhus EA, Nedergaard M. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep. 2013;3:2582. doi: 10.1038/srep02582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV. Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer’s disease: the role, regulation and restoration of LRP1. Front Aging Neurosci. 2015;7:136. doi: 10.3389/fnagi.2015.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johanson C, Stopa E, McMillan P, Roth D, Funk J, Krinke G. The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol. 2011;39:186–212. doi: 10.1177/0192623310394214. [DOI] [PubMed] [Google Scholar]
- 103.Sharma HS, Duncan JA, Johanson CE. Whole-body hyperthermia in the rat disrupts the blood-cerebrospinal fluid barrier and induces brain edema. Acta Neurochir Suppl. 2006;96:426–431. doi: 10.1007/3-211-30714-1_88. [DOI] [PubMed] [Google Scholar]
- 104.Carlos TM, Clark RS, Franicola-Higgins D, Schiding JK, Kochanek PM. Expression of endothelial adhesion molecules and recruitment of neutrophils after traumatic brain injury in rats. J Leukoc Biol. 1997;61:279–285. doi: 10.1002/jlb.61.3.279. [DOI] [PubMed] [Google Scholar]
- 105.Vinters HV, Ellis WG, Zarow C, Zaias BW, Jagust WJ, Mack WJ, Chui HC. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59:931–945. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]
- 106.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haglund M, Passant U, Sjobeck M, Ghebremedhin E, Englund E. Cerebral amyloid angiopathy and cortical microinfarcts as putative substrates of vascular dementia. Int J Geriatr Psychiatry. 2006;21:681–687. doi: 10.1002/gps.1550. [DOI] [PubMed] [Google Scholar]
- 108.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, Johnson K, Go RC, Growdon JH, Haines JL, Kukull WA, Farrer LA. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 109.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 110.Nordstrom P, Michaelsson K, Gustafson Y, Nordstrom A. Traumatic brain injury and young onset dementia: a nationwide cohort study. Ann Neurol. 2014;75:374–381. doi: 10.1002/ana.24101. [DOI] [PubMed] [Google Scholar]
- 111.de Ribeiro MC, Hirt L, Bogousslavsky J, Regli L, Badaut J. Time course of aquaporin expression after transient focal cerebral ischemia in mice. J Neurosci Res. 2006;83:1231–1240. doi: 10.1002/jnr.20819. [DOI] [PubMed] [Google Scholar]
- 112.Hirt L, Ternon B, Price M, Mastour N, Brunet JF, Badaut J. Protective role of early aquaporin 4 induction against postischemic edema formation. J Cereb Blood Flow Metab. 2009;29:423–433. doi: 10.1038/jcbfm.2008.133. [DOI] [PubMed] [Google Scholar]
- 113.Wang M, Iliff JJ, Liao Y, Chen MJ, Shinseki MS, Venkataraman A, Cheung J, Wang W, Nedergaard M. Cognitive deficits and delayed neuronal loss in a mouse model of multiple microinfarcts. J Neurosci. 2012;32:17948–17960. doi: 10.1523/JNEUROSCI.1860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu DC, Zador Z, Yao J, Fazlollahi F, Manley GTMDPD. Aquaporin-4 Reduces Post-Traumatic Seizure Susceptibility by Promoting Astrocytic Glial Scar Formation in Mice. J Neurotrauma. 2011 doi: 10.1089/neu.2011.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fukuda AM, Pop V, Spagnoli D, Ashwal S, Obenaus A, Badaut J. Delayed increase of astrocytic aquaporin 4 after juvenile traumatic brain injury: possible role in edema resolution? Neuroscience. 2012;222:366–378. doi: 10.1016/j.neuroscience.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke. 2013;44:S93–95. doi: 10.1161/STROKEAHA.112.678698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Black PM, Tzouras A, Foley L. Cerebrospinal fluid dynamics and hydrocephalus after experimental subarachnoid hemorrhage. Neurosurgery. 1985;17:57–62. doi: 10.1227/00006123-198507000-00009. [DOI] [PubMed] [Google Scholar]
- 118.Blasberg R, Johnson D, Fenstermacher J. Absorption resistance of cerebrospinal fluid after subarachnoid hemorrhage in the monkey; effects of heparin. Neurosurgery. 1981;9:686–691. doi: 10.1227/00006123-198112000-00012. [DOI] [PubMed] [Google Scholar]
- 119.Brinker T, Seifert V, Stolke D. Effect of intrathecal fibrinolysis on cerebrospinal fluid absorption after experimental subarachnoid hemorrhage. J Neurosurg. 1991;74:789–793. doi: 10.3171/jns.1991.74.5.0789. [DOI] [PubMed] [Google Scholar]
- 120.Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 121.Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Bruck W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365:2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, Klein RS. Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol. 2008;172:799–808. doi: 10.2353/ajpath.2008.070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–8064. doi: 10.4049/jimmunol.177.11.8053. [DOI] [PubMed] [Google Scholar]
- 124.Reboldi A, Coisne C, Baumjohann D, Benvenuto F, Bottinelli D, Lira S, Uccelli A, Lanzavecchia A, Engelhardt B, Sallusto F. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat Immunol. 2009;10:514–523. doi: 10.1038/ni.1716. [DOI] [PubMed] [Google Scholar]
- 125.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 127.Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, Mandler RN, Weinshenker BG, Pittock SJ, Wingerchuk DM, Lucchinetti CF. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 128.Wolburg-Buchholz K, Mack AF, Steiner E, Pfeiffer F, Engelhardt B, Wolburg H. Loss of astrocyte polarity marks blood-brain barrier impairment during experimental autoimmune encephalomyelitis. Acta Neuropathol. 2009;118:219–233. doi: 10.1007/s00401-009-0558-4. [DOI] [PubMed] [Google Scholar]
- 129.Iliff JJ, Goldman SA, Nedergaard M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015;14:977–979. doi: 10.1016/S1474-4422(15)00221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]