Abstract
Background
N-methyl-D-aspartate (NMDA) receptor antagonists have been shown to reduce perioperative pain and opioid use. We performed a meta-analysis to determine whether the use of perioperative dextromethorphan lowers opioid consumption or pain scores.
Methods
PubMed, Web of Science, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, pubget, and Embase were searched. Studies were included if they were randomized, double-blinded, placebo controlled trials written in English, performed on patients ≥12 years. For comparison of opioid use, included studies tracked total consumption of intravenous or intramuscular opioids over 24 to 48 hours. Pain score comparisons were performed at 1 hour, 4 to 6 hours, and 24 hours postoperatively. Difference in means (MD) was used for effect size.
Results
Forty studies were identified and 21 were eligible for one or more comparisons. In 848 patients from 14 trials, opioid consumption favored dextromethorphan (MD -10.51 mg intravenous morphine equivalents; 95% confidence interval [CI]: -16.48 mg to -4.53 mg; p = 0.0006). In 884 patients from 13 trials, pain at 1 hour favored dextromethorphan (MD -1.60; 95% CI: -1.89 to -1.31; p < 0.00001). In 950 patients from 13 trials, pain at 4-6 hours favored dextromethorphan (MD -0.89; 95% CI: -1.11 to -0.66; p < 0.00001). In 797 patients from 12 trials, pain at 24 hours favored dextromethorphan (MD -0.92; 95% CI: -1.24 to -0.60; p < 0.00001).
Conclusions
This meta-analysis suggests dextromethorphan use perioperatively reduces postoperative opioid consumption at 24-48 hours and pain scores at 1, 4-6, and 24 hours.
Introduction
N-methyl-D-aspartate (NMDA) receptor antagonists have become widely used adjuncts for postoperative analgesia.1-2 Ketamine, a well-studied NMDA antagonist, has been shown to decrease postoperative pain when administered preemptively,3-4 intraoperatively,5 and postoperatively,6-7 without causing an increase in sedation but with a notable increase in hallucinations and nightmares.8 Dextromethorphan, an NMDA antagonist that is most routinely used as an oral antitussive, has also been extensively studied for its use as a perioperative analgesic adjunct.9-29 Dextromethorphan has previously undergone systematic review without quantitative meta-analysis in which the authors determined that the drug was a potentially useful analgesic adjunct, but there still remained significant questions about the consistency of findings between studies.30 Since that systematic review was accepted for publication in 2005 there have been more than ten additional studies9-14,31-34 on dextromethorphan for postoperative pain control. A meta-analysis of the results of studies that investigate DM for its effect on postoperative pain and opioid reduction has not yet been published. We therefore performed a meta-analysis on the use of preoperative dextromethorphan and its effects on opioid consumption and postoperative pain scores.
Materials and Methods
This study is a meta-analysis of existing literature, did not involve the collection of new human or animal data, and is exempt from institutional review board review. The Cochrane specifications for systematic reviews was used to guide the construction of this meta-analysis.35 A systematic search was performed in PubMed, Web of Science, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Pubget, and EMBASE on August 4th, 2014. The following search terms were used: (dextromethorphan) AND acute pain; (dextromethorphan) AND postoperative pain; (dextromethorphan) AND pain. Trials were only included if they were randomized, double-blinded, placebo-controlled, and published in English. Unpublished abstracts and reports were excluded. Pediatric trials on patients less than twelve years of age were also excluded. Authors of the trials were not contacted for original data.
To ensure the quality of included trials, each was scored based on a modified validated scale previously used for meta-analysis.36 The scale was designed to evaluate the quality of placebo-controlled, randomized trials and includes the following parameters:
Randomization: a point was given for stating the trial was randomized. An additional point was given if randomization was described and appropriate, such as the use of a random number generator.
Blinding: a point was given if the trial was stated to be double-blind. If blinding method was described and appropriate, such as the use of identical placebo pills, an additional point was given.
Withdrawals: a point was given if patient withdrawals and the reasons for withdrawals were reported.
Pain intensity: to ensure that the trial evaluated clinically significant pain, a point was given if mean visual analog pain scores were greater than 30 mm or greater than 3 out of 10 on a numeric rating scale.
Power analysis: a point was given if sample size was determined through the use of a power analysis.
Thus, the minimum requirements for inclusion would be a score of 2 points and the maximum score would be 7 points.
To be included in the meta-analyses, we required all trials to have a treatment arm in which intravenous, intramuscular, or per os dextromethorphan was administered prior to surgery – if treatment groups also received intraoperative or postoperative doses of dextromethorphan (table 1) they were included as well. Only test groups from studies in which dextromethorphan was administered preoperatively were included for analysis. If test groups were administered dextromethorphan only intraoperative or postoperatively, they were not included for analysis. If multiple dextromethorphan dosages were administered in an included study, the highest dose group was used for the comparison. However, as a sensitivity analysis, all comparisons were recalculated, where possible, using the lowest dose groups.
Table 1.
list of studies included in one or more of the comparisons in the meta-analysis. DM = dextromethorphan, GA = general anesthesia, IM = intramuscular, IV = intravenous, mg/kg = milligrams per kilogram, mg/ml = milligrams per milliliter, NCA = nurse controlled analgesia (doses dependent on patient request and nurse administration on a PRN schedule), PACU = post-anesthesia care unit,PCA = patient controlled analgesia, PCEA = patient controlled epidural analgesia, PO = per os, POD = postoperative day, PRN = pro re nata (as needed), pts = patients.
| Study | Quality Score | Dextromethorphan pts | Control pts | Dextromethorphan dosing | Dextromethorphan dose timing | Surgery type | Anesthesia type | Comparisons tracked | PRN analgesic tracked | Tracked side effects | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entezary 2013 (9) | 3 | 54 | 58 | 1 mg/kg PO | Night before surgery | Knee arthroscopy | Spinal | Pain 1, 4h Total opioid 24h | Morphine NCA | Yes | |
| Suski 2010 (10) | 6 | 30 | 30 | 30 or 45 mg PO (weight based) ×4 | 1 hr pre-op and at 8, 20, and 32 hrs post-op | Scoliosis repair | General | Pain 1, 4/6, 24h Total opioid 24h | Morphine IV NCA | Yes | |
| Mahmoodzadeh 2009 (11) | 6 | 23 (in 45 group) 24 (in 90 group) | 22 | 45 or 90 mg PO | 2 hrs pre-op | Open chole | General | Pain 1, 6, 24h Total opioid 24h | Morphine IV NCA | No | Used 90 mg group for comparison |
| Chau-In 2007 (12) | 7 | 50 | 48 | 30 mg PO ×4 | 60 mins pre-op and 3 doses over first 24 hrs post-op | Total abdominal hysterectomy | General | Pain 1, 6, 24h Total opioid 24h | Morphine IV PCA | No | |
| Lu 2006 (13) | 6 | 20 DM 20 DM + ketorolac | 20 control 20 ketorolac | 40 mg IM | 30 mins pre-op | Vaginal hysterectomy | General | Pain 1, 4, 24h | Morphine IV PCA | Yes | Performed ketorolac and non-ketorolac comparisons |
| Yeh 2005 (14) | 4 | 30 DM plus epidural | 30 GA plus epidural; 30 GA only | 40 mg IM | 30 mins pre-op | Colon surgery | General + epidural | Pain 1, 4, 24h | 0.2% ropivacaine and 0.1 mg/mL morphine PCEA | Yes | Excluded GA only group as no direct DM comparison |
| Wu 2005 (15) | 5 | 25 DM 25 DM + lidocaine IV | 25 control 25 lidocaine IV | 40 mg IM | 30 mins pre-op | Lap chole | General | Pain 1, 4, 24h Total opioid 48h | Meperidine IM NCA | Yes | Performed lidocaine and non-lidocaine comparisons |
| Yeh 2004 (16) | 5 | 20 DM 20 DM + tenoxicam | 22 control 21 tenoxicam | 40 mg IM | 30 mins pre-op | Lap chole | General | Pain 1,4, 24h Total opioid 48h | Meperidine IM NCA | Yes | Performed tenoxicam and non-tenoxicam comparisons |
| Weinbroum 2004 (17) | 7 | 29 DM + PCA 28 DM + PCEA | 27 PCA 29 PCEA | 90 mg PO | 90 mins pre-op | Bone tumor resection | General or general + epidural | Pain 6, 24h | PCEA (ropivacaine 3.2 mg plus fentanyl 8 mcg/dose) or PCA (morphine 2mg/dose) only in PACU, then diclofenac | Yes | PCA only used in PACU so no 24h opioid comparison |
| Weinbroum 2003 (18) | 6 | 29 | 27 | 90 mg PO ×3 | 90 mins pre-op and on POD 1 and 2 | Bone tumor resection | General + epidural | Pain 1, 6, 24h | PCEA (1.6 mg ropivacaine plus 4 mcg/mL fentanyl) continuous and by demand | Yes | |
| Weinbroum 2002a (19) | 6 | 25 (in 60 group) 23 (in 90 group) | 24 | 60 or 90 mg PO ×3 | 90 mins pre-op and on POD1 and 2 | Bone tumor resection | General | Pain 1, 24h Total opioid 24h | Morphine IV PCA | Yes | Used 90mg group for comparison |
| Weinbroum 2002b (20) | 6 | 18 DM + epidural 20 DM + GA | 17 epidural 20 GA | 90 mg PO | 90 mins pre-op | Hernia repair or knee arthroscopy | General or epidural | Pain 1, 4/6h | Morphine IV PCA for 2 hours then diclofenac | Yes | Compared both epidural and GA groups |
| Weinbroum 2001 (21) | 6 | 16 (in 60 group) 17 (in 90 group) | 20 | 60 or 90 mg PO | 90 mins pre-op | Hernia repair or knee arthroscopy | Epidural | Pain 1, 6h | Morphine IV PCA in PACU and diclofenac at home | Yes | Used 90 mg group for comparisons as this was used in all the group's further studies |
| Helmy 2001 (22) | 5 | 20 (pre) 20 (post) | 20 | 120 mg IM | 30 mins before incision or 30 mins before end of surgery | Upper abdominal surgery | General | Total opioid 24h | Meperidine IV PCA | Yes | Excluded post group |
| Wadhwa 2001 (23) | 7 | 22 | 34 | 200 mg PO ×3 | 120 mins pre-op and 8 and 16 hrs post-op | Knee replacement or reconstruction | General | Total opioid 24h | Morphine IV PCA | Yes | |
| Liu 2000 (24) | 3 | 30 | 30 | 40 mg IM | 30 mins pre-op | Hemorrhoidectomy | Local | Total opioid 48h | Meperidine IM NCA | Yes | |
| Wu 2000 (25) | 3 | 15 (in 10 group) 15 (in 20 group) 15 (in 40 group) | 15 | 10, 20, or 40 mg IM | 30 mins pre-op | Upper abdominal surgery | General | Pain 1,4, 24h Total opioid 24h | Morphine IV PCA | Yes | Used 40 mg group for comparison as this was used in all the group's further studies |
| Wong 1999 (26) | 3 | 30 | 30 | 40 mg IM | 30 mins pre-op | Modified radical mastectomy | General | Total opioid 48h | Meperidine IM NCA | Yes | |
| Wu 1999 (27) | 2 | 30 (given pre) 30 (given intra) | 30 | 40 mg IM | Just prior to incision or intraoperative | Lap chole | General | Total opioid 48h | Meperidine IM NCA | Yes | Excluded intraoperative group |
| Grace 1998 (28) | 5 | 18 | 19 | 60 mg PO ×2 | Night before and 1 hour pre-op | Laparotomy | General | Total opioid 48h | Morphine IV PCA | No | |
| Kawamata 1998 (29) | 5 | 12 (in 30 group) 12 (in 45 group) | 12 | 30 or 45 mg PO | 60 mins pre-op | Tonsillectomy | General | Pain 24h | Diclofenac PO at home | Yes | Used 45 mg group for comparison |
The outcome variables we sought were postoperative opioid consumption, pain scores, and incidence of side effects. The investigation of published studies led to the a posteriori selection for analysis of total opioid consumption for 24-48 hours postoperatively, numeric pain scores at 1, 4-6, and 24 hours, and the incidence of opioid- and dextromethorphan-related side effects. For comparison of postoperative opioid use, studies were included if they tracked total use of opioids over a 24 or 48 hour period. If an opioid other than intravenous morphine was used, such as meperidine, the reported values were converted into IV morphine equivalents using an online calculator.37 Inclusion required sole use of opioids as a PRN analgesic. Comparisons between groups that received the same non-opioid intervention (such as a single dose of a non-steroidal anti-inflammatory agent in both control and dextromethorphan groups) were also included.
Studies were eligible for pain score comparisons if they reported pain scores on a standardized 0 to 10 numeric rating scale, such as the visual analog scale. Numeric pain score comparisons were performed at three time points: 1, 4-6, and 24 hours post-operatively. For the 1 hour group, studies were included if they reported pain scores within 1 hour post-operatively. Thus, studies were also included in this group if they did not report pain scores at 1 hour but did report in the first hour in the post-anesthetic care unit. For the 4 to 6 hour group, studies were included if they reported pain scores at 4 or 6 hours. If a study reported pain scores at both times, the score at 4 hours was used.
We intended to compare the incidence of opioid-related side effects, such as nausea and itching, as well as dextromethorphan-related side effects, such as nausea and euphoria, but this was not feasible due to the small number of events reported. Thus, rather than report meta-analysis of side effects, we systematically reviewed the included trials for reported side effects.
Statistical analyses were performed with Review Manager version 5.3 (The Nordic Cochrane Centre, Copanhagen, Denmark). All calculations required knowledge of the mean and standard deviation for the compared parameters. Some studies represented mean and standard deviation graphically – in these cases the computer program Plot Digitalizer38 was used to estimate values at the set time points. As mean and standard deviation were used for comparison calculation, the effect size is expressed as difference in means (MD). By convention, MDs favoring dextromethorphan were considered negative and those favoring control considered positive. To account for anticipated heterogeneity, a random-effects model39 was used for all calculations. We also utilized the I2 statistic to assess the degree to which differences between trials were due to heterogeneity.40 Alpha was set at 0.05 and, after performing a Bonferroni correction accounting for four total comparisons, the significance criterion set at 0.0125. All comparisons are presented graphically in this manuscript using forest plots.41
Results
Study selection
The selection process is summarized in figure 1. Table 1 lists all studies used in the comparisons including pertinent aspects of their design and subgroups. A total of 40 studies were identified and a total of 19 were excluded, leaving 21 studies which were used in at least one comparison. The median quality score of these studies was 5 out of 7 with an interquartile range of 2.
Figure 1.
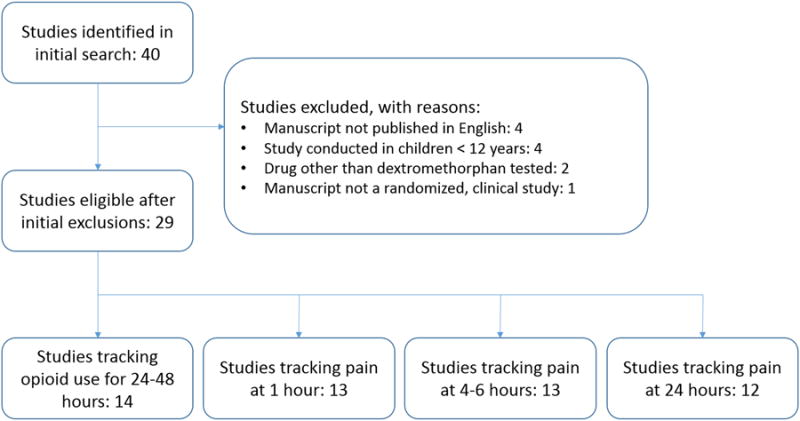
Diagram of study selection for each comparison. Note that exclusions on the comparison level, such as exclusion for not reporting mean and standard deviation for the specific comparison, are not shown.
Total opioid consumption
A total of 14 trials reported mean and standard deviation of opioid consumption for the first 24 or 48 hours post-operatively and a total of 848 patients were included in the comparison. MD favored dextromethorphan (MD: -10.51 milligrams [mg] of intravenous morphine equivalents; 95% confidence interval [CI]: -16.48 mg to -4.53 mg; p = 0.0006; Figure 2). Three studies in particular, Weinbroum et al. 2002a,19 Helmy et al. 2001,22 and Wu et al. 2000,25 were statistical outliers with a MD of less than -20. To ensure that these three studies alone had not resulted in the comparison's significance, a sensitivity analysis was performed with them excluded. The overall effect was lessened but the comparison remained significant (MD: -4.45 mg intravenous morphine equivalents; 95% CI, -7.47 mg to -1.43 mg; p = 0.004) and I2, although still high, was reduced from 97% to 88%.
Figure 2.
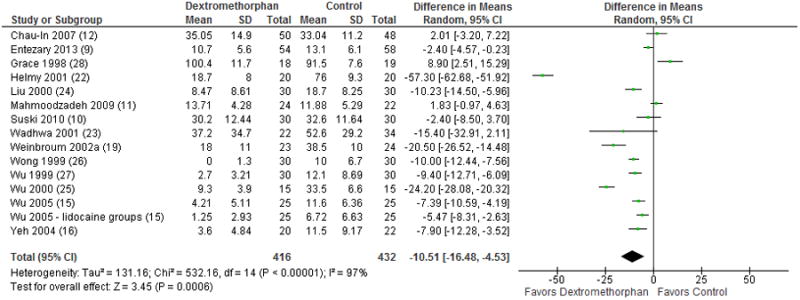
Forest plot for total opioid use over 24 or 48 hours. The table displays the study with reference number in parenthesis, mean, standard deviation, sample size, difference in means in milligrams of intravenous morphine with 95% confidence interval, heterogeneity, overall effect, and p-value. The Forest plot displays point estimate and 95% confidence interval. CI = confidence interval, SD = standard deviation.
Pain scores at 1, 4-6, and 24 hours
Pain scores at 1 hour were reported as mean and standard deviation in 13 studies with a total of 884 included patients. MD favored dextromethorphan (-1.60; 95% CI, -1.89 to -1.31; p < 0.00001; Figure 3). Weinbroum et al. 2002a19 was an outlier with a MD of less than -4. After exclusion, the overall effect was lessened but the comparison remained significant (-1.50; 95% CI, -1.78 to -1.22; p < 0.00001) and I2 decreased from 91 to 90%.
Figure 3.
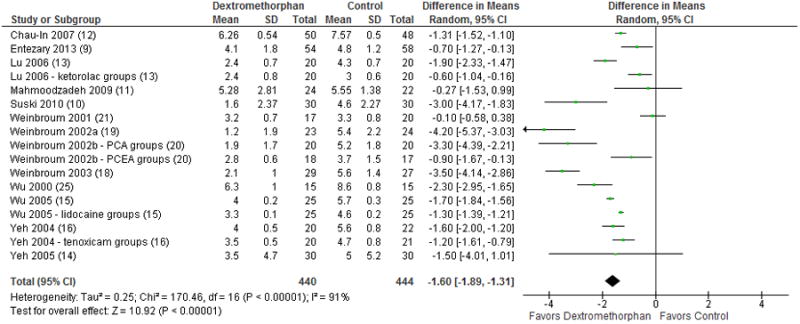
Forest plot for comparison of pain scores at 1 hour post-op. The table displays the study with reference number in parenthesis, mean, standard deviation, sample size, difference in means of visual analog scale with 95% confidence interval, heterogeneity, overall effect, and p-value. The Forest plot displays point estimate and 95% confidence interval. CI = confidence interval, PCA = patient-controlled analgesia, PCEA = patient-controlled epidural analgesia, SD = standard deviation.
Pain scores at 4 to 6 hours were reported in 13 studies with a total of 950 included patients. Three subgroups in two studies (Suski et al. 201010; Weinbroum 2002b20) reported pain scores at both 4 and 6 hours and the values recorded at the 4 hours were used in the comparison. MD favored dextromethorphan (-0.89; 95% CI, -1.11 to -0.66; p < 0.00001; Figure 4) with an I2 of 88%.
Figure 4.
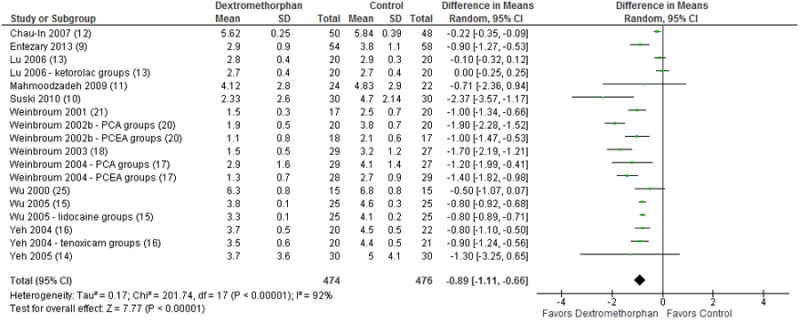
Forest plot for comparison of pain scores at 4 – 6 hours post-op. The table displays the study with reference number in parenthesis, mean, standard deviation, sample size, difference in means with 95% confidence interval, heterogeneity, overall effect, and p-value. The Forest plot displays point estimate and 95% confidence interval. CI = confidence interval, PCA = patient-controlled analgesia, PCEA = patient-controlled epidural analgesia, SD = standard deviation.
Pain scores at 24 hours were reported in 12 studies with 797 included patients. Dextromethorphan was also favored at this time point (MD: -0.92; 95% CI, -1.24 to -0.60; p < 0.00001; Figure 5) with an I2 of 92%.
Figure 5.
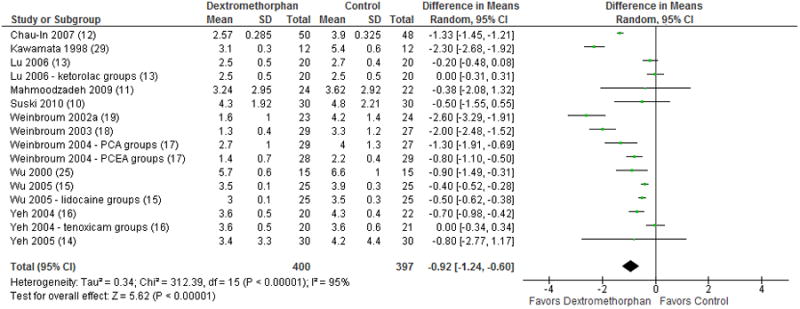
Forest plot for comparison of pain scores at 24 hours post-op. The table displays the study with reference number in parenthesis, mean, standard deviation, sample size, difference in means with 95% confidence interval, heterogeneity, overall effect, and p-value. The Forest plot displays point estimate and 95% confidence interval, CI = confidence interval, PCA = patient-controlled analgesia, PCEA = patient-controlled epidural analgesia, SD = standard deviation.
Comparisons using lower dose dextromethorphan groups
A total of 3 studies in the opioid consumption, 24 hour pain score, and 1 hour pain score comparisons and 2 studies in the 4-6 hour pain score comparison included multiple dosing regimens of dextromethorphan. Of note, two of these studies (Wu et al. 200025 and Weinbroum et al. 200121) were completed by groups that only used their highest dose in subsequent studies – thus their highest dosing groups best approximated the most common dextromethorphan doses in the study and were used for the initial comparisons. When comparisons were recalculated using the low dose instead of high dose groups for comparison, all results remained significant although with a lower magnitude of effect (opioid consumption MD -10.05 mg of intravenous morphine equivalents; 95% CI: -15.79 mg to -4.31 mg, p = 0.0006; pain at 1 hour MD -1.50; 95% CI: -1.79 to -1.21, p < 0.00001; pain at 4-6 hours MD -0.87; 95% CI: -1.11 to -0.64, p < 0.00001; pain at 24 hours MD -0.65; 95% CI: -0.95 to -0.35, p < 0.0001).
Incidence of side effects
Eighteen out of 21 trials included in our meta-analyses tracked the incidence of side effects, which for both opioids and dextromethorphan primarily consist of nausea, vomiting, dizziness, and lightheadedness. Ten studies reported either no side effects or a non-significant difference between groups.9-10,13-14,16,19-20,22,24,27,29 Five studies did, however, did report a decrease in side effects in groups receiving dextromethorphan.15,17-18,25-26 One study23 found a higher incidence of nausea in the dextromethorphan group, with rating mild to moderate nausea reported by patients at 31 time points in the dextromethorphan group compared to 20 time points in the control group, although no patients reported severe nausea at any time. Weinbroum et al. 200121 tracked sedation using a standardized scale and found an increase in sedation in the placebo group.
Discussion
A variety of study designs in multiple hospital settings and countries have attempted to elucidate the value of perioperative dextromethorphan as an adjunctive analgesic. In a prior report, these efforts were synthesized in a qualitative systematic review of NMDA receptor antagonists' role in decreasing postoperative pain and opioid consumption, which demonstrated a significant benefit from dextromethorphan in 67% of included studies.2 Additionally, a separate qualitative systematic review of dextromethorphan only that shared in common fifteen of the studies used in this analysis suggested that dextromethorphan had potential as an adjunct to postoperative opioid analgesics, but did note variability among the analyzed studies.30 Here we have systematically searched the published literature on the preoperative use of dextromethorphan to decrease postoperative pain and opioid use. Ultimately, we identified 21 trials published between 1998 and 2013 that addressed these metrics and were suitable for quantitative meta-analysis. The results of our meta-analyses suggest that, when used preoperatively, dextromethorphan significantly decreases pain and opioid use in the postoperative period.
To objectively index included trials by design quality, we scored each trial based on a quality index. The majority of studies in our meta-analyses scored in the 5 to 7 range. These studies demonstrated a high degree of transparency in their study designs and sampling processes. A minority of studies scored in the 2-4 range, with a score of 2 representing the minimum requirements of being a randomized, blinded trial. Although we did not weigh trials based on their scores, the average scores of the trials do reflect the on average high quality of the studies from which we draw our conclusions.
As an NMDA receptor antagonist,42 dextromethorphan has been proposed to exert its effects as a preemptive analgesic by preventing NMDA-mediated calcium current and subsequent modulation of nociception in spinal pain fibers and the central nervous system. This in turn prevents a pain phenomenon known as “windup” that results in amplified subsequent responses to painful stimuli and poorer responses to opioids.43-46 In previous trials, dextromethorphan has shown benefit in various chronic pain conditions including diabetic neuropathy, postherpetic neuralgia,47 and phantom limb pain.48-49 Effects on cancer pain have also been investigated in at least two trials with mixed results.50-51
Multimodal preemptive analgesic adjuncts including NMDA receptor antagonists, local anesthetic infiltration, non-steroidal anti-inflammatory agents (NSAIDs), epidural analgesia, and preemptive opioids and have been the subject of a prior meta-analysis.36 This study found benefit with preemptive NSAIDs, epidural analgesia, and local anesthetic infiltration, but its comparisons for both ketamine and dextromethorphan were equivocal. In contrast, the same year a meta-analysis of perioperative intravenous ketamine use reported a mean of 15.7 mg less morphine consumption at 24 hours and mean pain score improvements of 0.89 at 6 hours, 0.42 at 12 hours, 0.35 at 24 hours, and 0.27 at 48 hours.52 These results are remarkably similar to our own. A more recent meta-analysis of perioperative intravenous ketamine found benefits for opioid consumption and time to first analgesic, but did note increased hallucinations and nightmares.8 The statistic used for this analysis was the standardized mean difference rather than mean difference, making direct comparison to effect observed in our own study difficult.
However, while ketamine is widely used as a multimodal adjunct worldwide, our anecdotal experience from multiple institutions is that dextromethorphan does not appear to share the same level of popularity and is very rarely used as an adjunct for postoperative analgesia. Based on our findings, the use of dextromethorphan perioperatively could potentially provide similar benefits to preemptive ketamine therapy in a simple oral, IM, or IV formulation. Further investigation, particularly a head to head randomized trial alongside placebo, may help clarify whether the different NMDA antagonists provide similar levels of relief with a similar incidence of dysphoric or other side effects, or not. Additional research may also explore if there is benefit from the simultaneous use of more than one NMDA receptor antagonist as it is unclear if this would result in an additive, synergistic, or antagonistic effect.
Well-documented dextromethorphan side effects and concerns include dose-related tachycardia, respiratory depression, and gastrointestinal symptoms, as well as abuse potential.53 Although its recreational abuse potential is clear, dextromethorphan dependence has only been rarely described54-55 and its abuse is best described in adolescents.56-57 Recent work has described dose-dependent hallucinogenic properties of dextromethorphan as well as acute changes in memory and cognition,58-59 although these effects typically occurred at doses well in excess of those used in the included studies. Thus, it seems reasonable to avoid doses above 2 mg/kg PO, which has been described as a dose above which dissociative effects are typically seen,60 in order to prevent neurologic disturbances before surgery. However, there exists to our knowledge no evidence that a single dose of dextromethorphan for preemptive analgesia would increase potential for postoperative abuse, and indeed review of the included trials revealed a minimal incidence of dextromethorphan-related adverse effects.
Although opioids are a mainstay of effective perioperative analgesia, their use is nonetheless frequently associated with side effects that can increase hospital costs and length of stay.61 Multimodal analgesia has been proposed as a way to improve pain control while reducing side effects,62 but to date little evidence exists to link opioid-sparing analgesic regimens to reduced opioid-related adverse effects. The available studies were insufficient for meta-analysis on the incidence of side effects with dextromethorphan, but our qualitative review of the literature suggests that most studies saw minimal change in the incidence of side effects. Ketamine, in contrast, was shown in prior meta-analysis to increase the risk of hallucinations when administered in awake patients, although the incidence of opioid-related side effects was also unchanged.52 This difference highlights the fact that different NMDA receptor antagonists are not necessarily interchangeable, and therefore continued exploration into other agents like dextromethorphan and memantine are still warranted. Larger studies may clarify if opioid-sparing doses of dextromethorphan are able to quantitatively decrease the incidence of opioid-related side effects without causing hallucinations at similar rates to ketamine.
Similar to Duedahl et al.'s 2006 systematic review30 we observed a high degree of heterogeneity, with an I2 greater than 80% in each comparison. This is likely a reflection of the variability between study designs, such as differences in type of surgery, dextromethorphan dosing regimens, dextromethorphan administration routes, and post-operative analgesic regimens. We had anticipated this and therefore used a random-effects model for all of our calculations. The high heterogeneity does, nonetheless, demonstrate the variability in findings among dextromethorphan studies and highlights the need for a larger study with a standardized protocol to clarify dextromethorphan's role in the perioperative setting. Important details to clarify include the optimal perioperative dextromethorphan dose and duration of use, the incidence of side effects, and whether or not the perioperative use of dextromethorphan improves outcomes such as hospital length of stay.
Our analysis is also limited by the fundamental reliance of meta-analyses on the existing data and the reporting mechanisms of the original studies. Many high quality studies needed to be excluded from the quantitative analyses due to reporting results in forms other than mean and standard deviation, tracking opioid use over periods less than 24 hours, or reporting pain scores in forms other than fixed intervals (such as only reporting the worst recorded). In a small number of studies with multiple dextromethorphan dosing arms, we also had to exclude groups in order to avoid duplicating control patients in our comparisons. As a result our quantitative analyses do not necessarily represent the full body of literature on the perioperative use of dextromethorphan. In addition, due to the heterogeneity of published studies, this is an a posteriori derived analysis of total opioid consumption for 24 to 48 hours postoperatively and pain scores at 0 to 1 hours, 4 to 6 hours, and 24 hours postoperatively.
Despite these limitations, our comparisons do nonetheless represent a cross-section of several hundred patients in the available randomized controlled trials on the effects of preoperative dextromethorphan on postoperative pain control with significantly favorable results. To date no large randomized controlled trial has been conducted on this topic. Our quantitative meta-analyses of the existing randomized controlled studies of dextromethorphan for postoperative pain control demonstrated a significant reduction in postoperative opioid use for 24 to 48 hours after surgery as well as pain represented by pain scores up to 24 hours after surgery. Due to high heterogeneity between the existing trials and the lack of a single large randomized study on this topic, further evidence is required to definitively determine a benefit.
Final Box Summary Statement.
What we already know
Some NMDA receptor antagonists reduce postoperative pain and opioid requirements.
Dextromethorphan, a low-affinity noncompetitive NMDA receptor antagonist, may be beneficial in the perioperative setting.
What this article tells us that is new
This meta-analysis identified 21 studies describing the effects of dextromethorphan on postoperative pain and opioid consumption.
Dextromethorphan was found to reduce pain from 1 to 24 hours postoperatively, and was found to reduce morphine requirements 24-48 hours after surgery.
Acknowledgments
the authors are grateful to Hang Lee, Ph.D., Assistant Professor of Medicine, Harvard Medical School and the Massachusetts General Hospital Biostatistics Center, Boston, MA, USA, for statistical consultation.
Funding: This work was conducted with support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Research Resources and National Center for Advancing Translational Sciences, National Institutes of Health [Bethesda, Maryland] Award UL1 TR001102) and financial contributions from Harvard University (Cambridge, Massachusetts) and its affiliated academic healthcare centers.
Footnotes
Clinical Trials Registration: not applicable
Conflicts of interest: The authors declare no competing interests.
References
- 1.Suzuki M. Role of N-methyl-D-aspartate receptor antagonists in postoperative pain management. Curr Opin Anaesthesiol. 2009;22:618–22. doi: 10.1097/ACO.0b013e32832e7af6. [DOI] [PubMed] [Google Scholar]
- 2.McCartney CJ, Sinha A, Katz J. A qualitative systematic review of the role of N-methyl-D-aspartate receptor antagonists in preventive analgesia. Anesth Analg. 2004;98:1385–400. doi: 10.1213/01.ane.0000108501.57073.38. [DOI] [PubMed] [Google Scholar]
- 3.Roytblat L, Korotkoruchko A, Katz J, Glazer M, Greemberg L, Fisher A. Postoperative pain: the effect of low-dose ketamine in addition to general anesthesia. Anesth Analg. 1993;77:1161–5. doi: 10.1213/00000539-199312000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Launo C, Bassi C, Spagnolo L, Badano S, Ricci C, Lizzi A, Molinino M. Preemptive ketamine during general anesthesia for postoperative analgesia in patients undergoing laparoscopic cholecystectomy. Minerva Anestesiol. 2004;70:727–34. 734–8. [PubMed] [Google Scholar]
- 5.Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, Beach ML. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113:639–46. doi: 10.1097/ALN.0b013e3181e90914. [DOI] [PubMed] [Google Scholar]
- 6.Adriaenssens G, Vermeyen KM, Hoffmann VL, Mertens E, Adriaensen HF. Postoperative analgesia with i.v. patient-controlled morphine: effect of adding ketamine. Br J Anaesth. 1999;83:393–6. doi: 10.1093/bja/83.3.393. [DOI] [PubMed] [Google Scholar]
- 7.Zakine J, Samarcq D, Lorne E, Moubarak M, Montravers P, Beloucif S, Dupont H. Postoperative ketamine administration decreases morphine consumption in major abdominal surgery: A prospective, randomized, double-blind, controlled study. Anesth Analg. 2008;106:1856–61. doi: 10.1213/ane.0b013e3181732776. [DOI] [PubMed] [Google Scholar]
- 8.Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anaesth. 2011;58:911–23. doi: 10.1007/s12630-011-9560-0. [DOI] [PubMed] [Google Scholar]
- 9.Entezary SR, Farshadpour S, Alebouyeh MR, Imani F, Emami Meybodi MK, Yaribeygi H. Effects of preoperative use of oral dextromethorphan on postoperative need for analgesics in patients with knee arthroscopy. Anesthesiol Pain Med. 2014;4:e11187. doi: 10.5812/aapm.11187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suski M, Bujak-Gizycka B, Madej J, Kacka K, Dobrogowski J, Woron J, Olszanecki R, Korbut R. Co-administration of dextromethorphan and morphine: Reduction of post-operative pain and lack of influence on morphine metabolism. Basic Clin Pharmacol Toxicol. 2010;107:680–4. doi: 10.1111/j.1742-7843.2010.00559.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahmoodzadeh H, Movafegh A, Beigi NM. Preoperative oral dextromethorphan does not reduce pain or morphine consumption after open cholesyctectomy. Middle East J Anesthesiol. 2010;20:559–64. [PubMed] [Google Scholar]
- 12.Chau-In W, Sukmuan B, Ngamsangsirisapt K, Jirarareungsak W. Efficacy of pre- and postoperative oral dextromethorphan for reduction of intra- and 24-hour postoperative morphine consumption for transabdominal hysterectomy. Pain Med. 2007;8:462–7. doi: 10.1111/j.1526-4637.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- 13.Lu CH, Liu JY, Lee MS, Borel CO, Yeh CC, Wong CS, Wu CT. Preoperative cotreatment with dextromethorphan and ketorolac provides an enhancement of pain relief after laparoscopic-assisted vaginal hysterectomy. Clin J Pain. 2006;22:799–804. doi: 10.1097/01.ajp.0000210931.20322.da. [DOI] [PubMed] [Google Scholar]
- 14.Yeh CC, Jao SW, Huh BK, Wong CS, Yang CP, White WD, Wu CT. Preincisional dextromethorphan combined with thoracic epidural anesthesia and analgesia improves postoperative pain and bowel function in patients undergoing colonic surgery. Anesth Analg. 2005;100:1384–9. doi: 10.1213/01.ANE.0000148687.51613.B5. [DOI] [PubMed] [Google Scholar]
- 15.Wu CT, Borel CO, Lee MS, Yu JC, Liou HS, Yi HD, Yang CP. The interaction effect of perioperative cotreatment with dextromethorphan and intravenous lidocaine on pain relief and recovery of bowel function after laparoscopic cholecystectomy. Anesth Analg. 2005;100:448–53. doi: 10.1213/01.ANE.0000142551.92340.CC. [DOI] [PubMed] [Google Scholar]
- 16.Yeh CC, Wu CT, Lee MS, Yu JC, Yang CP, Lu CH, Wong CS. Analgesic effects of preincisional administration of dextromethorphan and tenoxicam following laparoscopic cholecystectomy. Acta Anaesthesiol Scand. 2004;48:1049–53. doi: 10.1111/j.1399-6576.2004.00455.x. [DOI] [PubMed] [Google Scholar]
- 17.Weinbroum AA, Bender B, Nirkin A, Chazan S, Meller I, Kollender Y. Dextromethorphan-associated epidural patient-controlled analgesia provides better pain- and analgesics-sparing effects than dextromethorphan-associated intravenous patient-controlled analgesia after bone-malignancy resection: a randomized, placebo-control. Anesth Analg. 2004;98:714–22. doi: 10.1213/01.ane.0000100151.56901.eb. [DOI] [PubMed] [Google Scholar]
- 18.Weinbroum AA, Bender B, Bickels J, Nirkin A, Marouani N, Chazam S, Meller I, Kollender Y. Preoperative and postoperative dextromethorphan provides sustained reduction in postoperative pain and patient-controlled epidural analgesia requirement: A randomized, placebo-controlled, double-blind study in lower-body bone malignancy-operated patients. Cancer. 2003;97:2334–40. doi: 10.1002/cncr.11330. [DOI] [PubMed] [Google Scholar]
- 19.Weinbroum AA, Gorodetzky A, Nirkin A, Kollender Y, Bickels J, Marouani N, Rudick V, Meller I. Dextromethorphan for the reduction of immediate and late postoperative pain and morphine consumption in orthopedic oncology patients: A randomized, placebo-controlled, double-blind study. Cancer. 2002;95:1164–70. doi: 10.1002/cncr.10784. [DOI] [PubMed] [Google Scholar]
- 20.Weinbroum AA. Dextromethorphan reduces immediate and late postoperative analgesic requirements and improves patients' subjective scorings after epidural lidocaine and general anesthesia. Anesth Analg. 2002;94:1547–52. doi: 10.1097/00000539-200206000-00032. [DOI] [PubMed] [Google Scholar]
- 21.Weinbroum AA, Lalayev G, Yashar T, Ben-Abraham R, Niv D, Flaishon R. Combined pre-incisional oral dextromethorphan and epidural lidocaine for postoperative pain reduction and morphine sparing: A randomised double-blind study on day-surgery patients. Anaesthesia. 2001;56:616–22. doi: 10.1046/j.1365-2044.2001.02088.x. [DOI] [PubMed] [Google Scholar]
- 22.Helmy SA, Bali A. The effect of the preemptive use of the NMDA receptor antagonist dextromethorphan on postoperative analgesic requirements. Anesth Analg. 2001;92:739–44. doi: 10.1097/00000539-200103000-00035. [DOI] [PubMed] [Google Scholar]
- 23.Wadhwa A, Clarke D, Goodchild CS, Young D. Large-dose oral dextromethorphan as an adjunct to patient-controlled analgesia with morphine after knee surgery. Anesth Analg. 2001;92:448–54. doi: 10.1097/00000539-200102000-00032. [DOI] [PubMed] [Google Scholar]
- 24.Liu ST, Wu CT, Yeh CC, Ho ST, Wong CS, Jao SW, Wu CC, Kang JC. Premedication with dextromethorphan provides posthemorrhoidectomy pain relief. Dis Colon Rectum. 2000;43:507–10. doi: 10.1007/BF02237195. [DOI] [PubMed] [Google Scholar]
- 25.Wu CT, Yu JC, Liu ST, Yeh CC, Li CY, Wong CS. Preincisional dextromethorphan treatment for postoperative pain management after upper abdominal surgery. World J Surg. 2000;24:512–7. doi: 10.1007/s002689910082. [DOI] [PubMed] [Google Scholar]
- 26.Wong CS, Wu CT, Yu JC, Yeh CC, Lee MM, Tao PL. Preincisional dextromethorphan decreases postoperative pain and opioid requirement after modified radical mastectomy. Can J Anaesth. 1999;46:1122–6. doi: 10.1007/BF03015519. [DOI] [PubMed] [Google Scholar]
- 27.Wu CT, Yu JC, Yeh CC, Liu ST, Li CY, Ho ST, Wong CS. Preincisional dextromethorphan treatment decreases postoperative pain and opioid requirement after laparoscopic cholecystectomy. Anesth Analg. 1999;88:1331–4. doi: 10.1097/00000539-199906000-00025. [DOI] [PubMed] [Google Scholar]
- 28.Grace RF, Power I, Umedaly H, Zammit A, Mersiades M, Cousins MJ, Mather LE. Preoperative dextromethorphan reduces intraoperative but not postoperative morphine requirements after laparotomy. Anesth Analg. 1998;87:1135–8. [PubMed] [Google Scholar]
- 29.Kawamata T, Omote K, Kawamata M, Namiki A. Premedication with oral dextromethorphan reduces postoperative pain after tonsillectomy. Anesth Analg. 1998;86:594–7. doi: 10.1097/00000539-199803000-00031. [DOI] [PubMed] [Google Scholar]
- 30.Duedahl TH, Rømsing J, Møiniche S, Dahl JB. A qualitative systematic review of peri-operative dextromethorphan in post-operative pain. Acta Anaesthesiol Scand. 2006;50:1–13. doi: 10.1111/j.1399-6576.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 31.Ehret GB, Daali Y, Chabert J, Rebsamen M, Wolff A, Forster A, Moursli F, Fritschy D, Rossier MF, Piguet V, Dayer P, Gex-Fabry M, Desmeules JA. Influence of CYP2D6 activity on pre-emptive analgesia by the N-methyl-D-aspartate antagonist dextromethorphan in a randomized controlled trial of acute pain. Pain Physician. 2013;16:45–56. [PubMed] [Google Scholar]
- 32.Abu-Samra MM, Ismaeil WA. Pre-emptive oral dextromethorphan reduced both postoperative and packing removal pain in patients undergoing nasal surgery. Saudi Med J. 2009;30:214–8. [PubMed] [Google Scholar]
- 33.Ali SM, Shahrbano S, Ulhaq TS. Tramadol for pain relief in children undergoing adenotonsillectomy: a comparison with dextromethorphan. Laryngoscope. 2008;118:1547–9. doi: 10.1097/MLG.0b013e318178272e. [DOI] [PubMed] [Google Scholar]
- 34.Aoki T, Yamaguchi H, Naito H, Shiiki K, Ota Y, Kaneko A. Dextromethorphan premedication reduced postoperative analgesic consumption in patients after oral surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:591–5. doi: 10.1016/j.tripleo.2005.10.060. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6. Chichester: John Wiley & Sons, Ltd.; 2006. updated September 2006. [Google Scholar]
- 36.Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005;100:757–73. doi: 10.1213/01.ANE.0000144428.98767.0E. [DOI] [PubMed] [Google Scholar]
- 37. [Accessed 2015 May 20];Opioid converter, opioid conversions, pain management. Available from: http://www.globalrph.com/narcoticonv.htm.
- 38.Plot Digitalizer. [Accessed 2015 July 20]; Available from: http://plotdigitizer.sourceforge.net.
- 39.Brockwell SE, Gordon IR. A comparison of statistical methods for meta-analysis. Stat Med. 2001;20:825–40. doi: 10.1002/sim.650. [DOI] [PubMed] [Google Scholar]
- 40.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis S, Clarke M. Forest plots: trying to see the wood and the trees. BMJ. 2001;322:1479–80. doi: 10.1136/bmj.322.7300.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Church J, Lodge D, Berry SC. Differential effects of dextrorphan and levorphanol on the excitation of rat spinal neurons by amino acids. Eur J Pharmacol. 1985;111:185–90. doi: 10.1016/0014-2999(85)90755-1. [DOI] [PubMed] [Google Scholar]
- 43.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 44.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 45.Woolf CJ, Chong MS. Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 46.Dickenson AH. Spinal cord pharmacology of pain. Br J Anaesth. 1995;75:193–200. doi: 10.1093/bja/75.2.193. [DOI] [PubMed] [Google Scholar]
- 47.Nelson KA, Park KM, Robinovitz E, Tsigos C, Max MB. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology. 1997;48:1212–8. doi: 10.1212/wnl.48.5.1212. [DOI] [PubMed] [Google Scholar]
- 48.Ben Abraham R, Marouani N, Kollender Y, Meller I, Weinbroum AA. Dextromethorphan for phantom pain attenuation in cancer amputees: a double-blind crossover trial involving three patients. Clin J Pain. 2002;18:282–5. doi: 10.1097/00002508-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Ben Abraham R, Marouani N, Weinbroum AA. Dextromethorphan mitigates phantom pain in cancer amputees. Ann Surg Oncol. 2003;10:268–74. doi: 10.1245/aso.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Mercadante S, Casuccio A, Genovese G. Ineffectiveness of dextromethorphan in cancer pain. J Pain Symptom Manage. 1998;16:317–22. [PubMed] [Google Scholar]
- 51.Katz NP. MorphiDex (MS:DM) double-blind, multiple-dose studies in chronic pain patients. J Pain Symptom Manage. 2000;19:S37–41. doi: 10.1016/s0885-3924(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 52.Elia N, Tramèr MR. Ketamine and postoperative pain–a quantitative systematic review of randomised trials. Pain. 2005;113:61–70. doi: 10.1016/j.pain.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 53.Bem JL, Peck R. Dextromethorphan. An overview of safety issues Drug Saf. 1992;7:190–9. doi: 10.2165/00002018-199207030-00004. [DOI] [PubMed] [Google Scholar]
- 54.Hinsberger A, Sharma V, Mazmanian D. Cognitive deterioration from long-term abuse of dextromethorphan: A case report. J Psychiatry Neurosci. 1994;19:375–7. [PMC free article] [PubMed] [Google Scholar]
- 55.Fleming PM. Dependence on dextromethorphan hydrobromide. BMJ. 1986;293:597. doi: 10.1136/bmj.293.6547.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyer EW. Dextromethorphan abuse. Pediatr Emerg Care. 2004;20:858–63. doi: 10.1097/01.pec.0000148039.14588.d0. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz RH. Adolescent abuse of dextromethorphan. Clin Pediatr. 2005;44:565–8. doi: 10.1177/000992280504400702. [DOI] [PubMed] [Google Scholar]
- 58.Reissig CJ, Carter LP, Johnson MW, Mintzer MZ, Klinedinst MA, Griffiths RR. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology. 2012;223:1–15. doi: 10.1007/s00213-012-2680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carter LP, Reissig CJ, Johnson MW, Klinedinst MA, Griffiths RR, Mintzer MZ. Acute cognitive effects of high doses of dextromethorphan relative to triazolam in humans. Drug Alcohol Depend. 2013;128:206–13. doi: 10.1016/j.drugalcdep.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Romanelli F, Smith KM. Dextromethorphan abuse: clinical effects and management. J Am Pharm Assoc. 2009;49:e20–5. doi: 10.1331/JAPhA.2009.08091. [DOI] [PubMed] [Google Scholar]
- 61.Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27:62–70. doi: 10.3109/15360288.2012.751956. [DOI] [PubMed] [Google Scholar]
- 62.White PF, Kehlet H. Improving Postoperative Pain Management: What Are the Unresolved Issues? Anesthesiology. 2010;112:220–5. doi: 10.1097/ALN.0b013e3181c6316e. [DOI] [PubMed] [Google Scholar]


