Abstract
Legacy environmental contaminants such as polybrominated diphenyl ethers (PBDEs) are widely detected in human tissues. However, few studies have measured PBDEs in placental tissues, and there are no reported measurements of 2,4,6-tribromophenol (2,4,6-TBP) in placental tissues. Measurements of these contaminants are important for understanding potential fetal exposures, as these compounds have been shown to alter thyroid hormone regulation in vitro and in vivo. In this study, we measured a suite of PBDEs and 2,4,6-TBP in 102 human placental tissues collected between 2010–2011 in Durham County, North Carolina, USA. The most abundant PBDE congener detected was BDE-47, with a mean concentration of 5.09 ng/g lipid (range: 0.12–141 ng/g lipid; detection frequency 91%); however, 2,4,6-TBP was ubiquitously detected and present at higher concentrations with a mean concentration of 15.4 ng/g lipid (range:1.31–316 ng/g lipid; detection frequency 100%). BDE-209 was also detected in more than 50% of the samples, and was significantly associated with 2,4,6-TBP in placental tissues, suggesting they may have a similar source, or that 2,4,6-TBP may be a degradation product of BDE-209. Interestingly, BDE-209 and 2,4,6-TBP were negatively associated with age (rs=−0.16; p=0.10 and rs=−0.17; p=0.08, respectively). The results of this work indicate that PBDEs and 2,4,6-TBP bioaccumulate in human placenta tissue and likely contribute to prenatal exposures to these environmental contaminants. Future studies are needed to determine if these joint exposures are associated with any adverse health measures in infants and children.
Keywords: Brominated flame retardants, polybrominated diphenyl ethers, thyroid hormone, placenta
Introduction
Polybrominated diphenyl ethers (PBDEs) have been used as additive flame retardants for decades in a variety of applications from polyurethane foams to high-impact polystyrene (HIPS). The presence of PBDEs in consumer products has led to their accumulation in indoor environments, and subsequent human exposure via inadvertent ingestion and/or inhalation of dust particles1,2 Particular attention has been given to a PBDE commercial mixture known as pentaBDE, which had a primary application in polyurethane foam used in residential furniture3,4. Studies have documented higher serum concentrations of PBDEs associated with pentaBDE in the US population relative to other regions of the world, likely due to the higher use of this mixture in residential furniture to meet a regional (state of California) flammability standard5. While the use of pentaBDE has now been banned or phased-out throughout the world, many older products in the home still contain these flame retardants, which will continue to leach into the indoor environment during the product lifetime. As a result, human exposure to PBDEs will continue for years to come, especially with the use of recycled foams and plastics in consumer products that may contain these phased-out chemicals. As such, PBDEs continue to be measured in human tissues such as serum, breast milk, umbilical cord blood, and placental tissues, suggesting that prenatal exposures to PBDEs occurs during pregnancy, and continues during infancy via breast feeding6–9.
In contrast, 2,4,6-tribromophenol (2,4,6-TBP) is widely used as an industrial chemical with an estimated US production volume of 4500 to 23,000 tonnes in 200610. 2,4,6-TBP has multiple applications, including use as an antifungal agent (e.g. as a replacement for pentachlorophenol) in wood applications, as a reactive brominated flame retardant (BFR), and as an intermediate in the production of other BFRs. 2,4,6-TBP can also be formed as a result of the photolytic degradation of tetrabromobisphenol-A (TBBPA), a widely used reactive BFR, and during the synthesis of 1,2-bis (2,4,6-tribromophenoxy) ethane (BTBPE)11. In addition to the anthropogenic sources of 2,4,6-TBP, there are natural sources of 2,4,6-TBP and other bromophenols from marine organisms and algae12. Few toxicity studies have examined the effects of 2,4,6-TBP in animal models. One study examined oral exposure to 2,4,6-TBP in adult zebrafish and observed reproductive toxicity in addition to perturbed gonadal morphology when exposed to spiked food at concentrations of 3300 ug/g dw13. Only a few studies have examined environmental levels and human exposure to 2,4,6-TBP. It has been measured in marine sediments at an average concentration of 3.02 ng/g dry weight and in riverine systems at 0.66 ng/g dry weight14. 2,4,6-TBP has also been measured in the indoor environment of Japanese homes, with indoor house dust concentrations ranging from 15–30 ng/g and indoor air concentrations between 220–690 pg/m3–15. Very few biomonitoring studies have included 2,4,6-TBP in the analyses of human tissues such as serum, cord blood, and/or breast milk. One Japanese study collected maternal serum and umbilical cord blood from a cohort of 16 mothers in 2006 for analysis of BFRs and PCBs. This study measured 2,4,6-TBP in maternal blood at a concentration of 22 pg/g wet weight and in cord blood at a concentration of 37 pg/g wet weight16. BFRs were also evaluated in Norwegian individuals working in electronics dismantling facilities, where 2,4,6-TBP was measured in plasma ranging from 0.17 to 81 ng/g lipid17. In a study measuring BFRs in a Canadian Inuit population from Nunavik, Quebec, plasma samples contained a geometric mean 2,4,6-TBP concentration of 9.4 μg/kg lipid, however, these concentrations were not correlated with PBDE concentrations18. Finally, Qiu et al. measured mean 2,4,6-TBP concentrations of 5.6 ng/g lipid in fetal plasma and 0.8 ng/g lipid in maternal plasma19.
PBDEs and 2,4,6-TBP share a chemical structure that is similar to endogenous thyroid hormones (THs), and have been demonstrated to disrupt TH homeostasis either in vitro or in animal exposure studies20,21. Concentrations of PBDEs in human serum have also been found to be significantly correlated with circulating levels of THs in adults, and are associated with adverse neurodevelopmental outcomes in children22,23. Early childhood represents a developmental period that is vulnerable to endocrine disruption. Development is a hormonally-regulated growth process that is sensitive to perturbations by environmental contaminants, like PBDEs and 2,4,6-TBP. The in utero stage of development also represents a highly vulnerable period of fetal growth that may be even more sensitive to endocrine disruption due to the underdeveloped nature of the fetus’ detoxification pathways, in addition to the myriad different growth and developmental processes that are occurring throughout gestation.
The placenta acts to facilitate the materno-fetal transfer of nutrients, gas, waste, and hormones throughout gestation and can act as a protective barrier against toxins and environmental contaminants24. In the case of PBDEs, passive diffusion and/or active uptake of these chemicals into the placenta occurs, and the placenta can act as a repository for these lipophilic chemicals. For example, one study looked at mother-child pairs in China and compared the placental transfer characteristics of various environmental endocrine disruptors, including PBDEs. Their results indicated that PBDEs can be transferred across the placenta from maternal circulation, and eventually reach the fetus25. Additionally, Frederiksen et al. used an experimental ex vivo human placenta perfusion system to show the differences in transplacental transfer of PBDEs based on degree of bromination26. Thus there is a need to better understand the accumulation of these contaminants in placental tissues, in order to understand fetal exposures. In this study, we present our findings from the analysis of 102 human placental tissues that were collected in North Carolina, USA. Tissue samples were analyzed for a suite of PBDEs and 2,4,6-tribromophenol in order to increase our understanding of exposures during pregnancy and their accumulation within the placenta.
Materials and Methods
Participant recruitment
Participants were recruited from within an observational prospective cohort study assessing the joint effect of social, environmental, and host factors on pregnancy outcomes (the Healthy Pregnancy, Healthy Baby (HPHB) Study conducted by the Children’s Environmental Health Initiative)27,28. The HPHB study enrolled pregnant women from the Duke Obstetrics Clinic and the Durham County Health Department Prenatal Clinic at the Lincoln Community Health Center in Durham, NC. Our analyses included a subset of women from the HPHB study that delivered at the Duke University Medical Center between March 2010 and December 2011. The intentional study design was to oversample women attending the Lincoln Community Health Clinic, in order to explore disparities in pregnancy outcomes by comparing African-American women with good outcomes to those with poor outcomes. As a result, the study population is predominantly African-American women with a lower socioeconomic standing and low educational attainment relative to the general US population. All aspects of this study were carried out in accordance with a human subjects research protocol approved by the Duke University Institutional Review Board.
Sample Collection
Consenting women had placenta tissue subsamples taken at the time of delivery at the Duke University Medical Center. Tissues (approximately 5–20 g) were stored in screwtop cryovials at −80°C until analysis.
Chemicals
All solvents used for the analysis were HPLC-grade or better. A fluorinated BDE standard, 2,3′,4,4′,6-tetrabromodiphenyl ether (FBDE-69)(Chiron Inc., Trondheim, Norway), 13C labeled 2,2′,3,4,5,5′-hexachlorinated diphenyl ether (CDE-141) (Cambridge Isotope Laboratories, Andover, MA), and labeled 13C-2,2′,3,3′,4,4′,5,5′,6,6′-decabromodophenyl ether (BDE-209) were used as internal and recovery standards for the BFR extractions. PBDE calibration standards were purchased from Accustandard and 2,4,6-tribromophenol was purchased from Cambridge Isotope Laboratories, Andover, MA.
BFR Analysis and Lipid Determination
Extractions were performed using between 2 and 17 grams of placenta tissue, depending on the sample and the amount collected during delivery. Tissues underwent 24 hours of lyophilization in order to completely dry the samples. The freeze-dried tissue samples were then homogenized into a fine powder with a pre-cleaned mortar and pestle before adding 15 mL of 1:1 hexane/dichloromethane (DCM) and letting the samples sit overnight, in order to allow for full solvent penetration. Samples were spiked with 1 ng of FBDE-69 and 13C-BDE-209 as internal standards. All glassware used for BFR analysis were cleaned by muffle furnace, in addition to triple-rinsing with hexane, DCM, and methanol solvents in order to minimize background contamination. Samples then underwent 20 minutes of water bath sonication followed by centrifugation, after which the solvent was decanted to a separate tube. The extraction step was then repeated twice (three times total), and the solvent extracts were combined in a clean 50 mL glass centrifuge tube. Following extraction, the samples were blown down under a gentle stream of N2 to a volume of 1 mL. A small aliquot of the extract was used for gravimetric lipid analysis and the remaining extract was passed through acidified silica columns for sample clean-up. Deactivated silica (4.0 g) was acidified using 40% by mass H2SO4, shaken, and loaded into a glass chromatography column. The columns were pre-cleaned by rinsing with hexane and acetone and then conditioned with 15 mL of the elution solvent mix. The extract was then loaded on to the column and eluted using 30 mL of 80:20 hexane/DCM. Sample extracts were then blown down under a gentle stream of N2 gas to a final volume of 100 uL. Samples were transferred to 200 uL glass vial inserts and spiked with 1 ng of 13C-CDE-141 as a recovery standard. Finally, PBDEs and 2,4,6-TBP were identified and quantified using authenticated standards and gas chromatography with electron capture negative ion mass spectrometry (GC/ECNI-MS).
Quality Control/Quality Assurance
Laboratory blanks (e.g. sodium sulfate) were included with each batch of tissue sample extractions beginning with lyophilization (one batch includes 10 tissues samples plus two lab blanks). All sample values were blank subtracted and MDLs were calculated as three times the standard deviation of the lab blank values for each analyte. Individual values were normalized to the measured lipid content of each tissue sample used for the extraction procedure to yield a final value in ng/g lipid.
Labeled internal standards were used as surrogates and internal standards in all samples and included F-BDE-69 and 13C-BDE-209 as internal standards (spiked prior to extraction) and 13C-CDE-141 as a recovery standard (spiked prior to GC/MS analysis). The recovery of the internal standards was calculated for all tissue samples and laboratory blanks in order to assess the recovery efficiency of the extraction methods. The mean recovery in the lab blanks for FBDE-69 was 82.5 ± 14%, while mean sample recovery was 60 ± 12 %.
Additionally, the BFR extraction method was validated using Standard Reference Material (SRM) 1947 (NIST, Gaithersburg, MD). SRM 1947 is a Lake Michigan fish homogenate with certified concentrations of PBDEs. The BFR extraction procedure previously described was used on a triplicate set of SRM 1947 samples. The concentrations of PBDE congeners of interest (BDE-47, -66, -99, -100, -153, and 154) were measured at 99%–116% of the certified values. Recovery of 2,4,6-TBP was evaluated by spiking 10 ng into a laboratory blank (in triplicate) and carrying it through the method. Recoveries averaged 87% (± 29%).
Statistical Analysis
Statistical analyses were performed using JMP Pro 11. ΣBDE was calculated by summing all PBDE congeners including BDE-47, -99, -100, -153, -154, and -209, while ΣBFR includes all PBDE congeners plus 2,4,6-TBP. Only analytes with ≥50% detection frequency were included in statistical analyses. Values below MDL were assigned a value equal to one-half the detection limit for statistical analyses. Preliminary analyses (Shapiro-Wilkes Test) indicated that the PBDE data were not normally distributed. As such, Spearman rank sum correlation analyses were used to assess the relationships between PBDE congeners in placenta and to assess their relationship with maternal age. It is important to note that the BFR concentrations were not significantly and positively associated with lipid content; however, we conducted all statistical analyses with both wet weight and lipid normalized concentrations. Results were similar using both methods. We present statistics using lipid normalized concentrations to facilitate comparison with other studies. Alpha < 0.05 was considered statistically significant.
Results
Population characteristics
Participant demographics are summarized in Table 1. Sixty-eight percent of the women in the study were non-Hispanic black. Most (58%) women were relatively young, between the ages of 18–24 years old (range 18–40). This was the first pregnancy for 45.5% of the women. Of all participants, 43.6% reported completing high school, and less than 10% of the women had private health insurance. Recruitment for this study used English literacy as an exclusion criteria, so the demographics of this study population are not entirely reflective of the population of women visiting the Prenatal Clinic at the Lincoln Community Health Center in Durham, NC. The population of women who most commonly use this prenatal clinic are Hispanic, while the women included in this study are predominantly non-Hispanic black women with a lower socioeconomic standing.
Table 1.
Cohort characteristics (n=101*)
| Characteristic | N (%) |
|---|---|
|
| |
| Maternal race | |
| Non-Hispanic white | 16 (15.8) |
| Non-Hispanic black | 69 (68.3) |
| Hispanic | 12 (11.9) |
| Other | 4 (4.0) |
| Maternal age | |
| 18–19 | 21 (20.8) |
| 20–24 | 38 (37.6) |
| 25–40 | 42 (41.6) |
| Parity | |
| First birth | 46 (45.5) |
| Male infant | 52 (51.5) |
| Maternal education | |
| Less than high school | 25 (24.8) |
| High school diploma | 32 (31.7) |
| More than high school | 44 (43.6) |
| Not married | 82 (81.2) |
| Smoked during pregnancy | 22 (21.8) |
| Private health insurance# | 9 (9.3) |
Demographic data was missing for one individual
No data is available on health insurance for four of the individuals
BFRs
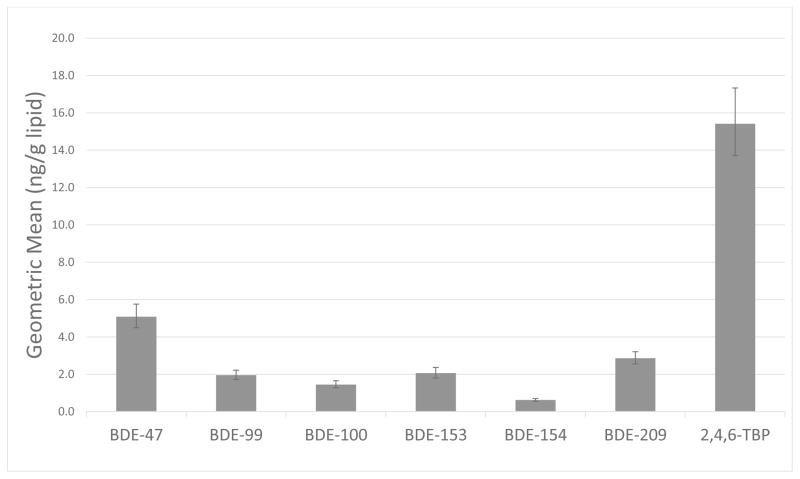
Detection frequencies for BDE-47, -100, -99, -154, -153, -209, and 2,4,6-TBP were all greater than 50% and are presented in Table 2 along with the range and distribution of concentrations measured. It is interesting to note that 2,4,6-TBP was detected in 100% of the samples and constituted 47.8% of the ΣBFR concentration measured in tissues. The most prominent PBDE measured was BDE-47, representing 34% of ΣBDE burden. The geometric mean concentration of 2,4,6-TBP was 15.4 ng/g lipid (range: 1.31 – 316 ng/g lipid), while the geometric mean concentration of BDE-47 was 5.09 ng/g lipid (range: 0.12 – 141 ng/g lipid). The PBDE congener ranking profile from highest geometric mean concentration to lowest geometric mean concentration is: BDE-47, -209, -153, -99, -100, -154 (Figure 1). Given the relatively homogenous distribution of our population, we were underpowered to examine associations between BFR exposures and race/ethnicity. However, we did examine associations with age. Interestingly, BDE-209 and 2,4,6-TBP were negatively associated with maternal age, rs=−0.16 (p=0.10) and rs=−0.17 (p= 0.08), respectively, although again the associations did not reach statistical significance at p<0.05. The remaining PBDE congeners showed no suggestion of associations with maternal age (p>0.20).
Table 2.
BFR concentrations (ng/g lipid) measured in placental tissues.
| Percentile | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Variable | MDL | Detection Frequency (%) | Geometric Mean | Min | Max | 25th | 50th | 75th | 95th |
|
| |||||||||
| PBDEs (n=102) | |||||||||
|
| |||||||||
| BDE-47 | 0.07 | 91.2 | 5.09 | 0.12 | 141 | 2.12 | 5.05 | 12.4 | 37.5 |
| BDE-99 | 0.07 | 68.6 | 1.95 | 0.09 | 223 | 0.62 | 1.95 | 4.43 | 17.1 |
| BDE-100 | 0.02 | 88.2 | 1.45 | 0.03 | 50.1 | 0.62 | 1.65 | 3.25 | 11.1 |
| BDE-153 | 0.01 | 93.1 | 2.06 | 0.02 | 513 | 1.21 | 2.36 | 4.15 | 16.9 |
| BDE-154 | 0.01 | 83.3 | 0.63 | 0.01 | 20.2 | 0.33 | 0.74 | 1.41 | 3.41 |
| BDE-209 | 0.17 | 52.9 | 2.86 | 0.16 | 50.4 | 1.55 | 2.64 | 6.83 | 17.3 |
| ΣPBDEs | 17.6 | 0.54 | 528 | 8.71 | 19.10 | 34.7 | 98.7 | ||
|
| |||||||||
| Phenolic compound (n=102) | |||||||||
|
| |||||||||
| 2,4,6-TBP | 0.05 | 100 | 15.4 | 1.31 | 316 | 6.25 | 15.0 | 32.7 | 171 |
| ΣBFRs | 37.3 | 2.18 | 568 | 18.3 | 38.1 | 75.6 | 317 | ||
ΣPBDE was calculated by summing all PBDE congeners (i.e. BDE-47, -99, -100, -153, -154, and -209), while ΣBFR includes the sum of all PBDE congeners plus 2,4,6-TBP.
Figure 1.
Geometric mean concentrations of BFRs measured in human placenta tissues (n=102; Error bars represent ±SEM)
Associations between PBDEs and 2,4,6-TBP
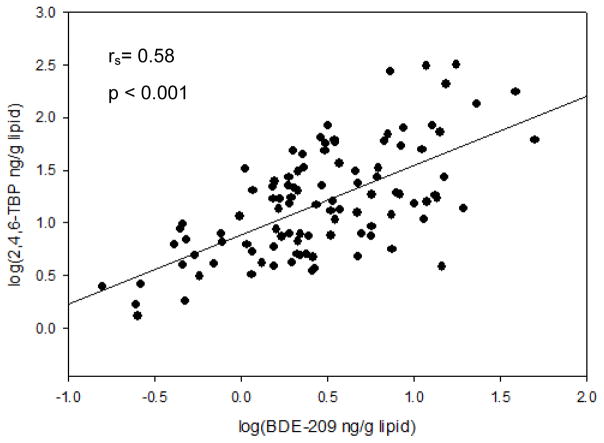
Correlation analyses are summarized in Table 3. All BFRs were significantly (p < 0.001) and positively correlated with each other and with ΣBFR concentrations. BDE-100 showed the strongest correlation (rs=0.89) with ΣBDE content followed by BDE-47 (rs=0.84). Interestingly, 2,4,6-TBP was significantly associated with all PBDE congeners. For example, 2,4,6-TBP showed a moderately strong correlation with BDE-209 (rs=0.58; p < 0.001; Figure 2).
Table 3.
Spearman correlation matrix for BFRs (ng/g lipid)
| BFR | BDE-47 | BDE-99 | BDE-100 | BDE-153 | BDE-154 | BDE-209 | 2,4,6-TBP | ΣPBDEs | ΣBFRs |
|---|---|---|---|---|---|---|---|---|---|
| BDE-47 | 1.00 | 0.48# | 0.88# | 0.58# | 0.61# | 0.49# | 0.50# | 0.84# | 0.73# |
| BDE-99 | 1.00 | 0.52# | 0.43# | 0.52# | 0.60# | 0.66# | 0.68# | 0.72# | |
| BDE-100 | 1.00 | 0.71# | 0.71# | 0.50# | 0.48# | 0.89# | 0.77# | ||
| BDE-153 | 1.00 | 0.71# | 0.50# | 0.38# | 0.77# | 0.66# | |||
| BDE-154 | 1.00 | 0.54# | 0.50# | 0.77# | 0.72# | ||||
| BDE-209 | 1.00 | 0.58# | 0.73# | 0.72# | |||||
| 2,4,6-TBP | 1.00 | 0.58# | 0.85# | ||||||
| ΣPBDEs | 1.00 | 0.89# | |||||||
| ΣBFRs | 1.00 |
p<0.001
Figure 2.
Scatterplot showing correlation between 2,4,6-TBP and BDE-209
Discussion
This is the first study to measure both PBDEs and 2,4,6-TBP in human placenta tissues, and observe a suggestive negative association with maternal age. It has often been assumed that the relative tissue concentrations of environmental contaminants within the placenta can be representative of fetal exposures for some contaminants29. In fact, numerous studies have examined the relationships of environmental contaminants, such as PBDEs, within maternal serum, umbilical cord blood, and placenta tissues. The results of these studies indicate that transplacental transfer (TPT) of PBDEs does occur, and leads to fetal exposure during gestation30–33. For example, a study by Frederiksen et al. found that PBDE exposure in the indoor environment, specifically from house dust ingestion, is linked to PBDE concentrations in maternal and umbilical cord plasma, which are additionally correlated with the PBDE concentrations measured in the paired placental tissues. This study also showed a decreased rate of transport of PBDE congeners across the placenta with increasing degree of bromination29. These results illustrate the placental transfer of PBDEs following maternal exposure to house dust and/or dietary sources of PBDEs, and also show that PBDEs are transferred to the fetal compartment during gestation. However, to our knowledge, no studies have examined TPT of 2,4,6-TBP, which should be addressed in future studies.
Additional research has also been conducted using a human ex vivo placenta perfusion system to study the kinetics and placenta transfer characteristics of BDE-47, -99, and -209. Significant accumulation was observed for all PBDE congeners tested, with placental transfer of BDE-47 being faster and more extensive than BDE-99 and BDE-20926. These results indicate that in utero exposure to PBDEs occurs during gestation as a result of placental transfer, with higher rates of transfer and exposure for the lower brominated congeners. In contrast to these results, Chen et al. measured higher ratios between fetal cord blood and maternal placenta (F/M ratio) for PBDEs with a higher degree of bromination, suggesting that TPT increases with increasing degree of bromination34. In addition, the ability of a chemical to bind to plasma transporter proteins will likely affect TPT characteristics. In the case of PBDEs, which have chemical structures similar to that of THs, their ability to bind TH transport proteins such as transthyretin (TTR) and/or TH membrane transporters such as OATPSs, MCTs, and LATs, may affect their TPT properties35. Different compounds exhibit different partitioning and transport behaviors, and the exact mechanisms of TPT are not fully understood, however, the presence of contaminants found in both maternal and fetal circulation is clearly indicative of fetal exposure.
To date, only two other studies have measured PBDEs in placenta tissue samples from US populations9,36. Additionally, placenta tissues from China, Japan, and European countries including Spain, Denmark, and Finland have been evaluated for PBDEs31,37–40. The results from these studies are summarized in Table 4. The median value for ΣPBDEs4-7 (tetra-through hepta-substituted congeners) in this present study was 13.8 ng/g lipid, and is similar to levels reported for placentae from individuals living near a Chinese e-waste site (19.5 ng/g lipid and 19.4 ng/g lipid), as well as in another US cohort that had a smaller sample size (n=42; 23.7 ng/g lipid). However, these values are much higher than the average ΣPBDE concentrations found in European samples (1.09 ng/g lipid), as well as placentae from Japan (0.25 ng/g lipid)41.
Table 4.
PBDE measurements in human placental tissues from various regions.
| Population Location | Year | # of placentae | # of BDE congeners | Median Σ3-7BDEs (ng/g lw*) | Median BDE-209 (ng/g lw*) | Lipid (%) | Congener concentration ranking | References |
|---|---|---|---|---|---|---|---|---|
| USA | 2010–2012 | 42 | 10 | 23.7 | NA | NA | 47>153=99>209 | Nanes et al., 20149 |
| USA | 2008–2010 | 102 | 7 | 13.8 | 2.64 | 1.09 | 47>209>153=99>100 | This study |
| USA | 2007–2008 | 5 | 42 | NA | NA | NA | 47=99>153 | Dassanayake et al., 200936 |
| China | 2012 | 69 | 8 | 19.4 | 3.30 | NA | 28>209>153>183>47 | Xu et al., 2015 (e-waste site)37 |
| China | 2012 | 86 | 8 | 1.9 | 2.08 | NA | 209>153>28>47>183 | Xu et al., 2015 (reference site)37 |
| China | 2012 | 30 | 17 | 9.96 | ND | 1.42 | 47>99>153 | Chen et al., 201440 |
| China | 2009–2011 | 31 | 9 | 3 | 2.64 | 1.38 | 209>197>153>47 | Zhao et al., 201343 |
| China | 2005–2007 | 130 | 39 | 0.54 | NA | NA | 47>153=99 | Ma et al., 201252 |
| China | 2005 | 5 | 36 | 19.5 | NA | NA | 47>153>99 | Leung et al., 2010 (e-waste site)53 |
| China | 2005 | 5 | 36 | 1.02 | NA | NA | 47>153>99 | Leung et al., 2010 (reference site) |
| China | NA | 6 | 7 | 2.73 | NA | NA | 47 | Zhang et al., 200854 |
| Japan | NA | 10 | 25 | 0.25 | 0.32 | 3.6 | 209>47>153 | Takasuga et al., 2007 |
| Spain | 2003–2004 | 30 | 15 | 0.65 | 1.0 | 0.7 | 209>47>153 | Gómara et al., 200731 |
| Denmark | 2007 | 50 | 12 | 1.22 | 1.14 | 1.21 | 209>47=153 | Frederiksen et al., 200939 |
| Denmark | 1997–2001 | 129 | 14 | 1.31 | NA | 1.09 | 153=47 | Main et al., 200738 |
| Finland | 1997–2001 | 56 | 14 | 1.18 | NA | 1.21 | 47>153 | Main et al., 200738 |
lw = lipid weight based
These current findings align with previous studies that have reported higher concentrations of PBDE congeners associated with the pentaBDE mixture in human samples from North America. North American concentrations are generally one to two orders of magnitude higher than those measured in European and Asian populations as a result of differences in fire regulatory standards, chemical regulatory and policy frameworks, and overall use and exposure to PBDEs42. In Europe, Japan, and China, BDE-209 is often found to be the most abundant congener, accounting for more than 50% of the total concentrations in human placentae 31,39,43. In the US, however, BDE-47 is the most prevalent congener measured in human and other biological tissues, and this is consistent with the higher concentrations of BDE-47 measured in indoor dust in the US compared to other countries. It is interesting to note that in this study, BDE-209 concentrations measured in placental tissues were approximately equal to measurements made in Chinese samples, twice as high as the Danish samples, and eight times higher than Japanese samples. Furthermore, the measured values for ΣPBDEs in American placentae from this study are relatively similar to those measurements found in samples from individuals living and working in Chinese e-waste recycling towns. E-waste dismantling and processing is an occupation that typically involves significant human exposure to flame retardants due to their higher contact with electronic components containing flame retardant chemicals. The concentration of PBDEs measured in placenta tissues from both populations suggests that the same level of PBDE exposure and accumulation occurs between the general US population and Chinese e-waste recycling town inhabitants, despite the stark discrepancy in their exposure scenarios, and likely exposure pathways. However, lower brominated PBDEs that are more commonly found in pentaBDE applications such as polyurethane foams sold in North America, were measured in high concentrations in the Chinese e-waste worker samples, despite the fact the pentaBDE has limited use in electronics. These may be the result of metabolic and/or abiotic debromination of BDE-209 and other higher brominated PBDEs that are more widely used in electronics and plastics.
In the current study, we also observed a suggestive negative association with maternal age for BDE-209 and 2,4,6-TBP, which to our knowledge, has not been observed previously. The explanation for this negative relationship is unclear, but may relate to differences in exposure based on difference in behavior with age (e.g. time spent in various micro-environments). The fact that both BDE-209 and 2,4,6-TBP were negatively associated with maternal age, and that both were correlated with each other, suggests that they may share a similar source (e.g. electronics). Usually, PBDE congeners within a single commercial mixture are more strongly correlated with one another than between commercial mixtures. However, our results are partially in agreement with a recent assessment of placental PBDE concentrations that measured significant correlations between BDE-209 and BDE-28, -47, -99, and -183, but not between BDE-209 and BDE-100, -153, and -15444. There are currently no other studies that have measured 2,4,6-TBP in human placenta tissue. The specific applications of 2,4,6-TBP as a reactive flame retardant remain unclear; however it appears that exposure to 2,4,6-TBP is common within our study population since it was detected in 100% of samples. 2,4,6-TBP was found to have a positive correlation with all PBDEs quantified, but was strongest for BDE-99 and BDE-209. 2,4,6-TBP and BDE-209 are not commonly analyzed together in biological tissues, likely due to the lower awareness of 2,4,6-TBP as an environmental contaminant of interest. As stated earlier, this unique relationship may be a result of these chemicals having related sources of exposure, or it may be indicative of a metabolic pathway that transforms BDE-209 into 2,4,6-TBP.
Previous work has explored the in vitro endocrine-disrupting potency of 2,4,6-TBP, as well as other BFRs, using a wide variety of assays. 2,4,6-TBP was found to be a potent inhibitor of estradiol sulfotransferase (ESULT) activity along with TBBPA and 6-OH-BDE-47, while the PBDEs showed much higher IC50 value (half maximal inhibitory concentration, or the concentration at which the enzyme activity is diminished by 50%) and/or no ESULT inhibition, indicating that ESULT inhibition potency is determined largely in part by the presence of a hydroxylated aromatic group. 2,4,6-TBP was also shown to be a very potent thyroxine competitor in the transthyretin (TTR)-binding assay, with a TTR-binding affinity 10.2 greater than the natural ligand, thyroxine45. Additionally, the ability of 2,4,6-TBP to inhibit thyroid hormone SULT activity in pooled human liver cytosol was evaluated, and 2,4,6-TBP was shown to have an IC50 value of 8.3 nM, which was more potent than any of the hydroxylated PBDEs profiled46. However, more research is necessary to understand the sources of 2,4,6-TBP in the indoor environment, as well as to examine potential adverse effects from exposure to 2,4,6-TBP among the general population.
It is well known that the first trimester of pregnancy is a critical period for fetal neurodevelopment, and that these neurodevelopmental processes are largely driven by the action of THs47. Animal exposure studies with PBDEs have shown permanent effects on spontaneous motor behavior (eg. hyperactivity) and decreased performance in learning and memory tests, implicating PBDEs as developmental neurotoxicants and endocrine disruptors48,49. Additionally, PBDEs have been shown to disrupt TH homeostasis; therefore, the presence of PBDEs in the placenta may impact the materno-fetal transfer of THs during gestation, leading to the disruption of TH-mediated processes in the fetal compartment. Furthermore, growing epidemiological evidence show associations between prenatal exposure to PBDEs and subsequent neurodevelopmental deficits measured in children50,23,51. Overall, flame retardant levels should continue to be closely monitored in the placenta, as well as their potential effects on fetal TH status and neurodevelopment.
One potential shortcoming of this study is the subsampling technique used in the collection of the placental tissue samples. Placental samples were collected at delivery and then sub-sampled to share among various studies. Therefore it was impossible to collect a whole placenta and homogenize the sample prior to sub-sampling. The placenta is a large, highly vascularized, heterogeneous organ. As a result, inconsistent or non-standardized subsampling techniques of the placenta organ may result in differences in our measurements of BFRs. For example, subsamples taken from the highly vascularized central region of the organ may contain different concentrations of BFRs than a peripheral subsample that has different vasculature and adipose composition. Normalization to lipid content may help control for some of these differences. However, we observed no significant correlations between BFR concentrations on a wet weight basis (ng/g ww) with percent lipid. But despite the inconsistencies in subsampling, the median values of PBDE concentrations from this study agree with PBDE measurements from previous studies9,34,44.
Results from this study indicate that PBDEs and 2,4,6-TBP bioaccumulate in human placenta tissues, and provide insight into fetal BFR exposure during pregnancy. This study also characterizes BFR exposures in a population of women from low socioeconomic backgrounds and represents a unique subpopulation of understudied women in the US that are not typically represented in other exposure studies. These data may provide useful comparisons to other study populations from different regions with different ethnic and socioeconomic backgrounds, and further our understanding of the exposure patterns across the US. Future studies should also consider investigating associations between adverse health outcomes and exposures to these mixtures of BFRs given their reported effects on endocrine function.
Highlights.
A suite of PBDEs and 2,4,6-TBP were measured in 102 placenta tissue samples.
BDE-209 was detected in more than 50% of the samples.
2,4,6-TBP was found in the highest concentrations in placenta tissue.
2,4,6-TBP was significantly correlated with PBDEs.
BDE-209 and 2,4,6-TBP were suggested to be negatively associated with maternal age.
Acknowledgments
The authors would like to thank all of our participants in this research study. Support for this project was provided by a grant from the National Institute of Environmental Health Sciences, R01ES020430.
Abbreviations
- 2,4,6-TBP
2,4,6-tribromophenol
- BFR
brominated flame retardant
- DI
deiodinase
- GC/ECNI-MS
electron capture negative ion mass spectrometry
- HIPS
high-impact polystyrene
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- PBDE
polybrominated diphenyl ether
- SPE
solid phase extraction
- SULT
sulfotransferase
- THs
thyroid hormones
- TPT
transplacental transfer
- TTR
transthyretin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between polybrominated diphenyl ether concentrations in house dust and serum. Environ Sci Technol. 2010;44:5627–32. doi: 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stapleton HM, Eagle S, Sjödin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ Health Perspect. 2012;120:1049–54. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stapleton HM, et al. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46:13432–9. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Diamond ML, Robson M, Harrad S. Sources, emissions, and fate of polybrominated diphenyl ethers and polychlorinated biphenyls indoors in Toronto, Canada. Environ Sci Technol. 2011;45:3268–74. doi: 10.1021/es102767g. [DOI] [PubMed] [Google Scholar]
- 5.Hites RA. Polybrominated Diphenyl Ethers in the Environment and in People: A Meta-Analysis of Concentrations. Environ Sci Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- 6.Zota AR, et al. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ Sci Technol. 2013;47:11776–84. doi: 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adgent MA, Hoffman K, Goldman BD, Sjödin A, Daniels JL. Brominated flame retardants in breast milk and behavioural and cognitive development at 36 months. Paediatr Perinat Epidemiol. 2014;28:48–57. doi: 10.1111/ppe.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdelouahab N, et al. Maternal and cord-blood thyroid hormone levels and exposure to polybrominated diphenyl ethers and polychlorinated biphenyls during early pregnancy. Am J Epidemiol. 2013;178:701–13. doi: 10.1093/aje/kwt141. [DOI] [PubMed] [Google Scholar]
- 9.Nanes JA, et al. Selected persistent organic pollutants in human placental tissue from the United States. Chemosphere. 2014;106:20–7. doi: 10.1016/j.chemosphere.2013.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covaci A, et al. Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour. Environ Int. 2011;37:532–56. doi: 10.1016/j.envint.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki G, et al. Identification of brominated and chlorinated phenols as potential thyroid-disrupting compounds in indoor dusts. Environ Sci Technol. 2008;42:1794–800. doi: 10.1021/es7021895. [DOI] [PubMed] [Google Scholar]
- 12.Gribble GW. The natural production of organobromine compounds. Environ Sci Pollut Res. 2000;7:37–49. doi: 10.1065/espr199910.002. [DOI] [PubMed] [Google Scholar]
- 13.Haldén AN, Nyholm JR, Andersson PL, Holbech H, Norrgren L. Oral exposure of adult zebrafish (Danio rerio) to 2,4,6-tribromophenol affects reproduction. Aquat Toxicol. 2010;100:30–7. doi: 10.1016/j.aquatox.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Sim WJ, Lee SH, Lee IS, Choi SD, Oh JE. Distribution and formation of chlorophenols and bromophenols in marine and riverine environments. Chemosphere. 2009;77:552–558. doi: 10.1016/j.chemosphere.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Takigami H, Suzuki G, Hirai Y, Sakai S. Brominated flame retardants and other polyhalogenated compounds in indoor air and dust from two houses in Japan. Chemosphere. 2009;76:270–7. doi: 10.1016/j.chemosphere.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Kawashiro Y, et al. Perinatal exposure to brominated flame retardants and polychlorinated biphenyls in Japan. Endocr J. 2008;55:1071–84. doi: 10.1507/endocrj.k08e-155. [DOI] [PubMed] [Google Scholar]
- 17.Thomsen C, Lundanes E, Becher G. Brominated flame retardants in plasma samples from three different occupational groups in Norway. J Environ Monit. 2001;3:366–70. doi: 10.1039/b104304h. [DOI] [PubMed] [Google Scholar]
- 18.Dallaire R, et al. Determinants of plasma concentrations of perfluorooctanesulfonate and brominated organic compounds in Nunavik Inuit adults (Canada) Environ Sci Technol. 2009;43:5130–6. doi: 10.1021/es9001604. [DOI] [PubMed] [Google Scholar]
- 19.Qiu X, Bigsby RM, Hites RA. Hydroxylated metabolites of polybrominated diphenyl ethers in human blood samples from the United States. Environ Health Perspect. 2009;117:93–8. doi: 10.1289/ehp.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noyes PD, Lema SC, Macaulay LJ, Douglas NK, Stapleton HM. Low level exposure to the flame retardant BDE-209 reduces thyroid hormone levels and disrupts thyroid signaling in fathead minnows. Environ Sci Technol. 2013;47:10012–21. doi: 10.1021/es402650x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabo DT, et al. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbstman JB, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–9. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eskenazi B, et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121:257–62. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James SR, Franklyn JA, Kilby MD. Placental transport of thyroid hormone. Best Pract Res Clin Endocrinol Metab. 2007;21:253–64. doi: 10.1016/j.beem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Li LX, et al. Exposure Levels of Environmental Endocrine Disruptors in Mother-Newborn Pairs in China and Their Placental Transfer Characteristics. PLoS One. 2013;8:e62526. doi: 10.1371/journal.pone.0062526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frederiksen M, Vorkamp K, Mathiesen L, Mose T, Knudsen LE. Placental transfer of the polybrominated diphenyl ethers BDE-47, BDE-99 and BDE-209 in a human placenta perfusion system: an experimental study. Environ Health. 2010;9:32. doi: 10.1186/1476-069X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda ML, Edwards SE, Swamy GK, Paul CJ, Neelon B. Blood Lead Levels Among Pregnant Women: Historical Versus Contemporaneous Exposures. Int J Environ Res Public Health. 2010;7:1508–1519. doi: 10.3390/ijerph7041508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swamy GK, Garrett ME, Miranda ML, Ashley-Koch AE. Maternal vitamin D receptor genetic variation contributes to infant birthweight among black mothers. Am J Med Genet A. 2011;155A:1264–71. doi: 10.1002/ajmg.a.33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frederiksen M, et al. Polybrominated diphenyl ethers in paired samples of maternal and umbilical cord blood plasma and associations with house dust in a Danish cohort. Int J Hyg Environ Health. 2010;213:233–42. doi: 10.1016/j.ijheh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Vizcaino E, Grimalt JO, Fernández-Somoano A, Tardon A. Transport of persistent organic pollutants across the human placenta. Environ Int. 2014;65:107–15. doi: 10.1016/j.envint.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Gómara B, et al. Distribution of polybrominated diphenyl ethers in human umbilical cord serum, paternal serum, maternal serum, placentas, and breast milk from Madrid population, Spain. Environ Sci Technol. 2007;41:6961–8. doi: 10.1021/es0714484. [DOI] [PubMed] [Google Scholar]
- 32.Chen A, et al. Hydroxylated polybrominated diphenyl ethers in paired maternal and cord sera. Environ Sci Technol. 2013;47:3902–8. doi: 10.1021/es3046839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JT, et al. Partitioning Behavior of Heavy Metals and Persistent Organic Pollutants among Feto Maternal Bloods and Tissues. Environ Sci Technol. 2015;49:7411–7422. doi: 10.1021/es5051309. [DOI] [PubMed] [Google Scholar]
- 34.Chen ZJ, et al. Polybrominated diphenyl ethers (PBDEs) in human samples of mother-newborn pairs in South China and their placental transfer characteristics. Environ Int. 2014;73C:77–84. doi: 10.1016/j.envint.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Patel J, Landers K, Li H, Mortimer RH, Richard K. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab. 2011;22:164–70. doi: 10.1016/j.tem.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Dassanayake RMAPS, Wei H, Chen RC, Li A. Optimization of the matrix solid phase dispersion extraction procedure for the analysis of polybrominated diphenyl ethers in human placenta. Anal Chem. 2009;81:9795–801. doi: 10.1021/ac901805d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu L, et al. Polybrominated diphenyl ethers in human placenta associated with neonatal physiological development at a typical e-waste recycling area in China. Environ Pollut. 2015;196:414–22. doi: 10.1016/j.envpol.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Main KM, et al. Flame retardants in placenta and breast milk and cryptorchildism in newborn boys. Environ Health Perspect. 2007;115:1519–1526. doi: 10.1289/ehp.9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frederiksen M, Thomsen M, Vorkamp K, Knudsen LE. Patterns and concentration levels of polybrominated diphenyl ethers (PBDEs) in placental tissue of women in Denmark. Chemosphere. 2009;76:1464–9. doi: 10.1016/j.chemosphere.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Chen ZJ, et al. Polybrominated diphenyl ethers (PBDEs) in human samples of mother-newborn pairs in South China and their placental transfer characteristics. Environ Int. 2014;73C:77–84. doi: 10.1016/j.envint.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Takasuga T, Kumar KS, Watanabe K. Accumulation profiles of organochlorine pesticides and PBDEs in mothers-blood,-breast milk,-placenta and umbilical cord: possible transfer to infants. Organohalogen …. 2006 at < https://scholar.google.com/scholar?hl=en&q=Accumulation+profiles+of+organochlorine+pesticides+and+PBDEs+in+mother%E2%80%99s+blood%2C+breast+milk%2C+placenta+and+umbilical+cord%3A+possible+transfer+to+infants&btnG=&as_sdt=1%2C34&as_sdtp=#0>.
- 42.Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs--a review of levels and sources. Int J Hyg Environ Health. 2009;212:109–34. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, Ruan X, Li Y, Yan M, Qin Z. Polybrominated diphenyl ethers (PBDEs) in aborted human fetuses and placental transfer during the first trimester of pregnancy. Environ Sci Technol. 2013;47:5939–46. doi: 10.1021/es305349x. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, et al. Polybrominated diphenyl ethers in human placenta associated with neonatal physiological development at a typical e-waste recycling area in China. Environ Pollut. 2015;196:414–22. doi: 10.1016/j.envpol.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Hamers T, et al. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92:157–73. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- 46.Butt CM, Stapleton HM. Inhibition of thyroid hormone sulfotransferase activity by brominated flame retardants and halogenated phenolics. Chem Res Toxicol. 2013;26:1692–702. doi: 10.1021/tx400342k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Préau L, Fini JB, Dubois GM, Demeneix B. Thyroid hormone signalling during early neurogenesis and its significance as a vulnerable window for endocrine disruption. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbagrm.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–67. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams AL, DeSesso JM. The potential of selected brominated flame retardants to affect neurological development. J Toxicol Environ Health B Crit Rev. 2010;13:411–448. doi: 10.1080/10937401003751630. [DOI] [PubMed] [Google Scholar]
- 50.Herbstman JB, Mall JK. Developmental Exposure to Polybrominated Diphenyl Ethers and Neurodevelopment. Curr Environ Heal reports. 2014;1:101–112. doi: 10.1007/s40572-014-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen A, et al. Prenatal Polybrominated Diphenyl Ether Exposures and Neurodevelopment in U.S. Children through 5 Years of Age: The HOME Study. Environ Health Perspect. 2014 doi: 10.1289/ehp.1307562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma J, Qiu X, Ren A, Jin L, Zhu T. Using placenta to evaluate the polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) exposure of fetus in a region with high prevalence of neural tube defects. Ecotoxicol Environ Saf. 2012;86:141–6. doi: 10.1016/j.ecoenv.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 53.Leung AOW, et al. Body burdens of polybrominated diphenyl ethers in childbearing-aged women at an intensive electronic-waste recycling site in China. Environ Sci Pollut Res Int. 2010;17:1300–13. doi: 10.1007/s11356-010-0310-6. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J, et al. Levels of PCDD/Fs, PCBs and PBDEs compounds in human placenta tissue. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42:911–8. [PubMed] [Google Scholar]