Abstract
The role that anergy, an acquired state of T cell functional unresponsiveness, plays in natural peripheral tolerance remains unclear. In this study, we demonstrate that anergy is selectively induced in fetal antigen-specific maternal CD4+ T cells during pregnancy. A naturally occurring subpopulation of anergic polyclonal CD4+ T cells, enriched in self antigen-specific T cell receptors, is also observed in healthy hosts. Neuropilin-1 expression in anergic conventional CD4+ T cells is associated with thymic regulatory T cell (Treg cell)-related gene hypomethylation, and this correlates with their capacity to differentiate into Foxp3+ Treg cells that suppress immunopathology. Thus, our data suggest that not only is anergy induction important in preventing autoimmunity, but it also generates the precursors for peripheral Treg cell differentiation.
Thymocytes that bind self antigen with high affinity are either deleted or selected to become suppressive Foxp3+ regulatory T cells (Treg cells)1. Nevertheless, not every self-antigen is expressed in the thymus. Consequently, some mature self-reactive CD4+ T cells emigrate to the periphery where they can recognize self peptide-Major Histocompatibility Complex class II (pMHCII) complexes. This necessitates peripheral tolerance mechanisms to prevent the development of autoimmune disease2.
Anergy has been postulated as one such peripheral tolerance mechanism wherein CD4+ T cells lose the capacity to produce autocrine growth factor and proliferate in response to antigen2,3. Multiple biochemical signaling defects have been ascribed to this inactivated state, including the up-regulation of counter-regulatory gene products such as Dgka, Rap1, Cblb, Itch, Rnf128, and Dtx12,3. In vivo, anergy is induced by repeated interactions with pMHCII complexes in the absence of infection or adjuvant, but is antagonized by mTOR pathway signaling4–7. For the case of T cell receptor (TCR)-transgenic CD4+ T cells, chronic recognition of self pMHCII in normal mice leads to anergy induction in association with the up-regulation of two surface markers: CD73 (Nt5e) and folate receptor 4 (FR4, Izumo1r)8,9. In contrast to normal hosts, the adoptive transfer of self-reactive CD4+ T cells into lymphopenic mice lacking Foxp3+ Treg cells fails to elicit the induction of anergy and instead leads to T effector/memory (Teff/mem) differentiation and severe immunopathology9–11.
Treg cells are also required for the maintenance of peripheral immune homeostasis to prevent immunopathology throughout the life span of an individual12–16. Most Foxp3+ Treg cells originate in the thymus (tTreg cells) and express neuropilin-1 (Nrp1), but some also differentiate in the periphery (pTreg cells) from conventional CD4+ T cells and appear to remain negative for Nrp1 expression17–20. Several studies have indicated that the expression of Foxp3 alone is not sufficient to maintain a stable Treg cell lineage. tTreg cells have a defined epigenetic signature (called tTreg-me) consisting of hypomethylated CpGs at four Treg cell-related genes: Tnfrsf18 (the gene encoding GITR), Ctla4, Ikzf4, as well as the Foxp3 conserved non-coding DNA sequence 2 (CNS2)20,21. Expression of Foxp3 protein and development of the tTreg-me epigenetic signature are independent and complementary events in tTreg cell differentiation. Demethylation of select Treg cell signature genes is associated with constitutive expression, whereas Foxp3 DNA-binding and transactivation at other signature genes occur only during Treg cell activation22–24. Notably, the loss of Foxp3 expression can occur leading to trans-differentiation of Treg cells to Teff/mem cells capable of causing autoimmunity25.
Despite this great potential for CD4+ T cell anergy and Treg cell suppression to act in concert to establish tolerance to peripherally expressed self-antigens, the investigation of anergy within a diverse auto-reactive polyclonal repertoire has been difficult, in part due to the lack of identifying markers. We now describe a novel subset of naturally occurring Foxp3−CD44hiCD73hiFR4hi polyclonal CD4+ T cells that is functionally anergic in healthy hosts. This anergic subset is enriched in self antigen-specific TCRs, and is also induced in response to fetal antigen recognition during pregnancy. Importantly, Nrp1+ anergic conventional CD4+ T cells demonstrate tTreg-me and preferentially give rise to functional Foxp3+Nrp1+ pTreg cells in vivo. Thus, anergy induction not only serves to reduce the proliferative responsiveness of potentially dangerous self-reactive CD4+ T cells, but also generates progenitors for pTreg cell differentiation.
RESULTS
Fetal antigen-reactive CD4+ T cells develop anergy
It was previously shown that KRN TCR-transgenic CD4+ T cells specific for ‘self’ glucose-6-phosphate isomerase (GPI) presented by MHC class II I-Ag7 undergo an abortive clonal expansion, up-regulate the expression of CD73 and FR4, and develop anergy when transferred into healthy H-2g7-strain hosts9. Subsequently it was reported that NOD mice also accumulate unresponsive Foxp3−CD44hiCD73hiFR4hi insulin-specific polyclonal CD4+ T cells in the pancreatic lymph node prior to frank diabetes onset (Supplementary Fig. 1)26. These results suggest that anergy might serve as a natural peripheral self-tolerance checkpoint and barrier to autoimmune disease development.
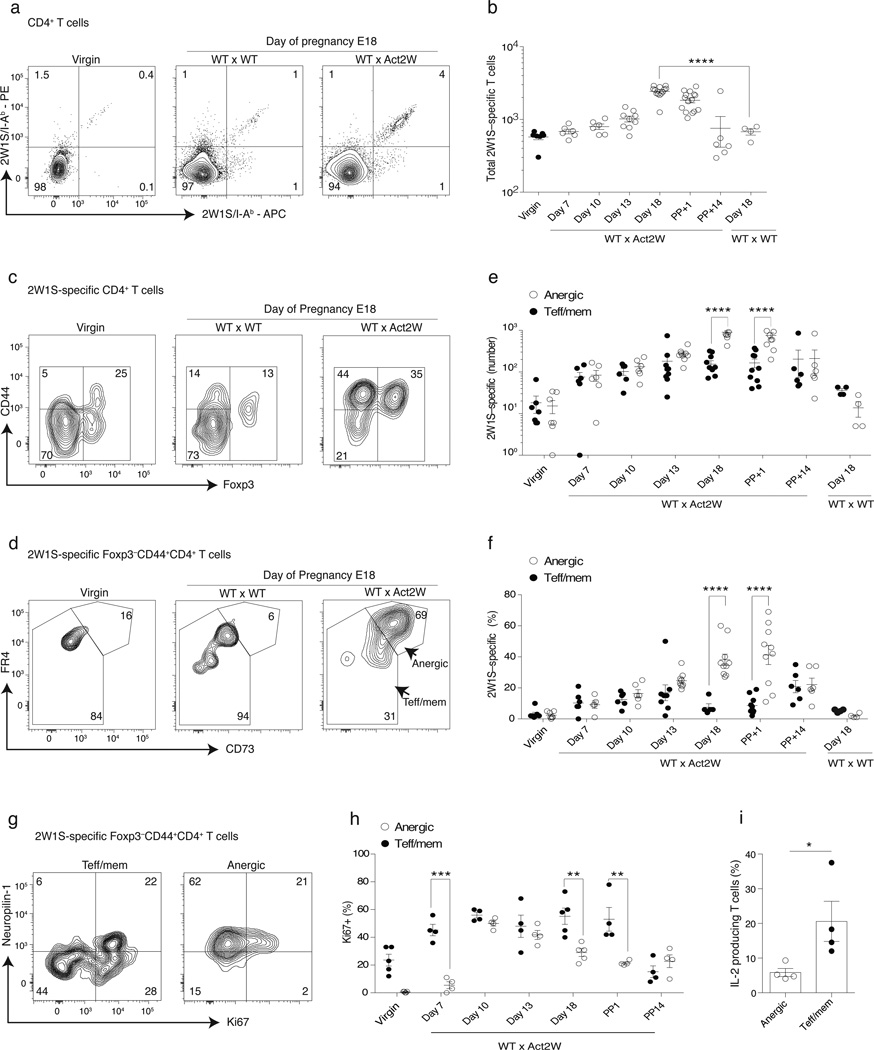
We therefore aimed to study anergy in a more physiological polyclonal setting, where the specificity of the self-antigen is known and a timeline for tolerance induction can be established. We made use of a model of fetal antigen-specific tolerance wherein male B6 Act2W transgenic breeder mice engineered to ubiquitously express a 2W1S55–68 (hereafter 2W1S) peptide are crossed with non-transgenic syngeneic B6 females27. Mothers were then examined for the development of tolerance in the 2W1S:I-Ab-specific polyclonal CD4+ T cell compartment, as defined using 2W1S:I-Ab tetramers28. A small population of 2W1S-specific CD4+ T cells (~400) was found in virgin females, and this number did not change by day 18 of gestation in pregnant females bred to non-transgenic males (Fig. 1a, b). However, pregnant hosts crossed to 2W1S-transgenic males demonstrated a small yet significant increase of 2W1S-specific Foxp3− CD44hi conventional CD4+ T cells that co-expressed CD73 and FR4, consistent with the hypothesis that they had been made anergic following recognition of fetus-derived 2W1S peptide (Fig. 1c–f). More than 80% of anergic T cells also specifically expressed Nrp1 by day 18 of pregnancy (Fig. 1g, and data not shown). Ki67 expression increased in both the anergic and non-anergic CD73loFR4lo (hereafter called Teff/mem) fractions of CD44hi 2W1S-specific T cells through day 10 of pregnancy, but at later time points Ki67 expression fell specifically in the anergic T cell group indicating the development of proliferative unresponsiveness (Fig. 1g–h). Consistent with proliferative anergy, at day 18 a significantly lower percentage of anergic phenotype 2W1S-specific CD4+ T cells retained the capacity to synthesize interleukin-2 (IL-2) in response to in vivo 2W1S peptide challenge, as compared to Teff/mem cells (Fig. 1i). By 14 d postpartum the anergic 2W1S:I-Ab–specific maternal CD4+ T cells demonstrated a sharp decline, suggesting that continuous antigen recognition and/or the pregnant state is required to maintain the phenotype or survival of CD4+ T cells made anergic to a fetal antigen (Fig. 1f). Taken together, these results validated the use of anti-CD73 and anti-FR4 as predictive biomarkers of polyclonal CD4+ T cell anergy induction in vivo.
Figure 1.
Maternal polyclonal CD4+ T cells specific for fetal Ag accumulate during gestation with an anergic phenotype. Wild-type B6 females were bred to Act2W-transgenic syngeneic B6 male mice, and then 2W1S:I-Ab tetramer-binding T cells were tracked at various times during and after gestation. (a) Representative flow cytometry plots showing CD4+ T cells from virgin or d18 pregnant females (WT × WT and WT × Act2W) that were immobilized and stained using a combination of PE and APC 2W1S:I-Ab tetramers for cells pooled from spleen and lymph nodes (inguinal, axillary, brachial, cervical, mesenteric and pancreatic). (b) Number of 2W1S:I-Ab tetramer-binding T cells at various time-points during pregnancy. Filled circles indicate virgin mice (c) Representative 2W1S:I-Ab tetramer-binding T cells stained for CD44 and Foxp3. (d) Identification of anergic (CD73hiFR4hi) and Teff/mem (CD73loFR4lo) cells within the Foxp3−CD44hi fraction of 2W1S:I-Ab tetramer-binding CD4+ polyclonal T cells. (e, f) Number and percentage of 2W1S:I-Ab tetramer-binding anergic and Teff/mem cells during pregnancy. (g) Representative Ki67 and Nrp1 staining for 2W1S:I-Ab tetramer-binding Teff/mem and anergic cells on day 18 of pregnancy (h) Percentage of 2W1S:I-Ab tetramer-binding anergic and Teff/mem cells that express Ki67 during pregnancy. (i) Pregnant females on day 18 of gestation were injected with 100 µg of 2W1S peptide for two hours. Tetramer enriched cells were stained intracellularly for IL-2. Mean data are representative of 3 to 4 independent experiments, n = 3 to 6 mice each experiment. Error bars represent the SEM. One-way ANOVA; * p < 0.05 **p < 0.01, *** p < 0.001, **** p < 0.0001 in (c, f, g, i). Unpaired one-tailed Student t-Test (j). Circles indicate individual mice.
Anergic polyclonal CD4+ T cells express CD73 and FR4
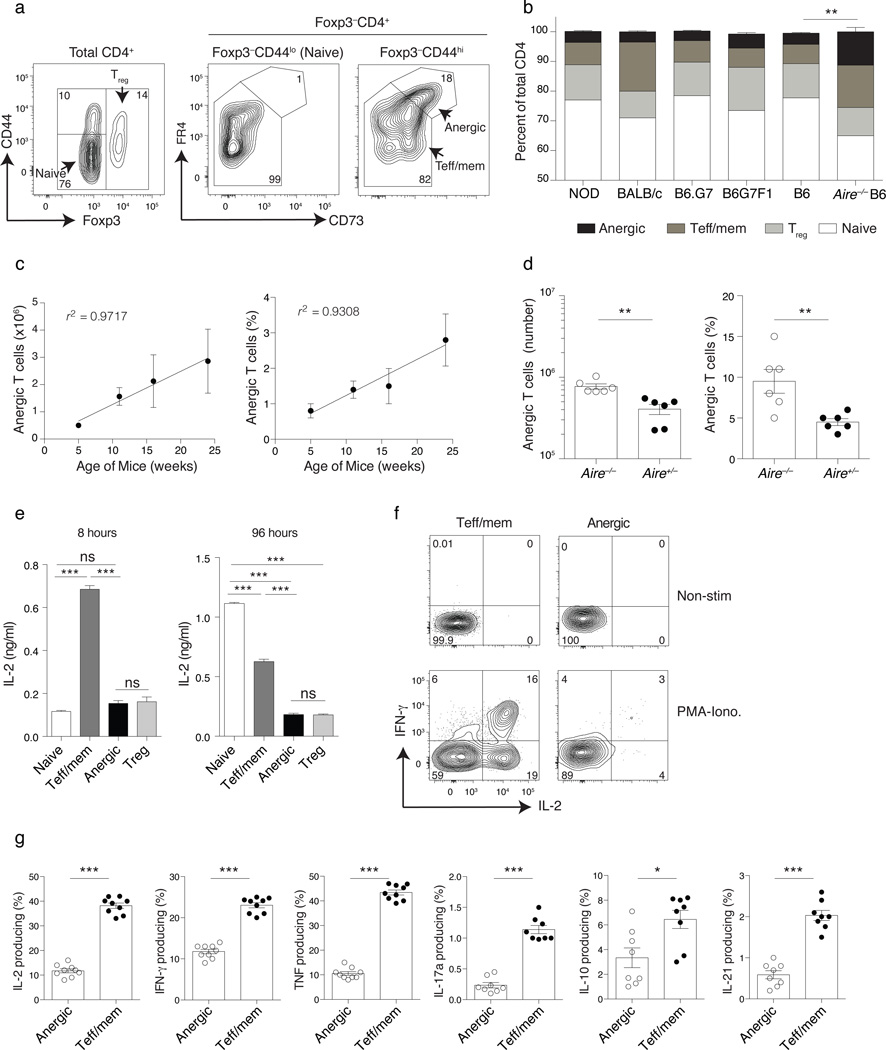
To investigate the general importance of in vivo anergy to peripheral self-tolerance we focused our attention on conventional CD4+ T cells that expressed CD44, CD73, and FR4 in combination within the secondary lymphoid organs. As naive Foxp3−CD44loCD4+ T cells in the lymph nodes and spleen express only low to intermediate CD73 and FR4, these cells were used to set flow cytometry analysis gates and then the Foxp3−CD44hi polyclonal CD4+ T cell repertoire was examined for evidence of high level CD73 and FR4 co-expression (Fig. 2a). A population of Foxp3−CD44hiCD73hiFR4hi polyclonal CD4+ T cells was identified in all healthy mouse strains tested, comprising approximately 2 – 5% of the total peripheral CD4+ T cell compartment (Fig. 2a,b). Both the frequency and absolute number of polyclonal anergic-phenotype CD4+ T cells rose with age (Fig. 2c). Similar to results reported for Bim-deficient mice29, Aire−/− mice defective for central T cell tolerance showed a significant increase in the number and frequency of anergic-phenotype CD4+ T cells in the lymph nodes and spleen, as compared to their littermate controls (Fig. 2b,d). Importantly, Foxp3−CD44hiCD73hiFR4hi polyclonal CD4+ T cells produced little IL-2 at either 8 or 96 hours following in vitro stimulation with the combination of anti-CD3 and anti-CD28 (Fig. 2e). In vitro proliferation in response to 96 h of CD3 and CD28 mAb stimulation was similarly reduced in anergic polyclonal T cells (average cell divisions = 1.0 ± 0.2) as compared to naive CD4+ T cells (4.0 ± 0.1 divisions, data not shown). For all other cytokines tested, including TNF, IFN-γ, IL-17a, IL-10, and IL-21, anergic cells showed little response to in vitro PMA+Ionomycin stimulation as compared to antigen-experienced Teff/mem cells (Fig. 2f,g). Therefore, the development of a peripheral Foxp3−CD44hiCD73hiFR4hi anergic CD4+ T cell phenotype is not an infrequent event in healthy mice, is increased in the setting of defective central tolerance, and is associated with a reduced capacity to produce IL-2 and proliferate.
Figure 2.
Foxp3−CD44hiCD73hiFR4hi anergic phenotype CD4+ polyclonal T cells accumulate in the secondary lymphoid organs. Cells pooled from spleen and lymph nodes (inguinal, axillary, brachial, cervical, mesenteric and pancreatic) (a) Representative analysis of Foxp3−CD44hi CD4+ polyclonal T cells for the expression of CD73 and FR4. (b) Percent Foxp3−CD44lo (naive), Foxp3+ (Treg cell), Foxp3−CD44hiCD73loFR4lo (Teff/mem) and Foxp3−CD44hiCD73hiFR4hi (anergic) of total CD4+ T cells in various mouse strains as indicated. Chi-Square test (c) Change in the mean total number and percentage of anergic CD4+ cells from secondary lymphoid organs per mouse over time, with Pearson correlation coefficient (r2) as indicated. (d) Number and percentage of anergic-phenotype CD4+ polyclonal T cells from Aire−/− and age-matched Aire+/− littermate controls. (e) Sorted polyclonal naive, Teff/mem, anergic, and Treg cell CD4+ T cell subsets from Foxp3DTR donors stimulated with a combination of anti-CD3 and CD28 for 8 or 96 h (as indicated) and then culture supernatants examined for secretion of IL-2 by ELISA. (f, g) Pooled spleen and lymph node CD4+ polyclonal T cell subsets stimulated with 1 µg/ml of PMA and 1µM ionomycin (PMA+Iono.) for 4 h or left unstimulated and then stained for intracellular IL-2, IFN-γ, TNF, IL-17a, IL-10, and IL-21. Mean data are representative of 2 independent experiments, n = 4 to 5 mice each experiment. Error bars represent the SEM. One-way ANOVA (e) and Unpaired one-tailed Student’s t-Test (d, g). * p < 0.05, **p < 0.01, *** p < 0.0001. Points denote individual mice.
Anergic cells are quiescent despite tonic pMHCII recognition
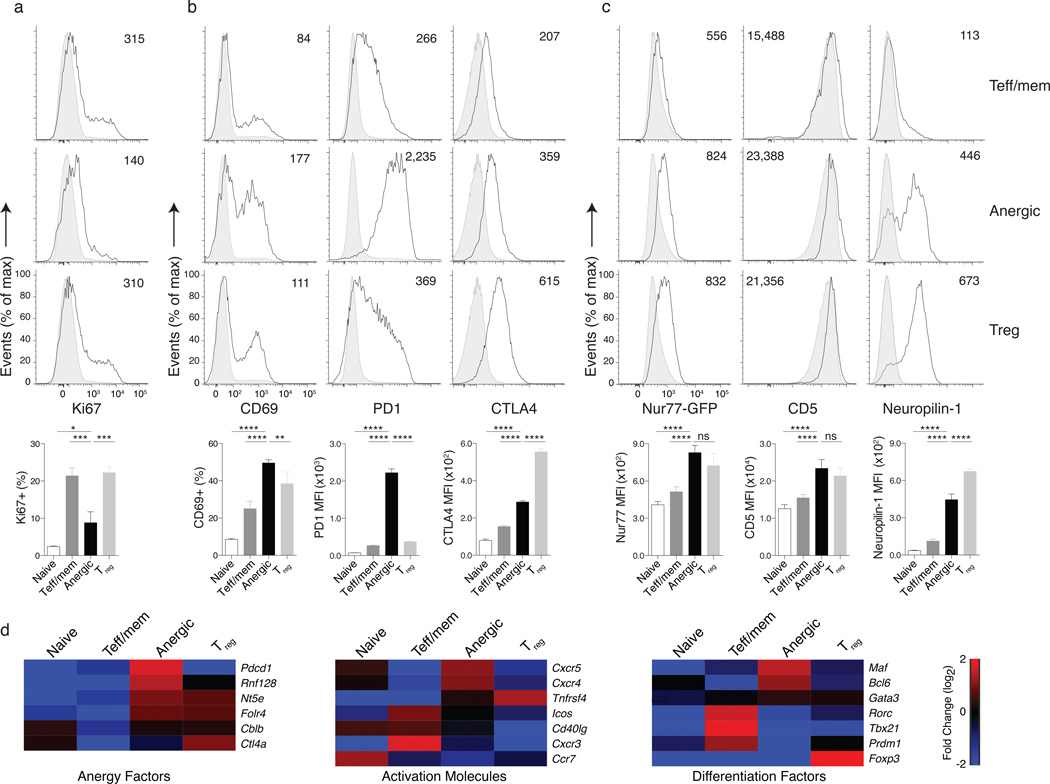
Consistent with the pregnancy model, naturally anergic CD4+ T cells did not appear to be in cell cycle, as evidenced by low expression of Ki67 and low forward scatter (Fig. 3a, and data not shown). Despite this proliferative quiescence, the expression of cell activation markers typically associated with strong TCR engagement (e.g., CD69, PD-1, CTLA4) was high on anergic T cells (Fig. 3b). Using a Nur77GFP reporter mouse to monitor TCR signal strength in vivo30 it was previously concluded that the high Nur77 found in Treg cells was a consequence of the expression of high affinity TCRs for self-pMHCII complexes. Using this Nur77GFP reporter mouse, we found that Nur77 expression in anergic T cells was comparable to Treg cells and higher than either naive or Teff/mem cells (Fig. 3c). CD5 and Nrp1 expression on anergic T cells was also similar to Treg cells but higher than both Teff/mem and naive cells. Consistent with these protein data, a survey of mRNA expression in these various T cell subpopulations confirmed a unique CD4+ T cell anergy gene signature different to either conventional Teff/mem cell or Treg cells, and including expression of the anergy-associated E3 ubiquitin ligase Rnf128 (known as GRAIL31) (Fig. 3d). Thus, anergic T cells in healthy mice show numerous signs of persistent self-pMHCII recognition and TCR engagement similar to Treg cells, yet appear non-proliferative.
Figure 3.
Anergic polyclonal CD4+ T cells are quiescent at steady state, yet show signs of continuous Ag encounter. Cells pooled from spleen and lymph nodes (inguinal, axillary, brachial, cervical, mesenteric and pancreatic) (a) Ki67 expression on Foxp3−CD44lo naive (shaded histograms), Foxp3−CD44hiCD73loFR4lo Teff/mem, Foxp3−CD44hiCD73hiFR4hi anergic, and Foxp3+ Treg cell (all three open tracings, as indicated) polyclonal CD4+ T cells from 8 to 11 week old B6 mice. The bar graphs below indicate the mean percentage of Ki67 expressing cells in each group. (b) Representative CD69, PD1, and CTLA4 expression, with bar graphs representing the mean percent CD69+ and MFI for PD1 and CTLA4. (c) CD4+ T cell subsets analyzed for Nr4a1 gene activation, CD5, and Neuropilin-1 in transgenic Nur77GFP reporter gene mice, with bar graphs below indicating the MFI. Mean data are representative of 2 independent experiments, n = 2 to 3 mice each experiment. Error bars represent the SEM. One-way ANOVA; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, non-significant (ns) (d) Gene expression data for individual sample groups are shown as the fold change (log base 2) relative to the mean of all samples, where red indicates up-regulation and blue indicates down-regulation of transcripts.
Reversal of anergy during T cell lymphopenia
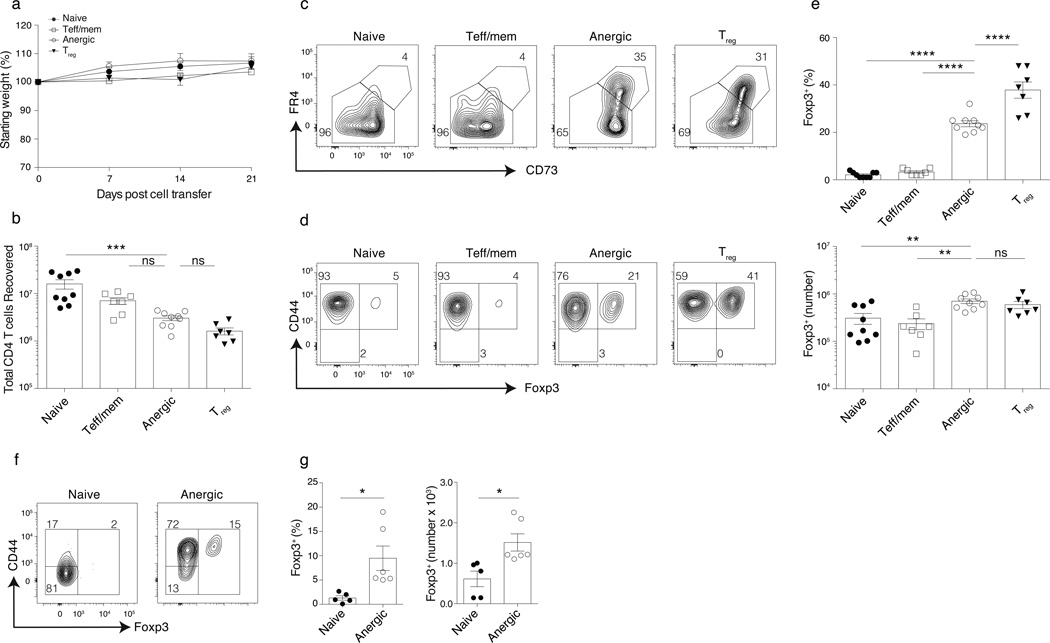
Naive GPI:I-Ag7–specific KRN CD4+ T cells cause arthritis when transferred to lymphopenic T cell receptor alpha deficient (Tcra−/−) GPI:I-Ag7–expressing B6G7F1 mice due to a failure of anergy induction in the absence of Treg cells9,32. We also found that KRN CD4+ T cells made anergic in GPI:I-Ag7–expressing wild-type mice inevitably lost their expression of CD73 and FR4 and caused arthritis and wasting disease following their adoptive transfer to lymphopenic Tcra−/− recipients (Supplementary Fig. 2). Therefore, we tested whether highly purified GFP−CD44hiCD73hiFR4hi anergic polyclonal CD4+ T cells from Foxp3DTR mice would also undergo anergy reversal and cause immunopathology following their adoptive transfer to Tcra−/− lymphopenic hosts (Supplementary Figure 3). Reversal of anergy did occur in lymphopenic mice, as most anergic T cells down-regulated CD73 and FR4 and underwent a clonal expansion consistent with a restoration of proliferative responsiveness. Nevertheless, anergy reversal failed to elicit any evidence of disease by day 21 (Fig. 4a–c). 20 ± 5% of the total donor-derived CD4+ T cells developed a Treg cell phenotype when anergic donor T cells were used (Fig. 4d,e). In contrast, the adoptive transfer of either naive or Teff/mem polyclonal CD4+ T cells failed to give rise to as many Treg cells in the Tcra−/− hosts (Fig. 4d,e). This differentiation of anergic conventional CD4+ T cells into Treg cells was not unique to lymphopenic hosts, as the adoptive transfer of purified anergic T cells into wild type B6 hosts also led to a conversion of 9.5 ± 6.3% of the cells to a Treg cell phenotype (Fig. 4f–g). Therefore, polyclonal anergic CD4+ T cells appear uniquely capable of differentiating into Treg cells.
Figure 4.
Reversal of anergy in polyclonal CD4+ T cells gives rise to Foxp3+ Treg cells. Cells pooled from spleen and lymph nodes (inguinal, axillary, brachial, cervical, mesenteric and pancreatic) or as specified. Sorted naive, Teff/mem, anergic, and Treg cell Foxp3DTR polyclonal CD4+ T cells were transferred (105) into syngeneic lymphopenic Tcra−/− B6 mice and analyzed 21 d later. (a) Weights of mice over time, relative to start. (b) Number of donor-derived spleen and lymph node CD4+ T cells recovered on day 21. Donor-derived CD4+ T cell expression of (c) CD73 and FR4, or (d) CD44 and Foxp3 on day 21. (e) Percent and number of Foxp3+ Treg cells among total donor CD4+ T cells on day 21. (f, g) Sorted naive and anergic Foxp3DTR polyclonal CD4+ T cells were transferred (5 × 105) into syngeneic lymphoreplete B6 mice and analyzed 30 d later. (f) Representative flow cytometry staining and (g) Percentage and number of Foxp3+ Treg cella among donor derived CD4+ T cells on day 30. Mean data shown are representative of 3 (a–e) and 2 independent experiments (f–g), with n = 2 to 3 mice per group. Error bars represent the SEM. One-way ANOVA; * p < 0.05 ** p < 0.01, *** p < 0.001, **** p < 0.0001, non-significant (ns). Points denote individual mice.
Deletion of anergy-derived Treg cells causes immunopathology
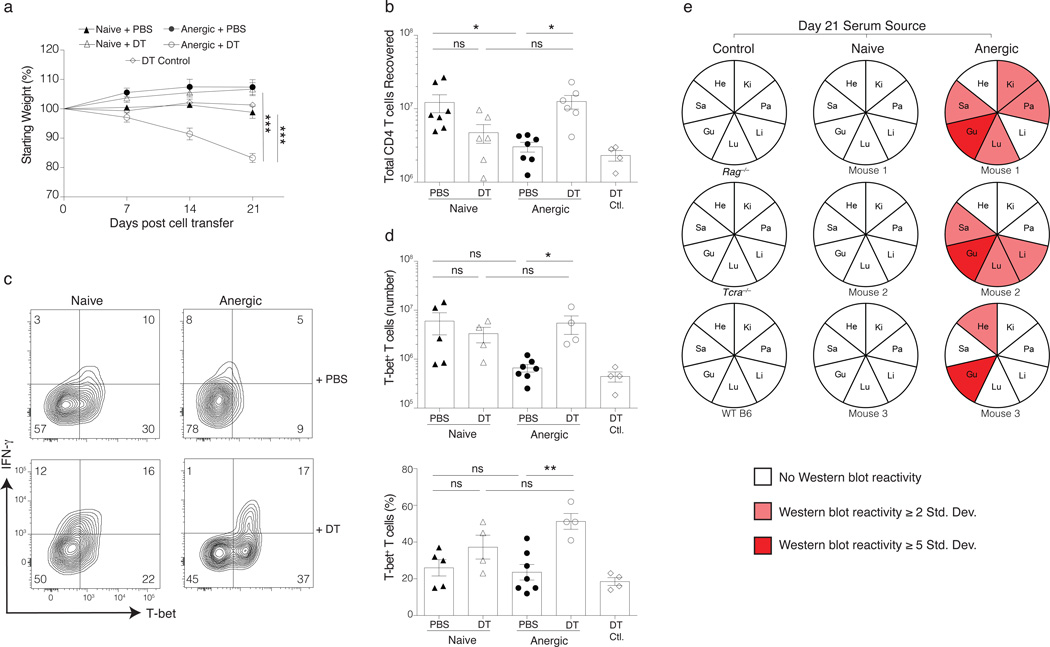
The resistance of the Tcra−/− hosts to immunopathology following the adoptive transfer of polyclonal anergic CD4+ T cells led us to the hypothesis that newly differentiated Treg cells inhibited the pathogenicity of other non-Treg cells following anergy reversal. To test this, we used diphtheria toxin (DT) to selectively ablate any Foxp3-expressing T cells that arise from purified anergic Foxp3DTR donor T cells during anergy reversal (Supplementary Fig. 4). The majority of Tcra−/− mice that received both anergic T cells and DT developed a severe wasting syndrome by day 21. This weight loss was not simply a result DT toxicity, as mice receiving anergic cells from a Foxp3GFP mouse and given the same DT regimen (DT Control) showed no signs of disease (Fig. 5a). Selective Treg cell ablation during anergy reversal also led to an increase in the number of T-bet-expressing Teff/mem cells as compared to the no DT (PBS) treated anergic group, consistent with a suppression of their clonal proliferation by anergy-derived Treg cells in the absence of DT (Fig. 5b–d). Additionally, sera obtained from DT-treated recipients of anergic Foxp3DTR T cells contained autoantibodies reactive to several tissues, indicating the development of systemic autoimmunity (Fig. 5e, Supplementary Fig. 5). In contrast to anergic T cell recipients, all lymphopenic mice receiving either naive or Teff/mem cells from Foxp3DTR donors remained healthy at day 21 even in the presence of DT (Fig. 5a–e, and data not shown). Thus, the anergic Foxp3−CD44hiCD73hiFR4hi polyclonal CD4+ T cell population in healthy mice naturally contains T cells with potentially dangerous TCRs, as well as conventional CD4+ T cells that can differentiate to protective Treg cells.
Figure 5.
Depletion of Treg cells derived from anergic cells leads to autoimmunity in lymphopenic mice. Sorted naive or anergic syngeneic Foxp3DTR polyclonal CD4+ T cells were transferred (105) into syngeneic lymphopenic Tcra−/− hosts and treated with either PBS or diphtheria toxin (DT), as indicated. Mice were monitored for weight loss and the experiment stopped if ~20% weight loss was observed. (a) Change in body weight of Tcra−/− mice receiving anergic T cells from Foxp3GFP mice and then treated with DT as a toxicity control (‘DT Control’), or else anergic or naive T cells from Foxp3DTR mice adoptively transferred in the presence or absence of DT. (b) Total donor-derived CD4+ T cells recovered on day 21. (c) Day 21 spleen and LN cells from adoptive transfer mice treated with DT (or PBS control) were stimulated with 1 µg/ml of PMA and 1µM ionomycin for 4 h and then examined for intracellular T-bet and IFN-γ levels in donor-derived naive and anergic CD4+ T cells. (d) Number and percentage of T-bet+ cells among total donor CD4+ T cells on day 21. Mean data shown are representative of 3 independent experiments, with n = 4 to 7 mice per group in total. Error bars represent the SEM. One-way ANOVA; * p < 0.05, ** p < 0.01, *** p < 0.0001. Points denote individual mice. (e) Sera obtained from recipient mice on day 21 were used to probe various Rag−/− tissue extracts (He = heart, Ki = kidney, Pa = pancreas, Li = liver, Lu = lung, Gu = gut, Sa = salivary gland) in an immunoblot. Detectable serum antibody reactivity to tissue antigen(s) is indicated in red.
Anergy-derived Treg cells suppress autoimmunity
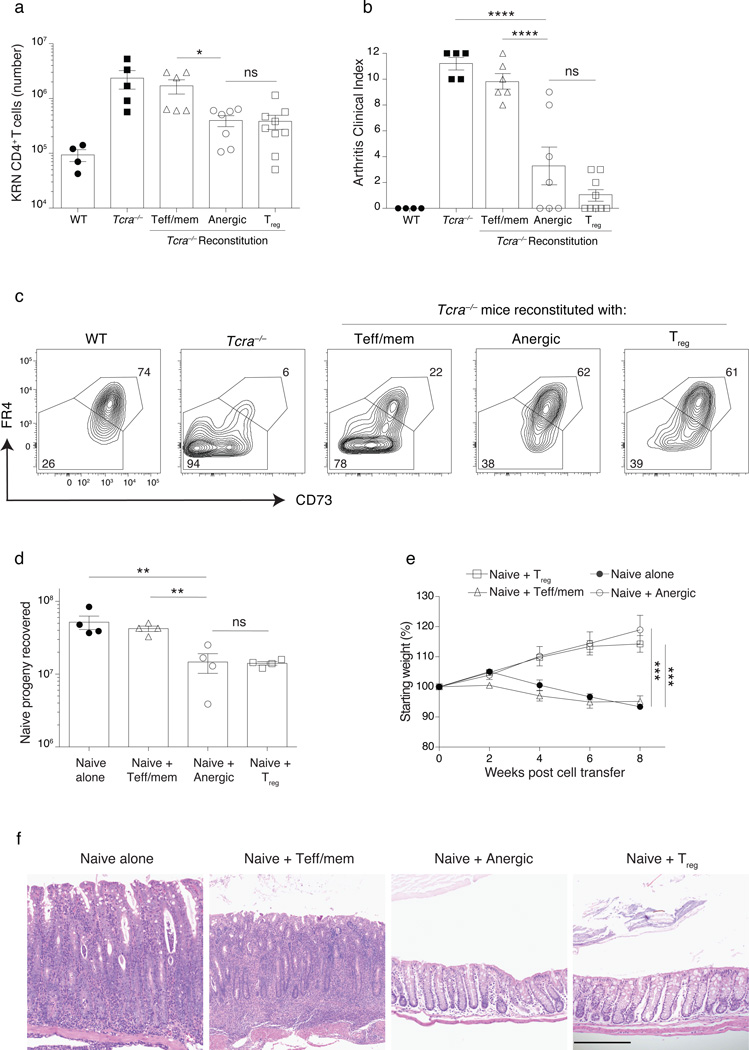
To formally test the suppressive capacity of anergy-derived Treg cells, lymphopenic Tcra−/− B6G7F1 hosts were first reconstituted with purified syngeneic polyclonal CD4+ anergic T cells for a period of 21 days and then were given a second adoptive transfer of KRN CD4+ T cells to elicit autoimmune arthritis. Anergic T cells again gave rise to Treg cells (Supplementary Fig. 6a–c). Consistent with the generation of strong in vivo Treg cell activity, reconstitution by anergy-derived Treg cells was associated with suppression of the KRN T cell clonal expansion and protection from severe arthritis (Fig. 6a,b). Reconstitution of Tcra−/− hosts by anergy-derived Treg cells also led to the up-regulation of CD73 and FR4 within the KRN T cells, consistent with their induction of anergy (Fig. 6c). Use of DT to selectively ablate newly generated Treg cells during anergy reversal increased arthritis (data not shown). In contrast to anergic cells, mice reconstituted with Teff/mem cells developed severe arthritis in association with higher KRN T cell numbers (Fig. 6a,b).
Figure 6.
Treg cells generated from anergic CD4+ polyclonal T cells prevent arthritis and colitis. (a–c) Lymphopenic Tcra−/− B6G7F1 mice were reconstituted with 1.5 × 106 sorted syngeneic Teff/mem, anergic, or Treg cell CD4+ polyclonal T cells from a Foxp3DTR mouse for a period of 21 d. On day 21, 104 naive KRN transgenic CD4+ T cells were transferred into these mice (as well as into control WT and Tcra−/− B6G7F1 hosts) and then recipients were monitored over 12 d for arthritis development as described9, with the (a) number of KRN cells recovered, and (b) clinical arthritis index score on day 12 after KRN cell transfer as indicated. (c) CD73 and FR4 expression on KRN T cells on day 33. (d–f) A total of 2 × 105 congenic–marked naive polyclonal CD4+ T cells were adoptively transferred into Tcra−/− B6 mice either alone or in combination with 4 × 105 Teff/mem, anergic, or Treg cell CD4+ polyclonal cells sorted from Foxp3GFP mice, and then recipients were monitored over 8 weeks for weight loss. (d) Number of donor naive cells recovered at the end of week 8. (e) Change in body weight of Tcra−/− B6 mice receiving naive cells alone, or naive plus Teff/mem, anergic or Treg cells. (f) Representative H&E staining of the colon (all panels same magnification, 200µm). Mean data shown are representative of 3 (a–c) or 2 (d–f) independent experiments, with n = 2 to 3 mice per group. Error bars represent the SEM. (a–c, d). Cells pooled from spleen and lymph nodes (inguinal, axillary, brachial, cervical, mesenteric and pancreatic). One-way ANOVA; * p < 0.05, ** p < 0.01, *** p < 0.001 **** p < 0.0001, non-significant (ns). Points denote individual mice.
By exploring a second model of TH17/TH1-dependent inflammatory bowel disease that is induced by the adoptive transfer of naive polyclonal CD4+ T cells into lymphopenic mice33,34, we discovered that a co-transfer of anergic polyclonal T cells together with the naive CD4+ T cell population allowed for the differentiation of anergy-derived Treg cells that suppressed the clonal expansion of the naive donor T cell progeny (Supplementary Fig. 6d,e and Fig. 6d). Furthermore, co-transferred anergic T cells protected all host mice against the development of chronic wasting disease, colitis, and typhlitis, similar to a co-transfer of bona fide Treg cells. In contrast, all Tcra−/− mice receiving naive syngeneic CD4+ T cells either alone or in combination with Teff/mem cells lost weight, and half of the mice demonstrated histological evidence of both colitis and typhlitis (Fig. 6e–f). Taken together, these results establish that anergy-derived Treg cells suppress CD4+ T cell-mediated immunopathology, based on their ability to block auto-reactive T cell clonal expansion, promote the induction of T cell anergy, and prevent the development of autoimmune arthritis and inflammatory bowel disease.
Some anergic cells have a unique tTreg-me
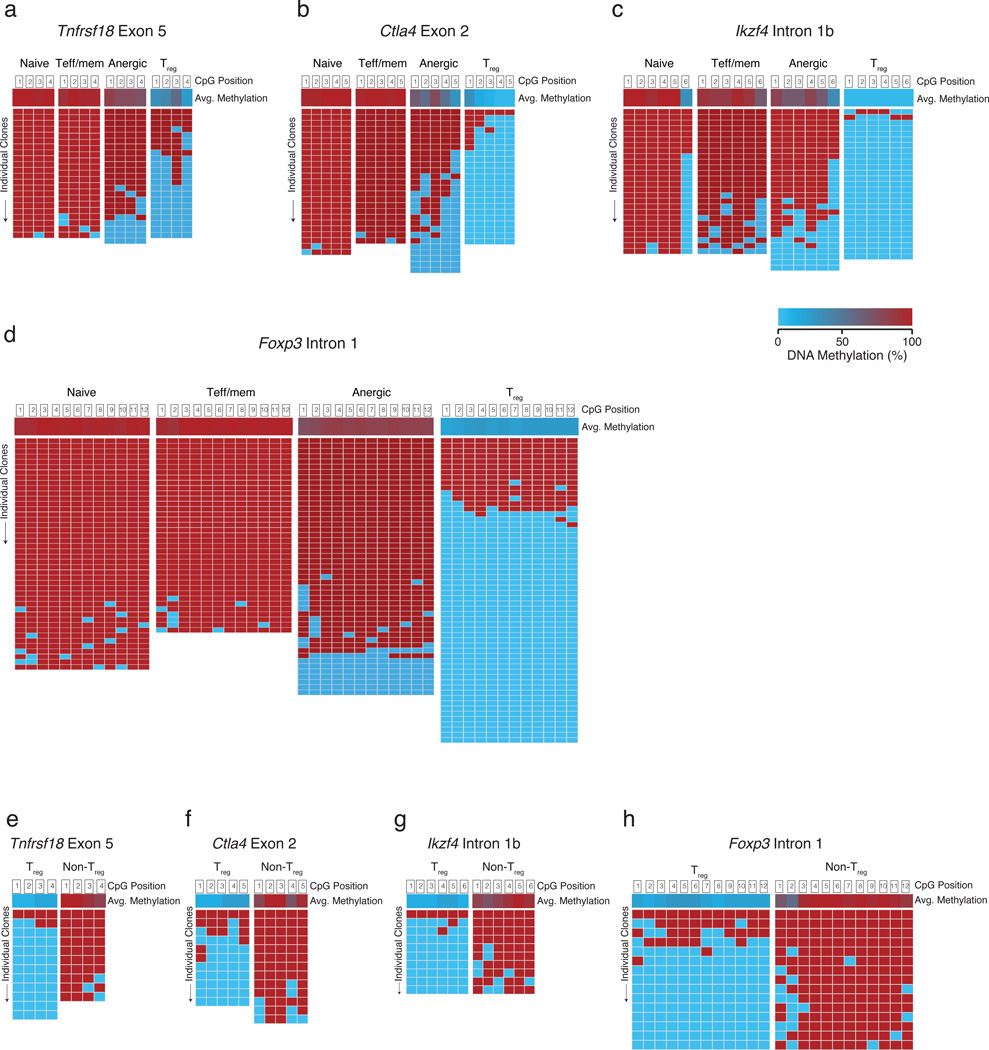
Why are anergic CD4+ T cells predisposed to Treg cell differentiation? The function and stability of tTreg cells is thought to depend on the expression of Foxp3 as well as the induction of an ‘tTreg -like epigenome’ (tTreg-me)21. This tTreg-me consists of hypomethylated CpGs at Tnfrsf18 (the gene encoding GITR) exon 5, Ctla4 exon 2, Ikzf4 intron 1b, and Foxp3 intron 1. Cells that express the tTreg-me but lack the expression of Foxp3 are proposed to be ‘potential Treg cells’20. To examine whether tTreg-me signature gene demethylation is involved in the differentiation of anergic T cells to Treg cells, bisulfite sequencing of purified naive, Teff/mem, anergic, and Treg cell DNA was performed. At Tnfrsf18 exon 5, 17% of anergic T cell DNA clones demethylated all four CpG positions in a pattern similar to the majority of Treg cells (Fig. 7a). The remainder of the Tnfrsf18 clones was heavily methylated similar to naive or Teff/mem cells. For the five CpG positions of Ctla4 exon 2, a total of 50% of the anergic T cell clones demonstrated at least 3 demethylations, whereas ≥3 demethylations were never observed in either naive or Teff/mem cells. In fact, 43% of the anergic cell clones showed a unique partial demethylation pattern not seen in any other naive, Teff/mem, or Treg cell group (Fig. 7b). At Ikzf4 intron 1b, a few clones (14%) were again fully demethylated like Treg cells, with the remainder more similar to naive or Teff/mem cells (Fig. 7c). Different from either naive or Teff/mem cells, 14 ± 12% (± S.D.) of anergic clones were completely demethylated at all twelve CpG positions of Foxp3 intron 1 similar to Treg cells, contributing to a total of 18% of the anergic clones with at least 3 demethylated CpGs (Fig. 7d). Consistent with peripheral conversion to a stable Treg cell lineage, anergy-derived Treg cells demonstrated the full tTreg-me demethylation signature (Fig. 7e–i). Note that only a very low frequency of exFoxp3 cells were observed within the anergic CD4+ T cell compartment, based on Foxp3 promoter-driven fate mapping35,36 (Supplementary Fig. 7a–c); therefore, this unique anergic T cell gene demethylation signature appears to reflect a predisposition to differentiate into a stable Treg cell lineage, rather than a history of previous Treg cell lineage commitment and subsequent loss of Foxp3 expression.
Figure 7.
Polyclonal anergic CD4+ T cells demonstrate unique gene methylations. Sorted naive, Teff/mem, anergic, and Treg cell populations were examined for CpG methylation status using bisulfite sequencing. Average methylation for each CpG probed is shown in the top row, followed by rows representing sequencing reactions of individual amplicons. Methylation patterns shown are for (a) Tnfrsf18 (GITR) exon 5, (b) Ctla4 exon 2, (c) Ikzf4 (Eos) intron 1b, and (d) Foxp3 intron 1. Data are pooled from 3 independent sequencing experiments, utilizing T cell subsets sorted from a total of 6 males and 6 females. (e–h) Foxp3+ Treg cells sorted on day 21 after transfer of polyclonal anergic cells (105) into syngeneic lymphopenic Tcra−/− B6 mice were examined for CpG methylation status using bisulfite sequencing. (e) Tnfrsf18 exon 5, (f) Ctla4 exon 2, (g) Ikzf4 intron 1b, and (h) Foxp3 intron 1.
Treg cell progenitors exist within the Nrp1+ anergic cells
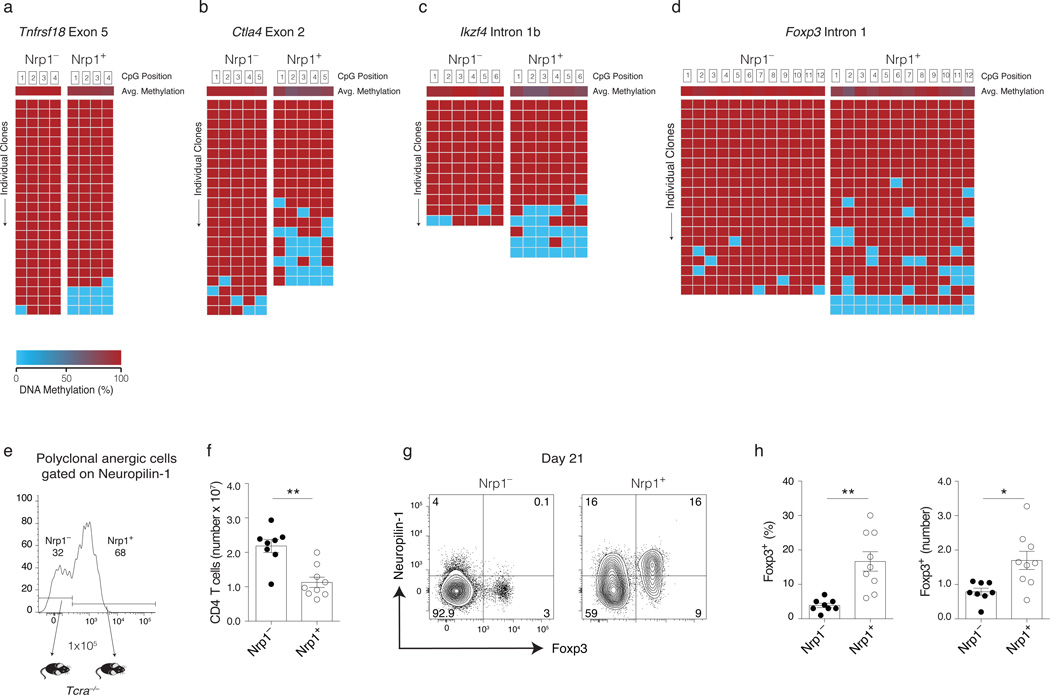
More than 60% of Foxp3−CD44hiCD73hiFR4hi anergic polyclonal CD4+ T cells expressed Nrp1 at steady state, and Nrp1 expression increased on fetal antigen-specific anergic T cells as pregnancy progresses (Fig. 1h and 3c, and data not shown). Given that Nrp1 is highly expressed on tTreg cells and its expression promotes Treg cell stability by inhibiting de-differentiation programs17–19, we asked whether its expression on certain anergic T cells predicts a greater capacity to differentiate to the Treg cell lineage. Bisulfite sequencing showed that Nrp1+ anergic T cells were more demethylated at the tTreg-me genes (Fig. 8a–d). Complete Foxp3 intron 1 demethylation was observed in 4 ± 7% of Nrp1+ clones, and this contributed to the total of 27% of the clones that demethylated at least 3 CpGs. Although the sample n-value is too low to detect small differences between the sorted populations, this number of fully demethylated Foxp3 genes in Nrp1+ anergic T cells was not significantly different from the bulk population (p = 0.15, unpaired one-tail t-test). Nrp1 expression on anergic T cells also predicted Ctla4 exon 2 hypomethlyation with 32% of Nrp1+ clones demethylated at ≥3 CpGs. In contrast, Nrp1− anergic clones never demonstrated ≥3 demethylations at either Foxp3 or Ctla4. Finally, adoptive transfer of purified Nrp1+ anergic T cells to lymphopenic Tcra−/− mice led to a reduced overall T cell clonal expansion after anergy reversal, yet an increased frequency and number of Treg cells, as compared to Nrp1− cells (Fig. 8e–h). Taken together, these data suggest that Nrp1+ anergic T cells are enriched in Treg cell precursors.
Figure 8.
Anergic cells expressing neuropilin-1 are enriched in Treg cell precursors. Sorted Neuropilin-1 positive (Nrp1+) and negative (Nrp1−) anergic populations were examined for CpG methylation status using bisulfite sequencing. Average methylation for each CpG probed is shown in the top row followed by rows representing successful sequencing reactions of individual amplicons (pooled from n = 3 individual sequencing experiments, using sorted T cell subsets from 6 mice). Methylation patterns shown are for (a) Tnfrsf18 exon 5, (b) Ctla4 exon 2, (c) Ikzf4 intron 1b, and (d) Foxp3 intron 1. (e) Sorted Nrp1+ and Nrp1− anergic cells from Foxp3GFP polyclonal CD4+ T cells were transferred (105) into syngeneic lymphopenic Tcra−/− B6 mice and analyzed 21 d later. (f) Total CD4+ T cells recovered and (g) Representative flow cytometry plots for Foxp3+ Treg cell proliferation and Neuropilin-1 expression in cells recovered on day 21 is shown from pooled spleen and lymph nodes cells (inguinal, axillary, brachial, cervical, mesenteric and pancreatic). (h) The percentage and number of Foxp3+ Treg cells on day 21 after Nrp1+ and Nrp1− polyclonal anergic cell transfer. Mean data shown are representative of 3 independent experiments, with n = 2 to 3 mice per group. Error bars represent the SEM. Student’s t-test (f, h). * p < 0.01, ** p < 0.001. Points denote individual mice.
DISCUSSION
We have investigated here a naturally occurring subpopulation of Foxp3−CD44hiCD73hiFR4hi polyclonal CD4+ T cells that demonstrates defective IL-2 production. High CD69, PD-1, Nrp1, CTLA4, CD5, Nur77 expression, and Rnf128 levels (and yet low Ki67 expression) suggest that they regularly come in contact with high affinity self-pMHCII complexes and develop anergy. We note that a previous study failed to identify anergic polyclonal CD4+ T cells based on high Nr4a1 (Nur77) gene activation yet poor CD3-triggered Ca2+ mobilization37. Nevertheless, Bim−/− mice defective for thymic T cell clonal deletion have an increased frequency of CD4+ T cells in the periphery with an anergic Nur77hiCD73hiFR4hi phenotype29. Here we have associated the up-regulation of CD73 and FR4 with anergy induction in polyclonal CD4+ T cells using multiple systems of immunological tolerance, and now establish the existence of anergy as a natural CD4+ T cell peripheral tolerance mechanism that may be particularly important when central tolerance fails.
The previous failure to identify anergic Nur77hi CD4+ T cells may relate to insufficient assay sensitivity, or more likely predicts that Ca2+ signaling remains undisturbed in anergic CD4+ T cells. Anergy to fetal antigen was lost postpartum, likely as a consequence of falling pMHCII expression36. Such a requirement for continuous antigen recognition predicts that at least some signaling pathways downstream of the TCR (perhaps including intracellular Ca2+ mobilization) remain intact in anergic T cells. Constant antigen recognition is similarly required for the optimal function and stability of Treg cells39,40. Other phenotypic characteristics shared between anergic CD4+ T cells and Treg cells (e.g., high PD-1, CTLA4, CD69, Nrp1, and Nur77) can also be taken as signs of recent and/or ongoing TCR engagement. Therefore, we propose that similar to Treg cells, repeated and chronic antigen encounter dictates the stability and survival of a diverse self-reactive TCR repertoire within the anergic CD73hiFR4hi T cell compartment.
During normal aging mice accumulate ‘senescent’ PD-1hi memory-phenotype (CD44hi) CD4+ T cells that have defects in cytokine production and proliferation41. Similar to these PD-1hi memory T cells, anergic Foxp3−CD44hiCD73hiFR4hiCD4+ T cells accumulate in older mice with increased expression of PD-1, CD69, Bcl6, Cxcr5, and Spp1 (data not shown). Osteopontin (the gene product of Spp1) can bind to Bcl6 and promote the differentiation of CXCR5+ T follicular helper (TFH) and T follicular regulatory (TFR) cells42. Whether anergy is an intermediate in the development of CD4+ T cell senescence remains unknown. Regardless, the Bcl6 upregulation that occurs during chronic TCR stimulation and anergy induction may be key to the differentiation of TFH cells versus Foxp3+ TFR cells, once anergy reverses.
Treg cells are essential to anergy induction in vivo9,10,43. Treg cells may also be important for anergy maintenance, given that the adoptive transfer of anergic CD4+ T cells into Tcra−/− hosts leads to down-regulation of CD73 and FR4 and their spontaneous clonal expansion. Following reversal of anergy, both KRN TCR-transgenic T cells (data not shown) and Nrp1− polyclonal CD4+ T cells took on a TH1 or TH17 effector phenotype and caused immunopathology in the absence of Treg cells. More remarkably, after anergy reversal the Nrp1+CD73hiFR4hi polyclonal conventional CD4+ T cell subset gave rise to pTreg cells carrying a tTreg-me epigenetic signature. For the case of tTreg cell development, the strength of TCR signaling induces Foxp3 expression, whereas the duration establishes the tTreg-me epigenetic signature20,21. It remains to be determined whether the chronicity of pMHCII recognition during the course of anergy induction also controls either Nrp1 expression or tTreg-me demethylation.
Finally, it remains unclear why anergic KRN TCR-transgenic CD4+ T cells failed to generate Treg cells after anergy reversal. Other TCR-transgenic CD4+ T cell lines (TCR-HA, DO11.10, OT-II) differentiate to CD25+Foxp3+ Treg cells following treatment of mice with anti-DEC-205 targeted peptide antigen—an activation regimen known to also induce anergy44–46. In these experiments, CD62lintCD69hiCD25+Foxp3− intermediates capable of fully differentiating to CD25+Foxp3+ Treg cells in response to exogenous IL-2 were also generated. KRN T cells made anergic after 6 days in wild-type B6G7F1 hosts generally do not express either CD25 or Foxp3 (data not shown). A polyclonal CD62LintCD69hiCD25+Foxp3− mature CD4+ T cell compartment has also been identified that contains Treg cell precursors46. Although Foxp3−Nrp1+CD44hiCD73hiFR4hi anergic CD4+ T cells described here do not express CD25 (data not shown), it is conceivable that these two Treg cell progenitor populations are related and that anergy induction and subsequent CD25 expression are key to the peripheral differentiation of stable Foxp3+ Treg cells. Regardless, anergy-derived Treg cells are potent and broadly functional, based on their capacity to suppress the clonal expansion of dangerous CD4+ T cells, induce them into an anergic state, and block autoantibody production, wasting disease, inflammatory bowel disease, and autoimmune arthritis. Given that TCR engagement, Nrp1 expression, and tTreg-me all serve to maintain optimal Treg cell stability, function, and survival19,21,39,40, we now speculate that self-pMHCII-reactive Nrp1+ anergic CD4+ T cells bearing hypomethylated tTreg-me loci are ideal progenitors for the peripheral differentiation of stable Foxp3+ pTreg cells.
METHODS
Mice
Mice were bred and housed in specific-pathogen free conditions in animal facilities at the University of Minnesota, Twin Cities. B6.g7 (H-2g7 congenic of B6) mice as well as B6 strain KRN mice that express a TCR transgene specific for GPI/I-Ag7 were gifts from Drs. Diane Mathis and Christophe Benoist (Harvard Medical School, Boston, MA) and the Institut de Genetique et de Biologie Moleculaire et Cellulaire (Strasbourg, France). B6 mice were purchased from Charles River Breeding Laboratories under a contract from the National Cancer Institute (Frederick, MD). B6 Tcra−/− (B6.129S2-Tcratm1Mom/J) and BALB/c mice were purchased from Jackson Laboratory (Bar Harbor, Maine). B6 Foxp3DTR gene knockin mice were a kind gift from Dr. Alexander Rudensky (Memorial Sloan-Kettering Cancer Center, New York, NY). B6 Foxp3GFP and Foxp3DTR CD45.1 mice were gifts from Dr. Sing Sing Way (University of Cincinnati, Cincinnati, OH). Cells from spleen and all the lymph nodes of Foxp3-Cre-GFP×R26-YFP mice were a gift from Dr. Jeffrey A. Bluestone (University of San Francisco, San Francisco, CA). The breeding of B6 strain Act2W transgenic mice that constitutively express 2W1S55–68 peptide:I-Ab complexes, B6 Nur77GFP transgenic reporter mice, Aire−/−, non-obese diabetic (NOD), B6 × B6.g7 F1 (B6G7F1), and B6G7F1 TCRα−/− mice was carried out in our facility. Experiments were reviewed and approved by the University of Minnesota Institutional Animal Care and Use Committee.
Polyclonal CD4+ T cell adoptive transfer
Spleen and LN cells (inguinal, axillary, brachial, cervical, mesenteric and pancreatic) from Foxp3DTR CD45.1 mice were harvested in PBS, 5% FBS, and 0.1% sodium azide, and then the CD4+ T cells were enriched by MACS negative selection. Flow cytometric cell sorting of the polyclonal T cell subsets was then performed using GFP as well as anti-CD44, -CD73, -FR4, and -CD25 antibodies, together with other non-T cell markers (B220, F4/80, CD8α, NK1.1, CD11c, and CD11b) as ‘dump’ reagents (eBiosciences, Inc, San Diego, CA). Cells were physically sorted on a BD FACSAria (BD Biosciences, San Jose, CA) at the University of Minnesota Flow Cytometry Core Facility. Purified CD4+ T cell subsets were then transferred (1 – 5 × 105) into syngeneic Tcra−/− B6 hosts. In some cases, Foxp3-expressing Treg cells were ablated following adoptive transfer by the i.p. injection of diphtheria toxin (DT; List Biological, Campbell, CA) at a concentration of 0.5 µg/mouse on day 1 after adoptive transfer of T cells, and then 0.2 µg/mouse every other until day 21. Weights were taken on the day of T cell transfer and every other day thereafter. Reported weights are relative to the initial measurement on the day of cell transfer.
Reverse transcriptase real-time quantitative PCR
RT real-time qPCR was used to compare gene expression levels in physically sorted purified CD4+ polyclonal T cell subsets, as previously described9. The primer sets used are shown in Supplementary Table. 1.
Arthritis model and Treg cell ablation
Arthritis was induced by the adoptive transfer of naive B6 KRN CD4+ T cells into lymphopenic Tcra−/− B6G7F1 hosts as previously described9. Arthritis severity was assigned a score from 0 – 3 for each paw based on swelling and erythema, resulting in a maximum Arthritis Clinical Index score of 12 for each mouse (Binstadt et al., 2006). Treg cells were ablated following adoptive transfer by the i.p. injection of diphtheria toxin (DT; List Biological, Campbell, CA) at a concentration of 0.2 µg/mouse on day 1 after adoptive transfer of T cells, and then 0.1 µg/mouse every other until day 21.
Bisulfite sequencing
Primers for bisulfite sequencing for Tnfrsf18 exon 5, Ctla4 exon 2, Ikzf4 intron 1b and Foxp3 intron 1 have been previously published21 and see Supplementary Table 1. Sodium bisulfite treatment of the extracted DNA was carried out using Qiagen Epitect® Bisulfite Kit (Cat. No. 59104). For cloning TOPO® TA Cloning Kit was used (Invitrogen K4500-40). Sequences were analyzed using BISMA web tool47.
Histological analysis
H&E-stained slides were prepared from 10% neutral buffered formalin-fixed tissues using standard methods, and were evaluated by an A.C.V.P.- board certified pathologist (M.G. O'S.) using light microscopy.
Flow cytometric phenotypic analysis
T cells were interrogated with allophycocyanin (APC)–conjugated anti-CD25 (PC61.5), eFluor 450-conjugated anti-CD73 (eBioTY/11.8), PE-Cy7–conjugated anti-FR4 (eBio12A5), Alexa Fluor 700–conjugated anti-CD44 (IM7), PerCP-Cy5.5-conjugated anti-CD45.1 (A20), V500–conjugated anti-CD4 (RM4-5) (eBiosciences). T cells suspensions were also stained with APC–eFluor780–conjugated anti-B220 (RA3-6B2), -CD11b (MI-70), -CD11c (N418) (all eBiosciences), and -F4/80 (BM8; Invitrogen, Carlsbad, CA), for use as dump channel reagents. Stained T cells were then treated with Foxp3 Fixation/Permeabilization Concentrate and Diluent and stained with APC–conjugated anti-Foxp3 (FJK-16s) (eBioscience). In some experiments cells were also stained with Pacific Blue–conjugated T-bet (BioLegend, San Diego, CA) following fixation/permeabilization.
Test for cytokine production and proliferation
For intracellular cytokine staining, T cells were incubated for 4 h at 37°C in RPMI medium 1640 + 10% FCS in the presence of 10 ng/ml PMA (Sigma-Aldrich, St. Louis, MO), 1 µM ionomycin (EMD Chemicals, Gibbstown, NJ) and with 10 µg/ml brefeldin A (Sigma-Aldrich, St. Louis, MO). Cells were then stained for surface markers as described above. Intracellular staining was preformed using the Cytofix/Cytoperm kit (eBioscience). Surface stained cells were fixed overnight and permeabilized for 45 m, then stained for APC-conjugated anti–IL2 (JESS-5H4), PE-Cy7–conjugated anti–IFNγ (XMG1.2), and eFluor450 anti_TNF (MP6-XT22) (eBiosciences). For in vitro proliferation and ELISA assays, sorted T cells were CFSE-labeled and then stimulated in plates coated (O/N) with CD3 and CD28 antibodies (both eBiosciences) at 1:10 ratio for 8 hours or 96 hours in complete media. The T cells were subsequently analyzed for their mean cell division response and supernatants from each stimulated population (Naive, Treg cell, Teff/mem, anergic) were assayed using ELISA and previously described10.
ELISA
IL2 ELISA was performed using purified anti-mouse IL2 (E05611-1573, eBioscience), biotin conjugated anti-mouse IL2 (JES6-5H4, eBioscience), SA-HRP (Cat: 554066, BD Pharmingen) and ABTS (Lot. 110185, KPL).
Immunoblots
Various tissues (heart, kidney, pancreas, liver, lung, gut, salivary gland) from RAG−/− mice were harvested and protein extraction was performed using freeze/thaw method. Briefly, harvested tissues were frozen in liquid nitrogen followed by thawing at room temperature and lysed using RIPA Lysis and Extraction buffer (Thermo Sci. No. 89900). The protein extracts were then used as substrate for immunoblot analysis. Mini-PROTEAN® TGXTM gels 4–15% were purchased from BIO-RAD (Ctl. No. 400083670). Mini Nitrocellulose iBlot® Gel Transfer Stacks were purchased from Novex (Lot. NM27023-01). Serum samples were probed with AF680 conjugated goat anti-mouse IgG Fcγ (JacksonImmuno Research, 11-625-071).
Statistical analysis
Mean Arthritis Clinical Index scores were compared using the Mann–Whitney U test. Other tests of significance shown represent the results of an unpaired one-tailed Student t-Test or One-Way Anova as indicated in the figure legends.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. Jeffrey A. Bluestone for spleen and lymph node cells from the Foxp3-Cre-GFP x R26-YFP mice, as well as for helpful discussion; Stephen C. Jameson, Matthew Mescher and Michael A. Farrar for helpful discussions; Philip J. Titcombe for technical support; Nisha Shah, Terri Martin and Jason Motl for assistance in cell sorting. This work was supported by a Within Our Reach: Finding a Cure for Rheumatoid Arthritis campaign grant from the Rheumatology Research Foundation (D.L. Mueller) and by the National Institutes of Health grant P01 AI35296 to D.L. Mueller, B.T. Fife, K.A. Hogquist, and M.K. Jenkins.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
L.A.K. and D.L.M. designed the experiments and analyzed the data; L.A.K performed most of the experiments. S.E.S., S.L.N., W.L., L.O.B., N.Z., G.L.S., D.P., K.E.P., J.L.L. performed experiments or provided technical help; M.G.O.S., scored the histology slides; B.T.F., K.A.H., M.K.J. provided scientific input; D.L.M. conceived the study and directed the research; L.A.K., and D.L.M. wrote the manuscript.
REFERENCES
- 1.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu. Rev. Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller DL. Mechanisms maintaining peripheral tolerance. Nat. Immunol. 2010;11:21–27. doi: 10.1038/ni.1817. [DOI] [PubMed] [Google Scholar]
- 3.Chappert P, Schwartz RH. Induction of T cell anergy: integration of environmental cues and infectious tolerance. Curr. Opin. Immunol. 2010;22:552–559. doi: 10.1016/j.coi.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 5.Vanasek TL, Khoruts A, Zell T, Mueller DL. Antagonistic roles for CTLA-4 and the mammalian target of rapamycin in the regulation of clonal anergy: enhanced cell cycle progression promotes recall antigen responsiveness. J. Immunol. 2001;167:5636–5644. doi: 10.4049/jimmunol.167.10.5636. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, et al. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J. Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 7.Delgoffe GM, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adler AJ, et al. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow-derived antigen-presenting cells. J. Exp. Med. 1998;187:1555–1564. doi: 10.1084/jem.187.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez RJ, et al. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J. Immunol. 2012;188:170–181. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanasek TL, Nandiwada SL, Jenkins MK, Mueller DL. CD25+Foxp3+ regulatory T cells facilitate CD4+ T cell clonal anergy induction during the recovery from lymphopenia. J. Immunol. 2006;176:5880–5889. doi: 10.4049/jimmunol.176.10.5880. [DOI] [PubMed] [Google Scholar]
- 11.Knoechel B, Lohr J, Kahn E, Abbas AK. The link between lymphocyte deficiency and autoimmunity: roles of endogenous T and B lymphocytes in tolerance. J. Immunol. 2005;175:21–26. doi: 10.4049/jimmunol.175.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 15.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 16.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 17.Bruder D. Neuropilin-1: a surface marker of regulatory T cells. Eur. J. Immunol. 2004;34:623–630. doi: 10.1002/eji.200324799. [DOI] [PubMed] [Google Scholar]
- 18.Yadav M, et al. 2012. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J. Exp. Med. 2012;209:1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delgoffe GM, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414–423. doi: 10.1016/j.immuni.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Ohkura N, et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785–799. doi: 10.1016/j.immuni.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 23.Hill JA, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Morikawa H, et al. Differential roles of epigenetic changes and Foxp3 expression in regulatory T cell-specific transcriptional regulation. Proc. Natl. Acad. Sci. U.S.A. 2014;111:5289–5294. doi: 10.1073/pnas.1312717110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pauken KE, et al. Cutting Edge: Type 1 Diabetes Occurs despite Robust Anergy among Endogenous Insulin-Specific CD4 T Cells in NOD Mice. The Journal of Immunology. 2013;191:4913–4917. doi: 10.4049/jimmunol.1301927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature. 2012;490:102–106. doi: 10.1038/nature11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stritesky GL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proc. Natl. Acad. Sci. U.S.A. 2013;110:4679–4684. doi: 10.1073/pnas.1217532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anandasabapathy N, et al. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 33.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int. Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 34.Ahern PP, et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polansky JK, et al. DNA methylation controls Foxp3 gene expression. Eur. J. Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489:160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J. Immunol. 1998;160:4719–4729. [PubMed] [Google Scholar]
- 39.Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 2014;15:1070–1078. doi: 10.1038/ni.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vahl JC, et al. Continuous T Cell Receptor Signals Maintain a Functional Regulatory T Cell Pool. Immunity. 2014;41:722–736. doi: 10.1016/j.immuni.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Shimatani K, Nakashima Y, Hattori M, Hamazaki Y, Minato N. PD-1+ memory phenotype CD4+ T cells expressing C/EBPalpha underlie T cell immunodepression in senescence and leukemia. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15807–15812. doi: 10.1073/pnas.0908805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leavenworth JW, Verbinnen B, Yin J, Huang H, Cantor H. A p85α-osteopontin axis couples the receptor ICOS to sustained Bcl-6 expression by follicular helper and regulatory T cells. Nat. Immunol. 2015;16:96–106. doi: 10.1038/ni.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kline J, et al. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin. Cancer Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 44.Hawiger D, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J. Exp. Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 46.Schallenberg S, Tsai PY, Riewaldt J, Kretschmer K. Identification of an immediate Foxp3(−) precursor to Foxp3(+) regulatory T cells in peripheral lymphoid organs of nonmanipulated mice. J. Exp. Med. 2010;207:1393–1407. doi: 10.1084/jem.20100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods Reference
- 47.Rohde C, et al. Bisulfite sequencing Data Presentation and Compilation (BDPC) web server--a useful tool for DNA methylation analysis. Nucleic Acids Res. 2008;36:e34. doi: 10.1093/nar/gkn083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.