Abstract
Objective
To determine if preeclampsia (PE) is associated with dysregulation of the neuropeptide Y (NPY) system.
Methods
The study enrolled 114 subjects either with normal pregnancy (NP) or with PE. Systolic blood pressure (SBP) was collected from patients using a standard sphygmomanometer. The PE patients were divided into two groups based on the gestational age (GA) at delivery – placental PE (PLPE, GA <34 weeks) or maternal PE (MTPE, GA ≥ 34 weeks). NPY was measured in platelet rich plasma (PRP), platelet poor plasma (PPP) and in the serum of NP and PE patients utilizing radioimmunoassay. Serum levels of soluble fms-like tyrosine kinase-1 (sFlt-1) and placental growth factor (PlGF) were measured in NP and PE subjects by ELISA.
Results
SBP was higher in PE compared to NP. Circulating NPY in serum and PRP, as well as NPY content per 100,000 platelets, but not its concentrations in PPP, were elevated in PE, as compared to NP. The highest NPY concentrations were observed in sera and PRP of patients with MTPE. PE patients had also elevated levels of sFlt-1, as compared to NP, although no difference between PLPE and MTPL groups were observed. There was no increase in P1GF in PE patients.
Conclusion
Systemic NPY is elevated in PE patients, as compared to NP. This increase is observed in blood fractions containing platelets, suggesting accumulation of the peptide in these cells. NPY concentrations are particularly high in patients with MTPE, underlying differences in etiology between PLPE and MTPE. Our study implicates NPY as a potential target in antihypertensive therapies for PE patients.
Keywords: preeclampsia, neuropeptide Y (NPY), vasoconstriction, soluble fms-like tyrosine kinase-1 (sFlt-1), placental growth factor (PlGF), stress
Introduction
The term preeclampsia (PE) refers to the new onset of hypertension (blood pressure higher than 140/90mmHg) and proteinuria after 20 weeks of gestation in previously normotensive, nonproteinuric women (Conrad, 1999). It is a unique form of human hypertension, one of the most common medical complications of pregnancy, and associated with increased maternal and neonatal morbidity and mortality (2000, NHLBI). Although the pathophysiologic mechanisms underlying PE still remain unclear, this condition is characterized by a marked increase in peripheral vascular resistance, leading to an increase in blood pressure that returns to normal after delivery (Cunningham and Lindheimer, 1992; Roberts and Redman, 1993; Schobel et al., 1996). Moreover, numerous studies have demonstrated decreased levels of angiogenic factors – vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) – in the blood of women with PE (Reuvekamp et al., 1999; Torry et al., 1998). This deficit in angiogenic factors is associated with high systemic concentrations of soluble fms-like tyrosine kinase-1 (sFlt-1), a releasable splice variant of the VEGF receptor type 1 (Maynard et al., 2003). Circulating sFlt-1 binds and deactivates VEGF and PlGF, depriving the endothelium of these essential growth factors and acting as an antiangiogenic molecule (Karumanchi and Bdolah, 2004). PE can be divided into 2 broad categories, depending on the gestational age (GA) at delivery: placental PE (PLPE or Type 1; GA < 34 weeks) and maternal PE (MTPE or Type 2; GA ≥ 34 weeks). While the first sub-type results from the poor development of early placenta, the second category is associated with an abnormal maternal response to pregnancy (Maynard et al., 2003).
Neuropeptide Y (NPY) is a sympathetic neurotransmitter co-released with norepinephrine. Changes in circulating NPY levels are associated with stress and depend on intensity of sympathetic nervous system (SNS) activation and its duration (Heilig, 2004; Zukowska-Grojec, 1995). Interestingly, such stress-induced increase in NPY levels is observed in platelet rich plasma (PRP), but not platelet poor plasma (PPP), suggesting its accumulation in platelets, rather than elevated levels of free circulating peptide (Kuo et al., 2007; Li et al., 2005; Li et al., 2011; Najafi et al., 2013; Tilan et al., 2013). Increases in NPY levels appear to be temporally associated with elevated blood pressure and vasoconstriction (Hashim and Tadepalli, 1995; Hauser et al., 1996; Zukowska-Grojec, 1995).
Cardiovascular effects of NPY are mediated by several NPY receptor subtypes. NPY Y1 receptor (Y1R) is the predominant receptor expressed on vascular smooth muscle cells, responsible for vasoconstrictive effects of NPY (Modin et al., 1999; Racchi et al., 1997). In contrast, Y2R is expressed on endothelial cells and is the main angiogenic receptor of NPY (Ekstrand et al., 2003; Lee et al., 2003). The balance between Y1R- and Y2R-mediated activity of NPY is regulated by dipeptidyl peptidase IV (DPPIV), a membrane protease expressed in variety of cells, including endothelium and immune cells (Kitlinska et al., 2003). DPPIV cleaves NPY to its shorter form, NPY3–36, which does not bind Y1R, but preserves its activity at Y2R (Mentlein et al., 1993). Thus, depending on the local DPPIV activity and ratio between Y1R and Y2R expression, NPY can act either as a vasoconstrictor or an angiogenic factor (Kitlinska et al., 2003).
Since the number of adrenergic nerve fibers in the uterine artery decreases in pregnancy, while the presence of NPY-containing nerves increases, it has been proposed that NPY takes over the role of the main uterine vasoconstrictor during pregnancy (Mione et al., 1990). Preliminary data on a small number of subjects indicated increased plasma concentrations of NPY in women with eclampsia and PE, as compared to normotensive pregnant women (Khatun et al., 2000). Moreover, systemic NPY concentrations in PE women have been shown to correlate with mean arterial pressure (MAP) before delivery (Kokot et al., 1999). The increased MAP found in this condition is consistent with hyperactivity of the SNS and increased plasma levels of NPY, which result in long-lasting vasospasms and hypertension (Schobel et al., 1996). In line with this, plasma NPY concentrations in PE women returned almost to reference values of normotensive nonpregnant women few days after delivery, showing no correlation between MAP and plasma NPY levels. In this context, the aim of this study was to extend the above finding by testing the levels of NPY in different sub-types of PE, as well as identifying the source of the peptide.
Materials and methods
Human subjects
The Institutional Review Board at the Georgetown University Hospital (GUH) approved the study protocol. Patients investigated were in the third trimester of pregnancy admitted to GUH from January 2004 until February 2007. The study enrolled 114 subjects either in the normal pregnancy (NP) group (n=57) or in the PE group (n=57) after being diagnosed by their physicians and interviewed by investigator (Tab. 1). Patients with NP had no history of any chronic disease or pregnancy related complications. PE was characterized by hypertension (BP>140/90mmHg) and proteinuria (protein excretion>300 mg/24hrs or >1+ protein by dipstick). The PE group was then classified as PLPE (n=28) when gestational age (GA) at delivery was <34 weeks or MTPE (n=29) when GA at delivery was ≥ 34 weeks. Appropriate informed consent was obtained from each participant.
Table 1.
Characteristics of the studied population
| Normal Pregnancy | Placental Preeclampsia | Maternal Preeclampsia | ||
|---|---|---|---|---|
| Maternal age at delivery (years) | 34 ± 5 | 31 ± 7 | 32 ± 7 | |
| Parity | Primipara | 23.6% | 48.3% | 51.9% |
| Multipara | 76.4% | 51.7% | 48.1% | |
| Number of offspring | Single | 96.4% | 40.7% | 72.4% |
| Multiple | 3.6% | 59.3% | 27.6% | |
| Medications | Anti- Hypertensive | 0 | 74.1% | 31% |
| MgSO4 | 0 | 85.7% | 65.5% | |
| Type of delivery | Natural vaginal | 49.2% | 10.7% | 28.6% |
| Cesarean section | 50.8% | 89.3% | 71.4% | |
| Gestational age at delivery (weeks) | 38 ± 1,3 | 30 ± 2,5 | 36 ± 1,6 | |
| Birth weight (g) | 3430 ± 532 | 1500 ± 520 | 2731 ± 556 | |
| Proteinuria (mg/24h) | * <300 | 1682.9 ± 255.6 | 1938.7 ± 221.1 | |
| Creatinine clearance (ml/min) | * 70–100 | 181.3 ± 92.9 | 141.2 ± 99.8 | |
| Serum creatinine (mg/dL) | * 0.6–1.2 | 0.7 ± 0.16 | 1.6 ± 0.2 | |
| Uric acid (mg/dL) | * 2.1–7.9 | 6.8 ± 1.4 | 6.7 ± 0.1 | |
| Platelets (100,000/mL) | * 1.44–3.87 2.2 ± 0.6 | 1.8 ± 0.4 | 1.7 ± 0.6 | |
| LDL (IU/L) | * 313–618 | 228.5 ± 49.9 | 249 ± 153.1 | |
| SOGT (IU/L) | * <40 | 52.8 ± 25.3 | 108.8 ± 131.1 | |
| SGTP (IU/L) | * <40 | 50.5 ± 40 | 83 ± 100.6 | |
| Total number of patients | 57 | 28 | 29 | |
#: Data analyzed correspond to subjects that had blood samples collected and assayed for serum NPY and sFlt-1. Data was expressed as the mean ± SEM and absolute value.
correspond to mean values in normal patients.
Exclusion criteria
Women with fever ≥38.3°C, ongoing bacterial infection (positive blood cultures, clinical suspicion of localized source of infection, or new need for antibiotics during the last 24h), or an operative blood loss of >800 ml were excluded from the study. Subjects who agreed to participate in the study were assigned a code number to maintain confidentiality.
Blood samples
10 ml samples of venous blood were collected from patients in the third trimester of pregnancy, after admission and prior to delivery. Blood samples were kept on ice and processed within 30 minutes after phlebotomy in order to obtain serum (tubes with no anticoagulant) and plasma (EDTA tubes). Blood for serum was allowed to clot and then centrifuged at 2000 rpm for 25 minutes. PRP was obtained by centrifugation of EDTA-treated blood at 850 rpm for 5 minutes. PRP was then collected to a separate tube and blood centrifuged again at 2300 rpm for 8 minutes to obtain PPP fraction. Serum and plasma samples were aliquoted and stored at −80° C.
NPY measurement
NPY level was measured in PRP, PPP and in the serum of pregnant women (NP and PE) utilizing EURIA-NPY RIA Kit (EURO diagnostica) according to the manufactures’ procedures.
sFlt-1 and PlGF measurement
Serum levels of sFlt-1 and PlGF were measured utilizing the ELISA Kit Quantikine® (R&D Systems) according to the manufactures’ procedures.
Systolic Blood pressure (SBP)
Measurements were collected from patients using a sphygmomanometer. The data used in this paper represent the highest measurement during the subjects’ admission.
PE severity score
In order to evaluate the possible association between serum NPY levels and the severity of PE, we created a PE severity score based on the assessment of the clinical symptoms that are most characteristic for PE. This system is used for clinical evaluation of patients at GUH and includes the following values: the highest systolic blood pressure (SBP, mmHg) during the subject’s admission, total proteinuria in a 24 hours urine collection (mg/24h), platelet levels (k/ml) using k=1×15, uric acid levels (mg/dl) and baby weight at birth (g). Each clinical variable obtained a score of 2, 4 or 6 points, depending on its severity (Table 2). The scores in each category were summarized to calculate total PE score and assign subjects to one of the following categories, Low (total score 0–10), Medium (11–20) and High Score (21–30).
Table 2.
Preeclampsia severity score.
| Preeclampsia severity score | |||
|---|---|---|---|
| Low (2 points per category) | Medium (4 points per category) | High (6 points per category) | |
| SBP (mmHg) | 140–145 | 146–150 | >150 |
| Proteinuria (mg/24h) | 300–500 | 501–1000 | >1000 |
| Platelet count (100,000/ml) | >1.45 | 1.2–1.44 | <1.2 |
| Uric acid (mg/dl) | <7.9 | 7.9–8.9 | >8.9 |
| Birth weight (g) | >2000 | 1600–1999 | <1600 |
The data was based on values used for clinical evaluation of patients at Georgetown University Hospital (GUH). The score for severity of preeclampsia (PE) was assigned based on the total number of points in all categories, as follows: Low (0–10 points), Medium (11–20) and High (21–30). SBP: systolic blood pressure.
Statistical analysis
SigmaStat® 2.03 software (SPSS Science, Chicago, IL) was used for all statistical analyses. Descriptive statistics were used to summarize the study population’s characteristics and Box plots were presented to show the distribution of the data. For values that are normally distributed, group means were compared using 2-sample t-test for 2 groups, or one-way analysis of variance (ANOVA) for more than two groups followed by Tukey method for post-hoc pairwise multiple comparisons. For the values that are not normally distributed, the nonparametric Mann-Whitney rank sum test was used to compare the group medians for 2 groups, and Kruskal-Wallis test was used for comparing more than 2 groups followed by Dunn’s test for post-hoc pairwise comparisons. p<0.05 was considered statistically significant.
Results
Demographic information
The total number of subjects in the study was 114: NP group (n=57) and PE group (n=57). The PE group was then subclassified as PLPE (n=28) and MTPE (n=29) based on GA at delivery. 31 blood samples were collected (NP=15; PLPE=13 and MTPE=13). Characteristics of the studied population are described in table 1.
Blood pressure
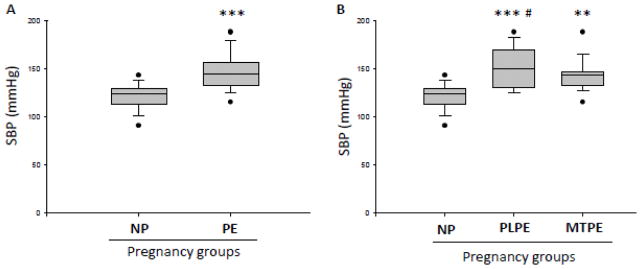
For blood pressure comparison, the highest SBP measurement during admission was used per each subject. SBP was significantly increased in PE (mean: SBP=147.1mmHg) compared to NP group (mean: SBP=121.2mmHg) (Fig. 1A) (p<0.001). Figure 1B shows the subdivision of PE groups. PLPE (mean: 151.5mmHg) and MTPE (mean: SBP=143.4mmHg) were significantly higher than control group (NP) (p<0.001 and p<0.01 respectively).
Figure 1. Systolic blood pressure in normal pregnancy and preeclampsia.
A) Comparison of systolic blood pressure (SBP) presented as the highest measurement during hospital admission per subject in normal pregnancies (NP) and preeclampsia (PE). B) SBP measured as above in NP and PE patients subdivided into placental PE (PLPE, gestational age at delivery < 34 weeks) and maternal PE (MTPE, gestational age at delivery ≥ 34 weeks). Data are presented as a mean and standard error of the mean (SEM). *** - p<0.001, as compared to control (NP) by t-test; ***# -p<0.001 and ** - p<0.01, as compared to control (NP) by one-way ANOVA followed by Tukey’s multiple comparison test.
Circulating levels of NPY in plasma, platelets and serum
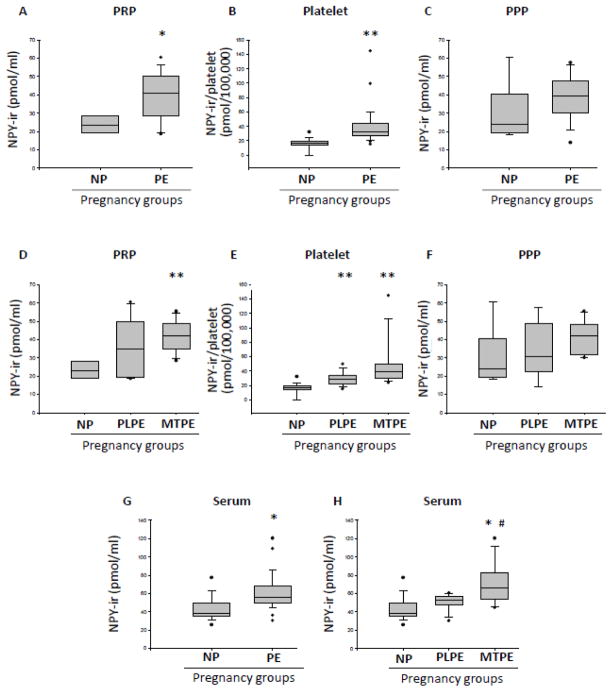
Based on the data distribution, the majority of NPY level comparisons was performed on median values. NPY immunorreactivity (NPY-ir) levels in PRP in PE group (median=40.90 pmol/ml) was significantly higher (p<0.05) than in NP group (median=23.20 pmol/ml), (Fig. 2A). The total NPY content per platelet was calculated using the ratio NPY/platelet (pmol/100,000 platelets) in PRP. PE group (median=22.13 pmol/100,000 platelet) was significantly higher (p<0.01) than NP group (median=10.43 pmol/100,000 platelets) (Fig. 2B). However, no significant difference in NPY levels between NP and PE patients was observed in PPP (Fig. 2C). When PE group was subdivided, the increase in PRP NPY levels over the NP control achieved statistical significance (p<0.01) for MTPE group (median=42.20 pmol/ml), but not PLPE subjects (median=34.75 pmol/ml) (Fig. 2D). However, when the NPY/platelet ratio was calculated, the increase in NPY/platelet ratio over the NP control remained statistically significant (p<0.01) for both PLPE and MTPE groups, with the highest values observed in MTPE patients (Fig. 2E). As observed for the combined PE group, no significant differences in PPP NPY levels were observed between NP and PLPE and MTPE subgroups (Fig. 2F). The serum NPY-ir levels in the PE group (median=55.50 pmol/ml) were significantly higher than in the NP group (median=38.60 pmol/ml), (p<0.05) (Fig. 2G). When PE group was subdivided, the statistically significant difference, as compared to the NP subjects, was observed for MTPE (median=66.60, p<0.01), but not PLPE group (median=54.70) (Figure 2H). Consistent with this observation, the difference in serum NPY levels between PLPE and MTPE group achieved statistical significance (p<0.05).
Figure 2. NPY levels in normal pregnancies and preeclampsia.
A) NPY immunoreactivity (NPY-ir) measured by radioimmunoassay in platelet rich plasma (PRP) of subjects with normal pregnancies (NP) and preeclampsia (PE). B) NPY-ir per 100,000 platelets in NP and PE. C) NPY-ir measured in platelet poor plasma (PPP) of subjects with NP and PE. D) NPY-ir in PRP of patients with NP or PE subdivided into placental PE (PLPE) and maternal PE (MTPE) groups. E) NPY-ir per 100,000 platelets in NP, PLPE and MTPE groups. F) NPY-ir in PPP of subjects with NP, PLPE and MTPE. G) NPY-ir in sera of subjects with NP and PE, H) Serum NPY-ir levels in NP, PLPE and MTPE. ** - p<0.01 and * - p<0.05, as compared to NP; # -p<0.05, as compared to PLPE.
Correlation between NPY levels and PE score of severity
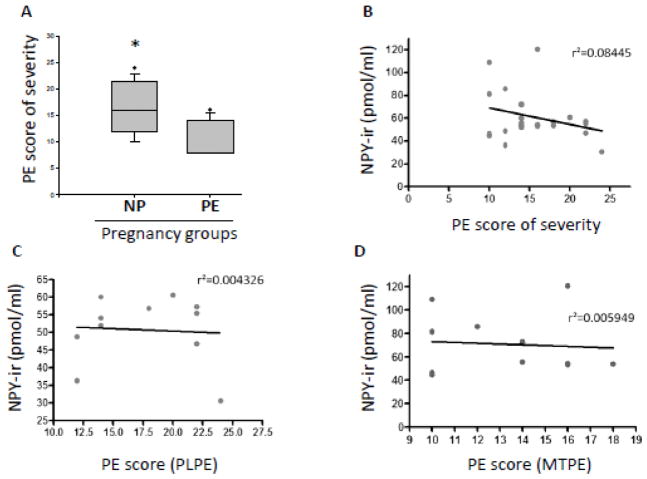
We used the PE severity score to evaluate the difference in severity of the disease for the PE group (N=33). The PE severity score in PLPE group (N=22 and mean=17.64 points score of severity) was significantly higher than in MTPE patients (N=11 and mean=13.33 points) (p<0.05) (Fig. 3A). However, we did not find a correlation between the serum values for NPY and the corresponding PE score of severity for each subject in PE group combined (N=23, r2=0.08445 and p=0.1786) (Fig. 3B) and PLPE (N=11, r2=0.004326 and p= 0.8476) (Fig. 3C) or MTPE alone (N=12, r2=0.005949 and p= 0.8117) (Fig. 3D). The slope deviation from zero was not statistically significant for any calculation above.
Figure 3. Correlation between serum NPY and severity of preeclampsia.
A) Preeclampsia (PE) score of severity for placental PE (PLPE) and maternal PE (MTPE). B) Correlation between serum NPY immunoreactivity (NPY-ir) measured by radioimmunoassay and PE score of severity when PLPE and MTPE are combined; C) Correlation between serum NPY-ir and PE score of severity for PLPE patients and D) Correlation between serum NPY-ir and PE score of severity for MTPE group. * - p<0.05 using unpaired t-test.
Serum levels of sFlt-1
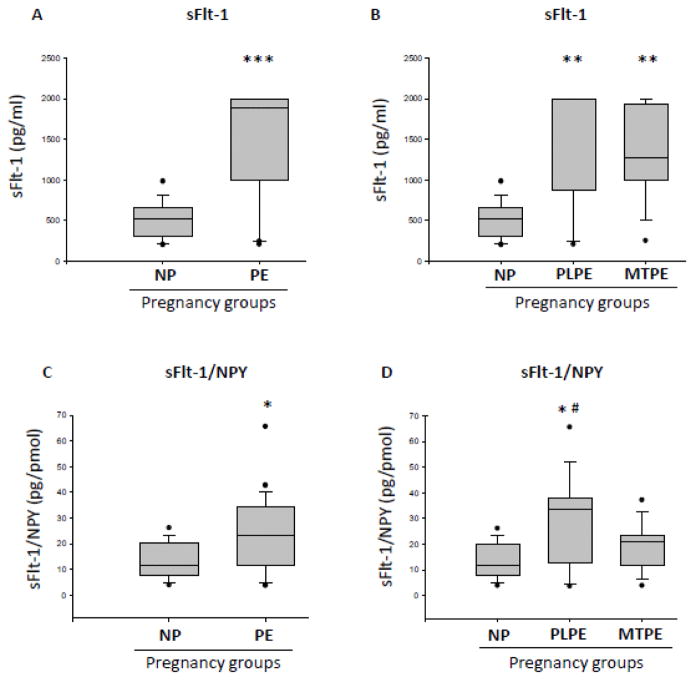
sFlt-1 levels were significantly higher in the PE group (mean=1307 pg/ml) compared to NP group (mean=513.8 pg/ml) (p<0.0001) (Fig. 4A). When PE group was sub-divided, sFlt-1 levels for PLPE (mean=1444 pg/ml) and MTPE patients (mean=1346 pg/ml) were significantly higher than for NP (p<0.01), and no difference between these two groups were observed (Fig. 4B).
Figure 4. Serum levels of sFlt-1 in normal pregnancies and preeclampsia.
A) Serum levels of sFlt-1 measured by ELISA in normal pregnancies (NP) and preeclampsia (PE). B) Serum levels of sFlt-1 in NP and PE patients subdivided into placental PE (PLPE) and maternal PE (MTPE). C) Ratio of serum sFlt-1 to serum NPY measured by radioimmunoassay in NP and PE. D) Serum sFlt-1/NPY ratio in NP, PLPE and MTPE. Data are presented as mean and standard error of the mean (SEM). *** - p<0.001 and * - p<0.05, as compared to control (NP) by t-test; ** - p<0.01 and *#- p<0.05, as compared to control (NP) by Newman-Keuls Multiple Comparison test.
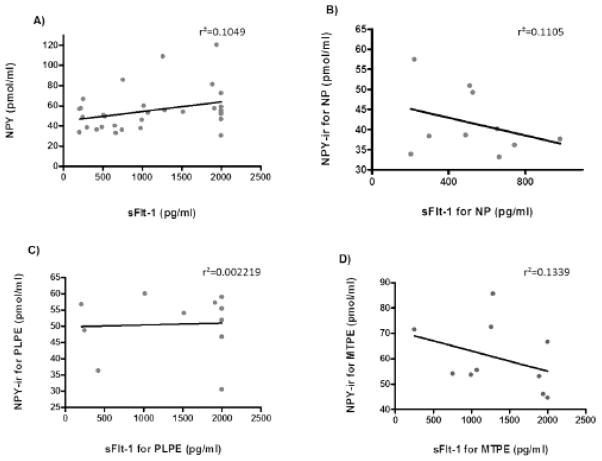
Ratios between sFlt-1 and NPY (sFlt-1/NPY) for the serum samples were calculated and analyzed. The sFlt-1/NPY ratio for PE group (mean=23.91 pg/ml) was significantly higher than the NP group (mean=12.73 pg/ml) (p<0.05) (Fig. 4C). When PE patients were subdivided, only the PLPE group (mean=28.70 pg/ml) was significantly higher than NP (p<0.05). The MTPE group (mean=19.34 pg/ml) had a higher ratio, but not statistically significant (Fig. 4D). We did not observe a correlation between the serum values for NPY and sFlt-1 in the study for each subject in NP, PLPE and MTPE groups combined (Fig. 5).
Figure 5. Correlation between serum NPY and sFlt-1 levels in pregnancy.
Correlation between levels of NPY immunoreactivity (NPY-ir) measured by radioimmunoassay and levels of sFlt-1 measured by ELISA in sera of: A) all subjects, including normal pregnancies (NP) and preeclampsia cases (PE); B) NP subjects; C) patients with placental PE (PLPE), and D) patients with maternal PE (MTPE).
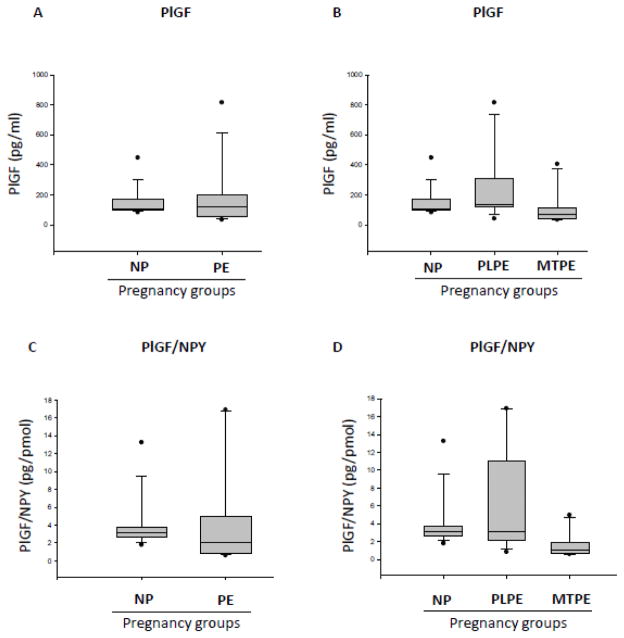
Serum levels of PlGF
There was no significant difference in PlGF levels between NP subjects (median= 105.0 pg/ml) and PE patients analyzed as a whole population (median=120.0 pg/ml) or subdivided into PLPE (median=134.0pg/ml) and MTPE (median=70.50pg/ml) groups (Fig. 6A, B). Similarly, no significant differences in the serum PlGF/NPY ratio were observed between the above groups - NP (median=3.165pg/poml), PE (median=2.083pg/pmol), PLPE (median=2.083pg/pmol) and MTPE (median=0.8031 pg/pmol) - although this value tended to be lower in MTPE patients (Fig. 6C, D).
Figure 6. Serum levels of PIGF in normal pregnancies and preeclampsia.
A) Serum levels of PIGF in normal pregnancies (NP) and preeclampsia (PE) measured by ELISA. B) Serum levels of PIGF in NP and PE patients subdivided into placental PE (PLPE) and maternal PE (MTPE). C) Ratio of PIGF to NPY levels measured by radioimmunoassay in sera of subjects with NP and PE. D) Serum PIGF/NPY ratio in NP PLPE and MTPE.
Discussion
PE is characterized by a marked increase in peripheral vascular resistance, which, in turn, causes the increase in blood pressure (Cunningham and Lindheimer, 1992; Roberts and Redman, 1993). This hypertension is strongly associated with sympathetic hyperactivity (Schobel et al., 1996). In spite of that, however, little is known about the role of the sympathetic neurotransmitter, NPY, in this condition. Here, we demonstrate elevated NPY-ir levels in serum and PRP of PE patients, as compared to NP subjects, with the highest increase observed in MTPE. Such an increase in systemic NPY levels in PE patients has been previously suggested by a small study performed on a limited number of patients (Khatun et al., 2000). Our current data provide further evidence for this association and implicate platelets as the main carrier of the peptide.
NPY can act as a vasoconstrictor via its Y1R expressed on vascular smooth muscle cells or as an angiogenic factor via endothelial Y2R (Ekstrand et al., 2003; Grundemar, 1993; Lee et al., 2003; Racchi et al., 1997). The association of the high systemic NPY levels with PE strongly suggests the prevalence of its vasoconstrictive activity in this disorder, as well as its neuronal origin. While placenta has also been shown to produce NPY and express DPPIV, an enzyme converting the peptide to the Y2R-specific agonist with angiogenic properties, the placental NPY mRNA and the levels of the peptide in umbilical blood are suppressed in PE (Dotsch et al., 1999; Kokot et al., 1998; Nishikawa et al., 2005). Therefore, our data and previous reports strongly suggest that while the local angiogenic actions of NPY are suppressed, its release from SNS and vasoconstrictive activities are elevated. This notion is supported by previous reports indicating correlations between plasma NPY levels and MAP before delivery in women with eclampsia and PE (Khatun et al., 2000; Kokot et al., 1999). Further studies are required to determine the mechanisms underlying this switch from the angiogenic to vasoconstrictive actions of NPY observed in PE patients. While local DPPIV activity in placenta appears to be increased in PE, nothing is known about the ratios between Y1R and Y2R expression in placentas from NP and PE subjects (Nishikawa et al., 2005). The follow-up studies may involve clinical investigations into the levels of NPY receptor expression in placenta, as well as experiments on animal models, which would allow for longitudinal assessment of changes in NPY system and mechanistic studies (Pennington et al., 2012).
Changes in circulating NPY levels in humans are associated with stress and relate to intensity of SNS activation and its duration. While NE is released during mild SNS stimulation, more intense activation is required for secretion of NPY (Zukowska-Grojec, 1995). Therefore, elevated circulating NPY levels have been proposed as a marker of severe chronic stress (Grundemar, 1993; Zukowska-Grojec, 1995). Previous reports suggested that elevated systemic levels of NPY in PE reflect SNS hyperactivity resulting in the long-lasting vasoconstriction and hypertension found in this condition. Such SNS overactivity can be triggered by uterine blood flow restriction, as hypoxia is a strong SNS activator (Rook et al., 2014). However, there are also epidemiological data indicating associations of psychosocial stress in pregnancy with increased risk of preeclampsia, suggesting that the elevated NPY levels observed specifically in MTPE may reflect maternal chronic stress (Klonoff-Cohen et al., 1996; Kurki et al., 2000; Paarlberg et al., 1995; Salvador-Moysen et al., 2012; Sandman et al., 1997; Wadhwa et al., 1996).
Our findings extend these observations even further indicating that platelets serve as a reservoir of NPY in this condition. We demonstrate significantly increased NPY-ir levels in PRP of PE patients, as well as their serum, which contains platelet-derived factors released during coagulation, but not in the PPP fraction devoid of platelets. In line with this, we observed a significant increase in the NPY-ir concentration per 100,000 platelets in both MTPE and PLPE patients, as compared to NP subjects. While the NPY/platelet ratio can be partially increased due to thrombocytopenia observed in PE patients, the difference in the platelet count between NP and PE subjects was not great enough to fully explain profound increase in amount of NPY per 100,000 platelets observed in this disorder. Thus, our results support the role for platelets in NPY storage and delivery.
The presence of NPY in platelets is intriguing, since these cells do not contain the peptide in normal healthy humans (Li et al., 2011). This indicates that platelets may accumulate NPY over prolonged periods of sympathetic hyperactivity and be therefore a marker and a “carrier” of the stress response (Li et al., 2005; Tilan et al., 2013). In addition to absorption of neuronal NPY by platelets, there is also evidence of its increased synthesis in megakaryocytes during chronic stress (Kuo et al., 2007; Li et al., 2005; Li et al., 2011; Najafi et al., 2013; Tilan et al., 2013). Such a phenomenon was previously found in certain strains of mice in association with autoimmune abnormalities (Schwarz et al., 1994). Since autoimmune factors have been also implicated in the pathogenesis of PE, particularly PLPE, this is an intriguing possibility which would warrant further studies. A similar stress-induced increase in platelet NPY has been observed in depression and chronic severe stress and shown to exacerbate atherosclerosis and obesity (Kuo et al., 2007; Li et al., 2005; Li et al., 2011; Najafi et al., 2013; Nilsson, 1996; Tilan et al., 2013). Likewise, in PE induction and/or accumulation of NPY in platelets may contribute to pathogenesis of the disease by delivering the peptide to the sites of placental remodeling where it may stimulate vasoconstriction.
To determine if systemic NPY levels affect severity of PE we correlated its serum concentrations with type and extent of the disease. We have found that the disease was more severe in PLPE patients, while the increase in NPY levels was more profound in MTPE patients. Consequently, there was no significant association between NPY concentration and disease severity score in individual patients. These data suggest that variability in NPY levels among PE patients most likely reflects differences in the disease etiology rather than its severity, implicating NPY as a marker of maternal pathology associated with MTPE. However, it is also important to remember that there are numerous confounding factors that could affect NPY measurements, including the different time of gestation of PLPE and MTPE groups, coexisting chronic conditions, such as hypertension, and different levels of stress in these patients. Also, the timing of blood sampling in relation to the last meal may have an effect on plasma levels of NPY-ir. In a study of hypertensive patients, elevated plasma NPY levels in comparison to normotensive people were only found after fasting (Solt, 1990). It is therefore remarkable that in spite of multiple factors that may differentially regulate circulating NPY, as well as the relatively small study cohort, significantly higher circulating serum and platelet levels of the peptide were consistently found in PE patients.
PlGF, a member of the VEGF family of angiogenic factors is required for development of vascularization of the placenta (Vrachnis et al., 2013). In PE, its angiogenic activities are diminished due to the presence of sFlt-1, a circulating soluble receptor and a binding protein (Kendall et al., 1996). For that reason, the elevated levels of sFlt-1 may be considered a marker of PE. We have shown that the serum levels of sFlt-1 in both forms of PE are equally elevated, as compared to NP, in accordance with previous reports (Levine et al., 2004; Zhou et al., 2002). In contrast, no significant changes were found in PlGF levels in the serum between PE and NP patients. However, the ELISA assay used in our study did not distinguish free PLGF from sFlt-1-bound form. Therefore, our results cannot be directly compared to previous studies indicating decreased levels of free PlGF protein, not bound by sFlt-1 (Levine et al., 2004). Moreover, PlGF levels are also affected by the GA and achieve their maximum in the first trimester of the pregnancy. Since NP patients delivered on average at 38 weeks of gestation, while PE patients delivered much earlier (30 weeks for PLPE and 36 weeks for MTPE), this variability might have contributed to lack of significant changes in PlGF levels between PE and NP.
In summary, our data indicate that PE is associated with increased systemic levels of NPY and suggests platelets as its main reservoir. Moreover, NPY concentrations are particularly high in MTPE, while no differences in sFlt-1 levels have been found between PLPE and MTPE patients, suggesting NPY as a differentiating factor reflecting various etiologies of these two forms of PE. Lastly, our results suggest NPY as a potential therapeutic target for preventing hypertension related to MTPE and warrant further investigations into the mechanisms of the increased peptide release and its actions in women with MTPE.
Highlights.
Systemic levels of neuropeptide Y are elevated in patients with preeclampsia.
In women with preeclampsia neuropeptide Y accumulates in platelets.
Neuropeptide Y concentrations are particularly high in maternal preeclampsia.
Neuropeptide Y is a potential target for antihypertensive preeclampsia therapy.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants HL067357 and HL055310 to Z.Z. and, in part, CA123211 to J.K.
The authors would like to acknowledge Dr. Zofia Zukowska, who was a leader in the fields of stress physiology and NPY biology and Dr. Sara Paiva’s mentor during her doctoral studies at Georgetown University.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 2.Conrad KP, Lindheimer MD. Renal and cardiovascular alterations. In: Lindheimer MD, Roberts JM, Cunningham FG, Stamford CT, editors. Chesley’s Hypertensive Dysorders in Pregnancy. 2. Appleton & Lange; 1999. pp. 263–362. [Google Scholar]
- 3.Cunningham FG, Lindheimer MD. Hypertension in pregnancy. N Engl J Med. 1992;326:927–932. doi: 10.1056/NEJM199204023261405. [DOI] [PubMed] [Google Scholar]
- 4.Dotsch J, Nusken KD, Knerr I, Kirschbaum M, Repp R, Rascher W. Leptin and neuropeptide Y gene expression in human placenta: ontogeny and evidence for similarities to hypothalamic regulation. J Clin Endocrinol Metab. 1999;84:2755–2758. doi: 10.1210/jcem.84.8.5892. [DOI] [PubMed] [Google Scholar]
- 5.Ekstrand AJ, Cao R, Bjorndahl M, Nystrom S, Jonsson-Rylander AC, Hassani H, Hallberg B, Nordlander M, Cao Y. Deletion of neuropeptide Y (NPY) 2 receptor in mice results in blockage of NPY-induced angiogenesis and delayed wound healing. Proc Natl Acad Sci U S A. 2003;100:6033–6038. doi: 10.1073/pnas.1135965100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundemar LaHR. Multiple neuropeptide Y receptors are involved in cardiovascular regulation. Peripheral and central mechanisms. Gen Pharmacol. 1993;24:785–796. doi: 10.1016/0306-3623(93)90151-m. [DOI] [PubMed] [Google Scholar]
- 7.Hashim MA, Tadepalli AS. Cutaneous vasomotor effects of neuropeptide Y. Neuropeptides. 1995;29:263–271. doi: 10.1016/0143-4179(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 8.Hauser GJ, Danchak MR, Colvin MP, Hopkins RA, Wocial B, Myers AK, Zukowska-Grojec Z. Circulating neuropeptide Y in humans: relation to changes in catecholamine levels and changes in hemodynamics. Neuropeptides. 1996;30:159–165. doi: 10.1016/s0143-4179(96)90083-9. [DOI] [PubMed] [Google Scholar]
- 9.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38:213–224. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology. 2004;145:4835–4837. doi: 10.1210/en.2004-1028. [DOI] [PubMed] [Google Scholar]
- 11.Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- 12.Khatun S, Kanayama N, Belayet HM, Bhuiyan AB, Jahan S, Begum A, Kobayashi T, Terao T. Increased concentrations of plasma neuropeptide Y in patients with eclampsia and preeclampsia. Am J Obstet Gynecol. 2000;182:896–900. doi: 10.1016/s0002-9378(00)70342-5. [DOI] [PubMed] [Google Scholar]
- 13.Kitlinska J, Lee EW, Li L, Pons J, Estes L, Zukowska Z. Dual role of dipeptidyl peptidase IV (DPP IV) in angiogenesis and vascular remodeling. Adv Exp Med Biol. 2003;524:215–222. doi: 10.1007/0-306-47920-6_26. [DOI] [PubMed] [Google Scholar]
- 14.Klonoff-Cohen HS, Cross JL, Pieper CF. Job stress and preeclampsia. Epidemiology. 1996;7:245–249. doi: 10.1097/00001648-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Kokot F, Ulman I, Wiecek A, Irzyniec T, Ulman J. Concentrations of leptin and neuropeptide Y in maternal plasma, umbilical cord blood and in amniotic fluid in pregnant women with EPH-gestosis. Arch Immunol Ther Exp (Warsz) 1998;46:311–316. [PubMed] [Google Scholar]
- 16.Kokot F, Ulman I, Wiecek A, Irzyniec T, Ulman J. Do leptin and neuropeptide Y influence blood pressure regulation in healthy pregnant women and women with preeclampsia? Pol Arch Med Wewn. 1999;101:385–390. [PubMed] [Google Scholar]
- 17.Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13:803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 18.Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95:487–490. doi: 10.1016/s0029-7844(99)00602-x. [DOI] [PubMed] [Google Scholar]
- 19.Lee EW, Grant DS, Movafagh S, Zukowska Z. Impaired angiogenesis in neuropeptide Y (NPY)-Y2 receptor knockout mice. Peptides. 2003;24:99–106. doi: 10.1016/s0196-9781(02)00281-4. [DOI] [PubMed] [Google Scholar]
- 20.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Jonsson-Rylander AC, Abe K, Zukowska Z. Chronic stress induces rapid occlusion of angioplasty-injured rat carotid artery by activating neuropeptide Y and its Y1 receptors. Arterioscler Thromb Vasc Biol. 2005;25:2075–2080. doi: 10.1161/01.ATV.0000179601.19888.19. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Najafi AH, Kitlinska JB, Neville R, Laredo J, Epstein SE, Burnett MS, Zukowska Z. Of mice and men: neuropeptide Y and its receptors are associated with atherosclerotic lesion burden and vulnerability. J Cardiovasc Transl Res. 2011;4:351–362. doi: 10.1007/s12265-011-9271-5. [DOI] [PubMed] [Google Scholar]
- 23.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mentlein R, Dahms P, Grandt D, Kruger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul Pept. 1993;49:133–144. doi: 10.1016/0167-0115(93)90435-b. [DOI] [PubMed] [Google Scholar]
- 25.Mione MC, Cavanagh JF, Lincoln J, Milner P, Burnstock G. Pregnancy reduces noradrenaline but not neuropeptide levels in the uterine artery of the guinea-pig. Cell Tissue Res. 1990;259:503–509. doi: 10.1007/BF01740777. [DOI] [PubMed] [Google Scholar]
- 26.Modin A, Malmstrom RE, Meister B. Vascular neuropeptide Y Y1-receptors in the rat kidney: vasoconstrictor effects and expression of Y1-receptor mRNA. Neuropeptides. 1999;33:253–259. doi: 10.1054/npep.1999.0755. [DOI] [PubMed] [Google Scholar]
- 27.Najafi AH, Aghili N, Tilan JU, Andrews JA, Peng X, Lassance-Soares RM, Sood S, Alderman LO, Abe K, Li L, Kolodgie FD, Virmani R, Zukowska Z, Epstein SE, Burnett MS. A new murine model of stress-induced complex atherosclerotic lesions. Dis Model Mech. 2013;6:323–331. doi: 10.1242/dmm.009977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson G, et al. Differences in the neuropeptide Y-like immunoreactivity of the plasma and platelets of human volunteers and depressed patients. Peptides. 1996;17:359–362. doi: 10.1016/0196-9781(96)00013-7. [DOI] [PubMed] [Google Scholar]
- 29.Nishikawa M, Itakura A, Ito M, Takeuchi M, Sato Y, Kajiyama H, Mizutani S, Kikkawa F. Changes in placental dipeptidyl peptidase IV in preeclampsia with intrauterine growth restriction. Horm Metab Res. 2005;37:408–413. doi: 10.1055/s-2005-870229. [DOI] [PubMed] [Google Scholar]
- 30.Paarlberg KM, Vingerhoets AJ, Passchier J, Dekker GA, Van Geijn HP. Psychosocial factors and pregnancy outcome: a review with emphasis on methodological issues. J Psychosom Res. 1995;39:563–595. doi: 10.1016/0022-3999(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 31.Pennington KA, Schlitt JM, Jackson DL, Schulz LC, Schust DJ. Preeclampsia: multiple approaches for a multifactorial disease. Dis Model Mech. 2012;5:9–18. doi: 10.1242/dmm.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Racchi H, Schliem AJ, Donoso MV, Rahmer A, Zuniga A, Guzman S, Rudolf K, Huidobro-Toro JP. Neuropeptide Y Y1 receptors are involved in the vasoconstriction caused by human sympathetic nerve stimulation. Eur J Pharmacol. 1997;329:79–83. doi: 10.1016/s0014-2999(97)00160-x. [DOI] [PubMed] [Google Scholar]
- 33.Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1999;106:1019–1022. doi: 10.1111/j.1471-0528.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 34.Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 35.Rook W, Johnson CD, Coney AM, Marshall JM. Prenatal hypoxia leads to increased muscle sympathetic nerve activity, sympathetic hyperinnervation, premature blunting of neuropeptide y signaling, and hypertension in adult life. Hypertension. 2014;64:1321–1327. doi: 10.1161/HYPERTENSIONAHA.114.04374. [DOI] [PubMed] [Google Scholar]
- 36.Salvador-Moysen J, Martinez-Lopez Y, Ramirez-Aranda JM, Aguilar-Duran M, Terrones-Gonzalez A. Genesis of preeclampsia: an epidemiological approach. ISRN Obstet Gynecol. 2012;2012:916914. doi: 10.5402/2012/916914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandman CA, Wadhwa PD, Chicz-DeMet A, Dunkel-Schetter C, Porto M. Maternal stress, HPA activity, and fetal/infant outcome. Ann N Y Acad Sci. 1997;814:266–275. doi: 10.1111/j.1749-6632.1997.tb46162.x. [DOI] [PubMed] [Google Scholar]
- 38.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia -- a state of sympathetic overactivity. N Engl J Med. 1996;335:1480–1485. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz H, Villiger PM, von Kempis J, Lotz M. Neuropeptide Y is an inducible gene in the human immune system. J Neuroimmunol. 1994;51:53–61. doi: 10.1016/0165-5728(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 40.Solt VB, Brown MR, Kennedy B, Kolterman OG, Ziegler MG. Elevated insulin, norepinephrine, and neuropeptide Y in hypertension. Am J Hypertens. 1990;3:48–52. doi: 10.1093/ajh/3.11.823. [DOI] [PubMed] [Google Scholar]
- 41.Tilan JU, Everhart LM, Abe K, Kuo-Bonde L, Chalothorn D, Kitlinska J, Burnett MS, Epstein SE, Faber JE, Zukowska Z. Platelet neuropeptide Y is critical for ischemic revascularization in mice. FASEB J. 2013 doi: 10.1096/fj.12-213546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–1544. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 43.Vrachnis N, Kalampokas E, Sifakis S, Vitoratos N, Kalampokas T, Botsis D, Iliodromiti Z. Placental growth factor (PlGF): a key to optimizing fetal growth. J Matern Fetal Neonatal Med. 2013;26:995–1002. doi: 10.3109/14767058.2013.766694. [DOI] [PubMed] [Google Scholar]
- 44.Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosom Med. 1996;58:432–446. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–1423. doi: 10.1016/S0002-9440(10)62567-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zukowska-Grojec Z. Neuropeptide Y. A novel sympathetic stress hormone and more. Ann N Y Acad Sci. 1995;771:219–233. doi: 10.1111/j.1749-6632.1995.tb44683.x. [DOI] [PubMed] [Google Scholar]