Abstract
Patients with severe tracheal obstruction in Morquio A syndrome are at risk of dying of sleep apnea and related complications. Tracheal obstruction also leads to life-threatening complications during anesthesia as a result of the difficulty in managing the upper airway due to factors inherent to the Morquio A syndrome, compounded by the difficulty in intubating the trachea. A detailed description of the obstructive pathology of the trachea is not available in the literature probably due to lack of a homogenous group of Morquio A patients to study at any one particular center. We present a series of cases with significant tracheal obstruction who were unrecognized due to the difficulty in interpreting tracheal narrowing airway symptoms. Our goal is to provide the guidelines in the management of these patients that allow earlier recognition and intervention of tracheal obstruction.
Sagittal MRI images of the cervical spine of 28 Morquio A patients (12 ± 8.14 years) showed that19/28 (67.9%) patients had at least 25% tracheal narrowing and that narrowing worsened with age (all 8 patients over 15 years had greater than 50% narrowing). Eight out of 28 patients were categorized as severe (>75%) tracheal narrowing when images were evaluated in neutral head and neck position. Of the 19 patients with tracheal narrowing, compression by the tortuous brachiocephalic artery was the most common cause (n = 15). Evidence of such tracheal narrowing was evident as early as at 2 years of age.
The etiology of tracheal impingement by the brachiocephalic artery in Morquio A appears to be due to a combination of the narrow thoracic inlet crowding structures and the disproportionate growth of trachea and brachiocephalic artery in relationship to the chest cavity leading to tracheal tortuosity.
In conclusion, tracheal narrowing, often due to impression from the crossing tortuous brachiocephalic artery, increases with age in Morquio A patients. Greater attention to the trachea is needed when evaluating cervical spine MRIs as well as other imaging and clinical investigations, with the goal of establishing a timely treatment protocol to reduce the mortality rate in this patient population.
Keywords: Morquio A syndrome, tracheal obstruction, MRI, brachiocephalic artery, disproportionate growth
Introduction
Morquio A syndrome (Mucopolysaccharidosis type IVA, MPS IVA) is an autosomal recessive lysosomal storage disorder caused by a deficiency of N-acetylgalactosamine-6-sulfate sulfatase (GALNS) (1,2). This enzyme deficiency leads to progressive accumulation of excessive glycosaminoglycans (GAGs) including keratan sulfate (KS) and chondroitin-6-sulfate (C6S) primarily in the lysosomes of bone, cartilage, and ligaments, as well as the extracellular matrix (ECM) of these tissues (1, 3–5). Deposition of GAGs impairs chondrogenesis and endochondral ossification, causing systemic skeletal dysplasia such as striking short trunk stature, cervical spinal cord compression, pectus carinatum, kyphoscoliosis, knock-knee, hypermobile joints, and an abnormal gait with an increased tendency to fall (5–9). Many patients become wheelchair-dependent in their second decade and undergo multiple surgeries to alleviate serious orthopedic complications. Individuals with Morquio A syndrome often do not survive beyond their twenties (5,6). The two most common causes of mortality in Morquio A syndrome are spinal cord compression and airway compromise (6).
Although the skeletal manifestations have been well described, the complex airway abnormalities caused by the interplay of restrictive chest deformity and accumulation of GAGs in the trachea have not been well studied. Imaging of the cervical spine, including x-rays and magnetic resonance imaging (MRI), is commonly acquired in Morquio A patients, although the airway is often not evaluated specifically. In fact, saturation bands are commonly placed across the anterior soft tissues of the neck to reduce motion artifact on cervical spine MRIs, sometimes at least partially to obscure the airway. Tracheal obstruction may go unrecognized or simply accepted as part of the natural course of Morquio A until life-threatening sleep apnea, cor pulmonale or anesthetic complications arise. Perioperative upper airway management and tracheal intubation can be extremely difficult during anesthetic management of the airway (10–16). Although tracheostomy can be used to rescue an otherwise unaccessible airway, performing a tracheostomy in a patient with Morquio A is challenging due to an extremely short neck, fixed cervical vertebrae with inability to hyperextend the neck, and a tortuous and redundant trachea.
We experienced two cases which were autopsied following death attributed to tracheal obstruction. Autopsy results showed marked infiltration of macrophages in the tracheal wall, indicating an inflammatory process (15, unpublished). In addition, we recently managed a 16-year-old boy who underwent 2.5 years of enzyme replacement therapy (ERT) but had worsening tracheal obstruction with severe respiratory distress, requiring urgent surgical intervention. His pre-operative imaging studies revealed two levels of tracheal obstruction. His surgical procedure included reimplantation of the brachiocephalic (or innominate) artery to relieve obstruction of the proximal trachea caused by vascular impingement, followed by excision of redundant distal trachea with end to end anastomosis [unpublished]. This case, in particular, prompted us to re-evaluate our population of Morquio A patients, focusing on the tracheal component of their airway obstruction. Tracheal obstruction poses a far greater risk to patients than other factors and timely recognition, and intervention may be a life-saving measure.
The purpose of this study is to evaluate the degree of tracheal narrowing in children and young adults with Morquio A that was evident but previously overlooked on magnetic resonance imaging (MRI) studies of the cervical spine.
Material and Methods
Subjects
Twenty-eight children and young adults (16 males) with Morquio A syndrome ranging in age from 1.0 through 35.8 years of age (mean 12 ± 8.14 years) evaluated at our tertiary care pediatric hospital who underwent cervical spine MRI were included in this IRB-approved retrospective study. Compared with a standard growth chart of Morquio A patients (17, 18), all 28 patients had a severe phenotype, with height ≦ 75th percentile (132 cm for male, 127 cm for female).
MRI Examination Technique
Fifty-four MRI scans were available in our Picture Archiving and Communication System for these 28 subjects. All MR studies of the cervical spine performed at our institution (23 examinations) were acquired on 1.5-T (GE Signa Hdxt, GE Healthcare, Waukesha, WI) or 3.0-T (GE Discovery MR750w, GE Healthcare) MR systems with dedicated spine coil for the purpose of evaluating spinal stenosis and spinal cord compression. The remainder of the studies were acquired at other facilities, within the Unites States and internationally, using varied MR platforms. All exams, regardless of an imaging facility, included sagittal T1 and T2-weighted sequences, typically with 3mm slice thickness (range 2–4mm). Image acquisition time was approximately 2.5 to 3.5 minutes for the sagittal sequences at our institution, with the patient free breathing. Axial images were not analyzed due to obscuration of the trachea by saturation bands used to decrease motion artifact.
The electronic medical record (EMR) was searched to determine the use of sedation for the MRI examinations, with documentation available for 22 studies. Among these 22 MRIs, 2 examinations were performed with general anesthesia with the airway secured with an endotracheal tube, and another 2 studies were performed using general anesthesia with laryngeal mask airway for maintaining a patent airway. Six MRIs were obtained with sedation and natural airway. 12 MRIs were acquired with no sedation. The remaining 32 examinations did not have sufficient information in the EMR to evaluate fully whether the MRIs were performed with sedation or without it.
Imaging Analysis
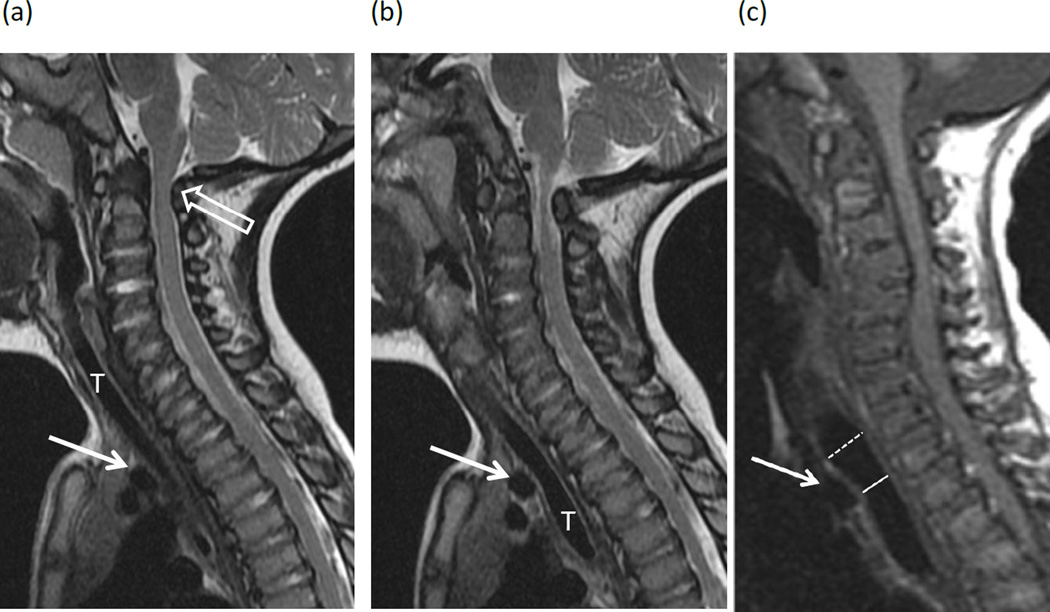
The anteroposterior diameter of the trachea was measured on sagittal T1 or T2-weighted images at three levels: at the level of greatest narrowing, the level of largest diameter above the narrowing, and the level of largest diameter below the narrowing. Multiple consecutive sagittal images were used to include all portions of the trachea. If flexion and extension positioning was available in addition to neutral neck position, these measurements were recorded separately. Percent narrowing was measured by comparing the narrowest tracheal diameter to the largest diameter above or below this level (narrowest diameter / largest diameter × 100). Severity of narrowing was assigned based on neutral images as follows: no narrowing (score 0), less than 25% narrowing; mild (score 1) ≥ 25% narrowing; moderate (score 2), ≥ 50% narrowing; and severe (score 3) ≥ 75% narrowing (Fig. 1). For patients with multiple studies, the highest score was recorded.
Figure 1. MR images in patients with Morquio A syndrome.
MR images of the cervical spine in patients with Morquio A syndrome illustrate the range of tracheal narrowing observed at the thoracic inlet in neutral positioning. (a, b) Two consecutive sagittal T2-weighted images in a 23 month-old girl show minimal anterior impression on the trachea (T) by the brachiocephalic artery (arrows), categorized in the “no narrowing” group. Note marked stenosis of the spinal canal at the C1–C2 level (open arrows), impinging on the spinal cord. (c) Sagittal T1-weighted image in a 7 year-old boy shows mild (25–50%) narrowing of the trachea with anterior impression by the brachiocephalic artery (arrow), decreasing in caliber from 11mm above the artery (dashed line) to 8mm at the level of the artery (solid line). (d) Sagittal T1-weighted image in a 15-year-old girl shows 50–75% narrowing of the trachea at the level of the brachiocephalic artery (solid line) compared to the trachea below this level (dashed line). In addition to the brachiocephalic artery (arrow), there is intermediate signal tissue between the artery and trachea adding to the mass effect. Axial T1-weighted image in the same child (e) confirms narrowing of the tracheal lumen with thickening of the tracheal wall (dashed arrow). The brachiocephalic artery is obscured by an anterior saturation band used to decrease motion artifact. (f, g) Two consecutive sagittal T1-weighted images in a 27 year-old man show severe narrowing (>75%) of the trachea (black arrows) with a marked buckled appearance as it crosses the level of the brachiocephalic artery (white arrow). Note capacious lumen of the subglottic trachea (dashed line) for comparison.
MR images were also evaluated for the anatomic cause of airway obstruction, noting presence or absence of a vascular impression on the trachea by the crossing right brachiocephalic (or innominate) artery, and thickening of the tracheal wall or surrounding soft tissues.
Statistical Analysis
Means and standard deviations of the total score were calculated by 4 age groups χ ≤ 5, 5 ≤ χ <10, 10 ≤ χ < 15, and 15 ≤ χ years of age. ANOVA-one way test was used to compare these four groups. Statistical analyses were performed using ANOVA test with SPSS v.16. The significance level was set at p < 0.05.
Results
Frequency of tracheal narrowing and severity score
On neutral MR imaging of the cervical spine, 19 of 28 (67.9%) patients had tracheal narrowing, with 8 of these patients showing severe narrowing (score 3) (Table 1).
Table 1.
Severity of tracheal narrowing on sagittal MR images of the cervical spine with neutral neck position.
| number | % | |
|---|---|---|
| MRI investigated | 28 | 100% |
| Obstruction | 19 | 67.9% |
| Score 0 | 9 | 32.1% |
| Score 1 | 3 | 10.7% |
| Score 2 | 8 | 28.6% |
| Score 3 | 8 | 28.6% |
Effect of neck position on tracheal narrowing
Flexion and extension imaging of the cervical spine was available in 19 of 28 subjects. Compared to neutral positioning, flexion caused increased tracheal narrowing in 6 patients, increasing the severity score by 1 category. Conversely, extension resulted in decreased tracheal narrowing in 2 patients and increased tracheal narrowing in 1 patient (Table 2, Fig. 2). In other words, increasing neck flexion (neutral to flexed or extended to neutral) caused worsening tracheal narrowing in 8 of 19 patients while the opposite was seen in only 1. No change in severity category was seen in the remaining 10 subjects.
Table 2.
Change in severity score of tracheal narrowing with flexion and extension sagittal MR images of the cervical spine.
| (a) Changed scores with flexion position | |
|---|---|
| Number | |
| No change | 11 |
| Increase | 6 |
| Decrease | 0 |
| Inadequate | 2 |
| Total | 19 |
| (b) Changed scores with extension position | |
|---|---|
| Number | |
| No change | 13 |
| Increase | 1 |
| Decrease | 2 |
| Inadequate | 3 |
| Total | 19 |
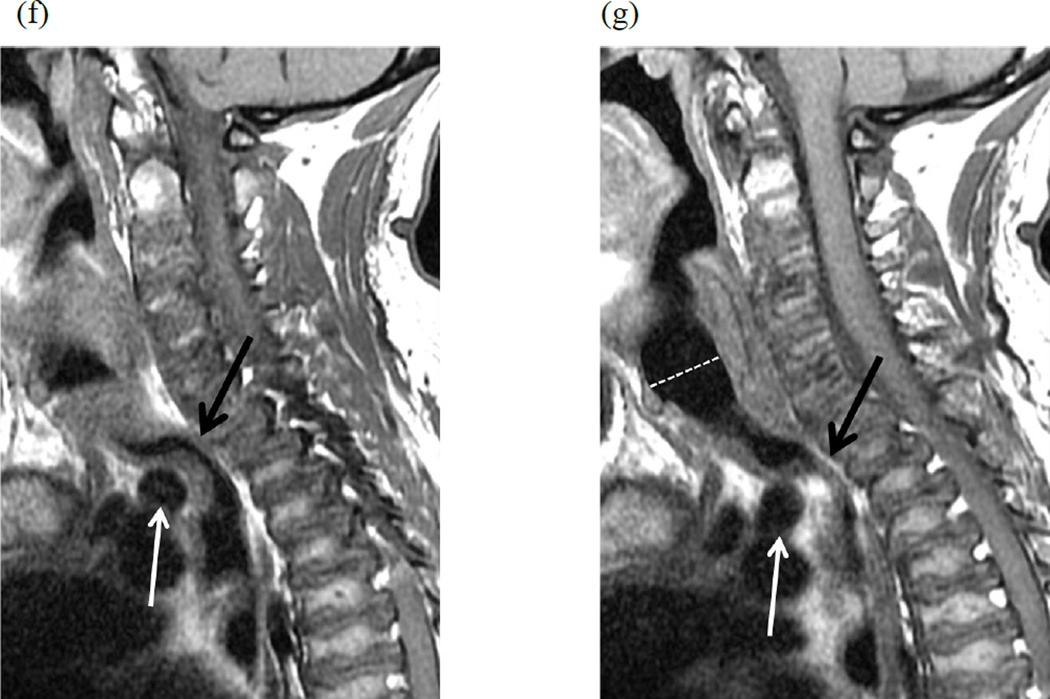
Figure 2. Flexion and extension MR imaging of the cervical spine in a 10-year-old female with Morquio A syndrome.
(a, b) Two consecutive sagittal T2-weighted images of the spine in flexion show 50–75% narrowing of the trachea at the level of the brachiocephalic artery (solid lines) compared to the cervical trachea (dashed line). Note the position of the brachiocephalic artery anterior to the trachea (arrows). (c) With the extension of the cervical spine, narrowing of the trachea is significantly improved (same annotations).
Anatomic cause of tracheal narrowing
The brachiocephalic trunk crossed from left to the right immediately anterior to the upper thoracic trachea in 24 of 28 subjects. In 2 cases, the brachiocephalic artery was not centered over the trachea but thickened, intermediate signal tracheal wall and surrounding tissue was seen at a similar level. In 2 other cases, both a crossing brachiocephalic artery and thickened tissue was seen. Of the 19 patients with score 1–3, the primary cause of tracheal narrowing was the crossing brachiocephalic artery in 15, both the artery and thickened tissue in 2, and thickened tissue only in 2 (Table 3, Fig. 3). In the 9 patients with score 0, the brachiocephalic artery passed anterior to the trachea but did not cause significant mass effect.
Table 3.
Anatomic cause of tracheal narrowing.
| (a) Material in front of airway | |
|---|---|
| Material | Number |
| Artery | 24 |
| Connective tissue | 2 |
| Both | 2 |
| Neither | 0 |
| (b) Material - only in narrowed cases | |
|---|---|
| Material | Number |
| Artery | 15 |
| Connective tissue | 2 |
| Both | 2 |
| Neither | 0 |
Artery: brachiocephalic artery. Connective tissue: thickening of the trachea and surrounding tissue.
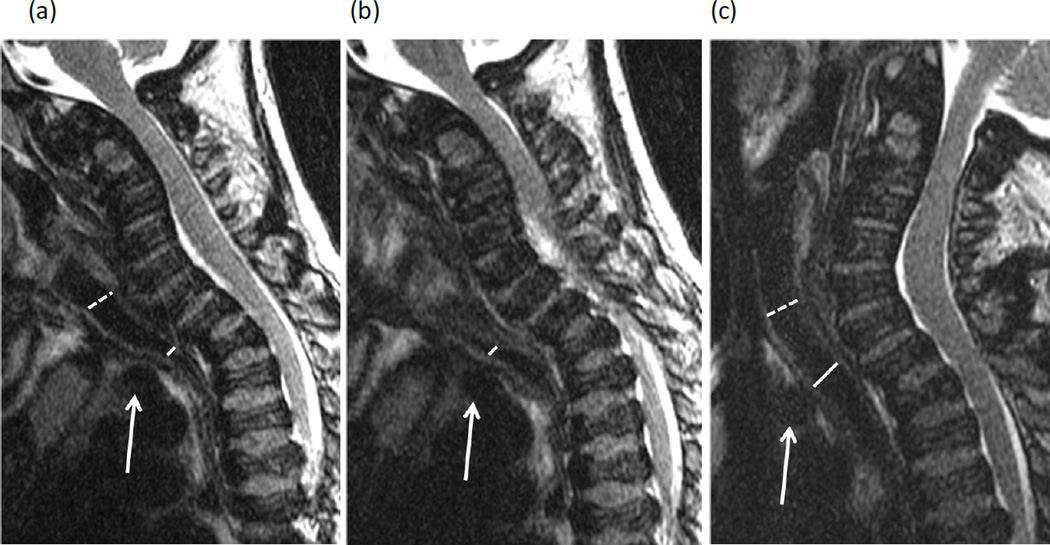
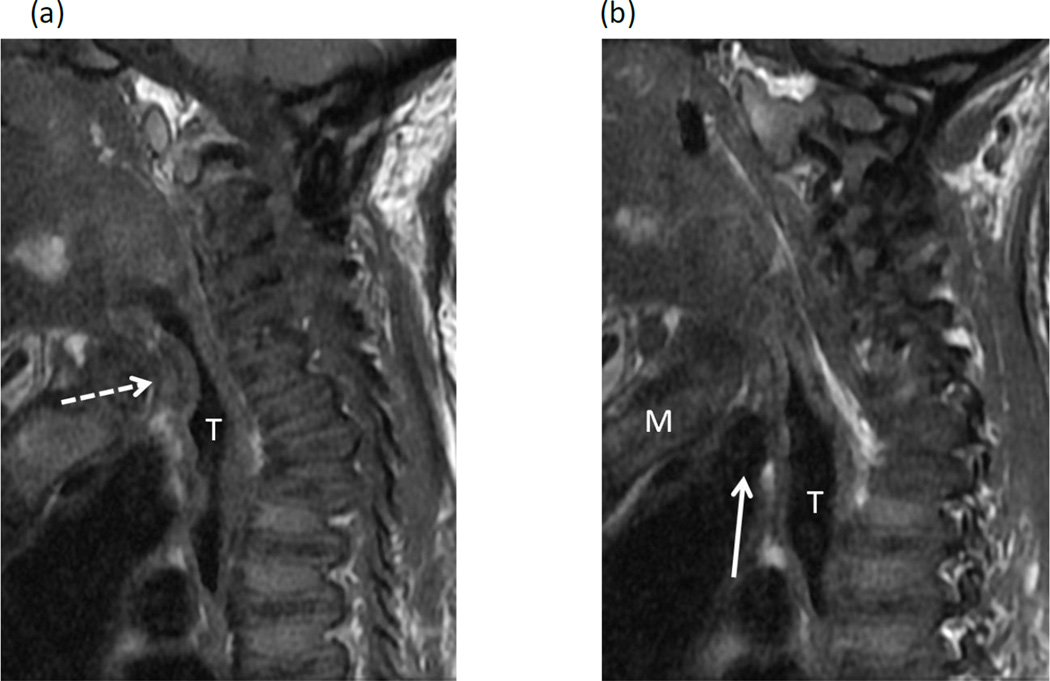
Figure 3. Narrowing of the trachea due to thickened tissue at the thoracic inlet in a 23-year-old female with Morquio A syndrome.
(a, b) Two consecutive sagittal T1-weighted MR images of spine show >75% narrowing of the trachea (T) at the thoracic inlet due to intermediate signal tissue (dashed arrow) along the anterior wall of the trachea. The origin of the brachiocephalic artery (solid arrow) is positioned below the manubrium (M) and below the level of tracheal narrowing.
Patient age and degree of tracheal narrowing
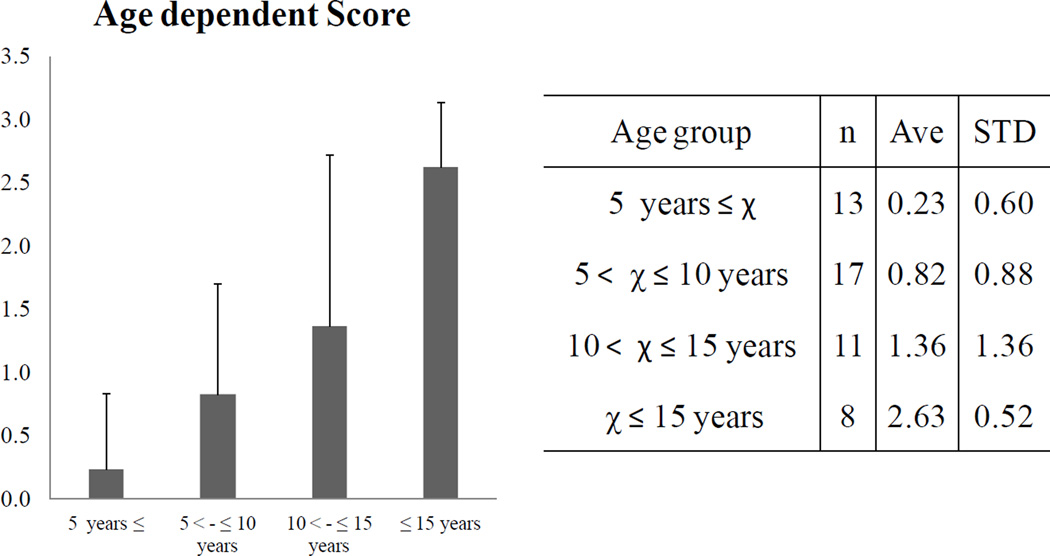
Trachea narrowing was seen in a child as young as 2 years of age. Narrowing increased with age, with all 8 patients over 15 years of age having greater than 50% narrowing (score 2 or 3) (Fig. 4). The score of the subjects over 15 years of age was nearly twice higher than the score of the 10–15 year-olds.
Figure 4. Age-dependent severity of tracheal obstruction in children and young adults with Morquio A syndrome.
Age and severity are significantly related to each other (p < 0.0001) using one-way ANOVA.
Discussion
Patients with Morquio A rarely live past their third decade of life due to severe spinal cord complications or a combination of obstructive and restrictive lung disease. As children with Morquio A grow older, they often assume a “look up to the sky” position to help alleviate airway obstruction (19, 20). Perioperative airway management in these patients, who often undergo multiple orthopedic procedures, can be very difficult due to the tortuous and narrowed airway. Despite these known features, very little attention has been given to imaging of the trachea in individuals with Morquio A syndrome.
In this article, we retrospectively evaluated the trachea in children and young adults who underwent cervical spine MRI for evaluation of spinal cord compression. Although not included as part of the original assessment, the airway could be visualized in all patients using a combination of T1 and T2-weighted sagittal images. Axial images were not included in this study, since most often the airway was obscured by a saturation band deliberately placed across the anterior soft tissues, used to help reduce motion artifact. A saturation band was also often employed for sagittal images, but enough of the airway was still visible for assessment. Due to the relatively small number of patients and incomplete medical records, the impact of sedation or anesthesia on the appearance of the airway could not be determined.
We found at least 25% caliber reduction of the upper thoracic trachea in 19 of 28 patients, starting as young as 2 years old. Tracheal narrowing progressively worsened with age, with all subjects over 15 years old having more than 50% tracheal narrowing. Eight of 28 individuals had greater than 75% tracheal narrowing. Most commonly, the crossing tortuous brachiocephalic artery caused a focal impression on the anterior wall of the trachea at the level of greatest narrowing (Brachiocephalic artery compression syndrome). Some patients also had thickening of the tracheal wall and surrounding tissue contributing to the airway narrowing, likely representing the excessive accumulation of GAGs and successive inflammation (15) in the tracheal cartilage and connective tissues. Dynamic narrowing of the airway, usually worse with neck flexion, was also observed in some patients.
Based on visualization of the trachea in only the sagittal plane, other features contributing to airway obstruction, such as tracheal tortuosity or kinking in the coronal plane and interplay with rib cage distortion, could not be evaluated in our study. The airway abnormalities in Morquio A patients are multifactorial, including restrictive and obstructive pathophysiologies (Fig. 5) (21, 22). Pectus carinatum, thoracic kyphosis and platyspondyly all contribute to restriction of lung volumes. Imbalanced growth of the trachea leading to tortuosity, a relatively large tongue, and vocal cord hypertrophy due to the accumulation of GAGs all contribute to obstructive symptoms. Keratan sulfate, the predominant GAG in Morquio A, accumulates in hyaline cartilage, which makes up the anterior ring of the trachea. Deposition of GAGs specifically in the cartilage of the trachea may cause floppiness and loss of linear and tensile strength of the tracheal wall, allowing for focal impression by the brachiocephalic artery and dynamic narrowing with neck flexion.
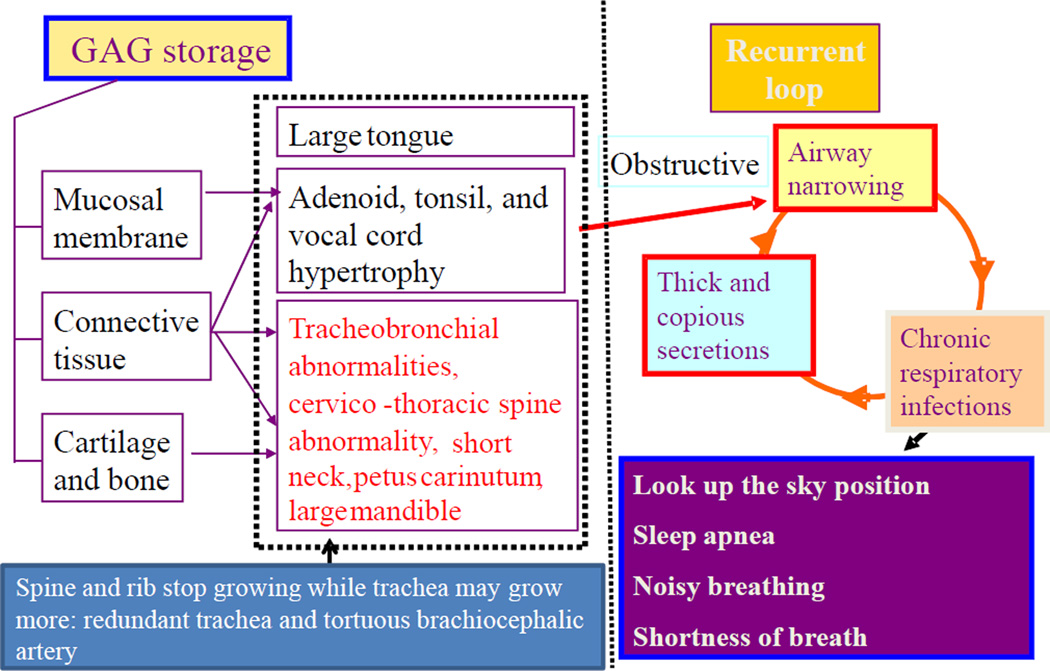
Figure 5. Pathophysiology of difficult airway in Morquio A.
The pathophysiology of Morquio A in the area of airway demonstrates the progressive problems and the existence of vicious cycles. Undegraded C6S and KS accumulation lead to alterations in the connective tissue and cartilage ground substance, distorting bone mass acquisition and perturbing the regular microarchitecture of cartilage. Pathohistological studies have indicated that patients with Morquio A present impaired cartilage quality because of distortion of geometric shape, collagen disposition in ECM, and remodeling, resulting in poor bone mineralization (15, 28, 29). The respiratory problems in Morquio A consist of restrictive and obstructive sequences. The restrictive lung is due to thoracic cage deformity while the obstructive disease is from tracheobronchial abnormalities (disproportionate growth in neck, chest cavity and trachea: redundant trachea), cervicothoracic spine abnormality, a tortuous brachiocephalic artery as well as large tongue and mandible and adenoidal, tonsillar, and vocal cord hypertrophy by accumulation of storage materials.
In addition to anatomical obstruction, Morquio A patients have small nasal passages by thickened mucous membranes and thick and copious secretions. Chronic upper respiratory tract infection further decreases the already diminished airway lumen. Patients with a severe obstruction show the clinical symptoms of sleep apnea, shortness of breath, look-up to the sky position, and noisy breathing, leading to sudden death and complications in anesthesia procedure.
The syndrome of brachiocephalic artery compression of the trachea was first reported in 1948 (23, 24, 25). Brachiocephalic artery compression of the trachea represents the most common cause of narrowed airway. Symptomatic patients show expiratory strider, cough, recurrent broncho pulmonary infections, and apnea (26). It was originally thought to be attributable to an aberrant brachiocephalic artery that originated anomalously on the left side of the aortic arch and coursed obliquely from left to right causing an anterior indentation of the trachea. In 1981, investigation of lateral radiographs from infants, children, and adolescents showed that the brachiocephalic artery originates at least partially to the left of the trachea in 95% of cases, suggesting that this location of origin is normal in children (27). In this study tracheal indentation was seen in 30% of patients younger than 2 years of age, with an increased incidence of indentation noted in children with congenital heart disease. The tracheal indentation was found to be age-related; the syndrome is rarely seen in children older than 1.5 years of age. Most patients with anterior tracheal compression fail to exhibit symptoms associated with respiratory compromise. There is no consensus as to why some patients are more prone to symptomatic brachiocephalic artery compression of the trachea; however, brachiocephalic artery compression syndrome is more likely to arise in patients with mediastinal crowding (27).
Unlike the known and speculated etiologies of brachiocephalic artery compression syndrome, Morquio A has distinctly different factors; 1) disproportionate growth between chest cavity, neck, brachiocephalic artery, and trachea, 2) a severe pectus carinatum that further narrows an already small thoracic inlet and thoracic cavity, 3) deposition of GAGs in airway. Redundant and twisted trachea, as well as a brachiocephalic artery, contributes to even greater crowding of structures in thoracic inlet contributing to worsening tracheal compression with age (Fig. 5). It is also noteworthy that not only tortuous brachiocephalic artery but also connective tissue was involved in the mass effect on the trachea. This connective tissue could arise directly from the GAG storage materials and/or inflammatory fibrosis.
Awareness of progressive tracheal narrowing in Morquio A syndrome by all members of the multidisciplinary care team is crucial for early recognition and timely intervention of tracheal obstruction, thus improving quality of life and perioperative safety for these patients. MRI of the cervical spine with flexion and extension offers a unique imaging opportunity to provide multifaceted information without ionizing radiation, including evaluation of spinal stenosis, atlantoaxial instability, spinal cord impingement, and dynamic airway narrowing. Judicious use of saturation bands in the axial and sagittal planes will allow for better characterization of the trachea while the addition of coronal images may also be helpful.
After review of the data presented in this paper, we have made these MRI protocol changes are our institution for patients with Morquio A syndrome and include a description of the airway in the radiology report.
We have corrected tracheal obstruction by a new surgical procedure on a16-year-old patient with worsening respiratory distress who underwent a 2.5 years of ERT. The surgical intervention has rescued his life and provided a better quality of life (unpublished). At present, there is no proof that ERT provides an impact on reduction of pre-existing tracheal obstruction among the patients investigated here since tracheal obstruction is caused primarily by anatomical complex, but more accumulation of cases will clarify this issue in a future long-term study. It is likely that the surgical intervention is the only way to correct the pre-existing severe tracheal obstruction.
Other imaging modalities for the assessment of the airway may also be indicated in Morquio A patients. Attention should be given to the airway on the routine lateral spine and plain chest radiographs, although the combination of thoracic kyphosis and overlapping shoulder tissues often hinders detailed evaluation. Dedicated airway radiographs or airway fluoroscopy are alternatives that might also suffer from similar problems. In those with critical airway symptoms or severe tracheal narrowing identified on MRI, contrast enhanced computed tomography angiography of the chest should be indicated to provide the greatest detail of airway morphology, depict the relationship of the trachea and brachiocephalic artery, and allow for 3-dimensional reconstructions.
Non-imaging investigations of the airway in Morqiuo A patients should be part of routine surveillance as well, including pulmonary function tests and exercise tolerance during gait analysis. Bronchoscopy may be needed for patients with severe airway symptoms, including bronchoscopy-assisted intubation prior to surgical procedures. Although tracheal obstruction leads to clinical signs and symptoms (shortness of breath, respiratory distress, loud breathing, look-up to the sky position, etc), relation between clinical severity and tracheal obstruction should be evaluated in the future studies such as pulmonary function, activity of daily living, and endurance test.
Conclusions
There is a high frequency of tracheal narrowing in children and young adults with Morquio A syndrome, which increases in severity with age. Cervical spine MRI is a feasible, non19 invasive method for detecting and monitoring tracheal narrowing in addition to the spine evaluation. Imaging protocols should include the trachea in the field of view, and the airway should become part of the standard imaging report. Findings of severe tracheal obstruction should alert clinicians of Morquio A patients at increased risk for anesthesiarelated morbidity and mortality and prompt appropriate intervention.
Highlights.
Tracheal obstruction is life-threatening in Morquio A.
High frequency of tracheal obstruction is observed.
The cause of tracheal obstruction is due to an imbalance of growth.
Brachiocephalic artery compresses trachea.
Patients over 15 years of age have over 50% of tracheal obstruction.
Acknowledgement
This work was supported by grants from the Austrian MPS Society and International Morquio Organization (Carol Ann Foundation). S.T. was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant number P20GM103464. The content of the article is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors. Editorial assistance to the manuscript was provided by Michelle Stofa at Nemours/Alfred I. duPont Hospital for Children.
Abbreviation
- C6S
chondroitin-6-sulfate
- ECM
extracellular matrix
- ERT
enzyme replacement therapy
- GALNS
N-acetylgalactosamine-6-sulfate sulfatase
- KS
keratan sulfate
- MR images
Magnetic resonance images
- MRI
Magnetic resonance imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
All the authors contributed to the Article and had no conflict of interest with any other party.
Shunji Tomatsu, Lauren W. Averill, Kazuki Sawamoto, William G. Mackenzie, Michael B. Bober, Christian Pizarro, Christopher J. Goff, Li Xie, Tadao Orii, and Mary Theroux declare that they have no conflict of interests.
Contributions to the project:
Shunji Tomatsu is a Principal Investigator for this article and has contributed to the concept and planning of the article, a collection of data, and reporting of the work described.
Lauren W. Averill contributed to the planning of the article, a collection of data on the obstructive airway, evaluation of MRI, and reporting of the work described.
Kazuki Sawamoto contributed to the planning of the article, analysis of data, and reporting of the work described.
William G. Mackenzie contributed to the planning of the article, treating Morquio A patients, a collection of data on obstructive airway and reporting of the work described.
Michael Bober contributed to the planning of the article, treating Morquio A patients, and reporting of the work described.
Christian Pizarro contributed to the planning of the article, treating a Morquio A patient who had a tracheal obstruction, and reporting of the work described.
Christopher J. Goff contributed to the planning of the article, a collection of data, and reporting of the work described.
Li Xie contributed to the planning of the article, statistical analysis of data, and reporting of the work described.
Tadao Orii contributed to the planning of the article, review of a collection of data, and reporting of the work described.
Mary Theroux is a Principal Investigator for this article and has contributed to the concept of the manuscript, planning of the article, a collection of data, and reporting of the work described.
References
- 1.Morquio L. Sur une forme de dystrophie osseuse familial. Archives de médicine des infants, Paris. 1929;32:129–135. [Google Scholar]
- 2.Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of inherited Disease. 8th ed. New York: McGraw-Hill; 2001. pp. 3421–3452. [Google Scholar]
- 3.Tomatsu S, Montaño AM, Oikawa H, et al. Handbook of Growth and Growth Monitoring in Health and Disease 1. New York: Springer; 2012. Impairment of Body Growth in Mucopolysaccharidoses; pp. 2091–2117. [Google Scholar]
- 4.Patel P, Suzuki Y, Maeda M, et al. Growth charts for patients with Hunter syndrome. Mol Genet Metab Rep. 2014;1:5–18. doi: 10.1016/j.ymgmr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomatsu S, Montaño AM, Oikawa H, et al. Mucopolysaccharidosis type IVA (Morquio A disease): clinical review and current treatment. Cur Pharm Biotech. 2011;12:931–945. doi: 10.2174/138920111795542615. [DOI] [PubMed] [Google Scholar]
- 6.Lavery C, Hendriksz C. Mortality in patients with morquio syndrome a. JIMD Rep. 2015;15:59–66. doi: 10.1007/8904_2014_298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohashi A, Montaño AM, Colón JE, et al. Sacral dimple: incidental findings from newborn evaluation. Mucopolysaccharidosis IVA disease. Acta Paediatr. 2009;98:768–769. doi: 10.1111/j.1651-2227.2009.01134.x. [DOI] [PubMed] [Google Scholar]
- 8.Montaño AM, Tomatsu S, Gottesman GS, Smith M, Orii T. International Morquio A Registry: clinical manifestation and natural course of Morquio A disease. J Inherit Metab Dis. 2007;30:165–174. doi: 10.1007/s10545-007-0529-7. [DOI] [PubMed] [Google Scholar]
- 9.Tomatsu S, Mackenzie WG, Theroux MC, et al. Current and emerging treatments and surgical interventions for Morquio A Syndrome: A review. Res Rep Endocr Disord. 2012;2012:65–77. doi: 10.2147/RRED.S37278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ransford AO, Crockard HA, Stevens JM, Modaghegh S. Occipito-atlanto-axial fusion in morquio-brailsford syndrome. A ten-year experience. J Bone Joint Surg Br. 1996;78:307–313. [PubMed] [Google Scholar]
- 11.Theroux MC, Nerker T, Ditro C, Mackenzie WG. Anesthetic care and perioperative complications of children with morquio syndrome. Paediatr Anaesth. 2012;22:901–907. doi: 10.1111/j.1460-9592.2012.03904.x. [DOI] [PubMed] [Google Scholar]
- 12.Pritzker MR, King RA, Kronenberg RS. Upper airway obstruction during head flexion in morquio's disease. Am J Med. 1980;69:467–470. doi: 10.1016/0002-9343(80)90021-2. [DOI] [PubMed] [Google Scholar]
- 13.Diaz JH, Belani KG. Perioperative management of children with mucopolysaccharidoses. Anesth Analg. 1993;77:1261–1270. doi: 10.1213/00000539-199312000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Drummond JC, Krane EJ, Tomatsu S, Theroux MC, Lee RR. Paraplegia after epidural-general anesthesia in a Morquio patient with moderate thoracic spinal stenosis. Can J Anaesth. 2015;62:45–49. doi: 10.1007/s12630-014-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yasuda E, Fushimi K, Suzuki Y, et al. Pathogenesis of Morquio A syndrome: an autopsied case reveals systemic storage disorder. Mol Genet Metab. 2013;109:301–311. doi: 10.1016/j.ymgme.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Apfelbaum JL, Hagberg CA, Caplan RA, et al. Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2013;118:251–270. doi: 10.1097/ALN.0b013e31827773b2. [DOI] [PubMed] [Google Scholar]
- 17.Montaño AM, Tomatsu S, Brusius A, Smith M, Orii T. Growth charts for patients affected with Morquio A Disease. Am J Med Genet A. 2008;146A:1286–1295. doi: 10.1002/ajmg.a.32281. [DOI] [PubMed] [Google Scholar]
- 18.Tomatsu S, Montaño A, Oikawa H, Giugliani R, Harmatz P, Smith M, Suzuki Y, Orii T. Impairment of Body Growth in Mucopolysaccharidoses. In: Preedy VR, editor. Handbook of Growth and growth monitoring in health and disease© Springer science+Businessmedia, LLC. 2012. pp. 2091–2116. http://dx.doi.org/10.1007/978-1-4419-1795-9_126. [Google Scholar]
- 19.Semenza GL, Pyeritz RE. Respiratory complications of mucopolysaccharide storage disorders. Medicine (Baltimore) 1988;67:209–219. doi: 10.1097/00005792-198807000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Pritzker MR, King RA, Kronenberg RS. Upper airway obstruction during head flexion in Morquio's disease. Am J Med. 1980;69:467–470. doi: 10.1016/0002-9343(80)90021-2. [DOI] [PubMed] [Google Scholar]
- 21.Shih SL, Lee YJ, Lin SP, Sheu CY, Blickman JG. Airway changes in children with mucopolysaccharidoses. Acta Radiol. 2002;43:40–43. doi: 10.1080/028418502127347628. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez ME, Mackenzie WG, Ditro C, Miller TL, Chidekel A, Shaffer TH. Skeletal dysplasias: evaluation with impulse oscillometry and thoracoabdominal motion analysis. Pediatr Pulmonol. 2010;45:679–686. doi: 10.1002/ppul.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross RE, Neuhauser EB. Compression of the trachea by an anomalous innominate artery; an operation for its relief. Am J Dis Child. 1948;75:570–574. doi: 10.1001/archpedi.1948.02030020585007. [DOI] [PubMed] [Google Scholar]
- 24.Friedman E, Kennedy A, Neitzschman HR. Innominate artery compression of the trachea: an unusual cause of apnea in a 12-year-old boy. South Med J. 2003;96:1161–1164. doi: 10.1097/01.SMJ.0000072420.60411.9F. [DOI] [PubMed] [Google Scholar]
- 25.Fawcett SL, Gomez AC, Hughes JA, Set P. Anatomical variation in the position of the brachiocephalic trunk (innominate artery) with respect to the trachea: a computed tomography-based study and literature review of Innominate Artery Compression Syndrome. Clin Anat. 2010;23(1):61–69. doi: 10.1002/ca.20884. [DOI] [PubMed] [Google Scholar]
- 26.Wiatrak BJ. Congenital anomalies of the larynx and trachea. Otolaryngol Clin North Am. 2000;33:91–110. doi: 10.1016/s0030-6665(05)70209-7. [DOI] [PubMed] [Google Scholar]
- 27.Strife JL, Baumel AS, Dunbar JS. Tracheal compression by the innominate artery in infancy and childhood. Radiology. 1981;139:73–75. doi: 10.1148/radiology.139.1.7010416. [DOI] [PubMed] [Google Scholar]
- 28.Turner AS, Nordin RW, Gaarde S, Connally HE, Thrall MA. Bone mineral density in feline mucopolysaccharidosis VI measured using dual-energy X-ray absorptiometry. Calcif Tissue Int. 1995;57:191–195. doi: 10.1007/BF00310257. [DOI] [PubMed] [Google Scholar]
- 29.McClure J, Smith PS, Sorby-Adams G, Hopwood J. The histological and ultrastructural features of the epiphyseal plates in Morquio type A syndrome (mucopolysaccharidosis type IV A) Pathology. 1986;18:217–221. doi: 10.3109/00313028609059462. [DOI] [PubMed] [Google Scholar]