Abstract
Objectives/Hypothesis
Vocal fold fibroblasts (VFF) are responsible for extracellular matrix synthesis supporting lamina propria in normal and diseased conditions. When tissue is injured, VFF become activated and differentiate into myofibroblasts to facilitate wound healing response. We investigated if vocal fold myofibroblasts can be utilized as surrogate cells for scarred VFF.
Study Design
In vitro.
Methods
Normal VFF cell lines from a 21-year-old male (N21), 59-year-old female (N59), and a scar VFF cell line from a 56 year-old female (S56) were used in this study. 10ng/mL TGFβ1 was applied for 5 days to normal VFF. Myofibroblast differentiation was determined with immunocytochemistry and western blot, measuring α-SMA. Cell growth, proliferation, contractile properties and gene expression profiles were evaluated.
Results
N21, N59 and S56 VFF presented elongated configuration. N21+ and N21− VFF demonstrated significantly greater proliferation compared to N59+, N59− and S56 VFF at 6 days. α-SMA was expressed in all cells. Fibronectin, α-SMA, CTGF and TIMP were the highest genes expression in VFF treated with TGFβ1. At 24 hours, S56 VFF showed lower contraction compared to N21+ and N59+ VFF, but at 60 hours, S56 VFF had lower collagen contraction compared to all cell groups. Highest collagen contraction matrices were measured with VFF treated with TGFβ1 at 24 hours and N59− VFF at 60 hours.
Conclusion
VFF treated with TGFβ1 (myofibroblasts) appear to have similar phenotypic characteristics but different genotypic behavior compared to scar VFF.
Keywords: vocal fold, fibroblasts, myofibroblasts, growth factor, remodeling, wound healing, in vitro
INTRODUCTION
Vocal fold lamina propria is composed of extracellular matrix (ECM) rich in collagen, elastin, hyaluronic acid, fibromodulin, versican, among other proteins, blood vessels and fibroblasts.1,2 Vocal fold fibroblasts (VFF) are responsible for ECM synthesis, playing a key role in support of the lamina propria in normal and diseased conditions. During tissue injury, VFF become activated and differentiate into myofibroblasts, initiating contractual properties and increasing catalysis of ECM in order to facilitate wound healing3-7 and promote tissue repair.8 Left unregulated, prolonged presence of myofibroblasts during wound healing can result in the development of fibroproliferative diseases, tumors9 and play a role in aging.10
One major limitation in the field of vocal fold biology is the lack of cell lines for normal and diseased states. There are no commercial VFF cells lines from patients who have vocal fold scarring or injury. Further in vitro vocal fold biology investigations and lamina propria biomaterial designs have generally been studied with normal VFF. Although this approach has yielded significant advances, it fails to capture several aspects of the in vivo diseased environment which critically impacts the quality and rate of VFF matrix synthesis. For instance, fibroblasts associated with chronic vocal fold scar often display myofibroblastic or fibrotic phenotype, whereas the VFF employed in most in vitro biomaterial studies are normal. Development of a surrogate cell type would be provide a more realistic in vitro environment for study of vocal fold wound healing.
In 2010, Vyas et al.5 developed a myofibroblast cell culture model to characterize and understand the molecular mechanism of VFF differentiation and function in injured vocal fold tissue. These authors treated VFF with transforming growth factor beta 1 (TGFβ1) for 7 days; smooth muscle actin (α-SMA) expression was demonstrated with TGFβ1 treatment indicating that VFF were capable of differentiating into myofibroblasts. More recently, Jetté et al7 evaluated morphology, kinetic growth, contractile properties, and α-SMA protein and gene expression between normal and scarred VFF as they were able to obtain rare human vocal fold scar tissue. Not surprisingly differences were measured between normal and scar VFF in terms of proliferation capacity and, α-SMA level and gene expression.
The objective of the present investigation was to confirm if the myofibroblast model developed by Vyas et al5 could be utilized as scarred VFF surrogate cells. We characterized and compared genotype and phenotype of normal VFF treated with TGFβ1 (myofibroblasts) to human primary scarred VFF. If successful, a reproducible, characterized method for obtaining VFF that behave as scarred VFF would have far reaching implications and impact on the field of biology of the vocal fold lamina propria as these would provide a valuable research tool.
MATERIAL AND METHODS
Fibroblasts from normal vocal folds were obtained from a 21-year-old male (N21) and 59-year-old female (N59), and a scar vocal fold from 56 year-old female (S56) as previously reported.11,12
Cell culture and treatment with TGFβ1
When cells reached 70-80% confluence, they are trypsinized and counted. Cells were sub-cultured into new plates and incubated at 37°C with 5% CO2. Passage 6-9 were utilized for this experiment, indicating the number of growth passages undertaken by these cells. Cells were plated on 10cm dishes (2×105cells/dish) for western blot, RNA and collagen contraction assays. For growth and proliferation assays, cells were plated in 24-well plates with 1.5 ×104cells/well in order to facilitate the cell counting and photos capturing. For immunocytochemistry, 1,000 cells were seated on coverslips placed inside the wells of a 12-well dish containing 5% fetal bovine serum (FBS; Sigma, St. Louis, MO, USA) for 24 hours followed by 24hrs of serum-free. Cells received either 5 days treatment of media 2.5% FBS + 10ng/ml TGF β1 (N21+ and N59+) or media 2.5% FBS with no added TGFβ1 (N21−, N59−, S56). To avoid contamination risk between cells treated with and without TGFβ1, we used separated supplies for the experiment.
Morphology and proliferation
Fibroblast categorization and identification has previously been reported.11 Morphological analysis photos were taken for 6 days using EVOS® Color Imaging microscope (AMG, Washington, USA). For growth and proliferation, after 5 days of with/without TGFβ1treatment, VFF were trypsinized and counted by hemocytometer. Cells from four random wells were counted each day for 6 days. Experiments were performed by quadruplicate.
Gene expression analysis
To analyze changes to ECM and growth factor genes after differentiation into myofibroblasts, we measured mRNA expression of collagen -I and -III, fibronectin, α-SMA, matrix metallopeptidase (MMP −1), metallopeptidase inhibitor (TIMP −1), connective tissue growth factor (CTGF), hepatocyte growth factor (HGF), TGFβ1, and vascular endothelial growth factor (VEGF). After TGFβ1 treatment, total RNA was extracted from VFF using RNeasy Mini Kit (Qiagen, Valencia, CA, USA). First strand cDNA was synthesized from 2μg of total RNA using an Omniscript Reverse Transcription Kit (Qiagen). SYBR Select Master Mix (Life Technologies, Carlsbad, CA, USA) was used with Applied Biosystem 7500 Fast System using a relative quantification standard curve. Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. Eight point standard curves were constructed with dilutions of PCR template with known mass concentrations. Amplified product was isolated from 1% agarose gel following electrophoresis using QAIquick gel extraction kit (Qiagen). Expression level of the target gene was normalized to the housekeeping gene. Experiments were done in triplicate.
Primer sequences, gene bank access numbers, and expected PCR product sizes are listed in Table 1 for CTGF and GAPDH. Other primers have been previously described.12-15 Each primer pair was confirmed by melting curves and PCR reactions, which showed a single peak and appropriate sized DNA band for each gene product.
Table 1.
Primer sequences and products of RT-PCR.
| Gene | GenBank # | Forward primer | Reverse primer | Size of PCR product (bp) |
|---|---|---|---|---|
| Collagen 114 | NM_000089 | 5’-AACAAATAAGCCATCACGCCTGCC-3’ | 5’-TGAAACAGACTGGGCCAATGTCCA- 3’ | 101 |
| Collagen 314 | NM_000090 | 5’-CCATTGCTGGGATTGGAGGTGAAA-3’ | 5’-TTCAGGTCTCTGCAGTTTCTAGCGG- 3’ | 187 |
| Fibronectin14 | NM_002026 | 5’-ACCTACGGATGACTCGTGCTTTGA-3’ | 5’-CAAAGCCTAAGCACTGGCACAACA-3’ | 116 |
| α-SMA17 | NM_001141945 | 5’-CCAGCAGATGTGGATCAGCAAACA-3’ | 5’-ACGAGTCAGAGCTTTGGCTAGGAA-3’ | 179 |
| MMP117 | NM_002421 | 5’-TGCAACTCTGACGTTGATCCCAGA-3’ | 5’-ACTGCACATGTGTTCTTGAGCTGC-3’ | 122 |
| TIMP17 | NM_000362 | 5’-TGATGCAGCACACACAATTCCC-3’ | 5’-AAGCTCTGTTATTCTGGCCTGGGT-3’ | 102 |
| CTGF | NM_001901.2 | 5’-GCTAGGATGTGCATTCTCC-3’ | 5’-CCGATTCCTGAACAGTGTC-3’ | 112 |
| HGF18 | NM_000601 | 5’-GGCCCACTTGTTTGTGAGCAACAT-3’ | 5’-TGGTGGGGTGCTTCAGACACACTTA-3’ | 84 |
| TGFβ116 | NM_000660 | 5’-TGCTCGCCCTGTACAACAGCA-3’ | 5’-CGTTGTGGGTTTCCACCATTAGCA-3’ | 126 |
| VEGF18 | NM_001025366 | 5’-ACACATTGTTGGAAGAAGCAGCCC-3’ | 5’-AGGAAGGTCAACCACTCACACACA-3’ | 179 |
| GAPDH | NM_002046.4 | 5’-TGAAGGTCGGAGTCAACGGATT-3’ | 5’-TTGACGGTGCCATGGAATTTGC-3’ | 172 |
α-SMA – Alpha Smooth Actin; MMP1 - Matrix Metallopeptidase; TIMP3- Metallopeptidase Inhibitor; CTGF- Connective Tissue Growth Factor; HGF- Hepatocyte Growth Factor; TGFβ1- Transforming Growth Factor β1; VEGF- Vascular Endothelial Growth Factor; GAPDH- Glyceraldehydes-3-phosphate dehydrogenase.
Immunocytochemistry
After VFF differentiation, coverslips were washed with phosphate buffered saline (PBS), fixed in 4% paraformaldehyde for 10 minutes at room temperature and washed three times with buffer composed by 1% Triton X-100 (Sigma) diluted in PBS. Coverslips were incubated in 0.5% Triton −100 buffer (Sigma). After 10 minutes, blocking buffer was added (2% bovine serum albumin (BSA) and 10% goat serum) for 30 minutes, followed by primary antibody anti- mouse α-SMA (Sigma) at 1:250 dilution with 2% BSA in PBS. After 90 minutes, cells were thoroughly washed in 1% Triton X-100 diluted in PBS. Secondary α-mouse (goat A488, Invitrogen, Carlsbad, CA, USA) antibody diluted to 1:200 was applied. After 1 hour, coverslips were washed and slides were prepared using Vectashield (Vector Laboratories, Burlingame, CA, USA) with 4',6-diamidino-2-phenylindole (DAPI). Images were captured by Nikon Eclipse E600 Fluorescence inverted microscope (Nikon, Tokyo, Japan).
Western Blot
VFF grown for 5 days in 10 cm dishes were washed with PBS and proteins were extracted using M-PER Protein kit (Pierce, Rockford, IL, USA). Total protein was quantified by BCA (Bicinchonic Acid Assay; Pierce, Rockford, IL, USA). For each protein sample, 1.5μg of total protein was characterized by electrophoresis on NuPage 10% Bis-Tris gels using Life technologies XCell SureLock mini-cell system (Invitrogen). Membranes were blocked overnight and probed with antibodies to SMA (mouse monoclonal α-SMA, 1:1,000; Sigma) and GAPDH (1:20,000; Sigma) using Life Technologies Western Breeze Chemilumescent Western Blot Immunodection kit.
Collagen contraction assay
After 5 days of TGFβ1 treatment, treated and untreated cells were plated at densities of 600,000 cells/well and seeded into wells each containing 0.5ml of collagen gel lattice (Biolabs, San Diego, CA, USA). After polymerization, 1ml of culture medium was added. After 2 days of culture, stressed matrices were released from the surrounding brim of wells using a sterile pipette tip. Photos of free-floating collagen gel lattices were taken immediately after the gel release (0 –control), 24 and 60 hours. Areas of collagen gel size were analyzed with ImageJ (National Institutes of Health, Bethesda, MD, USA) and normalized to areas of the well. Degree of contraction was evaluated by determining the area of the gel matrix. Assays were run in triplicate for each cell group (N21+, N21−, N59+, N59− and S56) and a cell-free collagen matrix was used as comparison control.
Statistical analysis
Analysis of variance (ANOVA) was used to analyze proliferation of cells across days with Fisher tests with statistical significance assigned at p<0.05. Degree of collagen contraction was compared across each time (0, 24 and 60 hours) and group (N21+, N21−, N59+, N59− and S56 VFF) using ANOVA and Fisher tests with statistical significance assigned at p <0.05. For gene expression, comparisons were made between the normalized gene expression values across the 5 different experimental conditions for all genes by Tukey-Kramer test. p <0.05 was considered statistically significant.
RESULTS
Morphology, Growth and Proliferation
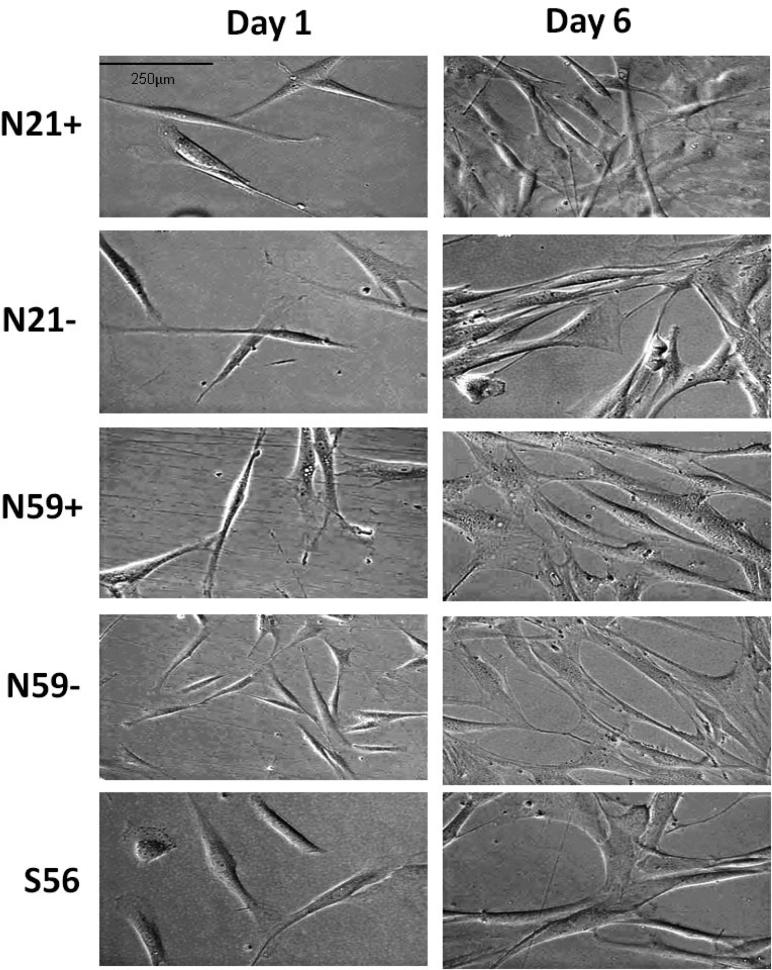
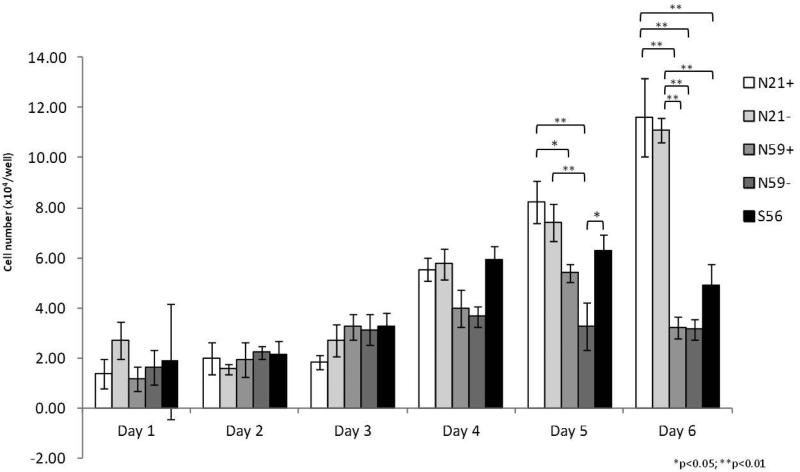
VFF (N21+, N21−, N59+, N59− and S56) presented a spindle shaped pattern (Figure 1). On day 1 scar VFF were larger. At day 6, N21+ and N59+ were bigger than N21− and N59−, and more similar to S56. Observing VFF growth curves (Figure 2), we found ascendent VFF proliferation. N21+ and N21− showed the greatest growth rate compared to N59+, N59− and S56. Differences in growth rate were measured on day five, with N21+ having significantly greater growth rate compared to N59− (p<0.0001) and N59+ (p=0.0059). N21− showed significantly higher growth rates compared to N59− (p<0.0001). S56 had significantly greater growth rate compared to N59− (p=0.0016). On day 6 N21+ and N21− maintained similar growth rates, distancing themselves even more from S56, N59+ and N59− (p<0.0001).
Figure 1.
Morphology of N21+, N21−, N59+, N59− and S56 VFF by culture day (40× magnification).
Figure 2.
Growth and proliferation curve of VFF from N21+, N21−, N59+, N59− and S56 during 6 days.
Gene expression
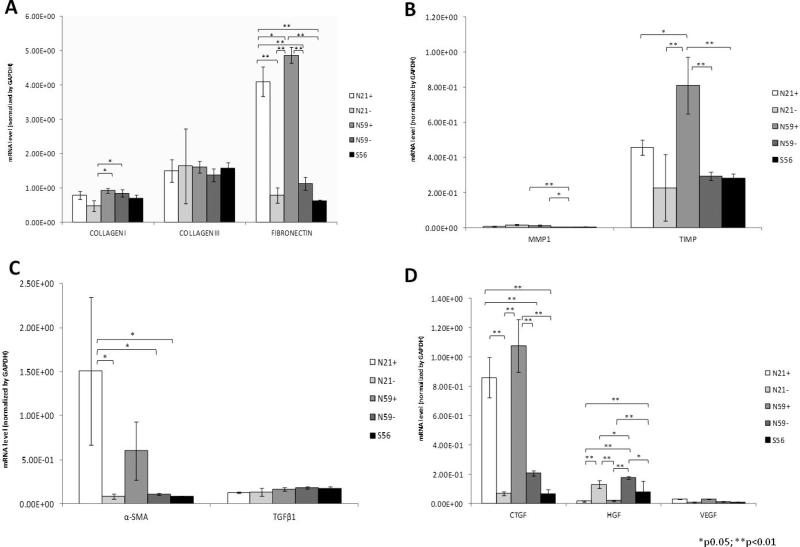
Gene expression for N21+, N21−, N59+, N59− and S56 VFF are shown in Figure 3. In summary, N21+ and N59+ VFF demonstrated the highest gene expression for fibronectin, TIMP, α-SMA and CTGF compared to those VFF not treated with TGFβ1 and S56 VFF.
Figure 3.
Gene expression of N21+, N21−, N59+, N59− and S56 VFF. (A) ECM genes; (B) myofibroblast expression genes; (C) Genes with link on tissue remodeling; (D) Growth factors genes expression. Values are the mean ±SD of results of quadruplicate test. All genes were normalized by GAPDH housekeeping gene.
Fibronectin was significantly higher in N59+ compared to S56, N59− and N21− (p<0.0001). Fibronectin was also significantly higher in N21+ compared to N21−, N59− and S56 (p<0.0001). No differences were measured in expression of collagen III between groups. Collagen type I was significantly higher in N59+ compared to N21− (p=0.0107) and N59− compared to N21− (p=0.0425).
α-SMA had significantly higher expression for N21+ compared to S56 (p=0.0103); the N21+ condition had significantly higher expression compared to the N21− (p=0.0104) and N59− (p=0.0116). No differences were measured for TGFβ1 expression between VFF groups.
TIMP was significantly higher in N59+ compared to N21− (p<0.0001), N59− (p=0.0009) and S56 (p=0008). MMP1 had significantly higher expression in N21− compared to N59− (p=0.0060) and N59+ compared to N59− (p=0.0170).
CTGF was significantly higher expressed in N59+ compared to N21−, N59−and S56 VFF (p<0.0001). HGF expression was significantly greater in N59− compared to N21+ (p<0.0001) and N59+ (p<0.0001). N59− also had significantly higher expression of HGF compared to S56 (p=0.0272) and with N21− (p=0.0475). No significant differences were seen in VEGF expression across groups.
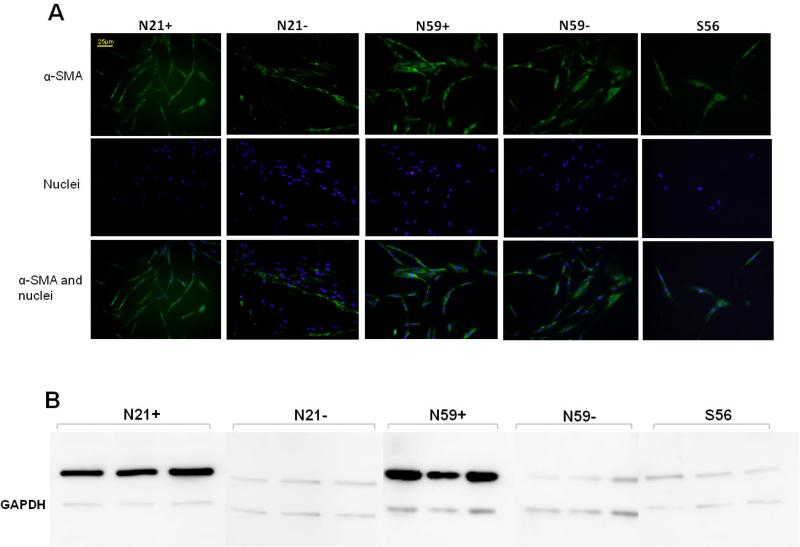
Immunocytochemistry and Western Blot
With immunocytochemistry, α-SMA expression was observed in all cell types. Differences were observed in N21+ and N59+ with higher α-SMA expression (Figure 4). Higher N21+ and N59+ α-SMA expression was also confirmed in western blot analysis (Figure 4), when compared with N21−, N59− and S56 VFF versus loading control.
Figure 4.
(A) Images by immunocytochemistry demonstrating α-SMA expression in N21+, N21−, N59+, N59− and S56 VFF. N21+ and N59+ VFF appear to have enhanced of α-SMA expression. Nuclei were stained with 4',6-diamidino-2-phenylindole (DAPI). (B) Western blot images showed evident difference of α-SMA expression in N21+ and N59+ compared to N21−, N59− and S56 VFF versus loading control (GAPDH).
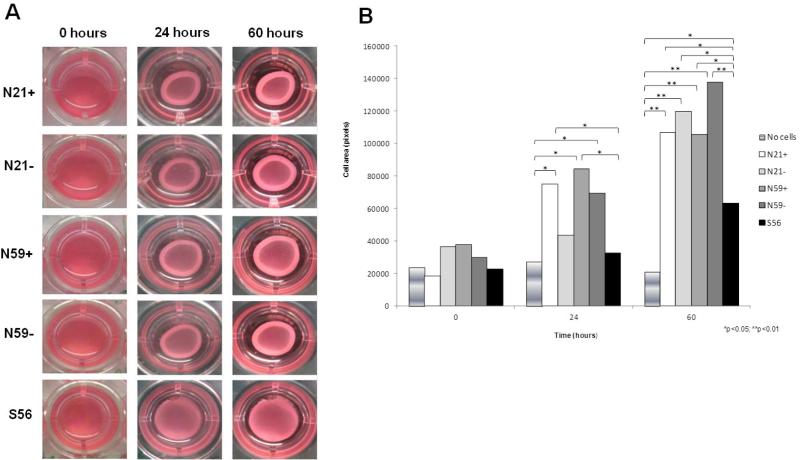
Collagen contraction
After edges were liberated from arrays wells, we observed collagen lattice contraction at 0 minutes, 24 and 60 hours (Figure 5). No differences in collagen contraction were observed at 0 minutes between any of the groups. At 24 hours, VFF treated with TGFβ1 had the highest collagen contraction compared to N21−, N59−, S56 and no cell. N59+ showed significantly higher collagen contraction matrix compared to no cell (p=0.0002) and S56 (p=0.0020). N21+ had significantly higher collagen contraction compared to no cell (p=0.0091) and S56 (p=0.0490). Higher collagen contraction was observed for the N59− group compared to no cell group (p=0.0462). At the 60 hour time point, N59− had significantly higher contraction of collagen lattice compared to no cell group (p<0.0001). N21−, N21+ and N59+ had higher collagen contraction matrices compared to no cell group (p<0.0001). S56 showed significantly lower collagen contraction matrix compared to N21+ (p=0.0290), N21− (p=0.0003), N59+ (p=0.0408) and N59− (p<0.0001). S56 showed difference when compared with no cell group (p=0.0497).
Figure 5.
(A) Collagen gel contraction assay. Representative gels of floating-populated collagen matrices after 0, 24 hours and 60 hours of no cells (control), N21+, N21−, N59+, N59− and S56 VFF. (B) Collagen contraction lattice mean of area (square pixels) of no cells (control), N21+, N21−, N59+, N59− and S56 VFF at 0 minutes, 24 hours and 60 hours. The assay was performed in triplicate.
DISCUSSION
VFF provide structural support to the lamina propria by producing growth factors and matrix fibers.1,16,17 In the presence of vocal fold scar, fibroblasts exhibit an unbalanced production of ECM components, leading to fibrosis.18 The ability of myofibroblasts to generate contractile forces is due to accumulation of cytoplasm and cellular fibronectin, resulting from ECM reorganization.19 Myofibroblasts which express α-SMA are considered as activated contractile fibroblasts, and primarily responsible for excessive ECM contraction deposition with scar.7
In this investigation we compared vocal fold myofibroblasts produced via treatment of normal VFF with TGFβ1 to VFF taken from vocal fold scar tissue. VFF treated with or without TGFβ1, showed similar elongated (fusiform) shape pattern confirming previously published reports.7,12,14,20 However, differences in proliferation indicate that age may be a more important factor for growth than treatment groups. Measured differences correlate with Jetté et al7 who demonstrated lower growth for a 56 year-old scar VFF donor compared to normal VFF. Decrease in fibroblast proliferation may be an integral part in the progression of the aging and fibrosis processes.21 Human aged larynges have less fibroblasts as compared with young adults,22 suggesting a decrease in the number of fibroblasts with age. An increase in the proliferation capacity demonstrated by the N21+ and N21− VFF in the present study, further demonstrate that age may influence cell growth and proliferation. Similar results were reported by Chen & Thibeault12 whereby higher growth and proliferation rates were measured from young VFF cells versus old.
Specific to fibroblast-myofibroblast differentiation, changes in gene expression may be a designate of an activated state19 that correspond to chronic vocal fold scarring. Results demonstrate that VFF treated with TGFβ1 had the highest gene expression levels compared to no treatment and scar groups. Fibronectin was highly expressed in VFF treated with TFGβ1, denoting that TGFβ1 increases the ability of fibroblasts to incorporate fibronectin into ECM.23 Fibronectin and α-SMA are directly linked to ECM structure and functionality. Fibronectin is a glycoprotein produced by fibroblasts in the lamina propria and basal membrane which participates in adhesion, differentiation and cell maintenance. It is also observed in higher expression in injured vocal folds by constant trauma such as in nodules.24 In scar, fibronectin accelerates wound healing providing collagen deposition and cell migration.25
α-SMA is a well-accepted marker of myofibroblast differentiation26 responsible for contraction during wound healing by its stress fibers.27,28 In rodents, Kumai et al29 evaluated expression of α-SMA in normal and scar vocal folds observing greater α-SMA expression in scar vocal folds. We found higher α-SMA gene expression in N21 and N59 VFF treated with TGFβ1. Scar VFF expression was similar to N21− and N59−. CTGF is a gene that participates in wound healing, stimulating cell proliferation and adhesion, angiogenesis and ECM production. During the repair process, TGFβ1 and CGTF work in partnership to stimulate matrix deposition.30 In this study, CTGF was expressed in all cells groups, with the highest expression in N21 and N59 treated with TGFβ1. It appears that CTGF expression was stimulated with TGFβ1 treatment and expressed concomitantly with α-SMA myofibroblast differentiation and collagen matrix contraction by TGFβ1 action as previously reported for corneal31 and kidney fibroblasts.32
Present immunocytochemistry and confirmation by western blot indicated that α-SMA was expressed in all cells, although the treatment of N21+ and N59+ had slightly higher staining in immunocytochemistry and strong signals in western blot. Myofibroblast phenotype expression in normal VFF, without TGFβ1, leads us to infer three reasons to explain this finding. α-SMA expression may be present during cell cycle progression in fibroblast culture7,33,34 particularly in monolayer cultures.5 Fibroblasts are subjected to various types of mechanical forces during phonation35 and vibration may stimulate α-SMA expression in response to tension.36 Myofibroblasts are a subset of specialized cells with fibroblast and smooth muscle cells characteristics, thus the main marker for these cells is α-SMA expression in cytoplasmic stress fibers. These cells maintain tension along their bodies by sustained myosin ATPase activity, ensuring tension and cell contraction.37 VFF may have myofilaments as stress fibers due to its tensional homeostasis. TGFβ1 may up-regulate α-SMA expression38 in VFF as in stimulated gingival and pulpal fibroblasts.39
It is well established that TGFβ1 is involved in myofibroblast differentiation to initiate the contractile apparatus40 that is necessary to generate forces for collagen-rich scar formation. For this reason, we performed a collagen contraction assay to investigate the degree of collagen contraction matrices. Analysis showed reduced contraction in S56 VFF, however, Jetté et al7 found no contraction differences between normal and scar VFF at 24 and 48 hours with a similar collagen contraction assay. In a dermal hypertrophic scar study, Zhang et al41 utilized decorin into fibroblast-populated collagen lattice and they reported when TGFβ1 was added, hypertrophic scar fibroblasts showed greater contraction of collagen gels than normal fibroblasts. Differences in collagen contraction lattices were found between scar and normal dermal VFF,42 however, these authors demonstrated that scar VFF had lower collagen contraction and higher α-SMA expression.
CONCLUSIONS
VFF treated with TGFβ1 (myofibroblasts) appear to have similar phenotypic characteristics but different genotypic behavior compared to scar VFF. VFF treated with or without TGFβ1, showed similar elongated (fusiform) shape feature. N21 VFF with or without TGFβ1 demonstrated significantly greater proliferation compared to N59+, N59− and S56 VFF. N21 and N59 VFF treated with TGFβ1 had the highest gene expression levels to fibronectin, α-SMA, CTGF and TIMP when compared to no treatment and scar groups. S56 VFF showed lower collagen contraction compared to N21 and N59 VFF treated with TGFβ1 at 24 hours, but at 60 hours, S56 VFF had lower collagen contraction compared to all cell groups.
Further investigation is warranted into differences in age of donor and different stages of wound healing to fully understand the role of the myofibroblasts in scar vocal folds. According the relationship between VFF treated with TGFβ1 and scarred VFF may not be as forthright as first expected.
ACKNOWLEDGMENTS
The authors thank Dr. Glen Leverson for statistical support.
Footnotes
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Will be presented as a poster at the American Laryngological Association Meeting, Boston, Massachusetts, U.S.A., April 22-23, 2015.
REFERENCES
- 1.Gray SD, Titze IR, Alipour F, Hammond TH. Biomechanical and histologic observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109:77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- 2.Madruga de Melo EC, Lemos M, Ximenes Filho JA, Sennes LU, Saldiva PHN, Tsuji DH. Distribution of collagen in the lamina propria of the human vocal fold. Laryngoscope. 2003;113(12):2187–2191. doi: 10.1097/00005537-200312000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Thibeault SL, Gray SD, Li W, Chen Z. Instability of extracellular matrix gene expression in primary cell culture of fibroblasts from human vocal fold lamina propria and tracheal scar. Ann Otol Rhinol Laryngol. 2002;111:8–14. doi: 10.1177/000348940211100102. [DOI] [PubMed] [Google Scholar]
- 4.Hirano S, Kishimoto Y, Suehiro A, Kanemaru S, Ito J. Regeneration of aged vocal fold: first human case treated with fibroblast growth factor. Laryngoscope. 2009;119(1):197–202. doi: 10.1002/lary.20004. [DOI] [PubMed] [Google Scholar]
- 5.Vyas B, Ishikawa K, Duflo S, Chen X, Thibeault SL. Inhibitory effects of HGF and IL-6 on TGF-β1 mediated vocal fibroblast-myofibroblast differentiation. Ann Otol Rhinol Laryngol. 2010;119(5):350–357. doi: 10.1177/000348941011900513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li B, Wang JH. Fibroblasts and myofibroblasts in wound healing: force generation and measurement. J Tissue Viabil. 2011;20(4):108–120. doi: 10.1016/j.jtv.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jetté ME, Hayer SD, Thibeault SL. Characterization of human vocal fold fibroblasts derived from chronic scar. Laryngoscope. 2013;123(3):738–745. doi: 10.1002/lary.23681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohno T, Yoo MJ, Swanson ER, Hirano S, Ossoff RH, Rousseau B. Regenerative effects of basic fibroblast growth factor on extracellular matrix production in aged rat vocal folds. Ann Otol Rhinol Laryngol. 2009;118:559–564. doi: 10.1177/000348940911800805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyden B, Banerjee SS, Shenjere P, Fisher C. The myofibroblast and its tumours. J Clin Pathol. 2009;62(3):236–249. doi: 10.1136/jcp.2008.061630. [DOI] [PubMed] [Google Scholar]
- 10.Vendrenne N, Coulomb B, Danigo A, Bonté F, Desmoulière A. The complex dialogue between (myo)fibroblasts and extracellular matrix during skin repair processes and ageing. Pathol Biol. 2012;60:20–27. doi: 10.1016/j.patbio.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Thibeault SL, Li W, Bartley S. A method for identification of vocal fold lamina propria fibroblasts in culture. Otolaryngol Head Neck Surg. 2008;139(6):816–822. doi: 10.1016/j.otohns.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Thibeault SL. Characteristics of age-related changes in culture human vocal fold fibroblasts. Laryngoscope. 2008;118(9):1700–1704. doi: 10.1097/MLG.0b013e31817aec6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Thibeault SL. Biocompatibility of a synthetic extracellular matrix on immortalized vocal fold fibroblasts in 3-D culture. Acta Biomater. 2010;6(8):2940–8. doi: 10.1016/j.actbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Thibeault SL. Response of fibroblasts to transforming growth factor-β1 on two-dimensional and in three-dimensional hyaluronan hydrogels. Tissue Eng Part A. 2012;18(23-24):2528–38. doi: 10.1089/ten.tea.2012.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Thibeault SL. Cell-cell interaction between vocal fold fibroblasts and bone marrow mesenchymal stromal cells in three-dimensional hyaluronan hydrogel. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1757. [Published online in Wiley Online Library (wileyonlinelibrary.com) DOI: 10.1002/term.1757] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sato K, Hirano M, Nakashima T. Age-related changes of collagenous. Ann Otol Rhinol Laryngol. 2002;111:15–20. doi: 10.1177/000348940211100103. [DOI] [PubMed] [Google Scholar]
- 17.Hirano S, Bless DM, Río AM, Connor NP, Ford CN. Therapeutic potential of growth factors for aging voice. Laryngoscope. 2004;114(12):2161–2167. doi: 10.1097/01.mlg.0000149450.37640.db. [DOI] [PubMed] [Google Scholar]
- 18.Gugatschka M, Ainodhofer H, Gruber HJ, Graupp M, Kieslinger P, Kiesler K, Saxena A, Hirano S, Friedrich G. Age effects on extracellular matrix production of vocal fold scar fibroblasts in rats. Eur Arch Otorhinolaryngol. 2014;271:1107–1112. doi: 10.1007/s00405-013-2722-7. [DOI] [PubMed] [Google Scholar]
- 19.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 20.Sato K, Umeno H, Ono T, Nakashima T. Histopathologic study of human vocal fold mucosa unphonated over a decade. Acta Oto Laryngol. 2011;131:1319–1318. doi: 10.3109/00016489.2011.615067. [DOI] [PubMed] [Google Scholar]
- 21.Barkauskas CE, Noble PW. Cellular mechanisms of tissue fibrosis. New insights into the cellular mechanisms of pulmonary fibrosis. Am J Physiol Cell Physiol. 2014;306:987–996. doi: 10.1152/ajpcell.00321.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano M, Sato K, Nakashima T. Fibroblasts in geriatric vocal fold mucosa. Acta Otolaryngol. 2000;120:336–340. doi: 10.1080/000164800750001215. [DOI] [PubMed] [Google Scholar]
- 23.Ignotz RA, Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261(9):4337–45. [PubMed] [Google Scholar]
- 24.Martins RHG, Defaveri J, Custódio Domingues MA, de Albuquerque E, Silva R, Fabro A. Vocal fold nodules: morphological and immunohistochemical investigations. J Voice. 2010;24(5):531–9. doi: 10.1016/j.jvoice.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Suehiro A, Hirano S, Kishimoto Y, Tateya I, Rousseau B, Ito J. Effects of basic fibroblast growth factor on rat vocal fold fibroblasts. Ann Otol Rhinol Laryngol. 2010;119:690–696. doi: 10.1177/000348941011901008. [DOI] [PubMed] [Google Scholar]
- 26.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159(3):1009–20. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 28.Darby IA, Hewitson TD. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007;257:143. doi: 10.1016/S0074-7696(07)57004-X. [DOI] [PubMed] [Google Scholar]
- 29.Kumai Y, Kobler JB, Park H, Galindo M, Herrera VL, Zeitels SM. Modulation of vocal fold scar fibroblasts by adipose-derived stem/stromal cells. Laryngoscope. 2010;120(2):330–7. doi: 10.1002/lary.20753. [DOI] [PubMed] [Google Scholar]
- 30.Daniels JT, Schultz GS, Blalock TD, et al. Mediation of transforming growth factor-beta(1)-stimulated matrix contraction by fibroblasts: a role for connective tissue growth factor in contractile scarring. Am J Pathol. 2003;163(5):2043–52. doi: 10.1016/s0002-9440(10)63562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrett Q, Khaw PT, Blalock TD, Schultz GS, Grotendorst GR, Daniels JT. Involvement of CTGF in TGF-β1–stimulation of myofibroblast differentiation and collagen matrix contraction in the presence of mechanical stress. Invest Ophthalmol Vis Sci. 2004;45:1109–1116. doi: 10.1167/iovs.03-0660. [DOI] [PubMed] [Google Scholar]
- 32.Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. FASEB J. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 33.Ehrlich HP, Desmouliere A, Diegelmann RF, et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105–113. [PMC free article] [PubMed] [Google Scholar]
- 34.Saed GM, Zhang W, Diamond MP. Molecular characterization of fibroblasts isolated from human peritoneum and adhesions. Fertil Steril. 2001;75:763–768. doi: 10.1016/s0015-0282(00)01799-4. [DOI] [PubMed] [Google Scholar]
- 35.Branski RC, Perera P, Verdolini K, Rosen CA, Hebda PA, Agarwal S. Dynamic biomechanical strain inhibits IL-1beta-induced inflammation in vocal fold fibroblasts. J Voice. 2007;21(6):651–60. doi: 10.1016/j.jvoice.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homcha C, Ehrlich HP. Fibroblast expression of alpha-smooth muscle actin, alpha2beta1 integrin and alphavbeta3 integrin: Influence of surface rigidity. Exp Mol Pathol. 2011;91(1):394–399. doi: 10.1016/j.yexmp.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adelstein RS. Regulation of contractile proteins by phosphorylation. J Clin Invest. 1983;72(6):1863–1866. doi: 10.1172/JCI111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-b1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- 39.Martinez EF, Araújo VC, Sousa SOM, Arana-Chavez VE. TGF-β1 enhances the expression of α-smooth muscle actin in cultured human pulpal fibroblasts: immunochemical and ultrastructural analyses. J Endodont. 2007;33(11):1313–1318. doi: 10.1016/j.joen.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 40.Van De Water L, Varney S, Tomasek JJ. Mechanoregulation of the myofibroblast in wound contraction, scarring, and fibrosis: Opportunities for new therapeutic intervention. Adv Wound Care. 2013;2(4):122–141. doi: 10.1089/wound.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z, Garron TM, Li XJ, Liu Y, Zhang X, Li YY, Xu WS. Recombinant human decorin inhibits TGF-b1-induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns. 2009;35(4):527–37. doi: 10.1016/j.burns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Boemi L, Allison GM, Graham WP, Krummel TM, Ehrlich HP. Differences between scar and dermal cultured fibroblasts derived from a patient with recurrent abdominal incision wound herniation. Plast Reconstr Surg. 1999;104:1397–1405. doi: 10.1097/00006534-199910000-00024. [DOI] [PubMed] [Google Scholar]