Conspectus
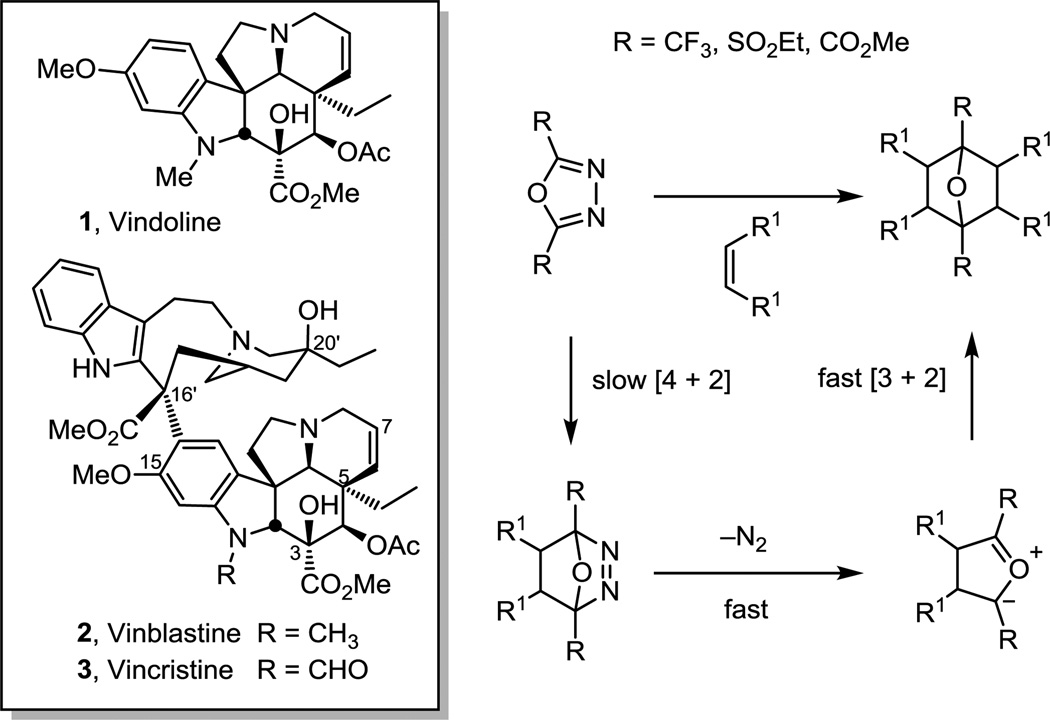
A summary of the development and initial studies on the scope of a powerful tandem intramolecular [4 + 2]/[3 + 2] cycloaddition cascade of 1,3,4-oxadiazoles is detailed and provides the foundation for its subsequent use in organic synthesis. Implemented with substrates in which both the initiating dienophile and subsequent dipolarophile are tethered to the 1,3,4-oxadiazoles, the studies expanded the scope of oxadiazoles that participate in the reaction cascade, permitted the use of differentiated dienophiles and dipolarophiles, extended their use to unsymmetrical dienophiles and dipolarophiles, provided exclusive control of the cycloaddition regioselectivities, and imposed exquisite control on the cycloaddition stereochemistry. As key reactivity and stereochemical features of the reactions were being defined, the cascade cycloaddition reaction was implemented in the total synthesis of a series of alkaloids including (−)-vindoline, (−)-vindorosine, the closely related natural products (+)-4-desacetoxyvindoline and (+)-4-desacetoxyvindorosine, natural minovine, (+)-N-methylaspidospermidine, (+)-spegazzinine, (−)-aspidospermine, and a number of key analogues. Most recently it was used in the divergent total syntheses of (+)-fendleridine, (−)-kopsinine, (−)-kopsifoline D, and (−)-deoxoapodine, in which four different strategic bonds in four different classes of the hexacyclic alkaloids were formed from a common cascade cycloaddition intermediate. A large number of vindoline analogues were prepared by variations on the cascade cycloaddition reaction for single step incorporation into analogues of vinblastine. These structural changes to vindoline permitted both systematic alterations to the peripheral substituents as well as deep-seated changes to the core structure and embedded functionality of vinblastine not previously accessible. Although explored initially for accessing vindoline and vinblastine, the use of the cycloaddition cascade in the total synthesis of an impressive range of additional natural products illustrate the power of the methodology. Alternative tethering strategies for the cascade cycloaddition reaction, combined intramolecular and intermolecular variants of either the initiating Diels–Alder reaction or the subsequent carbonyl ylide 1,3-dipolar cycloaddition, an expanded examination of the tethered dipolarophile scope, and applications to additional natural product classes represent attractive areas for future work.
Introduction
Cycloaddition reactions have long been powerful synthetic transformations in organic chemistry due to their ability to form multiple bonds with predictable stereochemistry, often providing access to the core cyclic structures of complex natural products. Tandem cycloadditions, or multiple cycloadditions that occur sequentially in the same reaction vessel, are especially powerful due to their ability to efficiently achieve high structural complexity, generating bridged or polycyclic structures containing several contiguous stereocenters, without the need to isolate intermediates before each subsequent reaction in the sequence.1–13 A special class of tandem cycloadditions that do not require the addition of reagents or modification of reaction conditions are deemed cascade cycloadditions.14–17 Cascade reactions are sometimes also referred to as domino reactions to emphasize that each step of the sequence depends on the functionality formed directly in the previous step.18–20
In exploration of a new synthetic approach to vinblastine and vincristine, we developed a powerful cycloaddition cascade inspired by the structure of vindoline, the more complex lower half of vinblastine. This tandem intramolecular [4 + 2]/[3 + 2] cycloaddition provided the pentacyclic core in a single step, incorporating all the embedded heteroatoms, substituents, functional groups and necessary stereochemistry required for conversion not only to vindoline, but to a series of Vinca and Aspidosperma alkaloid natural products. Originally inspired by the potential development of sequential intramolecular inverse electron demand Diels–Alder reactions of unsymmetrically substituted 1,2,4,5-tetrazines,21–24 our approach ultimately enlisted an alternative cascade cycloaddition reaction of electron-deficient five-membered heterocyclic azadienes using 1,3,4-oxadiazoles in the initiating inverse electron demand Diels–Alder reaction.25,26 Although a limited number of reports had described the cycloadditions of electron-deficient 1,3,4-oxadiazoles, they typically employed symmetrical oxadiazoles bearing two strong electron-withdrawing substituents (CF3, SO2Et, CO2Me) and all had been examined only in intermolecular reactions (Figure 1).27–47 Reactions of olefinic dienophiles were found to proceed through an initial [4 + 2] cycloadduct that loses N2 to provide a carbonyl ylide that subsequently reacted again with the same olefin in a 1,3-dipolar cycloaddition reaction to provide only 2:1 cycloadducts, limiting their synthetic utility.
Figure 1.
Tandem [4 + 2]/[3 + 2] cascade cycloaddition reaction of 1,3,4-oxadiazole.
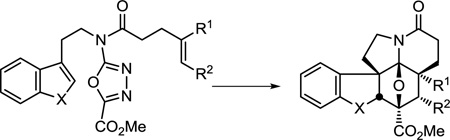
With the synthetic target vindoline (1) in mind, the tandem intramolecular Diels–Alder/1,3-dipolar cycloaddition reaction was implemented with substrates bearing both an initiating dienophile and a tethered indole to trap the in situ generated carbonyl ylide (Scheme 1). This work, representing the first examples of an intramolecular Diels–Alder and tandem intramolecular Diels–Alder/1,3-dipolar cycloaddition of 1,3,4-oxadiazoles, significantly expanded the scope of oxadiazoles that participate in the reaction cascade, permitted differentiated dienophiles and dipolarophiles to be incorporated, imposed control onto the cycloaddition stereochemistry, and provided exclusive control of the cycloaddition regioselectivities, thereby enhancing the synthetic utility of such cycloaddition reactions.25,26 In the case highlighted in Scheme 1, a single diastereomer of the cycloaddition cascade product is produced in which the relative stereochemistry is set by a combination of: (1) the dienophile geometry, and (2) exclusive endo [3 + 2] cycloaddition of the tethered indole sterically directed to the α-face of the 1,3-dipole opposite the newly formed fused lactam.48 Although it is tempting to attribute this latter endo cycloaddition diastereoselectivity as intrinsic to the [3 + 2] cycloaddition, comparable intermolecular reactions proceed with indole exo cycloaddition also directed to the sterically less hindered face.49 The endo addition observed with the substrates examined to date may be attributed to a conformational preference for the 1,3-dipolar cycloaddition transition state that is dictated by the dipolarophile tether since it also reflects the relative strain energy of the four possible products.26 The tandem cycloadditions construct three new rings with formation of four new C–C bonds and set all six stereocenters about the central six-membered ring in a single step typically without detection of a second diastereomer. Herein, we describe the key results of the initial exploration of the scope of this powerful intramolecular [4 + 2]/[3 + 2] cascade cycloaddition reaction and summarize its use to date in the total synthesis of natural products, including the total synthesis of analogues of vinblastine (2) and vincristine (3) that contain previously inaccessible deep-seated structural changes.
Scheme 1.
Scope of the Cycloaddition Cascade
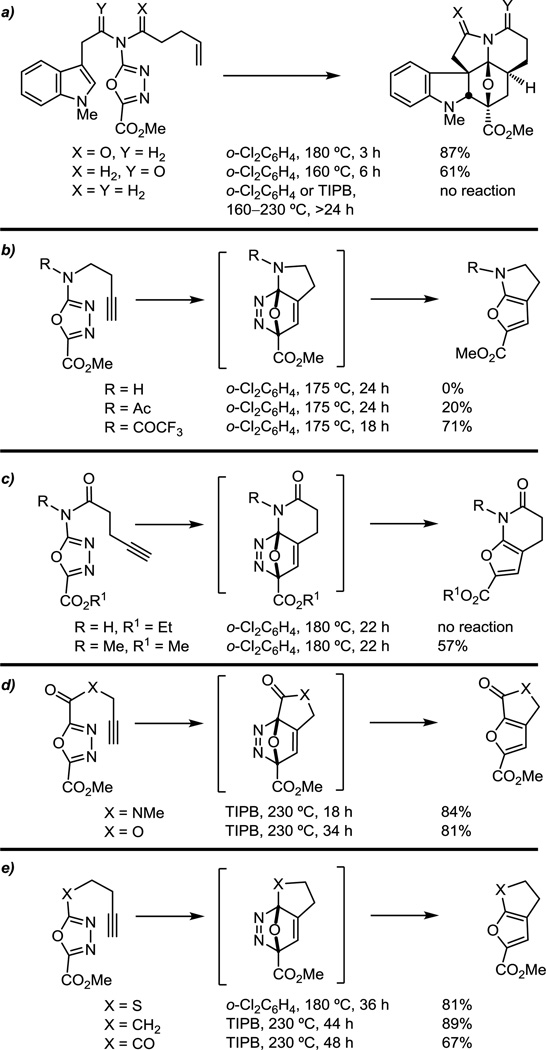
Although the substitution of the oxadiazoles that were examined stabilize the intermediate 1,3-dipole, these substrates are less electron-deficient than those previously known to participate in the initiating inverse electron demand Diels–Alder reactions (Figure 2).25,26 This results in a reduction of intrinsic reactivity for the initial [4 + 2] reaction that is compensated for by its use in an intramolecular Diels–Alder reaction. N-Acylation of the oxadiazole C2 amino group was found to be required for [4 + 2] cycloaddition reactivity (Figure 2a), although there is little distinction whether N-acylation is incorporated into the dienophile or dipolarophile tether. Expectedly, the reaction rate increases as the electron-withdrawing properties of the activating amide increases (R = COCF3 > Ac >> H, Figure 2b). Moreover, the studies suggest that the tertiary amide is required, which readily adopts a cis amide conformation required for [4 + 2] cycloaddition, as substrates bearing a secondary amide failed to participate in the initiating Diels–Alder reaction (Figure 2c). Thus, while the presence of such a tertiary amide is central in the synthetic approach to vindoline where it served as the tether site for both the dienophile and dipolarophile, its use is more integral to the success of the cycloaddition than might be appreciated.
Figure 2.
Impact of the amide substituent and alternatives to the amide.
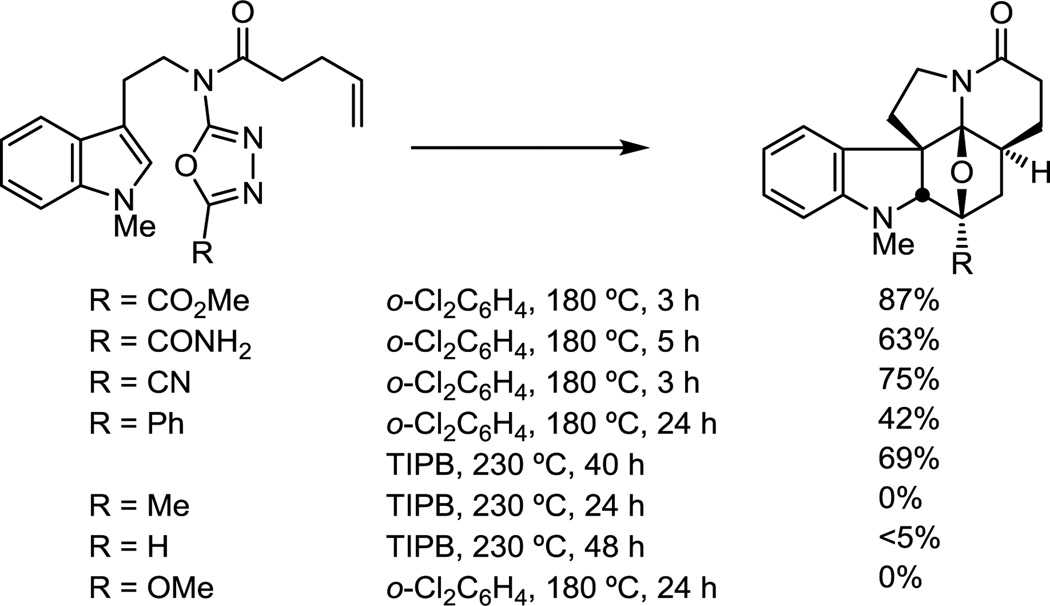
While the C5 methyl ester on the 1,3,4-oxadiazole was used to directly introduce the vindoline C3 methyl ester, other electron-withdrawing substituents effectively enhance the electron-deficient character of the oxadiazole, promoting the initiating inverse electron demand [4 + 2] cycloaddition reaction and stabilizing the intermediate 1,3-dipole. Thus, C5 electron-withdrawing substituents (CO2Me, CONH2, CN) provided oxadiazoles that participate effectively in the cycloaddition cascade, substrates bearing conjugating (Ph) substituents required more vigorous reaction conditions, and unactivating (Me, H) or electron-donating substituents (OMe) failed to support the tandem cycloaddition (Figure 3).
Figure 3.
Impact of oxadiazole electron-withdrawing substituent.
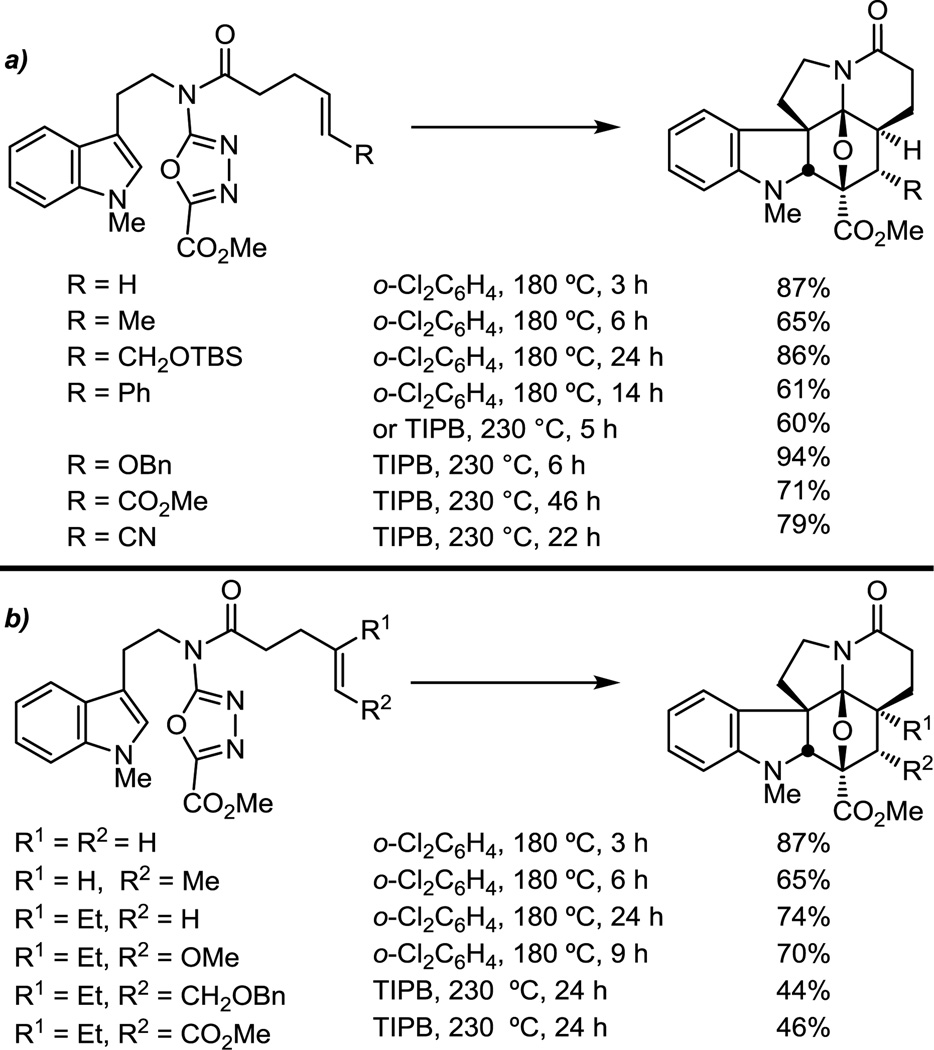
The relative ease of the tandem [4 + 2]/[3 + 2] cycloaddition is consistent with the cascade being initiated by an inverse electron demand Diels–Alder reaction, but even unactivated and electron-deficient dienophiles can effectively initiate the reaction (Figure 4a). Notably, the addition of substituents to the tethered dienophile has a significant impact on the ease of cycloaddition (Figure 4b), and even the addition of activating substituents at the alkene terminus slows the reaction relative to the unsubstituted terminal alkene, independent of the site of substitution. The exceptions to these generalizations include trisubstituted enol ethers, which remain suitably reactive to initiate the cycloaddition cascade via an inverse electron demand Diels–Alder reaction. Impressively, the cycloadditions remain diastereoselective, providing a single detectable product. Although early studies failed to observe reactions initiated by trisubstituted dienophiles containing neutral and electron-withdrawing substituents,50 recent studies have defined conditions where such substrates can be effectively used.51
Figure 4.
Impact of initiating dienophile substitution.
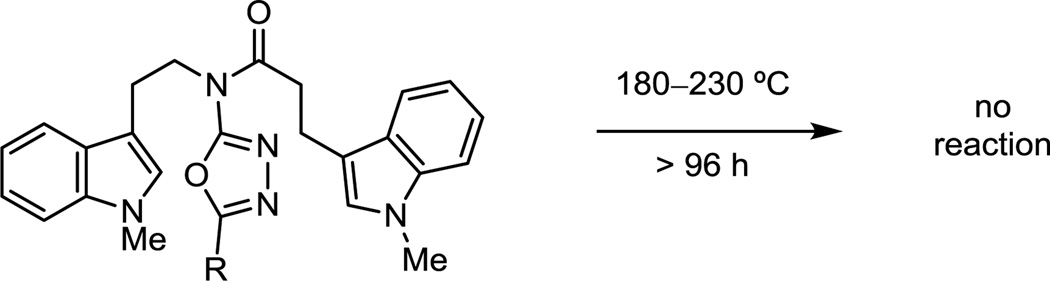
While it is possible that an indole [4 + 2] cycloaddition initiates the reaction cascade, attempts to detect such reactivity were not successful. If the reaction is prematurely worked up, only products derived from alkene [4 + 2] cycloaddition were detected, and the substrate that bears both an indole dienophile and dipolarophile failed to undergo reaction, even under forcing conditions (Scheme 2).
Scheme 2.
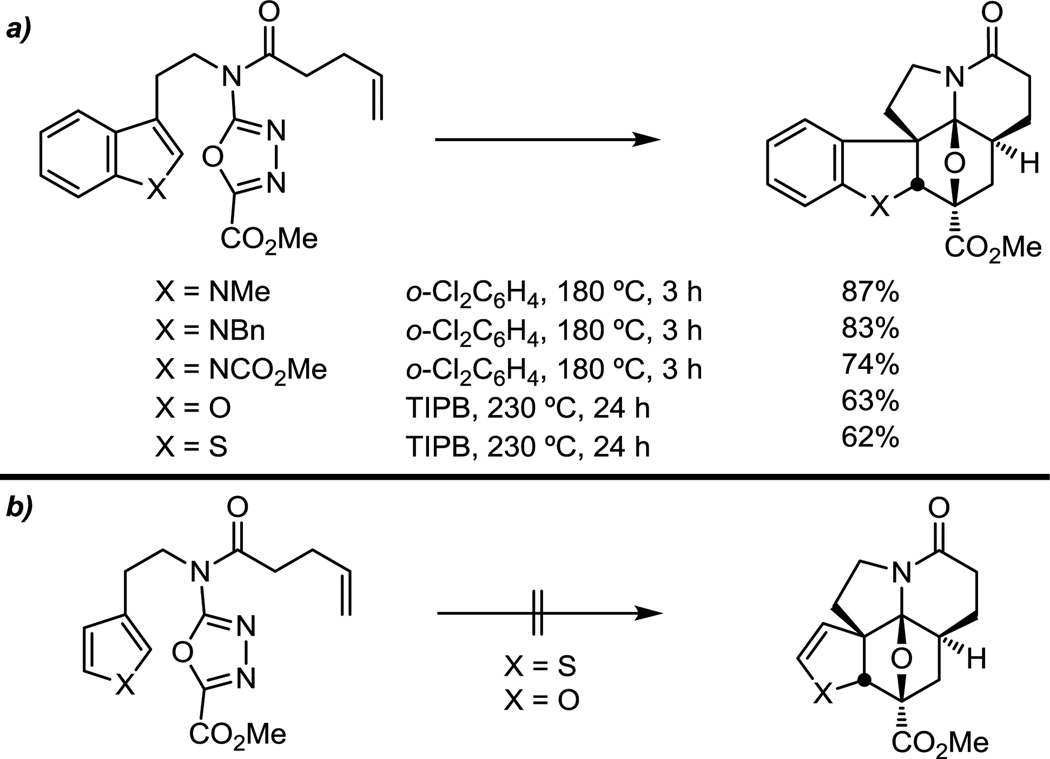
In order to explore the requisite reactivity of the tethered indole and to probe deep-seated modifications that may be incorporated into the vindoline/vinblastine structure, a series of closely related tethered dipolarophiles were examined. As expected, it was determined that the rate-limiting step of these reactions is typically the initiating [4 + 2] cycloaddition and that small differences in dipolarophile reactivity have no appreciable impact on the overall rate or yield (Figure 5a). Interestingly, tethered benzofurans and benzothiophenes (Figure 5a) participated effectively in the reaction cascade, while the corresponding furans and thiophenes (Figure 5b) failed to provide the analogous cycloadducts. The full scope of the tethered dipolarophiles that support the reaction has yet to be defined and represents a productive avenue for future studies.
Figure 5.
Tethered dipolarophile.
Alterations in the dienophile and dipolarophile tether lengths were shown to be possible and impact the reaction efficiencies in a predictable manner.26 Similarly, alternative tethering sites (e.g., C3 ester or C5 amide) create opportunities for the preparation of alternative fused ring systems and these likely represent productive avenues for future studies.26
Applications in the Total Synthesis of Natural Products
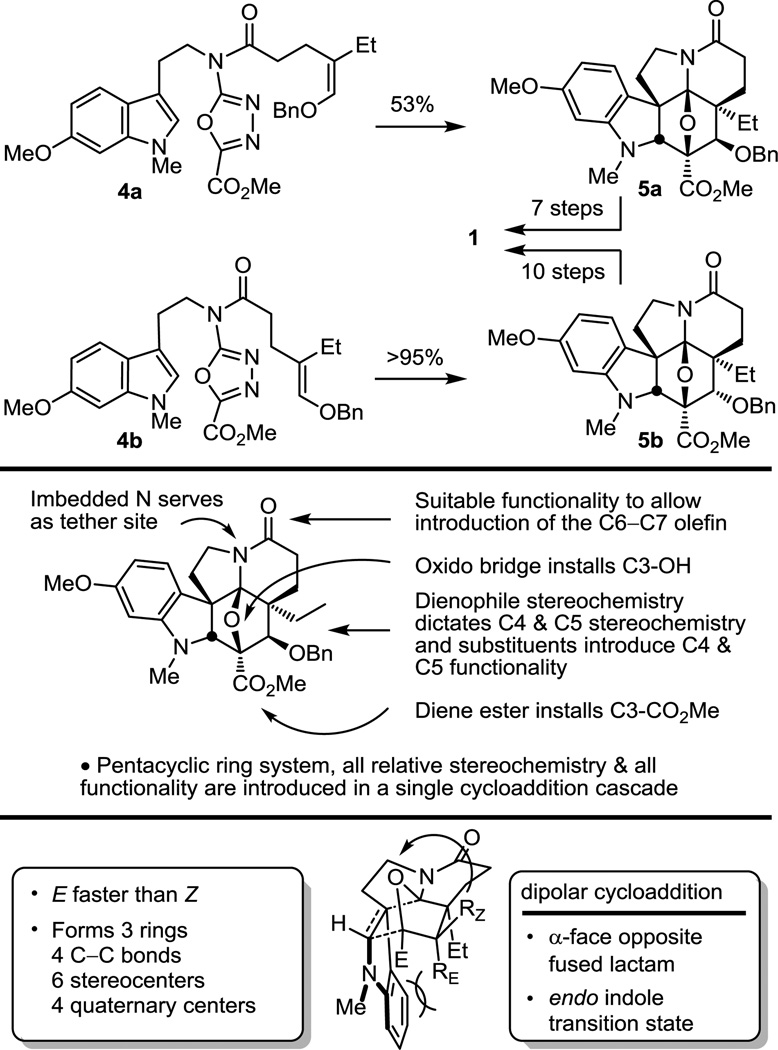
These studies set the stage for development of a concise 11-step total synthesis of (−)-vindoline (1).52,53 The cycloaddition cascade of either 4a and 4b provided complete control of the intrinsic stereochemistry found in its pentacyclic skeleton, establishing all six stereocenters about the central six-membered ring and was designed to introduce essentially all the functionality found in 1 (Figure 6).
Figure 6.
First generation total synthesis of (−)-vindoline.
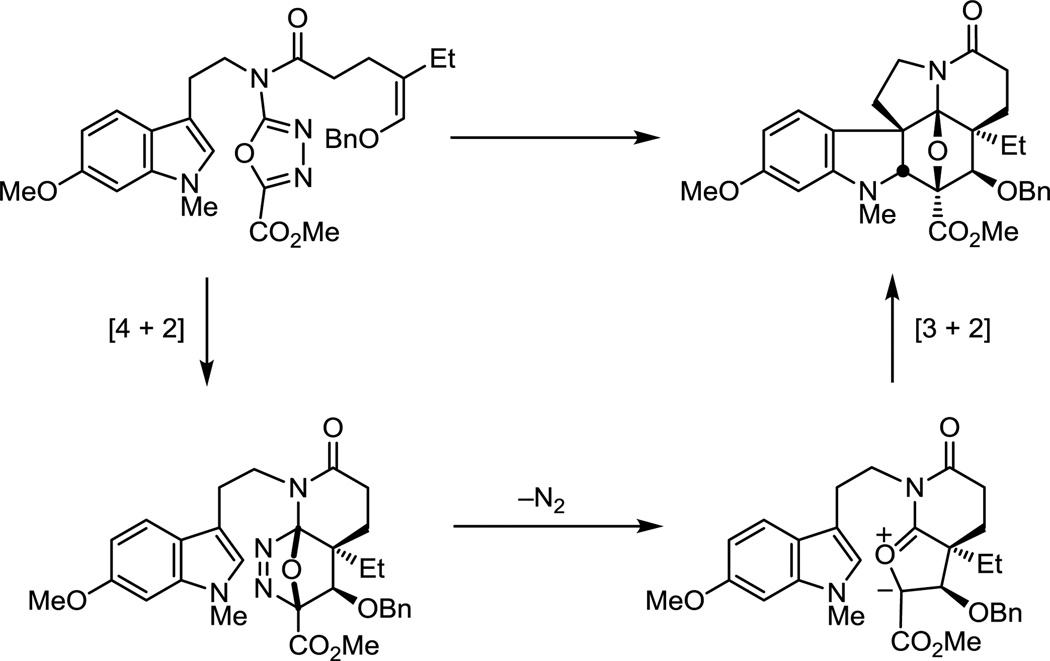
The distinction in the two cycloaddition reactions is that 4a permits the direct introduction of the naturally occurring C4-OAc stereochemistry, whereas 4b provides the C4 epimer, requiring an inversion of configuration at this center. Although 4a directly provides the preferred cycloadduct 5a for the use in the synthesis of 1, it proved to be more difficult to implement. In fact, it was observed that the [4 + 2] cycloaddition was the fast step in the reaction cascade and the subsequent [3 + 2] cycloaddition was the slow step for 4a, a reversal of what is observed with 4b and other typical substrates. The net consequence is that use of longer reaction times, more vigorous reaction conditions, and a more dilute reaction concentration led to substantial improvements in the conversion of 4a. This proved to be the result of the electronic character of the benzyloxy substituent of 4a that decelerates the typically fast 1,3-dipolar cycloaddition. Thus, the transition state derived from 4a suffers a destabilizing electrostatic interaction of its central oxygen with the (Z)-OBn substituent that decelerates the intramolecular dipolar cycloaddition reaction. With resolution of the cascade cycloadducts, both 5a and 5b permitted the expedient synthesis of (−)-1 in 7 or 10 subsequent transformations, respectively.52
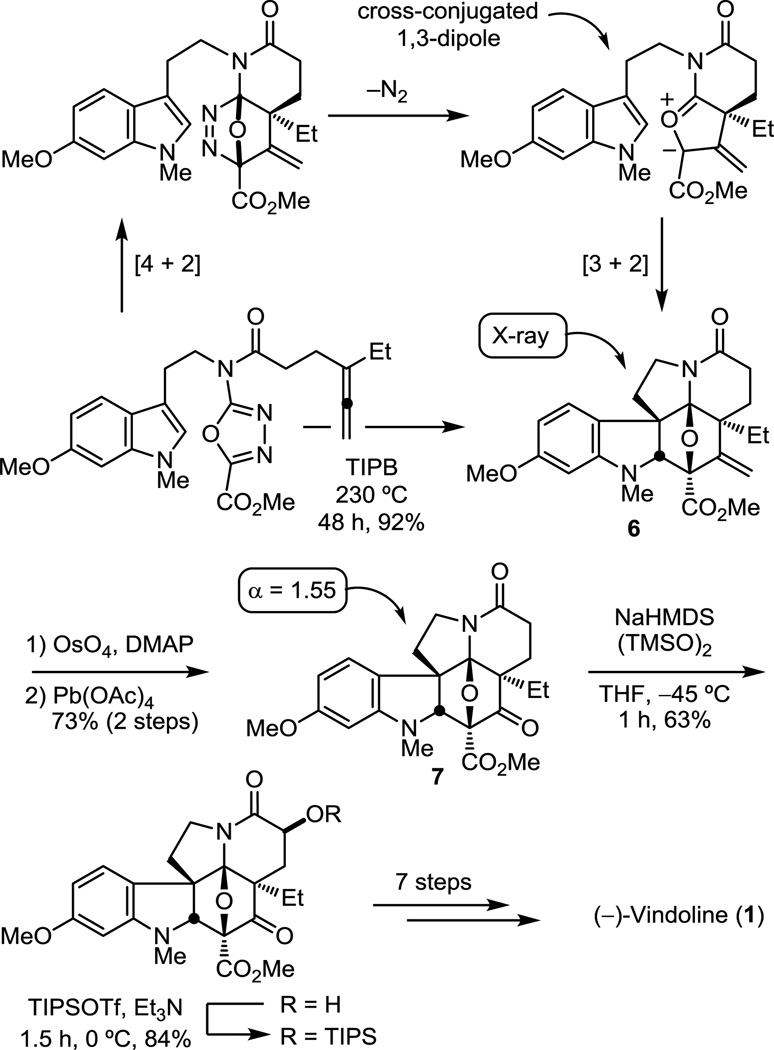
The continued examination of functionalized dienophiles tethered to the oxadiazole revealed that an allene not only initiates the oxadiazole cycloaddition cascade, but provides the desired cycloadduct in superb yields (Figure 7).54 The initiating Diels−Alder reaction affords an initial cycloaddduct that undergoes loss of N2 to provide an unusual cross-conjugated 1,3-dipole that most might judge to be an unmanageable intermediate. The ensuing 1,3-dipolar cycloaddition reaction proceeds remarkably well, providing 6 as a single diastereomer that results from an endo indole [3 + 2] cycloaddition. Corey dihydroxyation of 6 and subsequent Pb(OAc)4 diol cleavage provided ketone 7 (73%). After chiral phase HPLC resolution of 7 (α = 1.55), α-hydroxylation followed by in situ silyl ether formation provided an intermediate that we had previously converted to (−)-vindoline, formally completing an alternative total synthesis. Ketone 7 was also converted to (+)-4-epi-vindoline in a remarkably efficient manner, requiring only six isolated intermediates from the key cycloaddition cascade product, and used to prepare 4-epi-vinblastine in a single additional step.54
Figure 7.
Additional total synthesis of (−)-vindoline.
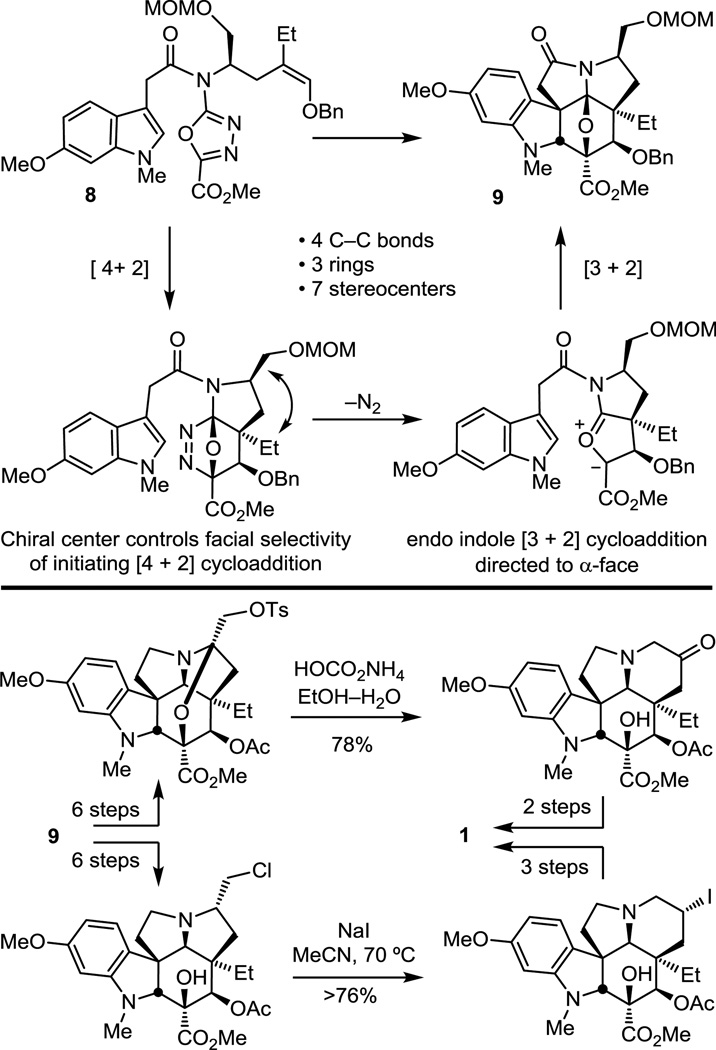
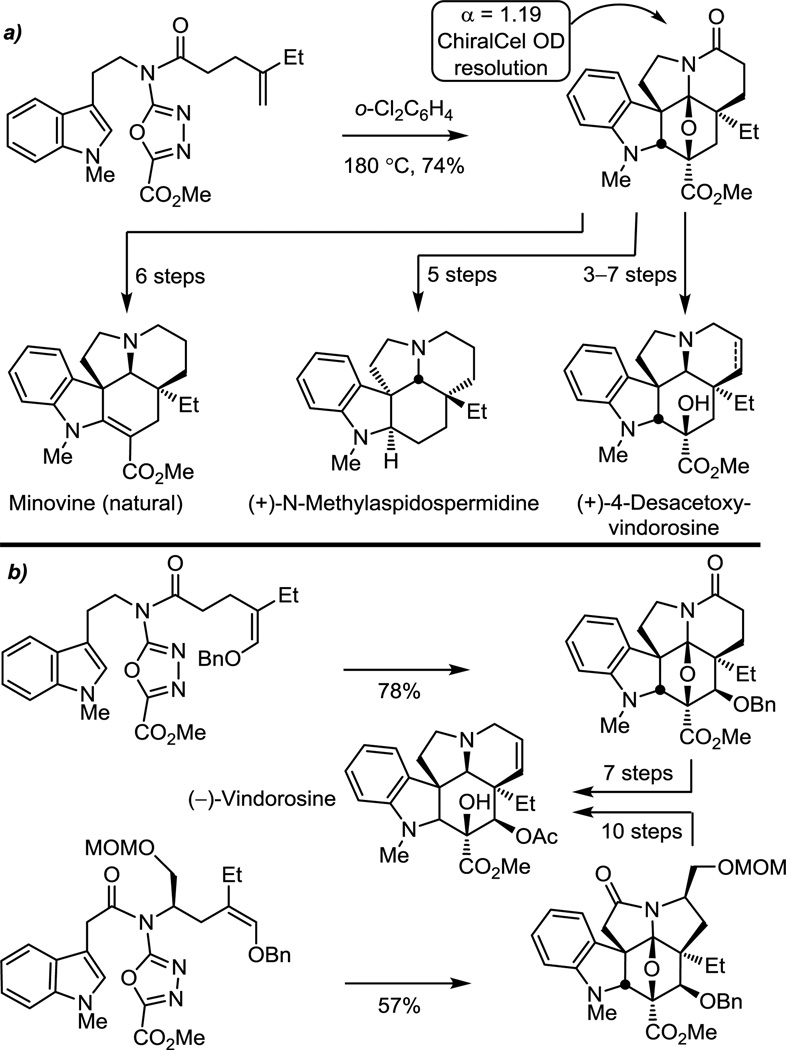
While the enantiomers of 5a (α = 1.70) and 5b (α = 1.19) as well as 7 (α = 1.55) were resolved by chiral phase HPLC to access both (−)-vindoline (natural) and ent-(+)-vindoline, an asymmetric synthesis was also developed based on an additional tandem [4 + 2]/[3 + 2] cycloaddition cascade in which the tether linking the initiating dienophile and oxadiazole bears a chiral substituent that sets the absolute stereochemistry of the remaining six stereocenters in the cascade cycloadduct (Figure 8).55,56 Cycloaddition of 8 proceeded cleanly, providing predominantly 9 in superb conversions, and only small amounts (0−13%) of a second diastereomer were occasionally isolated. The dienophile linking tether of 8 was reduced in length by one carbon relative to the earlier work, which not only permitted effective control of facial selectivity of the initiating Diels–Alder reaction but also allowed the reaction to be conducted under milder conditions than previously observed.52 The approach required that the activating acyl chain carbonyl reside in the dipolarophile tether and that the initiating Diels–Alder reaction provide a fused 5-membered ring, which must be suitably functionalized for subsequent ring expansion and introduction of the Δ6,7-olefin. Thus, the protected hydroxymethyl substituent of 8 not only effectively controls the stereochemical course of the cycloaddition cascade, but its use also provided two unique approaches to ring expansion.56 The first approach relied on a hydrolytic ring expansion of an activated N,O-ketal that is generated from the primary alcohol, while the second enlisted an intermediate aziridinium ion in a thermodynamically controlled reversible ring opening reaction. Together, the asymmetric [4 + 2]/[3 + 2] cycloaddition reaction cascade along with two approaches to ring expansion provided two distinct, concise asymmetric total syntheses of (−)-vindoline (1).55,56
Figure 8.
Asymmetric total synthesis of (−)-vindoline.
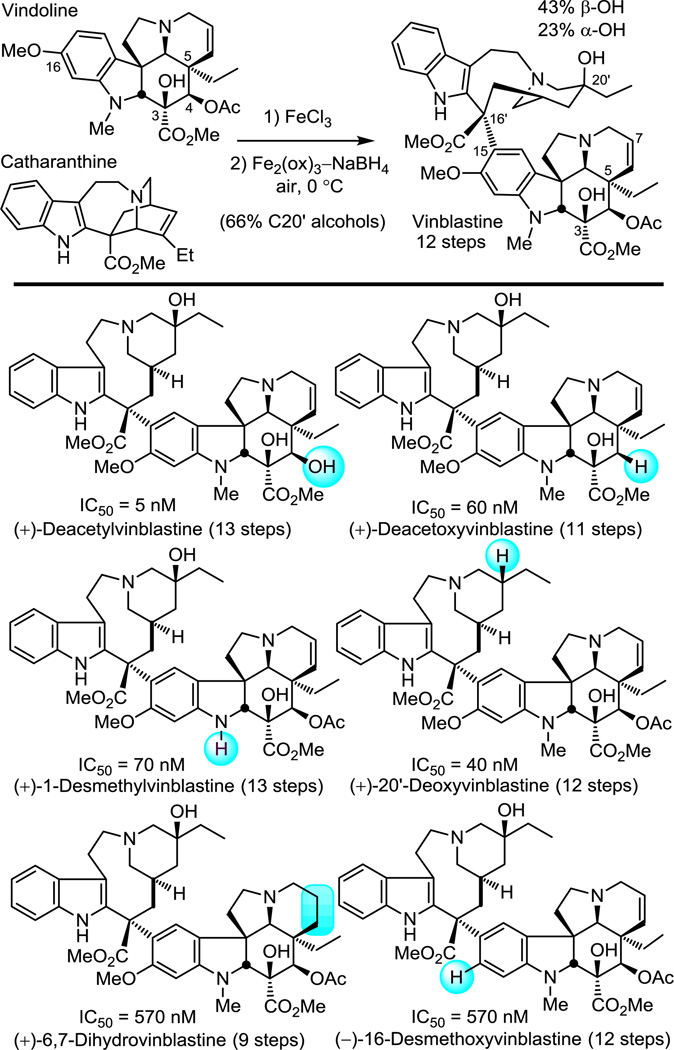
The subsequent development of a biomimetic57 Fe(III)-promoted coupling of vindoline with catharanthine58–60 allowed for their single step incorporation into total syntheses of vinblastine and related natural products (Figure 9). In addition to its use in the completion of the total syntheses of vinblastine (12 steps), vincristine, and a series of additional naturally occurring Vinca alkaloids, the approach also permitted the incorporation of vindoline analogues containing single site peripheral changes61 (Figure 9) as well as deep-seated changes to the vindoline core structure.62 As a result, a systematic exploration of the vindoline subunit structure of vinblastine was conducted, enlisting the cycloaddition cascade reaction to provide a series of vindoline analogues and their single step incorporation into the corresponding vinblastine analogues.
Figure 9.
Representative analogues probing vinblastine substituents.
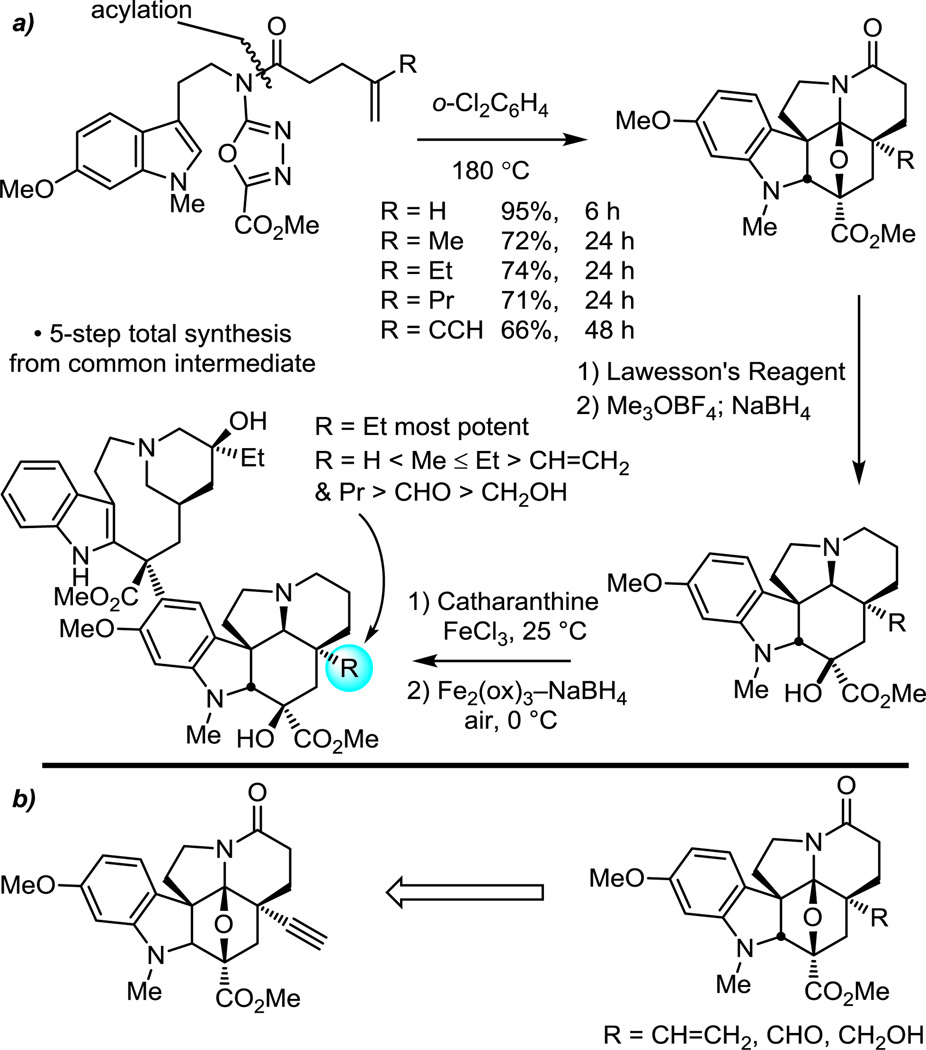
The introduction of alternative substitutions on the tethered dienophile allowed for systematic replacement of the vindoline C5 ethyl group, providing analogues that bear deep-seated structural changes at a site only accessible by total synthesis.63 The alternative substituents were well-tolerated in the cycloaddition cascade, leading to the exclusive formation of one diastereomer in superb yield (66–95%) consistent with expectations26,52 (Figure 10a). When incorporated into vinblastine analogues through their single step coupling with catharanthine (5 steps from an early stage common intermediate), this series provided the first systematic exploration of the impact of this site on biological activity and revealed that it is exquisitely sensitive to the presence, size, and polarity of the C5 ethyl group.
Figure 10.
Systematic examination of the embedded vinblastine C5 ethyl substituent.
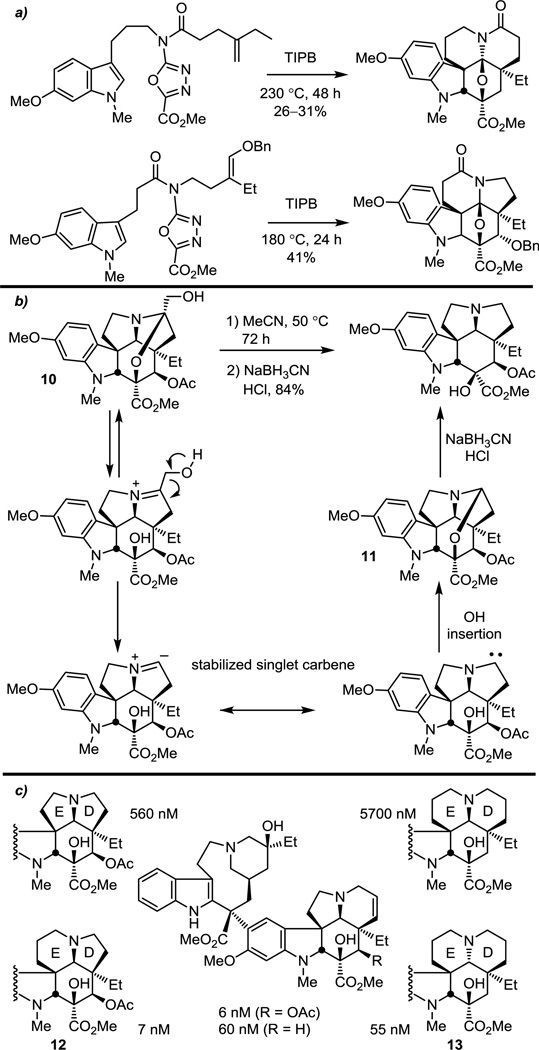
Alteration of the length of either the dienophile or dipolarophile tether provided the opportunity to modify the vindoline 6,5-D,E ring core architecture, affording the 5,5-, 6,6- and reversed 5,6-membered DE ring systems (Figure 11a).64 The 5,5-vindoline subunit analogue was accessed through an intermediate previously enlisted in the asymmetric synthesis of vindoline,56 where a remarkably facile thermal deformylation reaction of 10 generated 11 directly (50%).64 Presumably this unusual reaction occurs by reversible iminium ion formation followed by loss of formaldehyde to generate a stabilized singlet carbene that undergoes an insertion of the proximal C3 alcohol for reclosure of the N,O-ketal, affording the 5,5-D,E ring analogue of vindoline (Figure 11b). Increasing the length of the dipolarophile tether by one carbon and varying the length of the dienophile tether provided cycloadducts bearing the corresponding 6,5- and 6,6-DE ring system analogues. The vindoline analogues were found to be suitable substrates for the biomimetic Fe(III)-promoted coupling and subsequent HAT61,65 oxidation reaction, enabling access to vinblastine analogues that represent the largest departure from the structure of the vindoline subunit to date and that are not available from natural product sources. Most interesting in this probe was that it was the analogues that bore the greatest departure from the natural products (12 and 13) that exhibited the best activity, matching that of the comparison natural products.64 Unlike modifications to peripheral substituents, such core redesign is rarely explored in complex natural products, and was possible only because of the power of the oxadiazole cycloaddition cascade.
Figure 11.
Modification of the vindoline core structure in vinblastine.
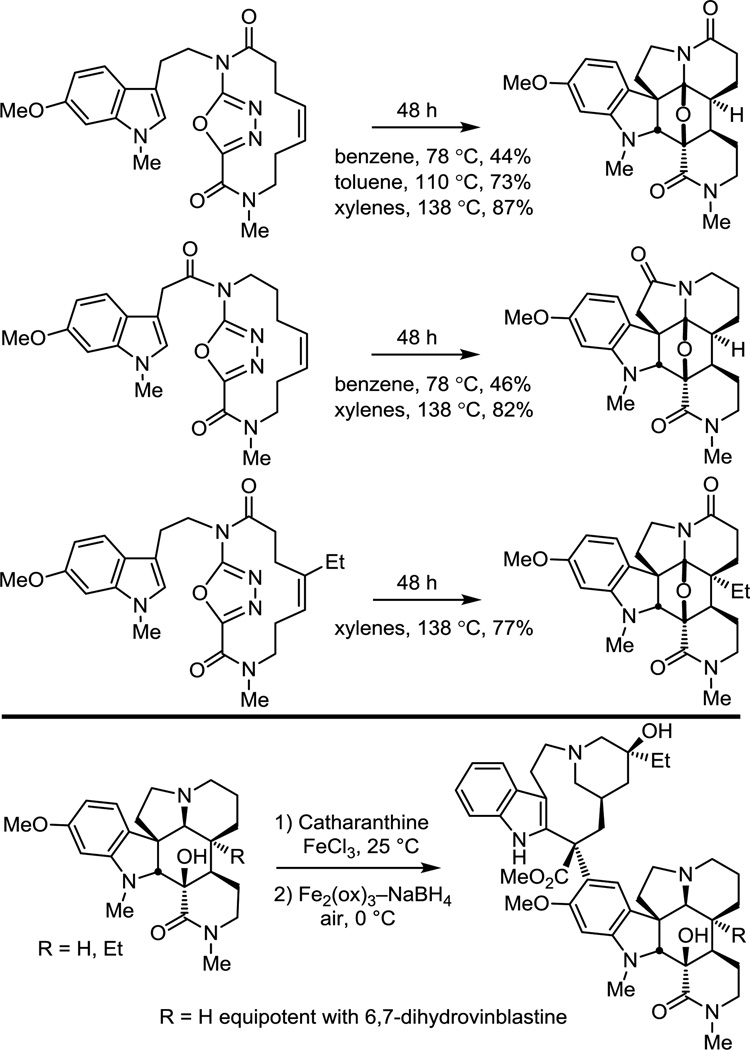
In the continued examination of key analogues of vinblastine, the scope of the key cycloaddition cascade was expanded to include substrates where the dienophile is constrained within a macrocycle, rendering the initiating [4 + 2] reaction transannular.66 Expectedly, the macrocyclic oxadiazoles undergo the tandem cycloaddition cascade at temperatures substantially lower than those of the related intramolecular reactions,26,52 forming an impressive four rings, four carbon–carbon bonds, and six stereocenters at temperatures as low as 80 °C (Figure 12). The resulting cycloadducts provided the basis for the synthesis of unique vinblastine analogues containing deep-seated modifications at the metabolically labile C3/C4 centers of the natural product.66
Figure 12.
Initiation of reaction cascade with a transannular [4 + 2] cycloaddition reaction.
As key elements of the scope and stereochemical features of the reaction were defined, a series of related natural products of increasing complexity were prepared by total synthesis. A total synthesis of natural and ent-minovine was completed in which questions surrounding the optical rotation, racemization potential, optical purity, and absolute configuration of the natural product were resolved.52 The same intermediate, albeit in the enantiomeric series, was enlisted in the total synthesis of (+)-N-methylaspidospermidine, which not only constituted its first total synthesis but also served to confirm its absolute configuration52 (Figure 13a). Similarly, the first total syntheses of 4-desacetoxy-6,7-dihydrovindorosine and 4-desacetoxyvindorosine,52 a late stage biosynthetic intermediate for (−)-vindorosine, were conducted along with efforts that provided a concise 11-step total synthesis of (−)- and ent-(+)-vindorosine52,67 (Figure 13b). In parallel, a total synthesis of the vindoline penultimate biosynthetic precursor (+)-4-desacetoxyvindoline was disclosed, representing its first total synthesis and the first instance of its characterization. Additionally, total syntheses of (+)-6,7-dihydrovindoline and (+)-4-desacetoxy-6,7-dihydrovindoline were completed.
Figure 13.
First and second generation total syntheses of (−)-vindorosine and related natural products.
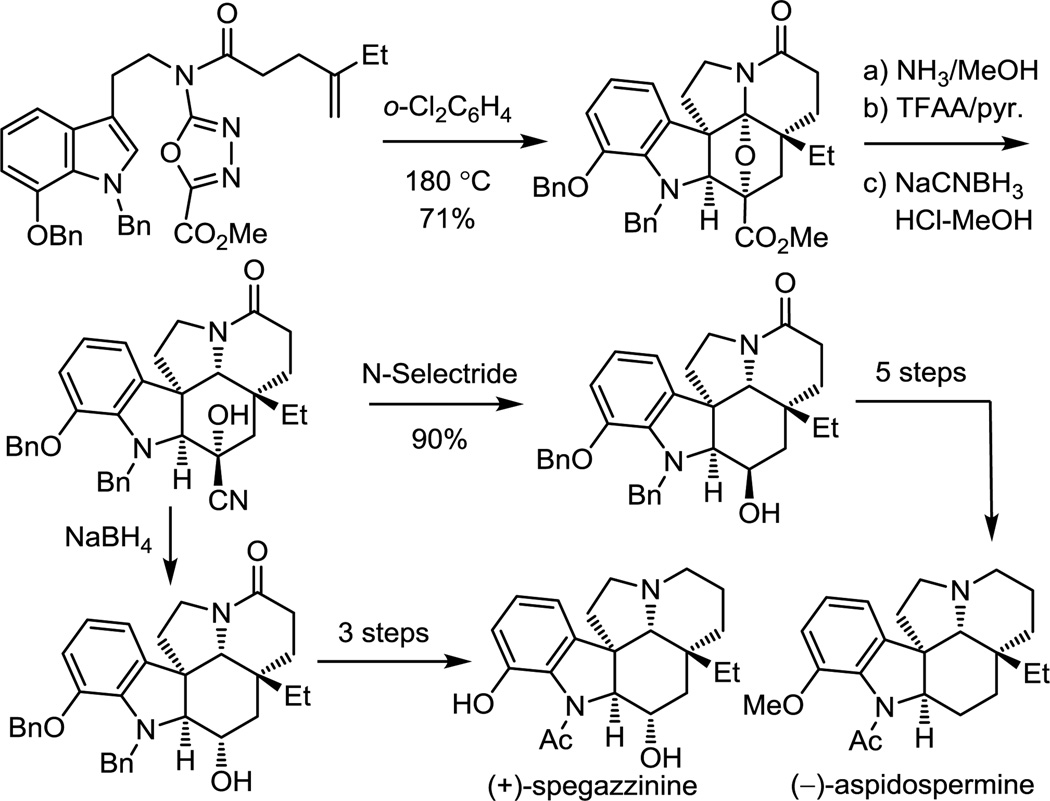
These studies were extended to the total synthesis of additional key members of the Aspidosperma alkaloids, (+)-spegazzinine and (−)-aspidospermine (Figure 14).69 This strategy was especially suited for spegazzinine, whose C3 alcohol was directly introduced upon reduction of a stable cyanohydrin formed after reductive oxido bridge cleavage of a nitrile derived from such a cycloaddition cascade product. An added bonus of the approach is that late stage modification of a route needed to define the spegazzinine C3 alcohol stereochemistry also permited a divergent synthesis of aspidospermine, entailing removal of the C3 alcohol. Significantly, this work constituted the first total synthesis of (+)-spegazzinine, serving to unambiguously assign its absolute configuration, and the first reported synthesis of (−)-aspidospermine that did not rely on a late-stage Fischer indole synthesis.69
Figure 14.
Divergent total synthesis of (+)-spegazzinine and (−)-aspidospermine.
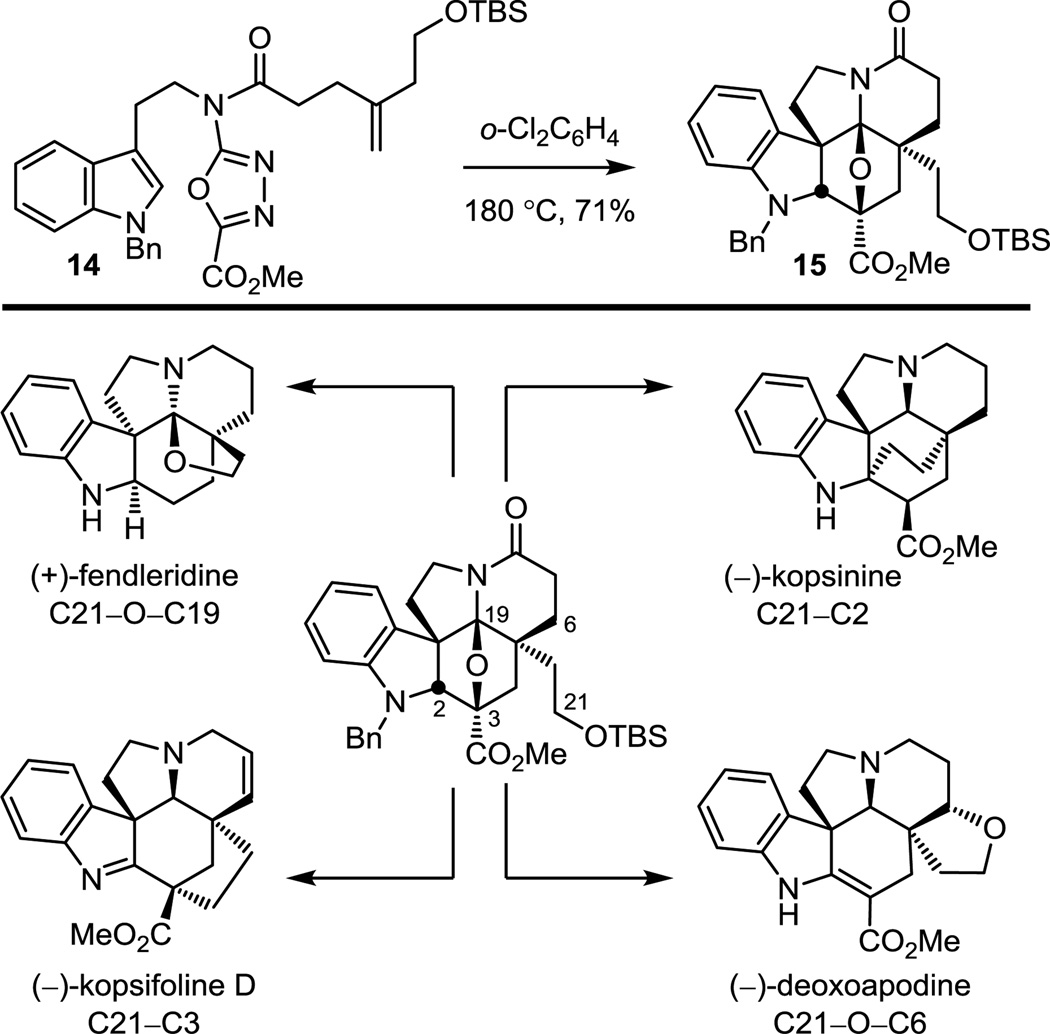
The cycloaddition cascade was also successfully implemented in the synthesis of alkaloids bearing more complex hexacyclic ring cores. The cycloadduct 15 derived from the tandem [4 + 2]/[3 + 2] cycloaddition cascade of 1,3,4-oxadiazole 14 served as an intermediate in the divergent synthesis of members of four different classes of natural products70–72 (Figure 15). This approach relied on late-stage divergent formation of four different key strategic bonds uniquely embedded in each natural product core structure.
Figure 15.
Divergent total synthesis of members of four different alkaloid classes from a common cascade cycloaddition product.
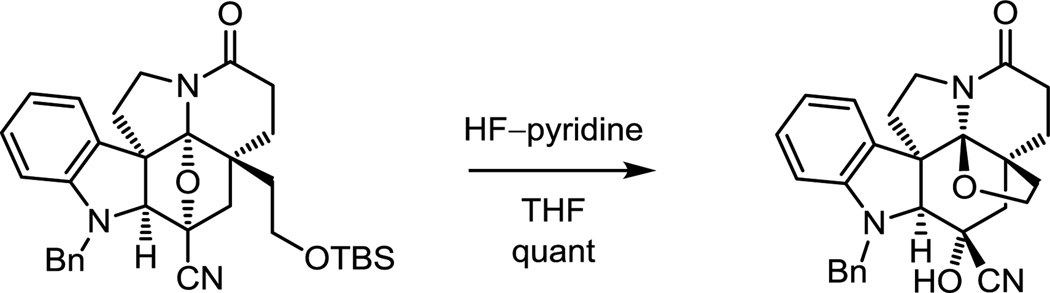
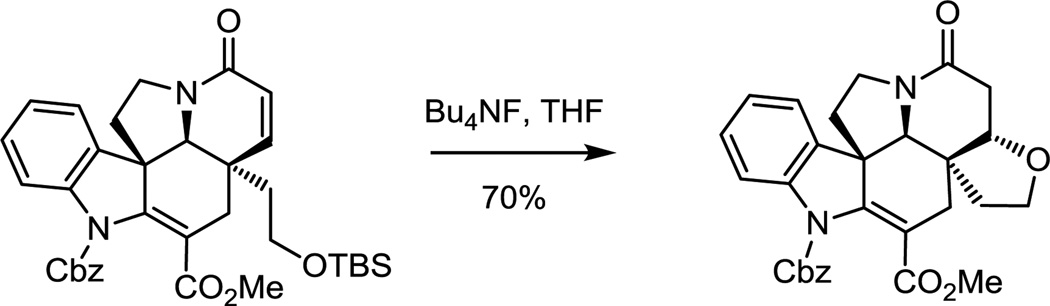
For (+)-fendleridine, the intrinsic oxidation state at C19 of 15 was exploited for closure of the tetrahydrofuran ring and with a modification that permits introduction of useful functionality (cyanohydrin) following cleavage of the oxido bridge.70 The reversibly generated iminium ion derived from oxido bridge opening is flanked by two quaternary centers, facilitating trap by the pendant alcohol (Scheme 3).
Scheme 3.
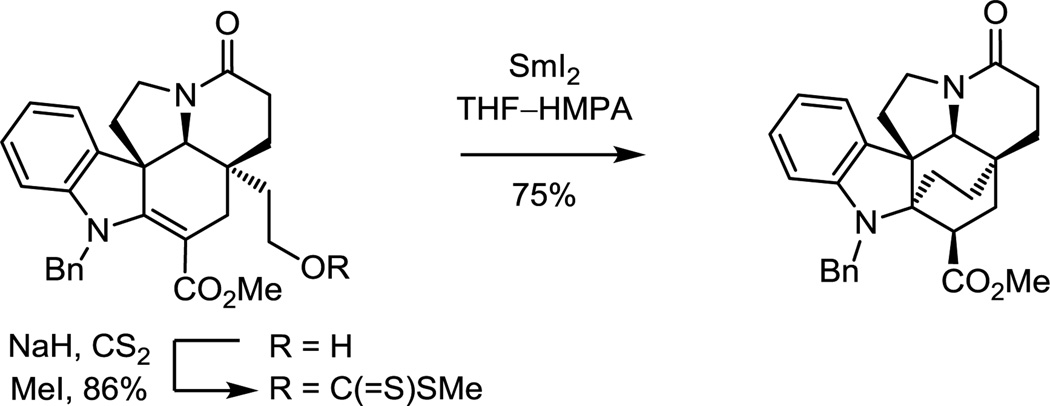
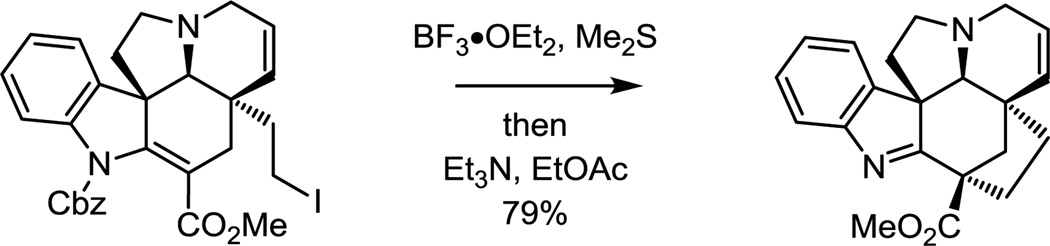
The approach to kopsinine enlisted a late stage C21–C2 bond formation that fundamentally differs from prior approaches.71 A SmI2-promoted transannular cyclization reaction provided the bicyclo[2.2.2]octane central to the kopsinine ring system. This unique transformation resulted from radical-mediated conjugate addition cyclization of the methyl dithiocarbonate followed by kinetic protonation of the further reduced ester enolate from the less hindered convex face (Scheme 4). Alternatively, the primary alcohol could be converted to the corresponding iodide and shown to undergo the SmI2-promoted transannular cyclization reaction even more effectively (85%).73
Scheme 4.
In the approach to (−)-kopsifoline D, a late-stage C21–C3 bond formation via a transannular enamide alkylation of an alcohol-derived C21 iodide was developed (Scheme 5) to complete the assemblage of the kopsifoline skeleton in a route that directly provides the natural product in its final indolenine oxidation state.72 This approach mimics what is the likely biosynthesis of the kopsifolines and represents the first total synthesis of a naturally occurring kopsifoline.
Scheme 5.
In complementary efforts, silyl ether deprotection prior to lactam carbonyl removal permitted C21–O–C6 bond formation by conjugate addition of the primary alcohol (Scheme 6), effecting closure of the tetrahydrofuran ring of (−)-deoxoapodine. This work represented the first synthesis of (−)-deoxoapodine in optically active form, confirming the absolute configuration assignment.72
Scheme 6.
These combined efforts represent the total syntheses of members of four different classes of natural products from a common intermediate, the cycloaddition cascade product of 1,3,4-oxadiazole 14, functionalized for late-stage formation of four different key strategic bonds uniquely embedded in each natural product core structure.
Conclusions and Outlook
Inspired by the structure of vindoline, the development of a powerful tandem intramolecular Diels–Alder/1,3-dipolar cycloaddition cascade of 1,3,4-oxadiazoles was conducted. Although its full scope remains to be established, its use in the total synthesis of vindoline, an extensive series of analogues, a set of related natural products including vindorosine, their incorporation into total syntheses of vinblastine and an extensive series of analogues, and an impressive range of additional natural products illustrate the power of the methodology. Alternative tethering strategies for the cascade cycloaddition reaction, combined intramolecular and intermolecular variants of either the initiating Diels–Alder reaction or the subsequent carbonyl ylide 1,3-dipolar cycloaddition, an expanded examination of the tethered dipolarophile scope, and applications to additional natural product classes represent attractive areas for future work.
Acknowledgments
We gratefully acknowledge the financial support of the National Institute of Health (CA115526 and CA042056, DLB).
Biographies
Dale L. Boger is the Richard & Alice Cramer Chair in Chemistry at The Scripps Research Institute (1990−present) where he serves as Chairman (2012−present) of the Department of Chemistry. He received his BS in Chemistry from the University of Kansas (1975) and his Ph.D. in Chemistry from Harvard University (1980). He was on the faculty at the University of Kansas and Purdue University before joining TSRI in 1990.
Justin E. Sears received his BS in Chemistry from Haverford College (2010) where he worked with Professor Frances Blase. He is currently a Ph.D. student at The Scripps Research Institute (2010−present) with Professor Boger.
Footnotes
The authors declare no competing financial interest.
References
- 1.Huisgen R, Scheer W, Huber H. Stereospecific conversion of cis-trans isomeric aziridines to open-chain azomethine ylides. J. Am. Chem. Soc. 1967;89:1753–1755. [Google Scholar]
- 2.Tsuge O, Wada E, Kanemasa S, Sakoh H. Diene-transmissive Diels–Alder cycloaddition reaction of bis(silyloxy) cross-conjugated trienes. Bull. Chem. Soc. Jpn. 1984;57:3221–3233. [Google Scholar]
- 3.Kanemasa S, Sakoh H, Wada E, Tsuge O. Diene-transmissive Diels–Alder reaction using 2-ethoxy-3-methylene-1,4-pentadiene and 2-(2-bromo-1-ethoxyethyl)1,3-butadiene. Bull. Chem. Soc. Jpn. 1985;58:3312–3319. [Google Scholar]
- 4.Kanemasa S, Sakoh H, Wada E, Tsuge O. Double Diels–Alder reaction of trans-3-methylene-1,4-hexadiene and 1-methyl-3-methylene-4-pentenyl tosylate. Bull. Chem. Soc. Jpn. 1986;59:1869–1876. [Google Scholar]
- 5.Kraus GA, Taschner MJ. Timed Diels–Alder reactions. J. Am. Chem. Soc. 1980;102:1974–1977. [Google Scholar]
- 6.Brokatzky-Geiger J, Eberbach W. Synthese grosser ringe durch intramolekulare cycloaddition von carbonyl-yliden. Tetrahedron Lett. 1982;23:4665–4668. [Google Scholar]
- 7.Donegan G, Grigg R, Heaney F, Surendrakumar S, Warnock WJ. Consecutive Diels–Alder - Michael addition - 1,3-dipolar cycloaddition processes. Tetrahedron Lett. 1989;30:609–612. [Google Scholar]
- 8.Nakano H, Date T, Okamura K, Tomisawa H, Hongo H. Double Diels–Alder cycloadditions of 2(1H)-pyridones acting as dienophiles. Chem. Pharm. Bull. 1991;39:2471–2473. [Google Scholar]
- 9.Minuti L, Selvaggi R, Taticchi A, Sandor P. Tandem Diels–Alder reaction of 4-oxo-2-cyclopentenyl acetate. A facile one-pot synthesis of hydrofluorenones. Tetrahedron. 1993;49:1071–1078. [Google Scholar]
- 10.Brodney MA, O'Leary JP, Hansen JA, Giguere RJ. Tandem intramolecular Diels–Alder (TIMDA) reactions: branched substrate studies and new synthetic pathways. Synth. Commun. 1995;25:521–531. [Google Scholar]
- 11.Hopf H, Sherburn MS. Dendralenes branch out: cross-conjugated oligoenes allow the rapid generation of molecular complexity. Angew. Chem. Int. Ed. 2012;51:2298–2338. doi: 10.1002/anie.201102987. [DOI] [PubMed] [Google Scholar]
- 12.Pronin SV, Shenvi RA. Synthesis of a potent antimalarial amphilectene. J. Am. Chem. Soc. 2012;134:19604–19606. doi: 10.1021/ja310129b. [DOI] [PubMed] [Google Scholar]
- 13.Denmark SE, Thorarensen A. Tandem [4 + 2]/[3 + 2] cycloadditions of nitroalkenes. Chem. Rev. 1996;96:137–166. doi: 10.1021/cr940277f. [DOI] [PubMed] [Google Scholar]
- 14.Broadwater SJ, Roth SL, Price KE, Kobaslija M, McQuade DT. One-pot multi-step synthesis: a challenge spawning innovation. Org. Biomol. Chem. 2005;3:2899–2906. doi: 10.1039/b506621m. [DOI] [PubMed] [Google Scholar]
- 15.Frost C, Chapman C. Tandem and domino catalytic strategies for enantioselective synthesis. Synthesis. 2007:1–21. [Google Scholar]
- 16.Ho T-L. Tandem Organic Reactions. New York: John Wiley & Sons; 1992. [Google Scholar]
- 17.Grossmann A, Enders D. N-Heterocyclic carbene catalyzed domino reactions. Angew. Chem., Int. Ed. 2012;51:314–325. doi: 10.1002/anie.201105415. [DOI] [PubMed] [Google Scholar]
- 18.Tietze LF, Brasche G, Gericke KM. Domino Reactions in Organic Synthesis. Weinheim, Germany: Wiley, VCH; 2006. [Google Scholar]
- 19.Padwa A. Domino reactions of rhodium(II) carbenoids for alkaloid synthesis. Chem. Soc. Rev. 2009;38:3072–3081. doi: 10.1039/b816701j. [DOI] [PubMed] [Google Scholar]
- 20.Baiazitov RY, Denmark SE. Methods and Applications of Cycloaddition Reactions in Organic Synthesis. John Wiley & Sons; 2014. [Google Scholar]
- 21.Boger DL. Diels–Alder reactions of azadienes. Tetrahedron. 1983;34:2869–2939. [Google Scholar]
- 22.Boger DL. Diels–Alder cycloaddition reactions of heterocyclic azadienes: scope and applications. Chem. Rev. 1986;86:781–793. [Google Scholar]
- 23.Boger DL, Weinreb SM. Hetero Diels-Alder Methodology in Organic Synthesis. San Diego: Academic; 1987. [Google Scholar]
- 24.Boger DL. Cycloaddition reactions of azadienes, cyclopropenone ketals and related systems: scope and applications. Chemtracts: Org. Chem. 1996;9:149–189. [Google Scholar]
- 25.Wilkie GD, Elliott GI, Blagg BSJ, Wolkenberg SE, Soenen DR, Miller MM, Pollack S, Boger DL. Intramolecular Diels–Alder and tandem intramolecular Diels–Alder/1,3-dipolar cycloaddition reactions of 1,3,4-oxadiazoles. J. Am. Chem. Soc. 2002;124:11292–11294. doi: 10.1021/ja027533n. [DOI] [PubMed] [Google Scholar]
- 26.Elliott GI, Fuchs JR, Blagg BS, Ishikawa H, Tao H, Yuan ZQ, Boger DL. Intramolecular Diels–Alder/1,3-dipolar cycloaddition cascade of 1,3,4-oxadiazoles. J. Am. Chem. Soc. 2006;128:10589–10595. doi: 10.1021/ja0612549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasilíev NV, Lyashenko YE, Kolomiets AF, Sokolskii GA. Cycloaddition of 2,5-bis(trifluoromethyl)-1,3,4-oxadiazole to olefins. Khim. Geterotsikl. Soedin. 1987:562. [Google Scholar]
- 28.Vasilíev NV, Lyashenko YE, Galakhov MV, Kolomiets AF, Contar AF, Sokolskii GA. 2,5-Bis(trifluoromethyl)-1,3,4-oxadiazole in cycloaddition reactions. Khim. Geterotsikl. Soedin. 1990:95–100. [Google Scholar]
- 29.Vasilíev NV, Lyashenko YE, Patalakha AE, Sokolskii GA. Perfluoro-1,3,4-oxadiazoles. J. Fluor. Chem. 1993;65:227–231. [Google Scholar]
- 30.Vasilíev NV, Romanov DV, Truskanova TD, Lyssenko KA, Zatonsky GV. New cycloaddition reactions of perfluoro-1,3,4-oxadiazoles. Mendeleev Commun. 2006:186–188. [Google Scholar]
- 31.Thalhammer F, Wallfahrer U, Sauer J. 1,3,4-Oxadiazole als heteroctglische 4π-komponenten in Diels–Alder-reaktionen. Tetrahedron Lett. 1988;29:3231–3234. [Google Scholar]
- 32.Seitz G, Wassmuth H. 2,5-Bis(trifluoromethyl)-1,3,4-oxadiazole and -thiadiazole as electron-poor diaza dienes in the Diels–Alder reaction with inverse electron demand. Chem.-Ztg. 1988;112:80–81. [Google Scholar]
- 33.Seitz G, Gerninghaus CH. syn-Facial hetero-bridged [n]polynorbornanes: a new class of polarofacial framework molecules composed of fused 7-oxa- and 7-azanorbornanes. Pharmazie. 1994;49:102–106. [Google Scholar]
- 34.Warrener RN, Butler DN, Liao WY, Pitt IG, Russell RA. The synthesis of polarofacial spacer molecules: a new twist in the coupling of ring strained olefins with oxadiazoles. Tetrahedron Lett. 1991;32:1889–1892. [Google Scholar]
- 35.Warrener RN, Groundwater P, Pitt IG, Butler DN, Russell RA. The synthesis of spacer molecules containing an alcohol group at each terminus. Tetrahedron Lett. 1991;32:1885–1888. [Google Scholar]
- 36.Warrener RN, Maksimovic L, Butler DN. The synthesis of internally functionalised cavity molecules using a cycloaddition strategy. J. Chem. Soc. Chem. Commun. 1994:1831–1832. [Google Scholar]
- 37.Warrener RN, Elsey GM, Russell RA, Tiekink ERT. Cage formation in the reaction 7-tert-butoxynorbornadiene with 2,5-bis(trifluoromethyl)-1,3,4-oxadiazole: X-Ray structure, AM1 calculations and mechanistic comments. Tetrahedron Lett. 1995;36:5275–5278. [Google Scholar]
- 38.Warrener RN, Wang S, Maksimovic L, Tepperman PM, Butler DN. New synthetic strategies for the production of rigid, internally functionalised cavity molecules. Tetrahedron Lett. 1995;36:6141–6144. [Google Scholar]
- 39.Warrener RN, Margetic D, Tiekink ERT, Russell RA. The 1,3,4-oxadiazole and 1,3,4-thiadiazole coupling of norbornenes and 7-oxanorbornenes under high pressure. New structures, mechanistic detail, and synthetic applications. Synlett. 1997:196–198. [Google Scholar]
- 40.Warrener RN, Butler DN, Russell RA. Building BLOCKs in synthesis. Part 2. Fundamental principles of BLOCK design and assembly in the production of large, rigid molecules with functional units (effectors) precisely located on a carbocyclic framework. Synlett. 1998:566–573. [Google Scholar]
- 41.Margetic D, Johnston MR, Tiekink ERT, Warrener RN. Building BLOCKs in synthesis. 11. Synthesis and modeling of novel rigid rods derived from a simple pentacyclic bis-norbornene. Tetrahedron Lett. 1998;39:5277–5280. [Google Scholar]
- 42.Butler DN, Shang M, Warrener RN. Alicyclophanes: a new range of cyclophanes containing rigid alicyclic subunits in place of the aromatic rings. Tetrahedron Lett. 2000;41:5985–5989. [Google Scholar]
- 43.Warrener RN, Margetic D, Foley PJ, Butler DN, Winling A, Beales KA, Russell RA. syn-Facial hetero-bridged [n]polynorbornanes: a new class of polarofacial framework molecules composed of fused 7-oxa- and 7-azanorbornanes. Tetrahedron. 2001;57:571–582. [Google Scholar]
- 44.Warrener RN, Butler DN, Liu L, Margetic D, Russell RA. Incorporation of a molecular hinge into molecular tweezers by using tandem cycloadditions onto 5,6-dimethylenenorbornene. Chem. Eur. J. 2001;7:3406–3414. doi: 10.1002/1521-3765(20010803)7:15<3406::aid-chem3406>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 45.Goli M, Johnston MR, Margeti D, Schultz AC, Warrener RN. Use of a 9,10-dihydrofulvalene pincer cycloadduct as a cornerstone for molecular architecture. Aust. J. Chem. 2006;59:899–914. [Google Scholar]
- 46.Margetic D, Eckert-Maksic M, Troselj P, Marinic Z. Reaction of 2,5-bis-trifluoromethyl-1,3,4-oxadiazole with 7-oxanorbornenes revisited: Experimental and quantum-chemical study of reaction stereoselectivity. J. Fluor. Chem. 2010;131:408–416. [Google Scholar]
- 47.Margetic D, Troselj P, Johnston MR. Tandem [4+2]/[3+2] cycloadditions of 1,3,4-oxadiazoles with alkenes. Mini-Rev. Org. Chem. 2011;8:49–65. [Google Scholar]
- 48.Padwa A, Price AT. Synthesis of the pentacyclic skeleton of the aspidosperma alkaloids using rhodium carbenoids as reactive intermediates. J. Org. Chem. 1998;63:556–565. doi: 10.1021/jo971424n. [DOI] [PubMed] [Google Scholar]
- 49.Muthusamy S, Gunanathan C, Babu SA. Novel regioselective synthesis of decahydrobenzocarbazoles using rhodium generated carbonyl ylides with indoles. Tetrahedron Lett. 2001;42:523–526. [Google Scholar]
- 50.We thank Dr. Scott Pollock for these early assessments.
- 51.Skepper CK, Yang S, Barker TJ, Sankar K, Boger DL. Unpublished studies [Google Scholar]
- 52.Ishikawa H, Elliott GI, Velcicky J, Choi Y, Boger DL. Total synthesis of (−)- and ent-(+)-vindoline and related alkaloids. J. Am. Chem. Soc. 2006;128:10596–10612. doi: 10.1021/ja061256t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi Y, Ishikawa H, Velcicky J, Elliott GI, Miller MM, Boger DL. Total synthesis of (−)- and ent-(+)-vindoline. Org. Lett. 2005;7:4539–4542. doi: 10.1021/ol051975x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sears JE, Barker TJ, Boger DL. Total Synthesis of (−)-vindoline and (+)-4-epi-vindoline based on a 1,3,4-oxadiazole tandem intramolecular [4 + 2]/[3 + 2] cycloaddition cascade initiated by an allene dienophile. Org. Lett. 2015;17:5460–5463. doi: 10.1021/acs.orglett.5b02818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kato D, Sasaki Y, Boger DL. Asymmetric total synthesis of vindoline. J. Am. Chem. Soc. 2010;132:3685–3687. doi: 10.1021/ja910695e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sasaki Y, Kato D, Boger DL. Asymmetric total synthesis of vindorosine, vindoline, and key vinblastine analogues. J. Am. Chem. Soc. 2010;132:13533–13544. doi: 10.1021/ja106284s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kutney JP. Plant cell culture combined with chemistry: a powerful route to complex natural products. Acc. Chem. Res. 1993;26:559–566. [Google Scholar]
- 58.Vukovic J, Goodbody AE, Kutney JP, Misawa M. Production of 3',4'-anhydrovinblastine: a unique chemical synthesis. Tetrahedron. 1988;44:325–331. [Google Scholar]
- 59.Ishikawa H, Colby DA, Boger DL. Direct coupling of catharanthine and vindoline to provide vinblastine: total synthesis of (+)- and ent-(−)-vinblastine. J. Am. Chem. Soc. 2008;130:420–421. doi: 10.1021/ja078192m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gotoh H, Sears JE, Eschenmoser A, Boger DL. New insights into the mechanism and an expanded scope of the Fe(III)-mediated vinblastine coupling reaction. J. Am. Chem. Soc. 2012;134:13240–13243. doi: 10.1021/ja306229x. See also: Gotoh H, Duncan KK, Robertson WM, Boger DL. 10'-Fluorovinblastine and 10'-fluorovincristine: synthesis of a key series of modified vinca alkaloids. ACS Med. Chem. Lett. 2011;2:948–952. doi: 10.1021/ml200236a.
- 61.Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. Total synthesis of vinblastine, vincristine, related natural products and key structural analogues. J. Am. Chem. Soc. 2009;131:4904–4916. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sears JE, Boger DL. Total synthesis of vinblastine, related natural products, and key analogues and development of inspired methodology suitable for the systematic study of their structure–function properties. Acc. Chem. Res. 2015;48:653–662. doi: 10.1021/ar500400w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Va P, Campbell EL, Robertson WM, Boger DL. Total synthesis and evaluation of a key series of C5-substituted vinblastine derivatives. J. Am. Chem. Soc. 2010;132:8489–8495. doi: 10.1021/ja1027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schleicher KD, Sasaki Y, Tam A, Kato D, Duncan KK, Boger DL. Total synthesis and evaluation of vinblastine analogues containing systematic deep-seated modifications in the vindoline subunit ring system: core redesign. J. Med. Chem. 2013;56:483–495. doi: 10.1021/jm3014376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Leggans EK, Barker TJ, Duncan KK, Boger DL. Iron(III)/NaBH4-mediated addition to unactivated alkenes: synthesis of novel C20' vinblastine analogues. Org. Lett. 2012;14:1428–1431. doi: 10.1021/ol300173v. Additionally see: Barker TJ, Boger DL. Fe(III)/NaBH4-mediated free radical hydrofluorination of unactivated alkenes. J. Am. Chem. Soc. 2012;134:13588–13591. doi: 10.1021/ja3063716. Leggans EK, Duncan KK, Barker TJ, Schleicher KD, Boger DL. A remarkable series of vinblastine analogues displaying enhanced activity and an unprecedented tubulin binding steric tolerance: C20' urea derivatives. J. Med. Chem. 2013;56:628–639. doi: 10.1021/jm3015684. Barker TJ, Duncan KK, Otrubova K, Boger DL. Potent vinblastine C20' ureas displaying additionally improved activity against a vinblastine-resistant cancer cell line. ACS Med. Chem. Lett. 2013;4:985–988. doi: 10.1021/ml400281w. Iwasaki K, Wan KK, Oppedisano A, Crossley SWM, Shenvi RA. Simple, chemoselective hydrogenation with thermodynamic stereocontrol. J. Am. Chem. Soc. 2014;136:1300–1303. doi: 10.1021/ja412342g. Lo JC, Yabe Y, Baran PS. A practical and catalytic reductive olefin coupling. J. Am. Chem. Soc. 2014;136:1304–1307. doi: 10.1021/ja4117632.
- 66.Campbell EL, Skepper CK, Sankar K, Duncan KK, Boger DL. Transannular Diels–Alder/1,3-dipolar cycloaddition cascade of 1,3,4-oxadiazoles: total synthesis of a unique set of vinblastine analogues. Org. Lett. 2013;15:5306–5309. doi: 10.1021/ol402549n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elliott GI, Velcicky J, Ishikawa H, Li Y, Boger DL. Total synthesis of (−)- and ent-(+)-vindorosine: tandem intramolecular Diels–Alder/1,3-dipolar cycloaddition of 1,3,4-oxadiazoles. Angew. Chem. Int. Ed. 2006;45:620–622. doi: 10.1002/anie.200503024. [DOI] [PubMed] [Google Scholar]
- 68.Wolkenberg SE, Boger DL. Total synthesis of anhydrolycorinone utilizing sequential intramolecular Diels–Alder reactions of a 1,3,4-oxadiazole. J. Org. Chem. 2002;67:7361–7364. doi: 10.1021/jo020437k. [DOI] [PubMed] [Google Scholar]
- 69.Lajiness JP, Jiang W, Boger DL. Divergent total syntheses of (−)-aspidospermine and (+)-spegazzinine. Org. Lett. 2012;14:2078–2081. doi: 10.1021/ol300599p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Campbell EL, Zuhl AM, Lui CM, Boger DL. Total synthesis of (+)-fendleridine (aspidoalbidine) and (+)-1-acetylaspidoalbidine. J. Am. Chem. Soc. 2010;132:3009–3012. doi: 10.1021/ja908819q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie J, Wolfe AL, Boger DL. Total synthesis of kopsinine. Org. Lett. 2013;15:868–870. doi: 10.1021/ol303573f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee K, Boger DL. Total syntheses of (−)-kopsifoline D and (−)-deoxoapodine: divergent total synthesis via late-stage key strategic bond formation. J. Am. Chem. Soc. 2014;136:3312–3317. doi: 10.1021/ja500548e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee K, Boger DL. Total synthesis of (−)-kopsinine and ent-(+)-kopsinine. Tetrahedron. 2015;71:3741–3746. doi: 10.1016/j.tet.2014.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]