Abstract
Infantile neuronal ceroid lipofuscinosis (INCL, Infantile Batten disease) is an invariably fatal neurodegenerative pediatric disorder caused by an inherited mutation in the PPT1 gene. Patients with INCL lack the lysosomal enzyme palmitoyl protein thioesterase-1 (PPT1, EC 3.1.2.22), resulting in intracellular accumulation of autofluorescent storage material and subsequent neuropathology. The Ppt1−/− mouse is deficient in PPT1 activity and represents a useful animal model of INCL that recapitulates most of the clinical and pathological aspects of the disease. Preclinical therapeutic experiments performed in the INCL mouse include CNS-directed gene therapy and recombinant enzyme replacement therapy; both seek to re-establish therapeutic levels of the deficient enzyme. We present a novel method for the histochemical localization of PPT1 activity in the Ppt1−/− mouse. By utilizing the substrate CUS-9235, tissues known to be positive for PPT1 activity turn varying intensities of blue. Presented here are histochemistry data showing the staining pattern in Ppt1−/−, wild type, and Ppt1−/− mice treated with enzyme replacement therapy or AAV2/9-PPT1-mediated gene therapy. Results are paired with quantitative biochemistry data that confirm the ability of CUS-9235 to detect and localize PPT1 activity. This new method complements the current tools for the study of INCL and evaluation of effective therapies.
Keywords: Batten disease, lysosomal storage disorder, histochemistry, palmitoyl protein thioesterase-1, enzyme replacement therapy
1. Introduction
Infantile neuronal ceroid lipofuscinosis (INCL, Infantile Batten disease, Cln1 disease) is a profoundly neurodegenerative lysosomal storage disorder (LSD) that affects children in early infancy. It is caused by mutations in the PPT1 gene which encodes the lysosomal enzyme palmitoyl protein thioesterase-1 (PPT1). This enzyme is primarily responsible for catalyzing the cleavage of thioester bonds that attach long-chain fatty acids to specific cysteine residues in polypeptides [1]. When the PPT1 gene is mutated, leading to a deficiency or complete lack of PPT1 activity, intracellular autofluorescent storage material accumulates in the central nervous system (CNS) and to varying degrees in many peripheral organs that express the mannose-6-phosphate receptor including kidney, liver, and heart. Concurrent with this accumulation are astrocytosis, cortical atrophy, microglial activation, apoptosis, and loss of gamma-aminobutyric acid (GABA) neurons [2]. Children with this disorder experience a rapid progression of symptoms, beginning at 6–12 months of age, that includes visual loss, motor impairments, seizures, and premature death as early as 6 years of age [3–7].
A useful animal model of INCL is found in the Ppt1-knockout mouse (Ppt1−/−). This animal is PPT1-deficient and recapitulates many key aspects of the human disease. Ppt1−/− mice experience visual deficits, motor abnormalities, myoclonic seizures, and premature death [8]. There is currently no known cure for INCL. However, pre-clinical experiments in the PPT1-deficient mouse suggest that some treatments may delay or lessen the severity of symptoms. All of these treatments address, either directly or indirectly, the central issue involved in INCL: lack of PPT1 enzyme activity. Until recently it has been impossible to detect PPT1 activity in situ. Polyclonal antibodies exist that are useful in localizing PPT1 protein; however, immunolocalization techniques may not correlate with enzyme activity. Localizing PPT1 activity in situ would greatly enhance our ability to develop and analyze novel therapies designed to replace PPT1. Here we describe the development and application of a histochemical stain (CUS-9235) for detecting PPT1 activity.
2. Methods
2.1 Ethics statement
All animal procedures were approved by the Institutional Animal Studies Committee at Washington University School of Medicine and were in accordance with the guidelines of the National Institutes of Health.
2.2 Animals
The Ppt1−/− mouse was created by eliminating the last exon of the PPT1 gene using a targeted disruption strategy [8]. This mutation was backcrossed to the C57BL/6 mouse for more than 10 generations, ultimately establishing colonies of Ppt1−/− and Ppt1+/+(WT) homozygotes that were maintained by MSS. Males and females of each genotype were used at various ages and under different treatment conditions for histochemical experiments. All animals were housed in an animal facility at Washington University School of Medicine in St. Louis, MO. Colonies were maintained under a 12 h light/dark cycle and had ad libitum access to food and water.
2.3 Enzyme therapy
Recombinant human PPT1 was expressed and purified as previously described [9]. Enzyme was stored at −80°C in aliquots at a concentration of 1.5 mg/mL and thawed only as needed. Adult (approximately 2 months of age) Ppt1−/− mice (n = 1 per dose) were injected with 250 μL of enzyme (corresponding to approximately 15 mg/kg) or 25 μL of enzyme (1.5 mg/kg) via tail vein. Neonatal Ppt1−/− mice (n = 4 per group) were injected on post-natal day (PND) 1 or 2 with 100 μL of enzyme via the superficial temporal vein. Mice were euthanized 2 h following enzyme injection. WT and Ppt1−/− mice were age-matched.
2.4 Gene therapy
The rAAV2/9-PPT1 vector used for these studies was produced as previously described [10, 11] with the key difference being that we used the AAV9 capsid protein instead of AAV5. The AAV9 capsid is reported to have widespread distribution throughout the brain following intracranial injection, primarily transduces neurons, and may be capable of crossing the blood-brain barrier (BBB) [12]. Ppt1−/− mice (n = 2) received systemic gene therapy (1 × 1011 vg/mouse) via tail vein injection at 28 days of age. Four weeks following injection, mice were euthanized via anesthesia overdose (Fatal-Plus Solution, Vortech Pharmaceuticals, Dearborn, MI, USA.
2.5 Tissue processing and histochemistry
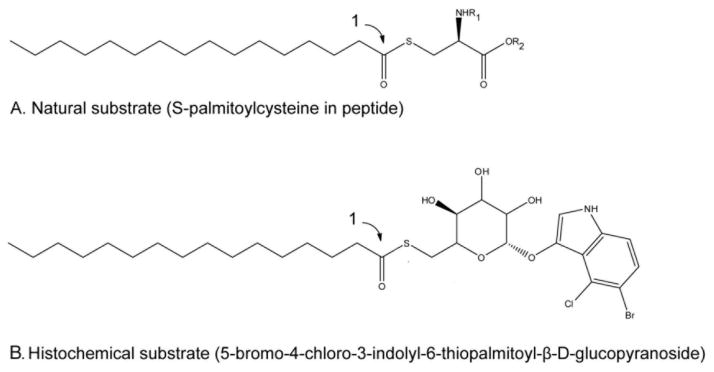
Following euthanasia, mice were dissected to acquire brain, kidney, liver, spleen, and heart for both histochemical and quantitative biochemical analyses. Unfixed tissues (whole kidney, one brain hemisphere, and samples of liver, spleen, and heart) were placed directly into Optimal Cutting Temperature Compound (OCT; Sakura Finetek USA, Inc., Torrance, CA), frozen on dry ice, and stored at −80°C prior to sectioning. In those cases where whole infant bodies were used, euthanized mice were submerged directly into OCT and frozen on dry ice. Sixteen micrometer cryosections were mounted directly onto Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA, USA). A water repellant barrier was created around the mounted tissue using the Super HT PAP pen (Biotium, Inc., Hayward, CA, USA) and tissue was fixed with 4% paraformaldehyde in PBS for 20 minutes at 4°C. Following fixation the slides were rinsed extensively with 0.2 M sodium acetate buffer (pH 4.5). After rinsing, each slide was covered with approximately 1 mL solution which consisted of: 250 μL 0.2 M sodium acetate, 628.5 μL sterile H2O, 10 μL 1% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA), 40 μL β-cyclodextrin (50 mg/mL), 25 μL 0.2 M ferrocyanide, 25 μL 0.2 M ferricyanide, 20 μL β-glucosidase (50 mg/mL) [13], 6.47 μL 1 M dithiothreitol, 20 μL dimethyl sulfoxide (DMSO), and 20 μL CUS-9235 (50 mg/mL in DMSO). This substrate (Figure 1) was custom synthesized by Matreya, LLC (State College, PA, USA) and is structurally similar to the fluorogenic PPT1 substrate previously described [13] with substitution of the 5-bromo-4-chloro-3-indole for the 4-methylumbelliferone (MU). Components of the CUS-9235 staining solution are best added in the order listed above so as to minimize precipitation. Slides were incubated in a humidified chamber overnight at 37°C. Approximately 16 h later slides were rinsed with copious amounts of H2O and counterstained for 2 min with Nuclear Fast Red (Sigma-Aldrich, St. Louis, MO, USA). After a final rinse with H2O, the slides were left at room temperature to dry overnight, then covered using microscope cover glass (Thermo Fisher Scientific, Waltham, MA, USA) and Cytoseal (Electron Microscopy Sciences, Hatfield, PA, USA).
Figure 1. Histochemical substrate for detecting PPT1 enzyme activity in situ.
Step (1) is performed by PPT1. (A) Natural substrate for PPT1; (B) Histochemical substrate. When the indole is released by added β-glucosidase, the indole oxidizes and polymerizes in the presence of added reagents and develops a blue color.
2.6 Biochemical assays
Samples of the same organs used for staining (whole kidney, one brain hemisphere, liver, spleen, and heart) were flash frozen in liquid nitrogen and homogenized in buffer as previously described [13]. Following centrifugation the supernatant was incubated with the fluorogenic substrate 4-methylumbelliferyl-6-thiopalmitoyl-β-glucoside in a 37°C water bath for 1 h. The reaction was stopped with 500 μL of a 0.1 M sodium carbonate/sodium bicarbonate buffer. Fluorescence emission was measured at 448 nm following excitation at 365 nm in a Hitachi F-2000 fluorescence spectrophotometer (Hitachi, Pleasanton, CA). Measurements were compared to a standard curve ranging from 0.5 to 5 mM of 4-methylumbelliferone (4-MU). PPT1 activity was normalized to total protein measured using a Coomassie dye-binding assay (Bio-Rad Laboratories, Hercules, CA) and data are expressed as nmol of substrate cleaved per mg of protein per h (nmol/mg/h).
3. Results
3.1 PPT1 distribution in normal and enzyme-treated newborn mice
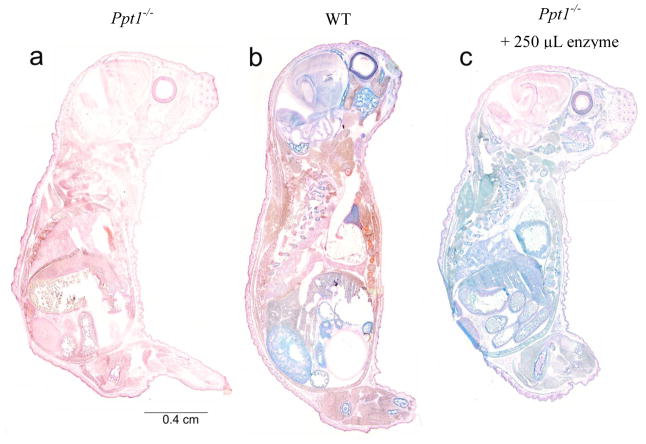
Entire newborn animals were stained for PPT1 activity (Figure 2). PPT1 activity is indicated by a blue color and the tissue was counterstained with nuclear fast red. Ppt1−/− mice (Fig. 2a) show no PPT1 activity in any organ, with red being the only color present across the entire body. In contrast, WT mice (Fig. 2b) exhibit a number of areas of intense blue staining. PPT1 activity is apparent in the brain, eye, bone tissue, thymus, gut, and kidney. There appears to be relatively little PPT1 present in the skin, brown fat, lung, and heart. Ppt1−/− mice treated with intravenous recombinant enzyme (Fig. 2c) have a different distribution of PPT1 activity. Intense blue staining is observed in enzyme-injected animals in liver, spleen, kidney, heart, eye, brown fat, skin, lung, thymus, bone marrow, and gut. However, there is relatively little PPT1 staining in the brain compared to peripheral tissues.
Figure 2. Histochemical stain for PPT1 activity in newborn mice.
Sagittal sections of entire newborn (PND1) mice were stained for PPT1 activity (blue) and counterstained with nuclear fast red. PPT1 activity is undetectable in untreated Ppt1−/− mice (a) and is indicated by discrete regions of intense blue in WT mice (b). Staining of tissue in enzyme-treated Ppt1−/− mice (c) is more uniformly blue, with the exception of the brain, indicating the presence of PPT1 activity. There also appears to be less intense staining in the retina of enzyme- treated animals compared to WT.
3.2 Quantitative enzyme assay in newborn tissues
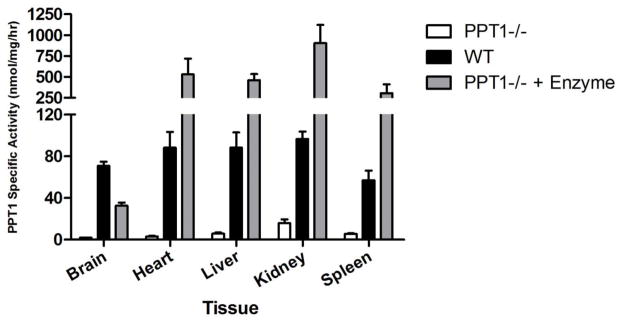
We performed PPT1 assays to determine the level of enzyme activity in tissues collected from neonatal WT, Ppt1−/− mice, and Ppt1−/− mice treated with intravenous enzyme (Figure 3). While enzyme activity is nearly undetectable in the Ppt1−/− infant brain, WT infant brains contain approximately 70.7 nmol/mg/hr. Intravenous enzyme treatment increases PPT1 activity in the Ppt1−/− infant brain to approximately 32.6 nmol/mg/hr. Treatment with enzyme increases PPT1 activity in the Ppt1−/− infant heart to 529.2 nmol/mg/hr, a five-fold increase over WT levels (88.2 nmol/mg/hr), while untreated Ppt1−/− heart tissue contain only 3.2 nmol/mg/hr. Enzyme activity in the livers of Ppt1−/− mice is very low (5.8 nmol/mg/hr), while treatment with enzyme increases this number to 459.6 nmol/mg/hr. This is a four-fold increase in the level of PPT1 activity compared to infant WT liver tissue (88.1 nmol/mg/hr). Kidney tissue from enzyme-treated Ppt1−/− mice contains 903.2 nmol/mg/hr PPT1 activity, nearly 10-times the amount in WT kidney (96.6 nmol/mg/hr) and over 50-times that seen in untreated Ppt1−/− kidney (15.7 nmol/mg/hr). Finally, intravenous enzyme treatment increases PPT1 activity in the spleen from 5.6 nmol/mg/hr (untreated Ppt1−/− infants) to 305.2 nmol/mg/hr in treated Ppt1−/− infants, while infant WT spleen contains 57.0 nmol/mg/hr. Of note, PPT1 activity in the Ppt1−/− brain was increased to 45% of WT levels 2 h after enzyme injection, whereas activity levels in peripheral tissues were much higher than WT, ranging from four-fold (liver) to over 10-fold (kidney) over normal tissues.
Figure 3. Quantitative PPT1 activity following enzyme therapy in neonatal Ppt1−/− mice.
Data are presented as mean (±SEM) in nmol/mg/h, n=4 for each group.
3.3 PPT1 distribution in normal and enzyme-treated adult mice
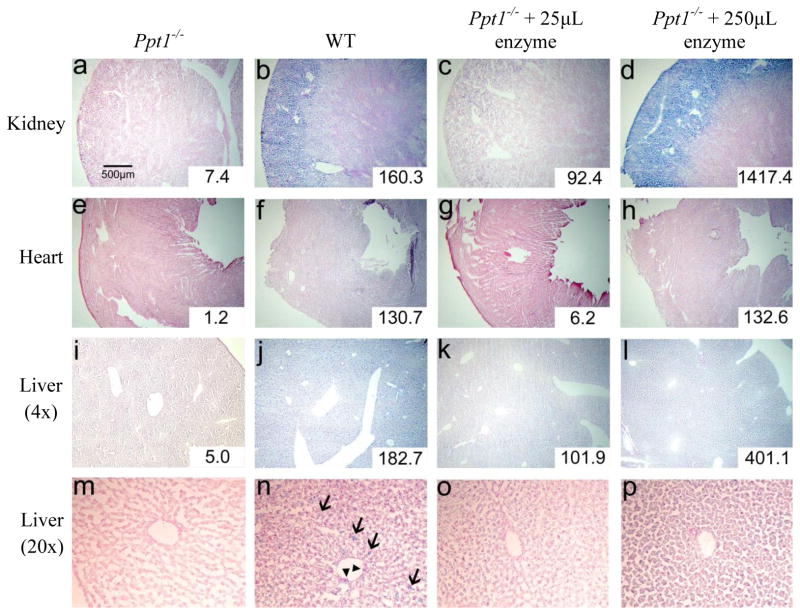
Ppt1−/− mice (Figure 4a) exhibit no apparent PPT1 staining in the kidney. WT mice (Fig. 4b) show staining throughout the renal cortex as well as the renal medulla, with the most intense stain present in the cortex among the glomeruli and associated tubules. In Ppt1−/− mice receiving 25 μL enzyme (1.5 mg/kg; Fig. 4c), some PPT1 activity is detected in the renal cortex but not the medulla; this staining clearly does not reach WT levels. In contrast, the kidney from Ppt1−/− mice receiving 250 μL of enzyme (15 mg/kg; Fig. 4d) exhibit intense blue staining in the renal cortex, clearly more intense than the stain in WT mice. However, even in those mice receiving the larger dose of enzyme, relatively little PPT1 activity is detected in the renal medulla.
Figure 4. PPT1 activity in adult tissues following enzyme therapy.
Kidney (a–d), heart (e–h), and liver (i–l) tissues were stained for PPT1 activity (blue) using CUS-9235, then counterstained with nuclear fast red. Panels m–p show the liver at high magnification (20×) and reveal a punctate, less uniform pattern of stain in the WT liver (n) compared to the liver treated with 250 μL of enzyme (p). Arrows (→) indicate cells in the liver parenchyma, likely to be Kupffer cells, and arrowheads (▶) indicate sinus-lining cells. Both cell types are revealed to contain PPT1 activity. From left to right in each row, groups are: untreated Ppt1−/−, WT, Ppt1−/− + 25μL enzyme, and Ppt1−/− + 250μL enzyme. Quantitative enzyme activity is shown in the inset for each tissue and is presented as nmol/mg/hr.
Staining in heart presents a somewhat similar pattern to the kidney. There appears to be no PPT1 activity in Ppt1−/− heart (Fig. 4e) and moderate activity in WT heart (Fig. 4f). The lower dose of enzyme therapy (1.5 mg/kg) did not result in detectable staining in the Ppt1−/− heart (Fig. 4g), and the full 250 μL dose (15 mg/kg; Fig. 4h) supplies PPT1 to the heart at a level similar to that seen in WT heart.
A similar picture emerges when examining sections of liver. There are no traces of blue stain to be found in the liver of Ppt1−/− mice (Fig. 4i), while PPT1 appears to be widely distributed throughout the liver of WT mice (Fig. 4j). Enzyme therapy clearly restored some PPT1 activity in the livers of Ppt1−/− mice with considerably more intense staining in mice receiving 250 μL (Fig. 4l) compared to 25 μL (Fig. 4k). An interesting difference in the distribution of PPT1 activity is observed between WT mice (Fig. 4n) and Ppt1−/− mice receiving 250 μL of enzyme (Fig. 4p) when viewed at higher magnification. Staining in WT liver appears to be spread throughout the tissue in a punctate pattern, indicating high levels of PPT1 activity in parenchymal cells (likely to be Kupffer cells) as well as some sinus lining cells. This is in distinct contrast to the pattern of staining seen in enzyme-treated Ppt−/− liver tissue which appears to be a uniform blue with the absence of a punctate pattern. In addition, sinus-lining cells in enzyme-treated Ppt1−/− liver appear largely devoid of PPT1 activity.
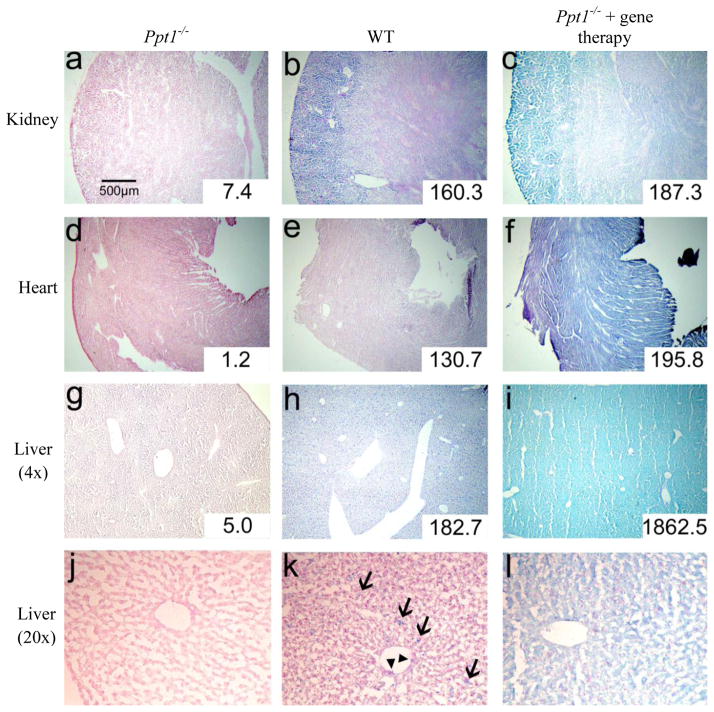
3.4 PPT1 distribution in AAV2/9-PPT1-treated mice
Compared to the same tissue from Ppt1−/− (Figure 5a) and WT mice (Fig. 5b), kidney tissue from Ppt1−/− mice injected intravenously 4 weeks prior with AAV2/9-PPT1 (Fig. 5c) stained a uniform, intense blue across the entire organ. In contrast to enzyme-treated mice, there appears to be little or no distinction in PPT1 activity between the renal cortex and renal medulla. PPT1 staining is considerably more robust in AAV-treated mice compared to untreated Ppt1−/− mice or WT mice. Histochemical staining with CUS-9235 reveals high levels of PPT1 activity in heart tissue from mice treated with AAV2/9 (Fig. 5f). Distribution appears to be uniform across the heart and more intense than WT (Fig. 5e) or Ppt1−/− tissue (Fig. 5d). This pattern is similar in liver tissue. Staining in Ppt1−/− mice treated with AAV2/9-PPT1 (Fig. 5i) is intense and uniform regardless of anatomy. There appears to be a distinct increase in liver PPT1 activity with AAV2/9 treatment when compared with knockout mice receiving no treatment (Fig. 5g) or WT mice (Fig. 5h).
Figure 5. PPT1 activity in adult tissues following AAV2/9-PPT1-mediated gene therapy.
Kidney (a–c), heart (d–f), and liver (g–i) tissues were stained for PPT1 activity (blue) using CUS-9235, then counterstained with nuclear fast red. Liver tissue at high magnification (20×) is shown in panels j–l. Arrows (→) indicate cells in the liver parenchyma, likely to be Kupffer cells, and arrowheads (▶) indicate sinus-lining cells. From left to right in each row, groups are: untreated Ppt1−/−, WT, Ppt1−/− + intravenous AAV2/9-mediated gene therapy. Quantitative enzyme activity is shown in the inset for each tissue and is presented as nmol/mg/hr. Note: stained sections from adult Ppt1−/− and WT animals are the same as presented in Fig. 4. Sections displayed were chosen because they represent staining in all animals within each group.S
3.5 Quantitative enzyme assay in adult tissues
Treatment with intravenous enzyme led to modest increases in PPT1 activity (23.2 – 44.9 nmol/mg/hr) in the brain compared to untreated Ppt1−/− adults (2.5 nmol/mg/hr), but neither small nor large dose restored activity to the level seen in the brain of WT mice (215.5 nmol/mg/hr). In the heart, however, 15mg/kg of intravenous enzyme in Ppt1−/− mice increased PPT1 activity to 132.6 nmol/mg/hr, compared to 130.7 nmol/mg/hr in WT heart (insets, Fig. 4). The lower dose resulted in a nearly undetectable difference from untreated Ppt1−/− heart tissue. Enzyme treatment with the large dose led to a two-fold increase in PPT1 activity in Ppt1−/− liver (401.1 nmol/mg/hr) compared to WT liver (182.7), while the lower dose increased activity to 101.9 nmol/mg/hr. Liver from untreated adult Ppt1−/− mice contained only 5.0 nmol/mg/hr. PPT1 activity in kidney tissue from untreated Ppt1−/− mice was very low (7.4 nmol/mg/hr) but was increased to 92.4 nmol/mg/hr with the lower dose of enzyme and 1417.4 nmol/mg/hr with the large dose of enzyme. Treatment with 15mg/kg enzyme increased PPT1 activity in the kidney of in Ppt1−/− mice nearly 9-fold compared to that seen in the kidney of WT mice (160.3 nmol/mg/hr). Finally, while the large and small doses of enzyme increased PPT1 activity in the spleen of Ppt1−/− mice (186.0 and 29.4 nmol/mg/hr, respectively) compared to untreated Ppt1−/− mice (7.61 nmol/mg/hr), neither increased PPT1 activity to WT level (517.3 nmol/mg/hr).
Treatment with intravenous AAV2/9-PPT1 led to higher PPT1 activity in Ppt1−/− mice compared to untreated Ppt1−/− mice in all tissues analyzed. PPT1 activity in the brain was slightly higher in treated Ppt1−/− mice (6.0 nmol/mg/hr) compared to untreated mice (2.5 nmol/mg/hr). However, the treatment did not increase enzyme activity to near WT levels (215.5 nmol/mg/hr). In contrast, AAV2/9 treatment led to normal or supernormal PPT1 activity in the heart, liver, and kidney of Ppt1−/− mice (insets, Fig. 5). In the heart, while PPT1 activity is nearly undetectable in untreated Ppt1−/− mice, gene therapy increased it to 195.8 nmol/mg/hr, greater than that seen in WT mice (130.7 nmol/mg/hr). A similar pattern is seen in the kidney: PPT1 activity in treated mice (187.3) was greater than activity in either untreated mice (7.4 nmol/mg/hr) or WT mice (160.3 nmol/mg/hr). The greatest increase in PPT1 activity resulting from AAV2/9 treatment was observed in the liver. Untreated Ppt1−/− mice were found to have very low liver PPT1 activity (5.0 nmol/mg/hr) compared to the levels found in WT liver (182.7 nmol/mg/hr). Treatment with gene therapy increased PPT1 activity in Ppt1−/− mice approximately 10-fold to 1862.5 nmol/mg/hr.
4. Discussion
The goal of the current study was to evaluate a novel method for detecting and localizing PPT1 activity in situ. Palmitoyl protein thioesterase-1 is a lysosomal enzyme that is absent in both humans who suffer from INCL as well as the murine model of this disease. Our results indicate that we are able to detect and precisely localize PPT1 activity in mouse tissue via histochemistry. The substrate CUS-9235 results in a reliable blue stain in those tissues that are confirmed by quantitative assay to contain PPT1 activity.
The function of PPT1 is to cleave long-chain fatty acids from cysteine residues [1]. CUS-9235 essentially mimics an endogenous fatty acid-cysteine substrate and takes advantage of PPT1’s action at thioester linkages, resulting in the precipitation of a blue substrate. The method described here utilizes standard histochemical techniques and the color development chemistry is similar to the well-established β-galactosidase assay [14]. Our protocol involves using fresh frozen tissue rather than fixed tissue; following anesthetic overdose the animals were not perfused and dissected tissues were placed directly in OCT and frozen over dry ice. After trials with different fixatives we determined that 4% paraformaldehyde in PBS was the ideal fixative to use on slides prior to staining. However using a chloral-formalin acetate fixative produced similar results. Other fixatives including 1%, 3%, and 5% neutral buffered formalin, 1% and 2% paraformaldehyde, and 70% acetone were tested but led to either severely desiccated tissues or suboptimal staining. We also tested the staining protocol on tissues from animals that were perfused with 4% PFA, post-fixed for 24h in 4% PFA, then transferred to 30% sucrose. The perfusion-fixed tissue stained with similar intensity and distribution as compared with unfixed tissue. As mentioned in the methods, it is important to heed the order of addition of stain components so as to minimize precipitation. Adding components in the order listed will still result in a very fine precipitate, as indicated by the clear, pale yellow solution turning cloudy as the final component (CUS-9235) is introduced. However, this does not negatively affect staining. Future iterations of this substrate with different aliphatic chain lengths (particularly, smaller) might provide improved solubility and more sensitivity. It is important to note that this stain does not allow for quantitative measurement of enzyme activity. The infant mice presented in Figure 1 are shown at 2× magnification and it appears that there is no blue staining in the brain of the Ppt1−/− mouse treated with a full dose of enzyme. However, our biochemical assay reveals that levels of PPT1 in brains of infant mice treated with enzyme is actually 45% of that seen in WT mice. Upon inspection at higher magnification (20× and 40×), blue stain becomes apparent in the brains of enzyme-treated mice, particularly in the ventricles and meninges. Though not quantitative in nature, the CUS-9235 protocol presented here does allow for the differential detection of low, intermediate, and high levels of PPT1 activity as revealed in liver, heart, and kidney tissues in Figures 4 and 5. Interestingly, it seems reasonable to speculate that even less intense or inconspicuous blue stain might correlate with decreased neuropathological markers. Two previous studies [9, 15] indicate that doses similar to ours result in relatively low brain PPT1 activity while still decreasing GFAP and CD68 and increasing lifespan. As such, highly intense blue staining may not be required to indicate therapeutic levels of PPT1 activity. Another important aspect of this protocol is maintaining the pH of tissues and staining solutions at approximately 4.5. We achieve this by using a pH 4.5 sodium acetate buffer as the base for our staining solution, and by soaking the slides in this buffer following 4% PFA fixation. Maintaining a low pH likely inactivates cytosolic thiolases that may or may not recognize and cleave our substrate.
While this study confirms that the substrate CUS-9235 and histochemical protocol described here allows us to visualize the enzyme, some interesting findings are also apparent. First is the widespread distribution of exogenously injected PPT1 throughout the entire corpus of the neonatal Ppt1−/− mouse, with the exception of the brain. Enzyme treatment in both infants and adults resulted in conspicuously low levels of PPT1 activity in the brain. This at least partially explains the lack of efficacy in the treatment of murine INCL using intravenous enzyme replacement alone [15] and confirms that PPT1 does not readily cross the BBB. This is not a surprising finding but indicates that while peripheral enzyme treatment may effectively treat the systemic disease associated with INCL [4], it cannot be expected to address the central nervous system aspects of the disease. However, repeated delivery of recombinant PPT1 directly to the CNS, via intrathecal or intracranial injection, could be a much more effective treatment.
Intravenous AAV2/9-mediated gene therapy to adult Ppt11−/− mice led to broad distribution of PPT1 activity with intense staining in the kidney, liver, and heart. Other PPT1-positive organs include spleen, eye, and lung (data not shown). A surprising finding following AAV2/9-mediated gene therapy was that little or no PPT1 activity was detectable in the brain. This is in contrast to an early description of AAV9 which reported its ability to cross the BBB [16]. It is possible that the accumulation of autofluorescent material or another as yet unidentified aspect (plasma membrane perturbation, altered plasma membrane receptor expression, etc.) of PPT1 deficiency inhibits the transport of AAV2/9 across the BBB. At least one report shows that specific disease states may alter the tropism of AAV2/9 [17].
5. Conclusions
We have described a sensitive and specific method for localizing PPT1 activity in tissues. This histochemical protocol complements the current tools for the study of INCL and development of effective therapies. The method described here will be useful in evaluating treatments such as enzyme replacement, gene therapy, stem cell transplant, and small molecule drugs (suppression of non-sense mediated decay, exon-skipping or chaperones) or other future therapies that are directed at increasing PPT1 activity.
Highlights.
Infantile Batten disease is an inherited pediatric disorder characterized by deficiency in the lysosomal enzyme PPT1
Lack of PPT1 results in intracellular accumulation of autofluorescent storage material and subsequent neuropathology
Treatments for infantile Batten disease aim to restore PPT1 function and include enzyme replacement and CNS-directed gene therapy
We present a novel substrate (CUS-9235) and method for localizing PPT1 activity and show results in diseased mice receiving various treatment conditions
Results indicate that this novel protocol complements the current tools for study of the disease and evaluation of effective therapies
Acknowledgments
This research was funded by NIH RO1 grant NS043205 (MSS), the Batten Disease Support and Research Association, the Batten Disease Family Association, and Taylor’s Tale (SLH). The authors would like to thank Kevin O’Dell for his expert technical assistance.
Abbreviations
- INCL
infantile neuronal ceroid lipofuscinosis
- PPT1
palmitoyl protein thioesterase-1
- AAV
adeno-associated virus
- WT
wild-type
- PBS
phosphate-buffered saline
- BBB
blood-brain barrier
- DMSO
dimethyl sulfoxide
- PND
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Camp LA, Hofmann SL. Purification and properties of a palmitoyl-protein thioesterase that cleaves palmitate from H-Ras. J Biol Chem. 1993;268:22566–22574. [PubMed] [Google Scholar]
- 2.Kielar C, Maddox L, Bible E, Pontikis CC, Macauley SL, Griffey MA, Wong M, Sands MS, Cooper JD. Successive neuron loss in the thalamus and cortex in a mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2007;25:150–162. doi: 10.1016/j.nbd.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shacka JJ. Mouse models of neuronal ceroid lipofuscinoses: useful pre-clinical tools to delineate disease pathophysiology and validate therapeutics. Brain Res Bull. 2012;88:43–57. doi: 10.1016/j.brainresbull.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Galvin N, Vogler C, Levy B, Kovacs A, Griffey M, Sands MS. A murine model of infantile neuronal ceroid lipofuscinosis-ultrastructural evaluation of storage in the central nervous system and viscera. Pediatr Dev Pathol. 2008;11:185–192. doi: 10.2350/07-03-0242.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munasinghe J, Zhang Z, Kong E, Heffer A, Mukherjee AB. Evaluation of neurodegeneration in a mouse model of infantile batten disease by magnetic resonance imaging and magnetic resonance spectroscopy. Neurodegener Dis. 2012;9:159–169. doi: 10.1159/000334838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santavuori P, Haltia M, Rapola J. Infantile type of so-called neuronal ceroid-lipofuscinosis. Dev Med Child Neurol. 1974;16:644–653. doi: 10.1111/j.1469-8749.1974.tb04183.x. [DOI] [PubMed] [Google Scholar]
- 7.Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- 8.Gupta P, Soyombo AA, Atashband A, Wisniewski KE, Shelton JM, Richardson JA, Hammer RE, Hofmann SL. Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc Natl Acad Sci U S A. 2001;98:13566–13571. doi: 10.1073/pnas.251485198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu JY, Hu J, Hofmann SL. Human recombinant palmitoyl-protein thioesterase-1 (PPT1) for preclinical evaluation of enzyme replacement therapy for infantile neuronal ceroid lipofuscinosis. Mol Genet Metab. 2010;99:374–378. doi: 10.1016/j.ymgme.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macauley SL, Roberts MS, Wong AM, McSloy F, Reddy AS, Cooper JD, Sands MS. Synergistic effects of central nervous system-directed gene therapy and bone marrow transplantation in the murine model of infantile neuronal ceroid lipofuscinosis. Ann Neurol. 2012 doi: 10.1002/ana.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts MS, Macauley SL, Wong AM, Yilmas D, Hohm S, Cooper JD, Sands MS. Combination small molecule PPT1 mimetic and CNS-directed gene therapy as a treatment for infantile neuronal ceroid lipofuscinosis. J Inherit Metab Dis. 2012 doi: 10.1007/s10545-011-9446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manfredsson FP, Rising AC, Mandel RJ. AAV9: a potential blood-brain barrier buster. Mol Ther. 2009;17:403–405. doi: 10.1038/mt.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Diggelen OP, Keulemans JLM, Winchester B, Hofman IL, Vanhanen SL, Santavuori P, Voznyi YV. A rapid fluorogenic palmitoyl-protein thioesterase assay: pre- and postnatal diagnosis of INCL. Mol Genet Metab. 1999;66:240–244. doi: 10.1006/mgme.1999.2809. [DOI] [PubMed] [Google Scholar]
- 14.Lojda Z. Indigogenic methods for glycosidases. II. An improved method for β-D-galactosidase and is application to localization studies of the enzymes in the intestine and in other tissues. Histochemie. 1970;23:266–288. doi: 10.1007/BF00306428. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Lu JY, Wong AM, Hynan LS, Birnbaum SG, Yilmaz DS, Streit BM, Lenartowicz EM, Thompson TC, Cooper JD, Hofmann SL. Intravenous high-dose enzyme replacement therapy with recombinant palmitoyl-protein thioesterase reduces visceral lysosomal storage and modestly prolongs survival in a preclinical mouse model of infantile neuronal ceroid lipofuscinosis. Mol Genet Metab. 2012 doi: 10.1016/j.ymgme.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen YH, Claflin K, Geoghegan JC, Davidson BL. Sialic acid deposition impairs the utility of AAV9, but not peptide-modified AAVs for brain gene therapy in a mouse model of lysosomal storage disease. Mol Ther. 2012;20:1393–1399. doi: 10.1038/mt.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]