Abstract
The human pathogenic Yersinia species cause diseases that represent a significant source of morbidity and mortality. Despite this, specific mechanisms underlying Yersinia pathogenesis and protective host responses remain poorly understood. Recent studies have shown that Yersinia disrupt cell death pathways, perturb inflammatory processes and exploit immune cells to promote disease. The ensuing host responses following Yersinia infection include coordination of innate and adaptive immune responses in an attempt to control bacterial replication. Here, we highlight current advances in our understanding of the interactions between the pathogenic yersiniae and host cells, as well as the protective host responses mobilized to counteract these pathogens. Together, these studies enhance our understanding of Yersinia pathogenesis and highlight the ongoing battle between host and microbe.
Introduction
Recognition of bacterial pathogens is fundamental for the mammalian immune system to mount a protective response. Consequently, pathogens must employ a variety of measures to counteract these host defenses and facilitate infection. This ever-changing interplay between host and microbe can be illustrated by the interactions of yersiniae and host cells.
Three species within the genus Yersinia are pathogenic for humans, Yersinia pestis, Yersinia pseudotuberculosis, and Yersinia enterocolitica. Y. pestis is the etiological agent of plague while Y. pseudotuberculosis and Y. enterocolitica typically cause a self-limiting gastroenteritis [1]. Despite the differences in clinical presentation of their infections, these pathogens share a propensity for colonization of lymphatic tissues. Common Yersinia virulence traits identified to date include mechanisms of resistance to complement and antimicrobial peptides, pathways for acquisition of essential nutrients such as iron, and a type III secretion system (T3SS) encoded on a plasmid [2–7]. These common virulence traits likely contribute to the tropism of pathogenic Yersinia species for lymphoid tissues. The plasmid-encoded T3SS exports Yersinia outer proteins (Yops), which are deposited into the host cell membrane or delivered directly into the cytosol of host cells to disrupt a multitude of cellular functions [8–12]. In addition to the above common virulence traits, there exist species-specific virulence determinants that play crucial roles during interaction with immune cells [7,13]. The combined actions of these virulence factors enable yersiniae to resist humoral immunity, acquire nutrients, and inject effectors into immune cells with the goal of neutralizing their responses. While the virulence determinants confer a significant advantage for these highly host-adapted pathogens, they do not come without a cost. Host cells are able not only to sense conserved structural features of pathogens, termed pathogen-associated molecular patterns (PAMPs) [14], but also pathogen-derived disruptions of cellular homeostasis as “patterns of pathogenesis” [15].
This review highlights recent developments regarding Yersinia and immunity, specifically how the pathogenic yersiniae evade both extracellular and intracellular immune defenses. To illustrate the dynamic process of host-pathogen interactions, we also cover current research on the protective innate and adaptive immune responses following Yersinia infection.
Yersinia evasion of complement
Following infection, yersiniae are confronted by a plethora of extracellular immune factors. One such factor is host complement, which is comprised of serum proteins and which can be stimulated through three distinct pathways: classical, lectin and alternative. Despite distinct mechanisms of activation, these pathways converge on the same set of effector molecules that results in opsonization of pathogens or bacteriolysis [16]. All three pathogenic Yersinia species are resistant to human serum, however there are differences in how surface structures of these pathogens promote this activity. In Y. enterocolitica, serum resistance requires the adhesin YadA, which binds factor H, while the adhesin Ail and LPS O-antigen play minor roles. In contrast, Ail is the primary serum resistance factor in Y. pestis and Y. pseudotuberculosis. Ail in Y. enterocolitica and Y. pseudotuberculosis is known to bind to C4b-binding protein (C4BP). A recent study shows that Ail in Y. pestis also mediates serum resistance by binding to C4BP and C4 [17], resulting in a blockade of classical and lectin pathways, which is likely critical for the bacterium to grow to high densities in the blood during plague.
Early interactions of Yersinia with host cells-important for dissemination and intracellular survival
Migration from the initial site of infection to deeper tissues is integral in Yersinia pathogenesis. Shortly after host entry, pathogenic yersiniae encounter an influx of immune cells, which they can either bypass or exploit to ensure survival and dissemination. Absence of the lipopolysaccharide (LPS) component O-antigen in Y. pestis is important for the invasion of dendritic cells expressing the C-type lectin Langerin [18]. Following inoculation into hind paws of mice, O-antigen-expressing derivatives are defective in spreading to sub-iliac lymph nodes, suggesting that exploitation of host Langerin is important for the efficient dissemination process associated with virulence in Y. pestis.
Intravital imaging of immune cell responses following intradermal [19] or flea-transmitted [20] Y. pestis infection in mice reveal neutrophils as the predominant cell population associated with the bacteria. However, depletion of neutrophils using an anti-GR1 antibody does not appear to alter dissemination of Y. pestis in an intradermal mouse model of infection [19], nor does depletion of neutrophils using a Ly6G antibody alter bacterial colonization during pneumonic plague [21]. Interestingly, a small subset of Y. pestis engulfed by human neutrophils is able to survive and replicate, and induces the production of the early apoptotic marker phosphatidylserine (PS) [22]. Neutrophils expressing PS on their cell surface are recognized and phagocytosed by macrophages via efferocytosis, thereby allowing for entry of Y. pestis into macrophages while simultaneously limiting production of proinflammatory mediators.
Later interactions of Yersinia with host cells-important for effector translocation by T3SS to counteract innate immunity
Infection with yersiniae triggers a variety of innate host responses, many of which are neutralized by the plasmid-encoded T3SS. For example, contact between the Y. pseudotuberculosis adhesive protein invasin and β1 integrin receptor on neutrophils induces extrusion of microbicidal DNA, via NETosis, a processes that is inhibited by effectors delivered by the T3SS (Figure 1A) [23]. In addition to stimulating host immune responses, adhesion of yersiniae to host cells facilitates Yop effector translocation by the T3SS into immune cells during infection, while factors in serum can restrict levels of translocation by shifting the specificity of Yop targeting [24–26]. Additional bacterial factors are important for fine-tuning effector translocation. The Yersinia cytotoxic necrotizing factor-γ produced by certain strains activates Rac-[27] and Rho-GTPases [28] to enhance Yop translocation into target immune cells, while structure-function studies reveal regions within the transolocon component YopD to be important for effector delivery [29–31].
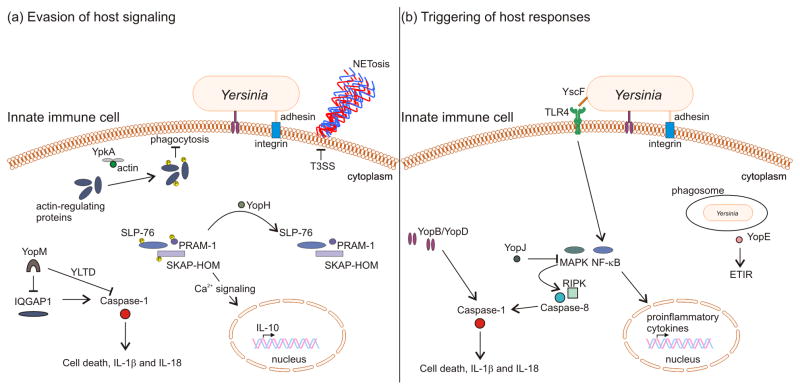
Figure 1. Yersinia vs. host immunity.
(a) Evasion of host signaling pathways by the type III secretion system. YpkA association with actin monomers targets actin-regulating proteins to inhibit phagocytosis. In neutrophils, delivery of YopH leads to dephosphorylation of SLP-76, PRAM-1 and SKAP-HOM, down-regulating calcium signaling and production of anti-inflammatory IL-10, while extrusion of microbicidal neutrophil DNA (NETosis) is inhibited in a T3SS-dependent manner. In macrophages, specific YopM isoforms utilize an internal motif (YLTD) within the protein to directly sequester caspase-1 while other YopM isoforms target caspase-1 via the host scaffolding protein IQGAP1. (b) Triggering of host responses by the type III secretion system. Toll-like receptor 4 detection of the needle protein YscF activates NF-κB, inducing expression and production of pro-inflammatory cytokines. In contrast, inhibition of NF-κB and MAP kinase pathways by YopJ drives RIP kinase- and caspase-8-dependent activation of inflammatory caspase-1. Activation of caspase-1 also occurs upon translocation of YopB and YopD into the host cell cytosol. Additionally, sensing of YopE GTPase-activating protein activity can trigger killing of phagosomal Yersinia.
Disruption of phagocytic signaling pathways by Yersinia T3SS effectors
The effectors delivered into immune cells by the T3SS provide yersiniae with a multifaceted and sophisticated mechanism to perturb host signaling pathways (Figure 1A). Recent progress has been made in identifying new host targets of these effectors, which in some cases have been validated by in vivo studies. In mice infected with Y. pseudotuberculosis, the tyrosine phosphatase YopH impairs neutrophil signaling by targeting the PRAM-1/SKAP-HOM and SLP-76 signaling axes, leading to an inhibition of calcium flux and a decrease in production of interleukin (IL)-10 [32]. An additional study showed that depletion of neutrophils in mice infected with Y. pseudotuberculosis leads to faster kinetics of dissemination by a yopH mutant [33], suggesting that inhibition of neutrophil signaling by YopH is critical for Yersinia virulence.
Blunting phagocytosis is a hallmark of pathogenic Yersinia species. Recent work identifies vasodilator-stimulated phosphoprotein (VASP), a regulator of actin dynamics, as a host target of YpkA (also known as YopO) [34,35]. YpkA induces phosphorlyation of VASP at serine residue 157, which is critical for disruption of actin polymerization [34]. Resolution of the YpkA crystal structure in complex with actin reveals the requirement of actin binding for sequestering of additional actin-regulating proteins to further impair phagocytosis [35]. Given the vastly different biochemical functions of Yop effectors, uncovering the molecular mechanisms by which they exert their effects remains an ongoing area of investigation.
Inhibition of inflammasome signaling and caspase-1 activation by Yersinia T3SS effectors
Activation of the cysteine protease caspase-1 in response to inflammasome assembly in the host cell cytosol drives maturation of cytokines IL-1β and IL-18 from their inactive precursors [36], and pyroptosis, a lytic form of cell death [37]. IL-1β and IL-18 are important for recruitment of innate immune cells while pyroptosis releases intracellular contents, further amplifying local immune responses. Thus, limiting activation of caspase-1 is an important step of bacterial pathogenesis. Both epithelial cells and macrophages have been shown to respond to Yersinia infection by activating caspase-1, but the mechanisms and outcomes are distinct. In human epithelial cells infected with Y. enterocolitica, invasin-β1 integrin interaction provides a priming signal for pro-IL-18 production, and the effectors YopH and YopE inhibit this response [38]. Recognition of LPS by TLR4 in murine macrophages provides a priming signal for production of pro-IL-1β and some inflammasome components (e.g. NLRP3), allowing for rapid activation of caspase-1 in response to Yersinia infection. In LPS-primed murine macrophages infected with Y. pseudotuberculosis, the leucine-rich repeat (LRR)-containing effector YopM inhibits activation of caspase-1 [39,40]. The virulence defect of yopM mutant Y. pseudotuberculosis is restored in Caspase-1/11−/− mice, emphasizing the importance of these caspase-dependent responses for killing of YopM-deficient Yersinia [39,40]. Different Yersinia strains encode distinct YopM isoforms that vary in the number and amino acid sequence of LRRs. A YopM isoform encoded by the Y. pseudotuberculosis strain YPIII contains a specific motif YLTD within its LRR region that appears to function as a pseudo-substrate inhibitor of caspase-1 [39]. Interestingly, YopM isoforms lacking the pseudo-substrate motif are still able to inhibit activation of caspase-1 [40], indicating that the variable LRR regions of distinct isoforms target different host proteins to inhibit inflammasome function. Consistent with this idea is the demonstration that the Y. pestis KIM YopM isoform interacts with the large scaffolding protein IQGAP1, and IQGAP1 is important for inflammasome activation in Yersinia-infected macrophages [40].
Immune responses to Yersinia pestis in the lung
The strikingly biphasic nature of pneumonic plague, characterized by limited initial inflammation followed by an overwhelming inflammatory host response, illustrates the unique immunological environment of the Y. pestis-infected lung. Increasing evidence suggests that Y. pestis uses several mechanisms to modulate host inflammatory response in the lung environment. Suppression of chemokine expression by the T3SS effector YopJ results in a delay of neutrophil influx to the lungs of infected mice [41], and induction of neutrophil migration to the lungs via exogenous supplementation of chemokines decreases bacterial burdens and promotes survival of mice challenged with Y. pestis. Consistently, IL-17 synthesized by neutrophils protects against pneumonic plague by coordinating macrophage-mediated immunity [42], demonstrating the protective host responses that are blunted during Yersinia infection.
Host cells evoke cell death pathways in response to bacterial infections. Thus, manipulation of these cell death programs constitutes an important mechanism of successful pathogens. Apoptotic cell death is initiated following ligation of Fas to its cognate receptor, Fas ligand (FasL), resulting in activation of caspase-3 and -7 [43]. These effector caspases subsequently mediate inflammatory, host protective responses. Recent work identifies FasL as a host target of the Y. pestis outer-membrane protease Pla in a mouse model of pneumonic plague [44]. Pla-dependent degradation of FasL perturbs caspase-3 and -7 activation. Moreover, the colonization defect of Pla deficient Y. pestis is restored in mice lacking functional FasL and treatment of mice with a caspase-3 and -7 specific inhibitor promotes outgrowth of the pla mutant in lungs. Together, these findings demonstrate the importance of Pla on limiting host defenses in the lung via disruption of the extrinsic pathway of apoptosis. Pla is absent in the enteric Yersinia species, and its acquisition by Y. pestis is tightly associated with the ability of modern strains to grow in the pulmonary compartment during pneumonic plague and to spread systemically during bubonic plague. Unexpectedly, a variant of Pla encoded by ancestral Y. pestis strains can promote bacterial colonization in lungs of infected mice, but is deficient in allowing dissemination during bubonic plague [45]. These finding raise the possibility that Y. pestis acquired the ability to cause pneumonic plague before evolving the capacity for invasive systemic infections [45]. Interestingly, the presence of Pla in outer membrane vesicles [46] may signify an important mechanism by which Y. pestis can enhance deployment of this virulence factor.
Initial events during pneumonic plague also include production of active of IL-1β; however, as discussed above there is an apparent absence of inflammation in the lungs of infected mice [47]. Y. pestis infection induces production of IL-1 receptor antagonist (IL-1RA) [47], which competes with IL-1β to limit IL-1 signaling. Neutralization of IL-1RA restores inflammation in the lung, increasing levels of proinflammatory cytokines and decreasing bacterial colonization. In contrast, bacterial mediators can drive aberrant inflammation and contribute to immunopathology of the lung. In vivo transcriptional profiling revealed the Yersinia gene ybtX as important for progression of pneumonic plague from the initial silent phase to the ensuing inflammatory response [48].
Innate immune responses against Yersinia based on recognition of T3SS components
Recognition of the Yersinia T3SS machinery and/or Yops can potentiate host innate immune signaling (Figure 1B). The T3SS needle protein YscF is sensed as a PAMP by the extracellular domain of Toll-like receptor 4 in human macrophage-like THP-1 cells, resulting in activation of nuclear factor kappa B (NF-κB) [49]. Removal of a small N-terminal region of YscF amplifies NF-κB activation [50], suggesting that Yersinia circumvents immune recognition of this necessary structural component. In addition to extracellular recognition, T3SS components that enter into the cytosolic compartment can be recognized by NLRC4 in conjunction with a NAIP protein. Needle proteins such as YscF are recognized by NAIP1/NLRC4 and inner rod proteins are recognized by NAIP2/NLRC4 in the cytosol of macrophages. Yersinia mutants lacking the T3SS effector YopK deliver increased amounts of the other effectors and the translocon proteins YopB and YopD into the cytosol of infected host cells. Inadvertent hyper-translocation of YopB and YopD into the murine macrophage cell cytosol is apparently sensed as a “pattern of pathogenesis”, consequently triggering robust activation of caspase-1 [51]. The patterns of pathogenesis concept can be further expanded on by the effector-triggered immune response (ETIR) [52], resulting from the biochemical functions of Yops on their cellular targets. YopJ acetylates MAPK kinases and the resulting inhibition of NF-κB and MAPK pathways drives caspase-8- and RIP kinase-mediated activation of caspase-1 in naive murine macrophages [53,54]. Mice deficient in RIPK3 and caspase-8 display increased susceptibility to Yersinia infection [53,54], indicating that activation of caspase-1 is an ETIR resulting from YopJ’s targeting of MAPK kinases. Likewise, YopE GTPase-activating protein (GAP) activity promotes intracellular killing of Y. pseudotuberculosis by naive murine macrophages [55]. Treatment of macrophages with the Clostridium difficile Rho GTPase inhibitor Toxin B restricts survival of Y. pseudotuberculosis expressing catalytically inactive YopE, suggesting that inactivation of Rho GTPases results in the production of an ETIR.
Protective adaptive immune responses mediated by T cells against Yersinia
The adaptive immune system is the highly specific branch of immunity that is mobilized following recognition of foreign peptides on the surface of antigen presenting cells (APCs). T cell receptor association with peptide-bound major histocompatibility complexes on APCs enables discrimination between self and non-self and promotes protective CD4+ and CD8+ T cell responses. Delivery of T3SS effectors into APCs infected with Yersinia affords the host an opportunity to process these proteins as antigens and generate CD8+ T cell responses to the corresponding peptides. By vaccinating C57BL/6 mice with attenuated Y. pestis, Lin et al. discovered that YopE contains a dominant H2Kb-restricted epitope that can prime a protective CD8+ T cell response against pneumonic plague [56]. This immunodominant epitope is also present in Y. pseudotuberculosis YopE, and confers protection against intragastric challenge of C57BL/6 mice with this enteric pathogen [57]. Primary infection of C57BL/6 mice with Y. pseudotuberculosis induces a surprisingly large YopE-specific CD8+ T cell response [58]. Importantly, a predominant population of these YopE-specific CD8+ T cells are positive for TNFα and IFNγ, indicating they are effector cells [58]. Production of TNFα and IFNγ by YopE-specific CD8+ T cells is important for protection against Yersinia, while CTL activity mediated by perforin is dispensable [59]. Moreover, in addition to its role in innate immunity, host fibrin is important for protection by T cells against Y. pestis [60]. It appears that fibrin cooperates with neutrophils to enhance the protective activity of T cells, demonstrating that multiple layers of immune defenses are important for resistance to plague. In contrast to the protective role of antigen-specific CD8+ T cells and CD4+ T cells, the Y. pseudotuberculosis superantigen (SAg) YPM drives expansion of a CD4+ T cell population that produces hepatotoxic molecules [61], demonstrating that unregulated adaptive responses can cause immunopathology.
Concluding remarks
While recent studies have provided remarkable insight on the interactions between pathogenic yersiniae and the host immune system, much is still unknown. Specific molecular events governing bacterial dissemination, subversion of immune defenses by bacterial effectors and subsequent recognition and protection mediated by innate and adaptive immunity are all open questions. The emergence of high-resolution imaging techniques and availability of knockout mice, coupled with the ease of genetic manipulation of Yersinia, enable researchers to shed light on these key fields of investigation. Further defining the mechanisms that these pathogens utilize to thwart host defenses remains an essential part of bacterial pathogenesis research and can lead to novel therapeutic strategies against infectious diseases.
Highlights.
Cell surface components mediate resistance of Yersinia to extracellular immune factors.
Interaction between Yersinia and host cells promotes dissemination and drives effector translocation.
Yersinia effectors, Pla protease and chromosomally-encoded gene products disrupt signaling pathways and modulate inflammatory environments.
Structural components of Yersinia and biochemical activities of effectors trigger protective host responses.
Innate and adaptive immune responses restrict Yersinia replication.
Acknowledgments
This work was supported by National Institutes of Health awards R01AI099222 to JB and T32AI007539 and F31AI118220 to LC, and by a Grant-In-Aid of Research award G201503151030819 from Sigma Xi, The Scientific Research Society, to LC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Literature of interest, published recently, are indicated as:
• of special interest
•• of outstanding interest
- 1.Wren BW. The yersiniae--a model genus to study the rapid evolution of bacterial pathogens. Nat Rev Microbiol. 2003;1:55–64. doi: 10.1038/nrmicro730. [DOI] [PubMed] [Google Scholar]
- 2.Revell PA, Miller VL. Yersinia virulence: more than a plasmid. FEMS Microbiol Lett. 2001;205:159–164. doi: 10.1111/j.1574-6968.2001.tb10941.x. [DOI] [PubMed] [Google Scholar]
- 3.Carniel E. Plasmids and pathogenicity islands of Yersinia. Curr Top Microbiol Immunol. 2002;264:89–108. [PubMed] [Google Scholar]
- 4.Heesemann J, Sing A, Trulzsch K. Yersinia’s stratagem: targeting innate and adaptive immune defense. Curr Opin Microbiol. 2006;9:55–61. doi: 10.1016/j.mib.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Perry RD, Fetherston JD. Yersiniabactin iron uptake: mechanisms and role in Yersinia pestis pathogenesis. Microbes Infect. 2011;13:808–817. doi: 10.1016/j.micinf.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelis GR. The Yersinia Ysc-Yop ‘type III’ weaponry. Nat Rev Mol Cell Biol. 2002;3:742–752. doi: 10.1038/nrm932. [DOI] [PubMed] [Google Scholar]
- 7.Leo JC, Skurnik M. Adhesins of human pathogens from the genus Yersinia. Adv Exp Med Biol. 2011;715:1–15. doi: 10.1007/978-94-007-0940-9_1. [DOI] [PubMed] [Google Scholar]
- 8.Viboud GI, Bliska JB. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol. 2005;59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- 9.Bliska JB, Wang X, Viboud GI, Brodsky IE. Modulation of innate immune responses by Yersinia type III secretion system translocators and effectors. Cell Microbiol. 2013;15:1622–1631. doi: 10.1111/cmi.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin S, Brodsky IE. The inflammasome: Learning from bacterial evasion strategies. Semin Immunol. 2015;27:102–110. doi: 10.1016/j.smim.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Plano GV, Schesser K. The Yersinia pestis type III secretion system: expression, assembly and role in the evasion of host defenses. Immunol Res. 2013;57:237–245. doi: 10.1007/s12026-013-8454-3. [DOI] [PubMed] [Google Scholar]
- 12.Trosky JE, Liverman AD, Orth K. Yersinia outer proteins: Yops. Cell Microbiol. 2008;10:557–565. doi: 10.1111/j.1462-5822.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 13.Caulfield AJ, Lathem WW. Substrates of the plasminogen activator protease of Yersinia pestis. Adv Exp Med Biol. 2012;954:253–260. doi: 10.1007/978-1-4614-3561-7_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 15.Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343:227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho DK, Skurnik M, Blom AM, Meri S. Yersinia pestis Ail recruitment of C4b-binding protein leads to factor I-mediated inactivation of covalently and noncovalently bound C4b. Eur J Immunol. 2014;44:742–751. doi: 10.1002/eji.201343552. [DOI] [PubMed] [Google Scholar]
- 18.Yang K, Park CG, Cheong C, Bulgheresi S, Zhang S, Zhang P, He Y, Jiang L, Huang H, Ding H, et al. Host Langerin (CD207) is a receptor for Yersinia pestis phagocytosis and promotes dissemination. Immunol Cell Biol. 2015 doi: 10.1038/icb.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shannon JG, Hasenkrug AM, Dorward DW, Nair V, Carmody AB, Hinnebusch BJ. Yersinia pestis subverts the dermal neutrophil response in a mouse model of bubonic plague. MBio. 2013;4:e00170–00113. doi: 10.1128/mBio.00170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Shannon JG, Bosio CF, Hinnebusch BJ. Dermal neutrophil, macrophage and dendritic cell responses to Yersinia pestis transmitted by fleas. PLoS Pathog. 2015;11:e1004734. doi: 10.1371/journal.ppat.1004734. This work uses state-of-the-art intravital imaging of infected mice to characterize for the first time the cellular immune responses following delivery of Y. pestis by its flea vector. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pechous RD, Sivaraman V, Price PA, Stasulli NM, Goldman WE. Early host cell targets of Yersinia pestis during primary pneumonic plague. PLoS Pathog. 2013;9:e1003679. doi: 10.1371/journal.ppat.1003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinner JL, Winfree S, Starr T, Shannon JG, Nair V, Steele-Mortimer O, Hinnebusch BJ. Yersinia pestis survival and replication within human neutrophil phagosomes and uptake of infected neutrophils by macrophages. J Leukoc Biol. 2014;95:389–398. doi: 10.1189/jlb.1112551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillenius E, Urban CF. The adhesive protein invasin of Yersinia pseudotuberculosis induces neutrophil extracellular traps via beta1 integrins. Microbes Infect. 2015;17:327–336. doi: 10.1016/j.micinf.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Maldonado-Arocho FJ, Green C, Fisher ML, Paczosa MK, Mecsas J. Adhesins and host serum factors drive Yop translocation by yersinia into professional phagocytes during animal infection. PLoS Pathog. 2013;9:e1003415. doi: 10.1371/journal.ppat.1003415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paczosa MK, Fisher ML, Maldonado-Arocho FJ, Mecsas J. Yersinia pseudotuberculosis uses Ail and YadA to circumvent neutrophils by directing Yop translocation during lung infection. Cell Microbiol. 2014;16:247–268. doi: 10.1111/cmi.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merritt PM, Nero T, Bohman L, Felek S, Krukonis ES, Marketon MM. Yersinia pestis targets neutrophils via complement receptor 3. Cell Microbiol. 2015;17:666–687. doi: 10.1111/cmi.12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolters M, Boyle EC, Lardong K, Trulzsch K, Steffen A, Rottner K, Ruckdeschel K, Aepfelbacher M. Cytotoxic necrotizing factor-Y boosts Yersinia effector translocation by activating Rac protein. J Biol Chem. 2013;288:23543–23553. doi: 10.1074/jbc.M112.448662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweer J, Kulkarni D, Kochut A, Pezoldt J, Pisano F, Pils MC, Genth H, Huehn J, Dersch P. The cytotoxic necrotizing factor of Yersinia pseudotuberculosis (CNFY) enhances inflammation and Yop delivery during infection by activation of Rho GTPases. PLoS Pathog. 2013;9:e1003746. doi: 10.1371/journal.ppat.1003746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams W, Morgan J, Kwuan L, Auerbuch V. Yersinia pseudotuberculosis YopD mutants that genetically separate effector protein translocation from host membrane disruption. Mol Microbiol. 2015;96:764–778. doi: 10.1111/mmi.12970. [DOI] [PubMed] [Google Scholar]
- 30.Solomon R, Zhang W, McCrann G, Bliska JB, Viboud GI. Random mutagenesis identifies a C-terminal region of YopD important for Yersinia type III secretion function. PLoS One. 2015;10:e0120471. doi: 10.1371/journal.pone.0120471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa TR, Amer AA, Farag SI, Wolf-Watz H, Fallman M, Fahlgren A, Edgren T, Francis MS. Type III secretion translocon assemblies that attenuate Yersinia virulence. Cell Microbiol. 2013;15:1088–1110. doi: 10.1111/cmi.12100. [DOI] [PubMed] [Google Scholar]
- 32.Rolan HG, Durand EA, Mecsas J. Identifying Yersinia YopH-targeted signal transduction pathways that impair neutrophil responses during in vivo murine infection. Cell Host Microbe. 2013;14:306–317. doi: 10.1016/j.chom.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westermark L, Fahlgren A, Fallman M. Yersinia pseudotuberculosis efficiently escapes polymorphonuclear neutrophils during early infection. Infect Immun. 2014;82:1181–1191. doi: 10.1128/IAI.01634-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ke Y, Tan Y, Wei N, Yang F, Yang H, Cao S, Wang X, Wang J, Han Y, Bi Y, et al. Yersinia protein kinase A phosphorylates vasodilator-stimulated phosphoprotein to modify the host cytoskeleton. Cell Microbiol. 2015;17:473–485. doi: 10.1111/cmi.12378. [DOI] [PubMed] [Google Scholar]
- 35.Lee WL, Grimes JM, Robinson RC. Yersinia effector YopO uses actin as bait to phosphorylate proteins that regulate actin polymerization. Nat Struct Mol Biol. 2015;22:248–255. doi: 10.1038/nsmb.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 37.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thinwa J, Segovia JA, Bose S, Dube PH. Integrin-mediated first signal for inflammasome activation in intestinal epithelial cells. J Immunol. 2014;193:1373–1382. doi: 10.4049/jimmunol.1400145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LaRock CN, Cookson BT. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe. 2012;12:799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Chung LK, Philip NH, Schmidt VA, Koller A, Strowig T, Flavell RA, Brodsky IE, Bliska JB. IQGAP1 is important for activation of caspase-1 in macrophages and is targeted by Yersinia pestis type III effector YopM. MBio. 2014;5:e01402–01414. doi: 10.1128/mBio.01402-14. The authors demonstrate that distinct isoforms of YopM can inhibit activation of caspase-1 in LPS-primed murine macrophages and identify IQGAP1 as a novel host target important for inflammasome modulation by YopM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vagima Y, Zauberman A, Levy Y, Gur D, Tidhar A, Aftalion M, Shafferman A, Mamroud E. Circumventing Y. pestis Virulence by Early Recruitment of Neutrophils to the Lungs during Pneumonic Plague. PLoS Pathog. 2015;11:e1004893. doi: 10.1371/journal.ppat.1004893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bi Y, Zhou J, Yang H, Wang X, Zhang X, Wang Q, Wu X, Han Y, Song Y, Tan Y, et al. IL-17A produced by neutrophils protects against pneumonic plague through orchestrating IFN-gamma-activated macrophage programming. J Immunol. 2014;192:704–713. doi: 10.4049/jimmunol.1301687. [DOI] [PubMed] [Google Scholar]
- 43.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 44.Caulfield AJ, Walker ME, Gielda LM, Lathem WW. The Pla protease of Yersinia pestis degrades fas ligand to manipulate host cell death and inflammation. Cell Host Microbe. 2014;15:424–434. doi: 10.1016/j.chom.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Zimbler DL, Schroeder JA, Eddy JL, Lathem WW. Early emergence of Yersinia pestis as a severe respiratory pathogen. Nat Commun. 2015;6:7487. doi: 10.1038/ncomms8487. This study reports the unexpected finding that a Pla isoform encoded by ancestral Y. pestis strains is able to promote pneumonic but not bubonic plague in mouse model, suggesting that the ability to cause the pulmonary form of the disease evolved first. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eddy JL, Gielda LM, Caulfield AJ, Rangel SM, Lathem WW. Production of outer membrane vesicles by the plague pathogen Yersinia pestis. PLoS One. 2014;9:e107002. doi: 10.1371/journal.pone.0107002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sivaraman V, Pechous RD, Stasulli NM, Miao EA, Goldman WE. Yersinia pestis activates both IL-1beta and IL-1 receptor antagonist to modulate lung inflammation during pneumonic plague. PLoS Pathog. 2015;11:e1004688. doi: 10.1371/journal.ppat.1004688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pechous RD, Broberg CA, Stasulli NM, Miller VL, Goldman WE. In vivo transcriptional profiling of Yersinia pestis reveals a novel bacterial mediator of pulmonary inflammation. MBio. 2015;6:e02302–02314. doi: 10.1128/mBio.02302-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jessen DL, Osei-Owusu P, Toosky M, Roughead W, Bradley DS, Nilles ML. Type III secretion needle proteins induce cell signaling and cytokine secretion via Toll-like receptors. Infect Immun. 2014;82:2300–2309. doi: 10.1128/IAI.01705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osei-Owusu P, Jessen Condry DL, Toosky M, Roughead W, Bradley DS, Nilles ML. The N terminus of type III secretion needle protein YscF from Yersinia pestis functions to modulate innate immune responses. Infect Immun. 2015;83:1507–1522. doi: 10.1128/IAI.02687-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zwack EE, Snyder AG, Wynosky-Dolfi MA, Ruthel G, Philip NH, Marketon MM, Francis MS, Bliska JB, Brodsky IE. Inflammasome activation in response to the Yersinia type III secretion system requires hyperinjection of translocon proteins YopB and YopD. MBio. 2015;6:e02095–02014. doi: 10.1128/mBio.02095-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stuart LM, Paquette N, Boyer L. Effector-triggered versus pattern-triggered immunity: how animals sense pathogens. Nat Rev Immunol. 2013;13:199–206. doi: 10.1038/nri3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky-Dolfi MA, Zwack EE, Hu B, Fitzgerald L, Mauldin EA, Copenhaver AM, et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc Natl Acad Sci U S A. 2014;111:7385–7390. doi: 10.1073/pnas.1403252111. These two papers characterize the mechanism by which YopJ activates caspase-1 in naïve macrophages, and go on to demonstrate that this “effector-triggered immune response” is protective against Yersinia infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK, Kelliher MA, et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A. 2014;111:7391–7396. doi: 10.1073/pnas.1403477111. These two papers characterize the mechanism by which YopJ activates caspase-1 in naïve macrophages, and go on to demonstrate that this “effector-triggered immune response” is protective against Yersinia infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Parashar K, Sitaram A, Bliska JB. The GAP activity of type III effector YopE triggers killing of Yersinia in macrophages. PLoS Pathog. 2014;10:e1004346. doi: 10.1371/journal.ppat.1004346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin JS, Szaba FM, Kummer LW, Chromy BA, Smiley ST. Yersinia pestis YopE contains a dominant CD8 T cell epitope that confers protection in a mouse model of pneumonic plague. J Immunol. 2011;187:897–904. doi: 10.4049/jimmunol.1100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y, Mena P, Romanov G, Lin JS, Smiley ST, Bliska JB. A protective epitope in type III effector YopE is a major CD8 T cell antigen during primary infection with Yersinia pseudotuberculosis. Infect Immun. 2012;80:206–214. doi: 10.1128/IAI.05971-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Zhang Y, Mena P, Romanov G, Bliska JB. Effector CD8+ T cells are generated in response to an immunodominant epitope in type III effector YopE during primary Yersinia pseudotuberculosis infection. Infect Immun. 2014;82:3033–3044. doi: 10.1128/IAI.01687-14. The authors demonstrate that the unusually large-scale YopE-specific CD8+ T cell response during primary infection of C57BL/6 mice with Y. pseudotuberculosis is associated with the production of effector T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Szaba FM, Kummer LW, Duso DK, Koroleva EP, Tumanov AV, Cooper AM, Bliska JB, Smiley ST, Lin JS. TNFalpha and IFNgamma but not perforin are critical for CD8 T cell-mediated protection against pulmonary Yersinia pestis infection. PLoS Pathog. 2014;10:e1004142. doi: 10.1371/journal.ppat.1004142. The authors demonstrate that YopE-specific CD8+ T cells protect against Yersinia infection by the production of TNFα and IFNγ, providing insight into how this arm of the adaptive immune system can counteract a largely extracellular pathogen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luo D, Lin JS, Parent MA, Mullarky-Kanevsky I, Szaba FM, Kummer LW, Duso DK, Tighe M, Hill J, Gruber A, et al. Fibrin facilitates both innate and T cell-mediated defense against Yersinia pestis. J Immunol. 2013;190:4149–4161. doi: 10.4049/jimmunol.1203253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goubard A, Loiez C, Abe J, Fichel C, Herwegh S, Faveeuw C, Porte R, Cayet D, Sebbane F, Penet S, et al. Superantigenic Yersinia pseudotuberculosis induces the expression of granzymes and perforin by CD4+ T cells. Infect Immun. 2015;83:2053–2064. doi: 10.1128/IAI.02339-14. [DOI] [PMC free article] [PubMed] [Google Scholar]