Bone marrow stromal cells (BMSC) in acute myeloid leukaemia (AML) contribute to extrinsic drug resistance, generally attributed to cell–cell contact or secreted cytokines (Jacamo et al, 2014). However, a recent report indicates that stromal protection may also occur via other soluble factors (Yang et al, 2014). Extracellular vesicles, such as exosomes, traffic protein and RNA between cells (Valadi et al, 2007). Stromal exosomes were recently shown to confer a proliferative advantage to multiple myeloma cells via transfer of microRNA (miRNA) (Roccaro et al, 2013). The functional impact of stromal exosomes in AML has not been studied. We therefore hypothesized that AML stroma release exosomes that protect leukaemia cells and traffic a distinct subset of miRNA and cytokines.
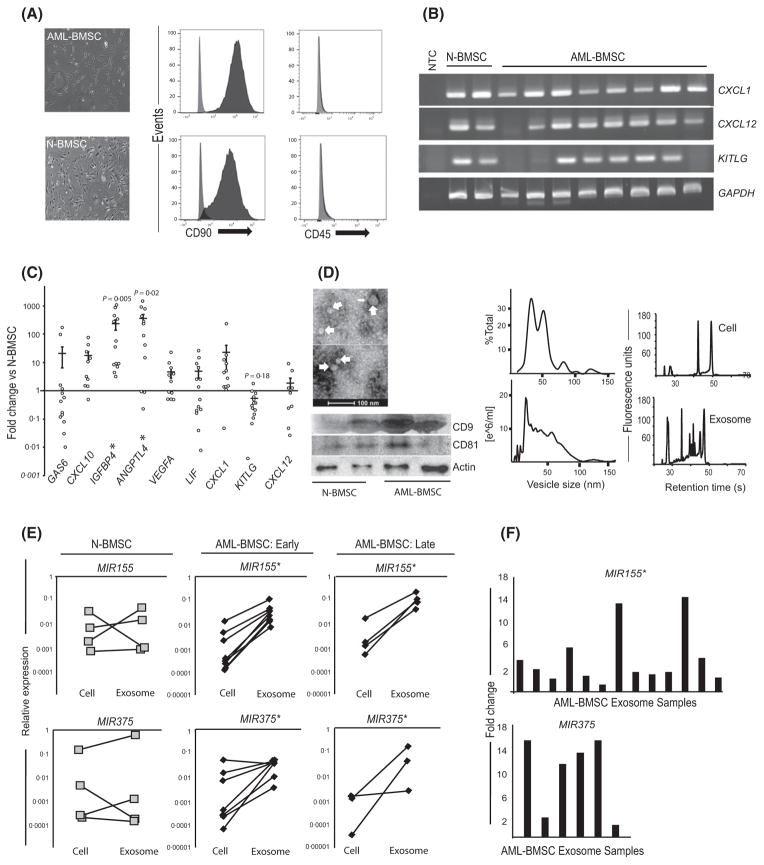
We isolated stromal cells from the marrow aspirates of 20 patients (AML-BMSC; see Table SI for patient characteristics) and five healthy controls (N-BMSC) according to a previously published protocol (Tyner et al, 2013). The cells were fibroblastic, adherent and expressed CD90, but not CD45 epitopes (Fig 1A). Reverse transcription polymerase chain reaction (RT-PCR) confirmed the expression of canonical stromal transcripts in both populations (Fig 1B) (Boxall & Jones, 2012). However, quantitative analysis revealed altered expression in AML-BMSC for CXCL12, KITLG and CXCL1, as well as for genes previously reported in modified stroma in myelodysplastic syndrome (IGFBP4, ANGPTL4) (Fig 1C) (Medyouf et al, 2014). The contribution of stromal-derived exosomes has been established in other malignancies (Roccaro et al, 2013). We isolated vesicles using ultracentrifugation and then used vesicle tracking analysis, electron microscopy and Western blotting to demonstrate a vesicle population conforming in size, morphology and tetraspanin membrane proteins to exosomes (Fig 1D) (Valadi et al, 2007).
Fig 1.
AML-BMSCs have altered gene expression profiles and release exosomes that are enriched in select mi-RNA. (A) Light micrograph and immunophenotype of primary AML-BMSCs and N-BMSCs showing adherent, fibroblastic morphology and positivity for CD90 and negativity for CD45 epitopes. (B) mRNA expression of stromal transcripts C-X-C Chemokine Ligands CXCL12, CXCL1 and KITLG (SCF). (C) Quantitative expression by polymerase chain reaction of select gene transcripts from AML-BMSCs (n = 13) normalized to N-BMSCs (n = 4, n = 3 for CXCL12). Expression is calculated relative to GAPDH, p-values reflect mean differences between N-BMSCs and AML-BMSCs for the genes shown. (D) Electron micrograph of 30–100 nm sized, cup-shaped vesicles (top left) showing the presence of the exosome markers CD9 and CD81 from both populations (bottom left). Vesicle size and concentration, by percentage of 100 vesicles measured from transmission electron microscope (middle top) and by nanovesicle tracking analysis (middle bottom) from AML-BMSC and N-BMSC. Bioanalyser electropherogram of RNA from stromal cells and their exosomes (right top and bottom), revealing enrichment of small RNA in stromal exosomes. (E) Graphs display the relative abundance of MIR155 and MIR375 in parent stromal cells versus the exosomes released during the same passage number, expressed relative to snRNA U6. Each cell-exosome pair is derived from a separate patient. ‘Early passage’ cells were used from their second to fourth passage, and ‘late-passage’ cells were used in their 10–12th passage; *P < 0·05 by Student’s t-test for mean differences between parent cells and their exosomes. (F) Each vertical bar represents fold increases of MIR155 (n = 13) or MIR375 (n = 6) 6from different primary AML-BMSC exosomes, normalized to the mean of 3 N-BMSC exosome isolates. Sample size differences between miRNA targets reflect senescence in samples between the timing of the two experiments; *P < 0·05 by Student’s t-test for mean differences in expression. AML-BMSC, bone marrow stromal cells from acute myeloid leukaemia patients; N-BMSC, normal bone marrow stromal cells; NTC, non-template control.
Exosome biogenesis allows for selective incorporation of different RNA species (Valadi et al, 2007). We determined the spectrum of BMSC exosome RNA using a Bioanalyser (Agilent, Santa Clara, CA, USA) and observed a relatively greater abundance of small RNAs compared with the parent cell (Fig 1D). Increased levels of MIR155 and MIR375 can independently identify AML patients at high risk for recurrence, with MIR155 contributing to the pathogenesis of several other haematological malignancies (Marcucci et al, 2013; Wang et al, 2013). Reasoning that stromal cell reprogramming in AML leads to unique exosomal miRNA incorporation, we used quantitative RT-PCR to compare the MIR155 and MIR375 levels in stromal cells from 12 patients (4 normal, 8 AML) and the exosomes released during the culture period. Strikingly, all eight AML-BMSC samples showed a statistically significant fold increase in incorporation of MIR155 and MIR375 in the exosomes relative to their parent cells, while no such increase was observed for N-BMSCs (Fig 1E). The comparative enrichment in AML-BMSC exosomes persisted even after 10 passages in tissue culture devoid of leukaemia cells, suggesting a durable change in stromal cell biology (Fig 1E). We then directly compared miRNA content between exosome isolates, independent of the parent cell content, and found MIR155, but not MIR375, was consistently elevated in AML-BMSC exosomes (Fig 1F). Taken together, the data indicate that exosomes released from AML-BMSCs are selectively enriched for miRNA that signify disease risk status in AML.
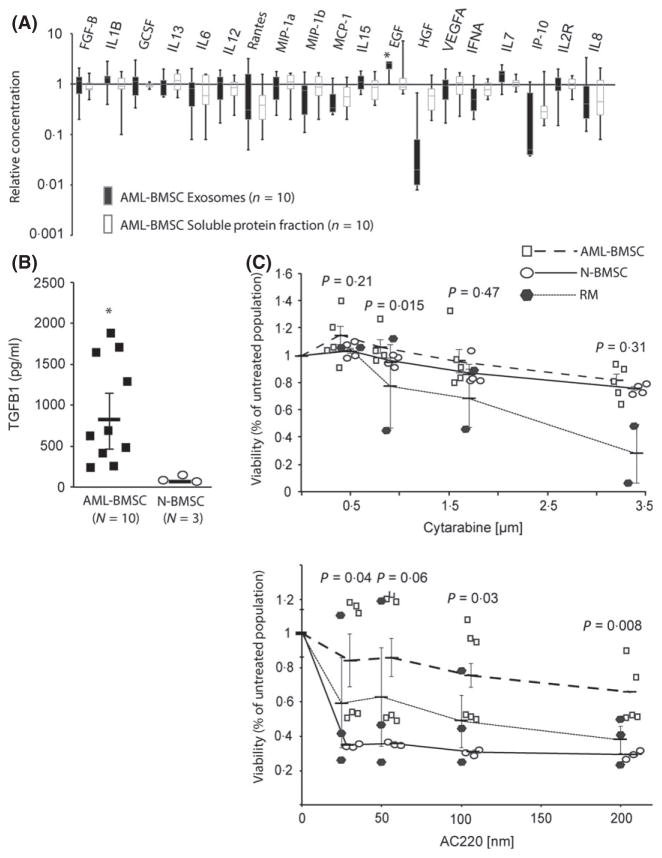
Cytokines and growth factors contribute to leukaemia niche function (Yang et al, 2014). We reasoned that exosome- associated and directly secreted cytokine alterations from AML-BMSCs exist. In a multiplex screening approach using bead-based technology (Luminex, Austin, TX, USA) and enzyme-linked immuno-sorbent-assay (ELISA) kits (Life Technologies, Carlsbad, CA, USA), we studied the concentration of cytokines in the exosomes and in the vesicle-depleted soluble protein fraction of conditioned media from AML-BMSCs (n = 10) and N-BMSCs (n = 3). Exosomal cytokine concentrations ranged from 1·6 pg/ml (B-FGF) to >10 000 pg/ml [interleukin (IL) 8], while other cytokines were undetected in exosomes or in the soluble protein fraction from BMSCs (IL2, IL5, IL17A, TNF, IFNG). We found statistically significant enrichment of epidermal growth factor (P < 0·05), as well as a relative depletion of hepatocyte growth factor (P = 0·08) in exosomes from AML-BMSC, changes that were not observed in the soluble protein fraction when compared to N-BMSCs (Fig 2A). Concentrations of other cytokines, whether exosome-associated or freely secreted, did not differ between the two populations. We separately analysed TGFB1, given its correlation with treatment response in AML (Hong et al, 2014). We found TGFB1 at concentrations ranging from 200 to 2000 pg/ml in exosomes from 10/10 AML-BMSC samples, but below the level of assay detection in exosomes from N-BMSCs (Fig 2B). Taken together, our results suggest that stromal cells in AML modify their exosome-associated cytokine concentrations in a manner independent from the directly secreted fraction.
Fig 2.
Exosomes from AML-BMSCs have altered cytokine levels and confer chemo-protection to AML cells: (A) Box-and-Whisker plot of a multiplex protein assay displaying the relative concentration of 19 cytokines in exosomes and in the vesicle-depleted soluble protein fraction of media conditioned from BMSCs of 10 AML patients, normalized to three healthy patients. All results obtained in biological triplicate; vertical bars represent the upper and lower quartile values for each cytokine, with the bisecting gray line reflecting the median value. Error bars reflect standard error of the mean; *P < 0·05 by Student’s t-test. (B) Enzyme-linked immunosorbent assay for TGFB1 showing high concentrations in AMLBMSC exosomes (n = 10), but undetectable in three separate N-BMSC exosome preparations. Horizontal bars represent mean [TGFB1], error bars reflect standard error of the mean; *P < 0·05 by Student’s t-test. (C) Protection of MOLM-14 cells from cytarabine or AC220 under BMSC exosome exposure, relative to exosome-free media (RM). Data was obtained in two (cytarabine) or three (AC220) separate experiments for each drug. Each individual symbol represents duplicate (cytarabine) or triplicate (AC220) data from stromal exosome samples of different patients: for cytarabine (n = 5 AML-BMSC, n = 4 N-BMSC) and for AC220 (n = 6 AML-BMSC, n = 3 N-BMSC). Horizontal bars represent mean viability of MOLM-14 cells for each exosome exposure, error bars reflect standard error of the mean. Student’s t-test P-values reflect the mean difference in viability between AML-BMSC and N-BMSC exosome exposure at each concentration. Protection of MOLM-14 cells is expressed at each drug concentration as the percentage of the non-drug treated population for that exosome exposure. AML, acute myeloid leukaemia; BMSC, bone marrow stromal cells.
Our findings, along with existing reports on general stromal protection in AML, led us to hypothesize that stromal exosomes alter chemo-resistance in AML cells. Representing a standard component of AML therapy, we treated MOLM-14 FLT3 internal tandem duplication (FLT3-ITD+) AML cells with the nucleoside analogue cytarabine after exposure to exosomes from AML-BMSC, N-BMSC or control media (Fig 2C). The data provide the first evidence, to our knowledge, that exosomes from both AML patients and controls (Total n = 10, 6 AML-BMSC, 4 N-BMSC) protect AML cells from cytarabine. Further, when AML cells were treated with the FLT3 inhibitor AC220 after exposure, only AML-BMSC exosomes significantly protected AML cells (n = 6), while N-BMSC exosomes provided no such protective effect (n = 3) (Fig 2C).
Our data in aggregate suggest that stromal cells in AML patients undergo modification that includes alterations in function as well as the protein and RNA present in the exosomes they release. We report a first demonstration that stromal cells in AML release exosomes enriched for known clinical risk factors, including TGFB1, MIR155 and MIR375. Finally, our data add stromal exosome trafficking as a candidate mechanism for extrinsic chemo-resistance within the niche in AML, with differential protection against kinase pathway inhibition observed only by AML-BMSC exosomes. Such unique protection could occur, for example, by exosomal miRNA-directed down-regulation of promoters of apoptosis or cell differentiation, thereby releasing the leukaemia cell from kinase pathway dependence. Future studies in this area may uncover new mediators of such resistance.
Supplementary Material
Acknowledgments
SV, PK, ET, NH and JH designed the experiments. SV and AA performed the experiments. SV analysed all data and wrote the manuscript. PK, JT and BJ provided essential resources. We are indebted to Dr. Oleh Taratula for assistance with nanoparticle tracking analysis. This project was supported in part by CCSG Knight Cancer Institute, P30CA069533, Druker, PI.
Footnotes
Competing interests
The authors have no competing interests to disclose.
Additional Supporting Information may be found in the online version of this article:
Table SI. AML patient characteristics.
References
- Boxall SA, Jones E. Markers for characterization of bone marrow multipotential stromal cells. Stem Cells International. 2012;2012:975871. doi: 10.1155/2012/975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CS, Muller L, Whiteside TL, Boyiadzis M. Plasma exosomes as markers of therapeutic response in patients with acute myeloid leukemia. Frontiers in Immunology. 2014;5:160. doi: 10.3389/fimmu.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacamo R, Chen Y, Wang Z, Ma W, Zhang M, Spaeth EL, Wang Y, Battula VL, Mak PY, Schallmoser K, Ruvolo P, Schober WD, Shpall EJ, Nguyen MH, Strunk D, Bueso-Ramos CE, Konoplev S, Davis RE, Konopleva M, Andreeff M. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-kappaB mediates chemoresistance. Blood. 2014;123:2691–2702. doi: 10.1182/blood-2013-06-511527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucci G, Maharry KS, Metzeler KH, Volinia S, Wu YZ, Mrozek K, Nicolet D, Kohlschmidt J, Whitman SP, Mendler JH, Schwind S, Becker H, Eisfeld AK, Carroll AJ, Powell BL, Kolitz JE, Garzon R, Caligiuri MA, Stone RM, Bloomfield CD. Clinical role of microRNAs in cytogenetically normal acute myeloid leukemia: miR-155 upregulation independently identifies high-risk patients. Journal of Clinical Oncology. 2013;31:2086–2093. doi: 10.1200/JCO.2012.45.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medyouf H, Mossner M, Jann JC, Nolte F, Raffel S, Herrmann C, Lier A, Eisen C, Nowak V, Zens B, Mudder K, Klein C, Oblander J, Fey S, Vogler J, Fabarius A, Riedl E, Roehl H, Kohlmann A, Staller M, Haferlach C, Muller N, John T, Platzbecker U, Metzgeroth G, Hofmann WK, Trumpp A, Nowak D. Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell. 2014;14:824–837. doi: 10.1016/j.stem.2014.02.014. [DOI] [PubMed] [Google Scholar]
- Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, Anderson KC, Scadden DT, Ghobrial IM. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. The Journal of Clinical Investigation. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner JW, Yang WF, Bankhead A, 3rd, Fan G, Fletcher LB, Bryant J, Glover JM, Chang BH, Spurgeon SE, Fleming WH, Kovacsovics T, Gotlib JR, Oh ST, Deininger MW, Zwaan CM, Den Boer ML, van den Heuvel-Eibrink MM, O’Hare T, Druker BJ, Loriaux MM. Kinase pathway dependence in primary human leukemias determined by rapid inhibitor screening. Cancer Research. 2013;73:285–296. doi: 10.1158/0008-5472.CAN-12-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hong Z, Gao F, Feng W. Upregulation of microRNA-375 is associated with poor prognosis in pediatric acute myeloid leukemia. Molecular and Cellular Biochemistry. 2013;383:59–65. doi: 10.1007/s11010-013-1754-z. [DOI] [PubMed] [Google Scholar]
- Yang X, Sexauer A, Levis M. Bone marrow stroma-mediated resistance to FLT3 inhibitors in FLT3-ITD AML is mediated by persistent activation of extracellular regulated kinase. British Journal of Haematology. 2014;164:61–72. doi: 10.1111/bjh.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.