Abstract
We often encounter setbacks while pursuing our goals. Success requires that we cope with these negative outcomes and choose to persist in spite of them. For example, learners may be more likely to continue a course after failing an assessment if they control their emotional reactions to the setback and study harder. However, the ability to effectively cope with the negative emotion inherent in such setbacks can be compromised by acute stress present in daily life (e.g., struggles in the household), which can subsequently lead to problems with persisting with a goal. The present study examined whether increasing the perception of control over setbacks (e.g., belief that a setback was caused by a correctable mistake rather than uncontrollable factors) can guard against the influence of a prior acute stressor on reactions to setbacks. Participants underwent a socially-evaluated cold water stress or a non-stress control procedure. Afterwards, they performed a behavioral task designed to measure persistence through controllable and uncontrollable setbacks. We observed that exposure to an acute stressor led to a detrimental effect on decision making by decreasing persistence behavior. Importantly, we also observed that the perception of control protected against the effect of preexisting stress and helped promote persistence. That is, stress impaired persistence through uncontrollable setbacks, but the impairment was alleviated by presenting setbacks as controllable. The findings demonstrate a potential avenue for improving the maintenance of goals aimed at behavior change, which can be susceptible to effects of stress.
Keywords: negative affect, failure, perseverance, aversive, adversity
Success often depends on the ability to cope with inevitable negative outcomes, or setbacks, and to persist through the adversity. Unfortunately, we sometimes encounter setbacks while already affected by a prior, often unrelated, stressor. For example, an online learner might fail an assessment and face an immediate decision to persist with a learning goal or quit, and the learner may already be under preexisting stress from an unrelated problem at home when this decision occurs. Stress can pose an obstacle to goal pursuit, correlating with critical failures of persistence such as dropping out of difficult academic programs (Beasley & Fischer, 2012) or failing to maintain a goal of behavioral change, such as drug abuse abstinence (Sinha, 2007). However, the causal effect of preexisting stress on persistence behavior is poorly understood. The goal of the present study was to examine how preexisting acute stress influences emotional reactions to setbacks and decisions to persist in spite of such setbacks. A second goal was to understand how the perception of a setback can guard against potentially detrimental effects of stress.
Stress affects biological and psychological factors that can be instrumental in coping with setbacks and pursuing a goal. The experience of an acute stressor causes a neuroendocrine response extended over time. Cortisol, a marker of the stress response, typically peaks 15 to 30 minutes after the stressor (Joëls & Baram, 2009; Schwabe, Haddad, & Schachinger, 2008). Importantly, this stress response can last over an hour following the termination of a stressor, and has the potential to influence how people respond to subsequent events that may be unrelated to the original stressor (Schwabe et al., 2008). For example, pre-exposure to acute stress increases avoidance of negative information and causes individuals to favor rigid stimulus-response associations instead of more flexible goal-directed decision-making strategies (Arnsten, 2015; Ellenbogen, Schwartzman, Stewart, & Walker, 2002; Maren & Holmes, 2015; Otto, Raio, Chiang, Phelps, & Daw, 2013; Petzold, Plessow, Goschke, & Kirschbaum, 2010; Porcelli & Delgado, 2009; Putman, Hermans, & van Honk, 2010; Schwabe & Wolf, 2009, 2013). Taken together, this research suggests that an individual who encounters a setback in a preexisting stressed state may be less able to regulate negative emotions and more likely to respond with a strategy aimed at avoiding stimuli associated with the setback (e.g., abandon a goal rather than persist through the setback).
The effects of stress can be pervasive, but what factors might counteract the extended detrimental effects of an acute stressor? Perceiving control over one’s outcomes has positive effects on emotion regulation, motivation, and learning (Andrews & Debus, 1978; Grolnick & Ryan, 1987; Hartley, Gorun, Reddan, Ramirez, & Phelps, 2013; Janssen, Spinhoven, & Arntz, 2004; Leotti, Iyengar, & Ochsner, 2010; Perry, Stupnisky, Hall, Chipperfield, & Weiner, 2010), and thus can influence the way that people respond to negative outcomes. For example, training students to focus on controllable determinants of academic outcomes (e.g., insufficient studying caused a failed exam rather than unfair questions) promotes improvement from initially poor performance (Perry et al., 2010). Thus, a second goal of this experiment was to probe whether increasing perceived control can guard against the impairing effect of stress on the ability to cope with setbacks. That is, preexisting stress may impair a student’s ability to persist through a failed exam, but perceiving control over the failure may reduce the influence of stress on persistence. Finally, if the perception of control mitigates effects of preexisting stress then people under stress may have an increased preference for controllable situations. That is, stressed individuals may prefer to make their own choices rather than have choices made for them. A third goal of the experiment was to examine effects of preexisting stress on preferences for controllable situations.
The present research examines the effect of a prior acute stressor on the ability to persist through controllable and uncontrollable setbacks. Participants first underwent an acute stress or non-stressful procedure (socially-evaluated cold-pressor test; Schwabe et al., 2008), then completed a Persistence-After-Setbacks task (PAS task) during the time period following the acute stressor when the cortisol response was expected to peak (15 to 30 minutes after the stressor). Following the PAS task, participants completed a behavioral assessment of their preference for free-choice compared to no-choice situations. We hypothesized that the perception of control would moderate the effect of preexisting stress on behavior in the PAS task. That is, we predicted that exposure to acute stress would increase setback-elicited negative affect and decrease decisions to persist with goals, but that this effect would be attenuated during trials where perceived control was emphasized. We further examined the effect of preexisting stress on preference for free-choice compared to no-choice situations, and whether this preference related to persistence through controllable versus uncontrollable setbacks.
Methods
Participants
Eighty-three Rutgers University undergraduates were recruited to participate in the study for course credit. Three participants failed to complete the study (2 chose to discontinue the cold water procedure before the minimum amount of time required, and 1 did not understand instructions for the first behavioral task). Thus, 80 participants were included in the analysis reported (57 female, age 18–52 years, M = 21.5, 95% CI = [20.28, 22.92]). The sample size of 40 participants in each group was predetermined to yield greater than 80% power to detect an effect of comparable size to previously demonstrated effects of acute stress on emotion regulation and decision-making (Otto et al., 2013; Porcelli & Delgado, 2009; Raio, Orederu, Palazzolo, Shurick, & Phelps, 2013). There were additional exclusions for specific analyses: 2 were excluded from cortisol analysis only (insufficient quantity of saliva for measurement) and 11 were excluded from skin conductance analysis (responses on less than a third of trials). Participants provided informed consent and were recruited through the university’s online experiment signup system. Procedures were approved by the institutional review board of Rutgers University.
Timeline of Experimental Procedures
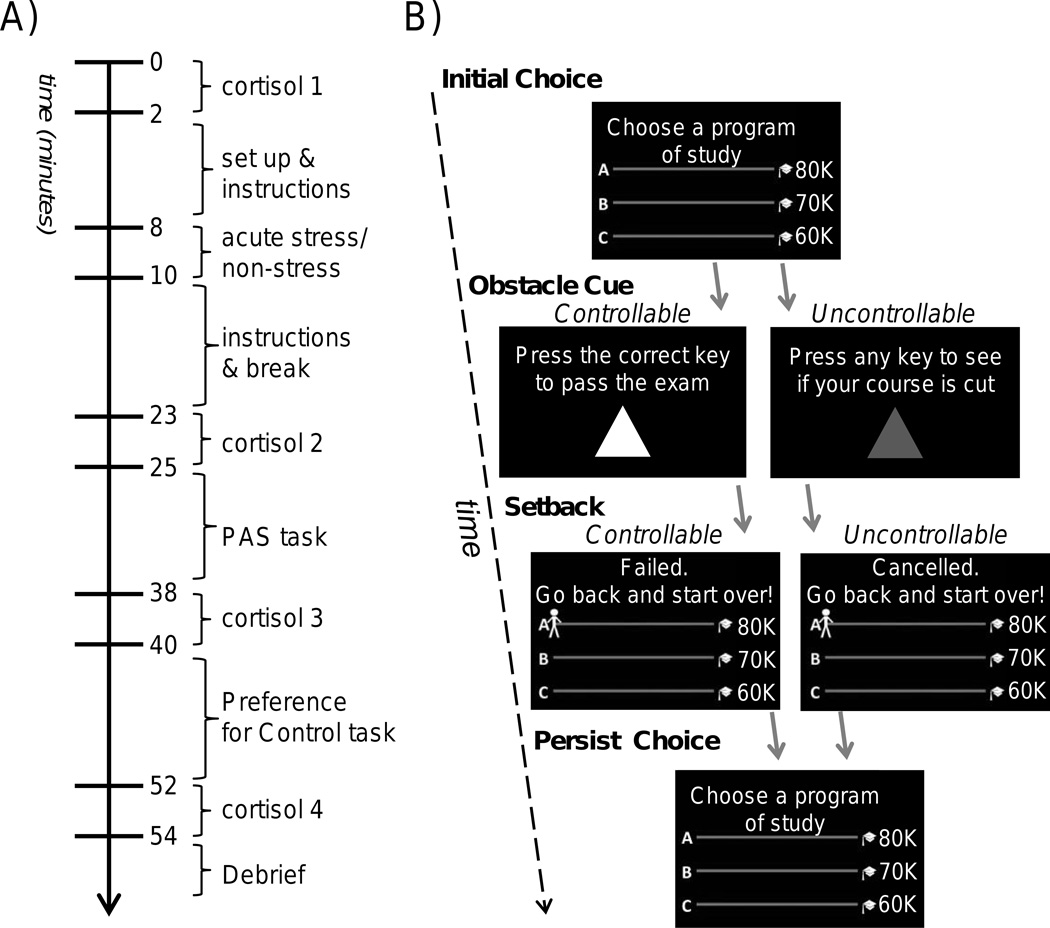
The experiment began with participants providing informed consent and receiving general instructions. Participants were then randomly assigned to the stress or non-stress control group. Next, the first salivary cortisol sample was taken less than 5 minutes after the participant’s arrival to the lab and all following events were timed in reference to this moment (Figure 1A). Participants then a) underwent the acute stress or non-stress procedure (Schwabe et al., 2008), b) completed questionnaires (Duckworth & Quinn, 2009), then performed two cognitive tasks during the time period of expected peak cortisol response to the acute stressor: c) the PAS task (Bhanji & Delgado, 2014) designed to measure persistence behavior after setbacks, and d) the Choice Preference task (Leotti & Delgado, 2011) designed to measure their preference for controllable situations. Salivary cortisol samples were taken at four time points (see Figure 1A).
Figure 1.
Experimental timeline and Persistence After Setbacks (PAS) task. A) Experiment timeline: Saliva samples were taken to measure cortisol at four time points during the study, labeled cort 1–4. B) Key components of a PAS task trial were the initial choice, obstacle cue, setback, and then the choice to persist with the initial choice or not. Initial Choice: Participants chose a path to pursue then advanced by encountering progress cues (“class meetings” not shown here). Obstacle Cue: Participants encountered controllable or uncontrollable obstacles on the chosen path. Setback: Participants avoided (not shown) or received setbacks, which sent them back to the beginning of the path. Persist Choice: Participants chose to persist or not persist with the originally chosen path. A blank screen with a fixation dot at the center appeared between each event for 4s or 6s, allowing for measurement of skin conductance responses to setbacks. A round ended if the participant reached the end of a path (three steps) or if the participant “ran out of time” (i.e., after a predetermined number of setbacks, which was unknown to participants).
Acute Stress Manipulation
Participants assigned to the stress group underwent a socially evaluated cold-pressor test (Schwabe et al., 2008) where they were videotaped by an experimenter wearing a white lab coat while they submerged their right hand in 2–3° C ice water for 2 minutes. The two minute duration of our procedure differed from the three minute duration typically used (Schwabe et al., 2008). The decision to use a shorter duration was based on pilot research (n = 15) showing more than 20% of participants could not keep their hand in the cold water past the two minute mark. Despite the shortened exposure duration, the procedure was effective (see Results). The experiment was discontinued if participants were unable to keep their hand submerged in the ice water for at least 30 seconds. The experimenter wore no lab coat for interactions with the control group participants, who submerged their right hand in lukewarm water for 2 minutes and were not videotaped during the procedure. Immediately following the cold stressor or control task, participants rated the subjective unpleasantness, stressfulness, and painfulness of the procedure on a scale from 0 to 100 (with 10-point intervals). The three ratings were averaged to provide a measure of the subjective stressfulness of the procedure.
Salivary Cortisol Measurements
Saliva samples were taken at baseline (3–5m after participant arrived), then at three additional time points (see Figure 1A) throughout the study to assess the rise and fall of cortisol over the experimental timeline. The experiment was run between 1pm and 4pm, and participants were asked to avoid eating or drinking (anything other than water) for 2 h prior to the beginning of the experiment. To acquire salivary cortisol data, participants placed a Salimetrics oral swab underneath the tongue for 2 min. The swab was then placed in an individual centrifuge tube and then frozen in cold storage at −10°C. Samples were then packed with dry ice and sent to Salimetrics Laboratory (State College, PA) for duplicate biochemical assay analysis. Cortisol levels (µG/dL) were log transformed to correct for positive skew and area under the curve with respect to increase (AUCI) was computed to summarize the increase and decrease relative to the baseline (first) sample over time (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). This measure removes information about the starting cortisol level that participants entered with, and differs from area under the curve with respect to ground (AUCG), which incorporates not just the rise from each participant’s starting point, but also the absolute height of the response. For the purpose of classifying participants as cortisol responders or non-responders in a binary manner, we computed the percent increase from baseline (first sample) to peak (maximum value of the samples) and the minimum threshold for cortisol responders was set at 15.47% as recommended by Miller and colleagues (2013). Cortisol data for 2 participants (1 stress group, 1 control group, both female) were unavailable due to insufficient samples. Analyses involving cortisol were conducted on the remaining 78 participants.
Skin Conductance Measurement
Skin conductance data were acquired throughout the experiment, allowing for measurement of arousal states during the two minute acute stressor/control procedure, as well as arousal responses specifically elicited by setbacks in the PAS task. A BIOPAC conductance module and AcqKnowledge software were used to collect and analyze data. Skin conductance levels (SCL) during the acute stressor/control procedure were computed as the average level of skin conductance (in microsiemens, µS) during the time that the participant’s hand was submerged in water. Skin conductance responses (SCR) specific to setbacks in the PAS task were computed using the Ledalab software toolkit using continuous decomposition analysis to compute the skin conductance response between 1s and 4s after each setback (Benedek & Kaernbach, 2010). Responses less than the minimum threshold of .01 µS in amplitude were entered as zero. SCR data were excluded from analysis if a participant failed to show supra-threshold SCRs on at least one third of trials in the PAS task (11 participants). SCL data from the stressor/control procedure were excluded from the same participants. Mean SCLs during the stressor/control procedure and SCRs for controllable and uncontrollable setbacks in the PAS task were computed for each participant and then log transformed to correct for positive skew.
PAS task
Participants performed a behavior assay of their tendency to persist with a goal through setbacks framed as controllable or uncontrollable. This task has been used in prior research examining neural responses associated with persistence through setbacks (Bhanji & Delgado, 2014). Participants were instructed they would play repeated rounds of an “academic degree decision game” in which they would choose a path (a “program of study”) and try to earn the points at the end of the path (a “diploma” with an assigned point value) by progressing through obstacles to reach the end of the path. To start each round, participants chose between three paths with a distinct point value at the end (80, 70, or 60 points; Figure 1B). Participants then encountered obstacles while taking steps toward the end of their chosen path. When participants encountered controllable obstacles (“midterm exams”), they pressed one of four buttons, knowing that only one was correct (and would “pass the exam”). The correct button remained the same for all controllable obstacles within a round, thus the correct “exam answer” could be learned by trial and error (Delgado, Jou, Ledoux, & Phelps, 2009). An incorrect response (“failed exam”) sent the participant back to the beginning of the path but a correct response avoided the setback and moved the participant one step toward the end of the chosen path. When participants encountered uncontrollable obstacles (“course cancellations”), participants pressed any button to see if their course was randomly selected to be cancelled. A cancelled course sent the participant to the beginning of the path, otherwise the setback was avoided and the participant moved forward one step. Thus, “failed exams” represented controllable setbacks because they were the result of a correctable action, whereas “cancelled courses” represented uncontrollable setbacks because they were due random chance rather than a participant’s action. Notably, setbacks impeded participants’ progress towards their chosen goal, but did not directly result in lost points, unlike negative outcomes in many decision-making paradigms.
Critically, after a controllable or uncontrollable setback participants were presented with a decision to persist with their previously chosen path or choose a different path. Persistence through controllable and uncontrollable setbacks was calculated as the percent of choices for the same path immediately after experiencing a setback on that path. This operationalization defines persistence as the continuance of a course of action to achieve a goal, despite difficulty (Andrews and Debus, 1978; Di Paula and Campbell, 2002). This interpretation of persistence does not depend on the value of the goal (e.g., a student might persist through difficulty to pursue a Ph.D. in mathematics despite more lucrative opportunities in finance), only whether a person chooses to continue to pursue the goal after a setback. Thus, the measure of persistence included instances where participants chose to persist with any path where they encountered the setback (i.e., lower or higher value paths). An alternative view of persistence might require that the current course of action be optimal for a choice to represent persistence. A supplementary analysis incorporated this alternative view of persistence and examined only setbacks where participants had initially chosen the highest value path.
Participants were instructed to try to earn as many points as possible, but did not receive monetary compensation. Participants were explicitly instructed that path difficulty was not necessarily related to point value. A round included either controllable or uncontrollable setbacks, but not both, and the path point values remained the same for the round (participants were instructed on these details). A round ended when the player reached the end of a path (three steps) or time ran out (after a pseudorandomly determined number of events). An end-of-round screen showed either “You graduated!” with the point value, or “You ran out of time before graduating.” To facilitate participants achieving goals, they were occasionally presented with a third cue signaling a “class meeting,” which allowed participants to move forward. Participants received 12 setbacks in each condition. The distribution of setbacks was predetermined to ensure that every participant had the same amount of trials and chances to persist in each condition. A post-experimental probe showed that no participants suspected that the setbacks were predetermined. After completing the task, participants rated their affective responses (valence: How negative or positive did you feel? intensity: How intense was your feeling?) to each type of setback on 5-point scales. The PAS task took on average 11.66 minutes (95% CI = [11.51, 11.80]) for participants to complete.
Choice Preference Task
Following the PAS task, we assessed participants’ preference for controllable situations by behaviorally measuring their preference for stimuli that led to free-choice versus no-choice gambles of equivalent value (Choice Preference task; Leotti & Delgado, 2011). This measure of control-seeking behavior was used to test whether preexisting stress caused participants to increase their preference for controllable situations. The task consisted of repeated trials, each made up of 3 steps. In step 1, participants chose between two stimuli on the screen: a black circle and a white circle. In step 2, depending on which stimulus was chosen in step 1, participants either (a) saw two stimuli on the screen (a dotted circle and a striped circle) and made a free choice between them, or (b) saw only one stimulus (a dotted or striped circle) available on the screen and faced a no choice (i.e., select the only available stimulus). One of the stimuli in step 1 always led to free-choice situations, and one of the stimuli always led to no-choice situations. In step 3, participants saw an outcome of 0, 1, or 5 points won. Importantly, all stimuli had equal expected value, thus, the free-choice stimulus led to an equal number of points as the no-choice stimulus. Participants were first given 75 practice trials and told to see what happens when they select different stimuli, and then completed 25 trials under instructions to earn as many points as possible. The number of practice trials allowed participants to experience outcomes associated with each stimulus before we measured choice preference. The number of times that participants chose the free choice stimulus in the last 25 trials was taken as the behavioral measure of preference for control.
Data Analysis
A 2 (stress group) × 2 (setback controllability) ANOVA was used to assess effects of stress and setback controllability on behavior in the PAS task (percentage of choices to persist and response time). Planned t-tests of the stress manipulation effect were used to characterize significant interactions. Tests were two-tailed except where noted for one-tailed tests of the directional hypothesis of acute stress decreasing persistence behavior. Participant gender was included as a covariate in all analyses to control for gender differences in stress responses. Gender did not exhibit a significant effect in any analyses (all p > .10). Bootstrap bias corrected accelerated 95% confidence intervals (CI) were computed for means in the text (DiCiccio & Efron, 1996).
Results
Acute Stress Increases Physiological and Subjective Markers of Stress
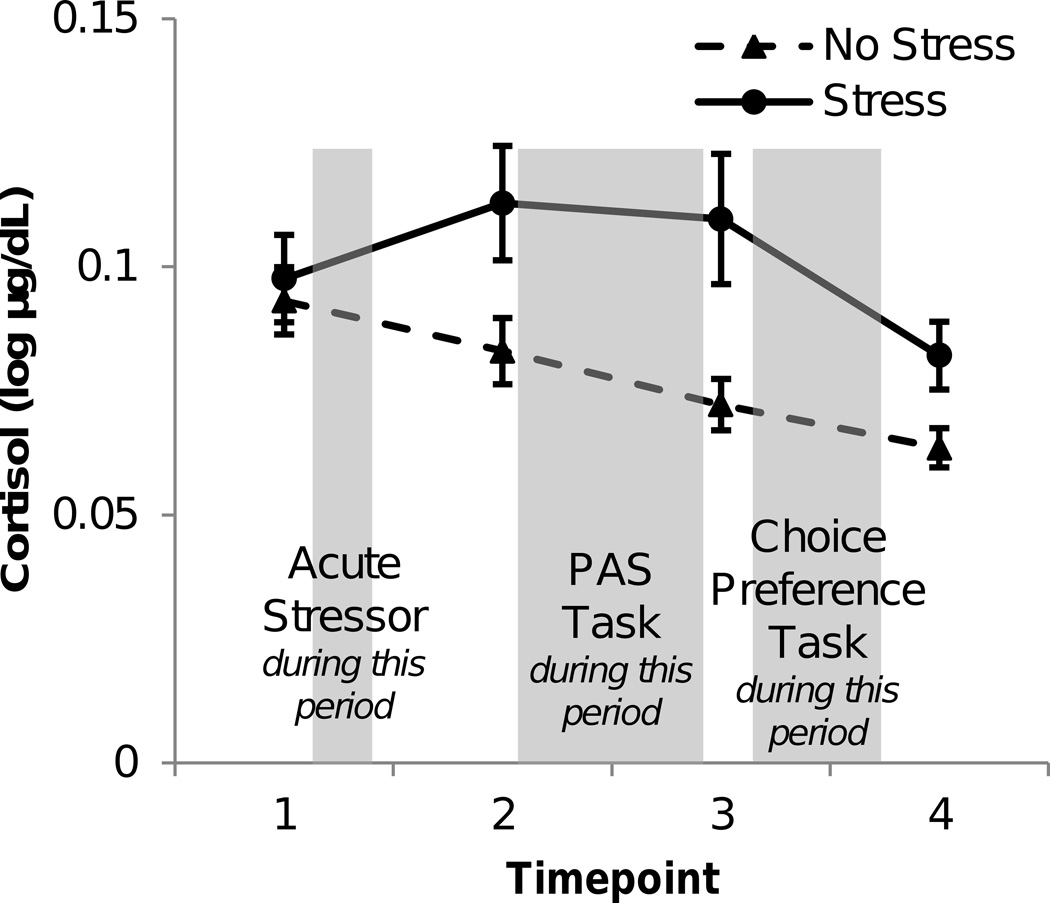
The socially evaluated cold-pressor test successfully elicited an extended elevation in salivary cortisol and an immediate increase in skin conductance levels and subjective ratings of stress. Area under the curve with respect to increase (AUCI) was computed to characterize the rise and fall of cortisol over time in relation to the baseline cortisol measure at the first time point. As predicted, cortisol AUCI was increased in the stress group compared to the non-stressed group (t(75) = 2.92, p = .005, d = .672; see Figure 2). The effectiveness of the stress procedure was further validated by two additional measures. First, mean skin conductance levels were elevated during the socially evaluated cold-pressor test (M = .88 log10µS, 95% CI = [.82, .94]) compared to the warm water control (M = .75 log10µS, 95% CI = [.69, .81]; t(77) = 2.85, p = .006, d = .707). Second, subjective stress ratings were higher after the socially evaluated cold-pressor test (M = 61.67, 95% CI = [53.51, 70.25]) compared to the warm water control (M = 4.58, 95% CI = [2.75, 6.63]; t(77) = 12.24, p < .001, d = 3.314).
Figure 2.
Salivary cortisol levels during the experiment. Salivary cortisol increased in acutely stressed compared to non-stressed participants during the time period when participants completed the PAS and Choice Preference Tasks. Error bars indicate SEM across participants.
Acute Stress Impairs Persistence Through Uncontrollable Setbacks
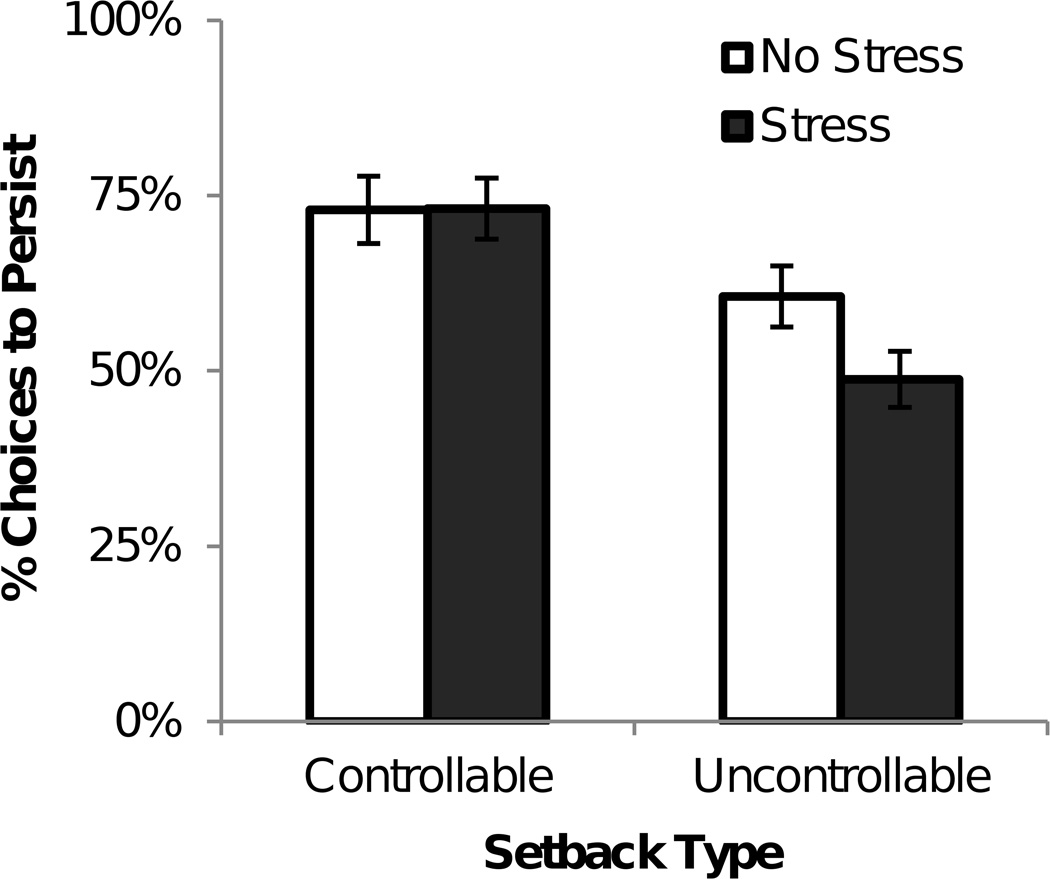
Pre-exposure to acute stress had a detrimental effect on persistence in the PAS task – an effect that was specific to trials with uncontrollable setbacks. That is, setback controllability moderated the effect of stress on persistence (stress group × setback controllability: F(1,77) = 4.06, p = .047; main effect of setback controllability: F(1,77) = 10.17, p = .002; stress group main effect was not significant: F(1,77) = 1.18, p > .250; see Figure 3). More specifically, pre-exposure to acute stress decreased persistence in the uncontrollable setback condition (stressed M = 48.75%, 95% CI = [39.17, 58.64]; non-stressed M = 60.63%, 95% CI = [51.46, 70.21]; t(77) = −1.81, p = .037, one-tailed, d = .410). In contrast, persistence did not differ between groups in the controllable setback condition (stressed M = 73.13%, 95% CI = [65.21, 80.42]; non-stressed M = 72.92%; 95% CI = [64.17, 81.04]; t(77) = .03, p > .250, one-tailed). The main effect of setback controllability was in accordance with prior research demonstrating a general beneficial effect of perceived control on persistence (Andrews & Debus, 1978; Bhanji & Delgado, 2014; Perry et al., 2010). However, a critical new finding was that perceived control demonstrated a protective effect against the detrimental effect of preexisting stress on persistence. That is, stress impaired persistence when setbacks were uncontrollable, but not when setbacks were perceived as controllable.
Figure 3.
Persistence as a function of preexisting stress and setback controllability. Prior acute stress impairs persistence through uncontrollable setbacks, but spares persistence through controllable setbacks. Error bars indicate SEM across participants.
Pre-exposure to stress did not significantly influence response times for the decisions to persist (stress group main effect and interaction with setback controllability Fs < 1, p > .250). A main effect of setback controllability (F(1,77) = 4.33, p = .041), however, showed that participants took longer to make persistence decisions following uncontrollable (M = 1132 ms, 95% CI = [1026, 1245]) compared to controllable setbacks (M = 1043 ms, 95% CI = [943, 1148]; d = .270). While decisions after uncontrollable setbacks took longer, the actual responses that participants made to try to avoid the controllable obstacles (M = 1509ms, 95% CI = [1378, 1639]) took longer than for uncontrollable obstacles (M = 1317ms, 95% CI = [1211, 1416]; main effect of setback controllability F(1,77) = 12.09, p = .001, d = .471).
Stress Influences Affective Responses to Setbacks
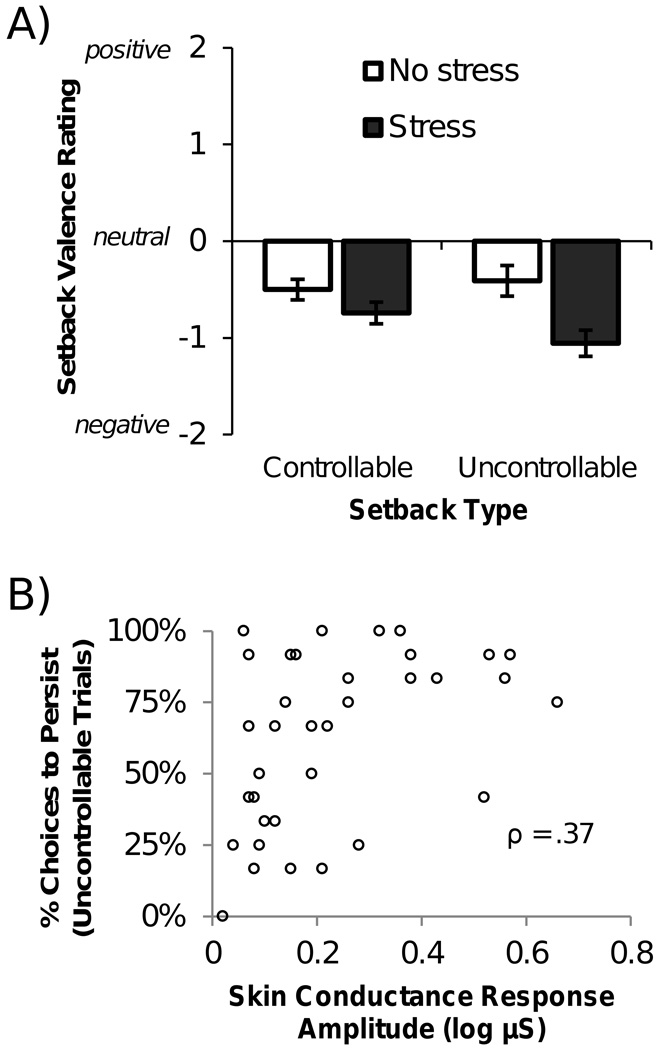
Affective responses in the PAS task were measured by self-report ratings of setback experiences made immediately after the task and by online measurements of SCRs to setbacks in the task. Analyses of affective responses were restricted to those participants who exhibited SCRs to at least one-third of setbacks (see Methods). Valence ratings of setbacks were influenced by a main effect of stress group (F(1,66) = 7.14, p = .010), and notably, an interaction of stress group and setback controllability (F(1,66) = 4.32, p = .042; Figure 4A). Intensity ratings were not significantly influenced by the manipulations (Fs < 1, p > .250). Planned comparisons showed that stressed participants rated the experience of uncontrollable setbacks more negatively (M = −1.06, 95% CI = [−.79, −1.34]) than non-stressed participants (M = −.41, 95% CI = [−.12, −.69], t(66) = −2.95, p = .004, d = .747) but did not differ significantly for controllable setbacks (stressed: M = −.74, 95% CI = [−.53, −.95]; non-stressed: M = −.50, 95% CI = [−.34, −.69]; t(66) = −1.32, p = .193, d = .382).
Figure 4.
Affective and physiological responses to setbacks. A) Participants reported how negative/positive they felt when they experienced setbacks in the PAS task. The chart shows these valence ratings as a function of preexisting stress and setback controllability. Error bars indicate SEM across participants. B) Skin conductance responses were recorded immediately following each setback in the PAS task. In the no stress group, increased SCRs to uncontrollable setbacks correlate with greater persistence.
Physiological responses were also assessed via SCRs to uncontrollable and controllable setbacks. SCRs were not influenced by the stress or setback controllability manipulations (main effects of stress group, controllability, and interaction: F < 1, p > .250). SCRs to uncontrollable setbacks were marginally correlated with the valence ratings of uncontrollable setbacks (indicating larger SCRs related to ratings that were less negative: Spearman’s ρ = .23, 95% CI = [−.03, .41], p = .058), but there was no significant relation to intensity ratings (ρ = −.20, 95% CI = [−.38, .01], p = .108). SCRs to controllable setbacks were not significantly related to negative valence (ρ = −.14, 95% CI = [−.33, .06], p > .250) or intensity ratings (ρ = −.16, 95% CI = [−.35, .04], p = .190). However, SCRs to uncontrollable setbacks correlated positively with persistence (ρ = .24, 95% CI = [.04, .41], p = .050), which is consistent with prior research demonstrating that greater affective responses to uncontrollable setbacks predict greater persistence (Bhanji & Delgado, 2014). But how is this relation influenced by preexisting stress?
We hypothesized that preexisting stress would disrupt the relation between affective reactions and persistence, due to a stress-induced impairment of the ability to adaptively interpret negative affect (Raio et al., 2013). Therefore, we examined the relation between uncontrollable setback SCRs and persistence separately in stressed and non-stressed participants. In the non-stressed group, SCRs to uncontrollable setbacks correlated with persistence (ρ = .37, 95% CI = [.09, .60], p = .032; Figure 4B), consistent with prior work with the PAS task (Bhanji & Delgado, 2014). Critically, this relation was not significant in the stress group (ρ = .01, 95% CI = [−.28, .30], p > .250). The difference in the strength of the relation between the groups did not reach significance (Fisher’s r-to-z (Cohen & Cohen, 1983; Preacher, 2002): z = 1.18, p > .250).
One potential explanation for this non-significant difference between groups is that preexisting stress disrupted the relation between SCRs and persistence only among participants who exhibited a physiological stress response to the socially evaluated cold-pressor test, rather than among all stress group participants. To examine this possibility, participants were grouped into cortisol responders (n = 23) and non-responders (n = 45) according to a baseline to peak increase criterion (Lewis, Porcelli, & Delgado, 2014; Miller, Plessow, Kirschbaum, & Stalder, 2013). This classification was made without regard to stress/non-stress group status (18 cortisol responders were from the stress group). SCRs correlated with persistence among cortisol non-responders (ρ = .40, 95% CI = [.17, .59], p = .006) but not among cortisol responders (ρ = −.19, 95% CI = [−.51, .18], p > .250). This correlation significantly differed between cortisol non-responders and responders (using Fisher’s r-to-z test for independent correlations with unequal sample sizes (Cohen & Cohen, 1983; Preacher, 2002): z = 2.27, p = .023). This exploratory finding suggests that, under normal (non-stressed) conditions, people experiencing setback-related arousal (to uncontrollable setbacks) are able to interpret those responses in a manner that leads to persistence, but a prior stressor can disrupt that ability. Taken together, these findings demonstrate that prior stress alters emotional responses to uncontrollable setbacks, and disrupts the relation between physiological arousal and persistence behavior.
Acute Stress Increases Preference for Control
An interesting possibility is that preexisting stress also increases individual preferences for controllable events themselves. We assessed individuals’ preference for controllable situations to examine this possibility and assess the association with persistence in the PAS task. Preference for control was behaviorally measured by a preference for choice task (Leotti & Delgado, 2011) administered shortly after the PAS task. The task measures behavioral preference for free-choice compared to no-choice prospects of equivalent expected value. Stress group participants showed a marginally significant increased preference for control compared to non-stressed participants (stress group M = 70.60% free-choice selections, 95% CI = [66.50, 74.96]; non-stress group M = 64.00%, 95% CI = [59.35, 68.56]; t(77) = 1.87, p = .065). This preference for control, however, was only marginally related to persistence through controllable setbacks (r = .20, p = .085) and not related to persistence through uncontrollable setbacks (r = .06, p = .58). The effect of preexisting stress on persistence through uncontrollable setbacks remained significant after controlling for participants’ preference for control (t(76) = −1.98, p = .026, one-tailed). These findings suggest that preexisting stress may increase preferences for controllable situations, but this effect is separable from its effect on persistence through uncontrollable setbacks.
Persistence-Related Self-Views Predict Persistence Among Non-Stressed Participants
An exploratory analysis examined the possibility that pre-exposure to stress might influence participants’ views of themselves as generally persistent, and whether these persistence-related self-views were related to behavior in the PAS task. Persistence-related self-views were measured with the 8-item Grit questionnaire (Duckworth & Quinn, 2009), which probes participants’ views concerning their tendency to persist with real-life goals (e.g., “setbacks don’t discourage me”). Under normal conditions, Grit is expected to be stable within an individual over time (Duckworth & Quinn, 2009), however, participants here completed the questionnaire after pre-exposure to stress or non-stress conditions. Consequently, we expected that pre-exposure to stress might decrease participants’ tendency to see themselves as persistent. Indeed, stress group participants (M = 3.38 on a scale from 1 to 5, 95% CI = [3.22, 3.52]) reported lower Grit compared to non-stressed participants (M = 3.63, 95% CI = [3.47, 3.79], t(77) = −2.46, p = .016). Interestingly, the non-stressed sample (but not the stress group) demonstrated a relation between higher Grit scores and persistence in the PAS task. That is, non-stressed participants reporting higher Grit were more persistent through uncontrollable setbacks (r = .35, 95% CI = [.09, .61], p = .03, partial correlation controlling for their persistence behavior in the controllable setback condition), but there was no significant association among stress group participants (r = −.19, 95% CI = [−.51, .17], p = .261). This association between Grit and persistence significantly differed between the non-stressed and stressed groups (using Fisher’s r-to-z (Cohen & Cohen, 1983; Preacher, 2002): z = 2.37, p = .018). This exploratory analysis suggests that preexisting stress decreases participants’ perceptions of themselves as persistent, and alters the association between self-perceived persistence and actual persistence behavior in the PAS task.
Discussion
Setbacks are in some cases inevitable during goal pursuit. To meet goals, it is critical that people cope and persist in spite of such setbacks. However, the ability to cope with negative affect can be impaired by a preexisting stressed state (Raio et al., 2013). The current study shows (a) that pre-exposure to acute stress decreases decisions to persist with a goal following a setback, and (b) this effect can be buffered by increased perception of control over setbacks.
The selective influence of stress in the current study prompts the question of how the decision to persist through an uncontrollable setback differs from a controllable setback. We suggest that controllable and uncontrollable setbacks pose distinct challenges for an individual. Perceiving a setback as controllable allows a person to actively cope with the setback and concomitant negative affect by correcting the action that led to the setback. Thus, people will persist to the extent to which they feel they have corrected the mistake. However, if setbacks are uncontrollable then there are no mistakes to correct, thus this avenue of active coping is unavailable. In this way, uncontrollable setbacks can place greater emphasis on flexibly interpreting the negative affective experience in a manner that allows persistence (e.g., interpret negative affect as temporary or likely to change) rather than something to be avoided (e.g., unchangeable).
This distinction between coping mechanisms has been referred to as active or problem-focused coping in contrast to emotion-focused coping (Folkman & Lazarus, 1988; LeDoux & Gorman, 2001; Troy, Shallcross, & Mauss, 2013). Consistent with the different demands that controllable and uncontrollable setbacks place on an individual, distinct brain regions appear involved in responding to these setbacks. Ventral striatum activity, typically linked to reinforcement and avoidance learning (Delgado, Li, Schiller, & Phelps, 2008; Schönberg, Daw, Joel, & O’Doherty, 2007; Tricomi & Fiez, 2008), relates to persistence through controllable setbacks regardless of negative affect. On the other hand, ventromedial prefrontal cortex activity, previously linked to emotion regulation (Roy, Shohamy, & Wager, 2012; Schiller & Delgado, 2010) and skin conductance responses (Nagai, Critchley, Featherstone, Trimble, & Dolan, 2004), mediates the relation between increased negative affect from uncontrollable setbacks and increased persistence (Bhanji & Delgado, 2014). One possibility consistent with the current findings is that a prior acute stressor can have extended effects on prefrontal activity, including ventromedial prefrontal cortex (Arnsten, 2015, Maren & Holmes, 2015), which is involved in adaptively appraising emotion (Roy, Shohamy, & Wager, 2012; Schiller & Delgado, 2010) and persisting through uncontrollable setbacks (Bhanji & Delgado, 2014).
The association between arousal responses to uncontrollable setbacks and persistence behavior is consistent with the interpretation that acute stress specifically impairs emotion-focused coping. Non-stressed participants showed a positive relation between arousal (SCR) elicited by the uncontrollable setbacks and persistence through those setbacks, but this association was disrupted by responses to a prior acute stressor. This finding suggests that adaptive responses do not always require the reduction of negative affect and arousal, although arousal can be maladaptive if too high (Yerkes & Dodson, 1908). Negative affect can be an indicator of motivation or value placed on a goal (Carver, 2004) and coping well may not always require directly reducing negative affect but instead formulating an adaptive interpretation. For example, the student who is more upset by an unjustly (due to uncontrollable factors) failed exam might be more persistent than a student who is not upset, because the negative affect indicates a greater motivation or value placed on completing the class. Negative affect may play a lesser role in persistence through controllable setbacks because the decision to persist may be driven by how much one has learned from the setback rather than how motivated they are by the setback. For uncontrollable setbacks, persistence relies more on the ability to flexibly interpret the negative affective experience.
The flexible interpretation of negative affective experiences likely involves activity in prefrontal cortex that may be deprioritized in the wake of the acute stressor (Arnsten, 2015; Bhanji & Delgado, 2014; Otto et al., 2013; Raio et al., 2013; Schwabe & Wolf, 2013). Accordingly, participants whose cortisol levels rose in response to the prior stressor showed no relation between arousal and persistence, suggesting that the acute stressor disrupted a positive relation between arousal and persistence. In other words, under effects of a prior acute stressor, arousal was no longer helpful in the PAS task. These findings highlight the importance of individual differences both in how people respond to a stressor (i.e., cortisol) and how people respond to a setback (i.e., arousal and behavior). While there is no measure of exactly how participants interpret their negative affect experience in the current study, we speculate that participants might interpret the experience as temporary and likely to change, allowing them to move on from the negative experience without necessarily altering the magnitude of arousal (Di Paula & Campbell, 2002; Weiner, 1985). This lack of connection between arousal (i.e., skin conductance) responses to setbacks during the task and negative affect (self-reported after the task) presents a further challenge to understanding the role of negative affect in persistence decisions. The increased uncontrollable setback-related negative affect in the stress group’s retrospective reports may indicate a difference in recalling and reflecting on negative affect as much as it may indicate a difference in the online experience of negative affect. Affective self-reports were not taken during the PAS task in the current study out of concern that requiring reflection on negative affect might influence the measure of behavioral persistence, which was a priority.
Persistence behavior through controllable setbacks was resistant to effects of the prior stressor. This finding follows from demonstrations that stress prioritizes more automatic, less resource-demanding modes of responding under stress (Arnsten, 2015; Otto et al., 2013; Raio et al., 2013; Schwabe & Wolf, 2009, 2013). Persistence decisions after controllable setbacks appeared to place less demand on resources compared to uncontrollable setbacks (i.e., shorter response time for persistence decisions following controllable setbacks). This demand was not a function of response rules to avoid the setbacks themselves (i.e., responses to avoid the controllable setbacks took longer than responses to uncontrollable setbacks). Instead, the longer response time for persistence decisions after uncontrollable setbacks may be explained by the different demands of coping with uncontrollable (emotion-focused: adaptive interpretation of negative affect) compared to controllable setbacks (problem-focused: assessing whether anything was learned from the setback). If the effect of preexisting stress is due to the greater resource demand of persistence decisions following uncontrollable setbacks, then institutions (e.g., academic) might seek to ameliorate effects of stress on persistence in two manners. First, institutions might give feedback emphasizing controllable interpretations of setbacks (e.g., corrective comments given with a failing grade). Second, institutions might try to train adaptive interpretations of negative affect so that they are more automatic and less resource demanding. There is evidence that this first strategy can be successful in the real world (Perry et al., 2010), but future research will be important to determine how emotion regulation training could influence persistence and vulnerability to preexisting stress.
An alternative explanation for the effect of prior acute stress on persistence is that stressed participants are more likely to see uncontrollable setbacks as something to avoid, perhaps because they are associated with greater subjective uncertainty. This possibility is consistent with the increased negative valence ratings of uncontrollable setbacks for stressed compared to non-stressed participants. Greater negative affect or uncertainty associated with uncontrollable setbacks may have caused stress group participants to devalue the path on which they experienced the setback, and explore alternative paths. However, previous work using a variant of the PAS task showed that decreasing the cost of exploration (i.e., increasing the relative value of alternative paths) did not influence persistence after uncontrollable setbacks (Bhanji & Delgado, 2014). This data is consistent with the interpretation that persistence in the task is a function of how participants cope with the setbacks rather than their tendency to explore alternatives. Nonetheless, acute stress may have an effect on variables not measured in the current study such as exploration or tolerance for uncertainty and these are important factors for further research. Indeed, acute stress can influence the perception of risk, and perceived risk likely plays a role in deciding whether to persist with a goal or try an alternative (Buckert, Schwieren, Kudielka, & Fiebach, 2014; Porcelli & Delgado, 2009). The uncertainty or ambiguity over how many more chances one might have before time ran out in the PAS task round also likely factored into risk perceptions. However, an important feature of the task was that the frequency of setbacks and the length of rounds was equal across the uncontrollable and controllable conditions, and the effect of preexisting stress was specific to persistence through uncontrollable setbacks.
The current study focuses on beneficial aspects of control but there are variables not considered here that may determine the benefits of perceived control and the benefits of persistence. Persistence with the highest value goal was advantageous in the PAS task because setbacks were equally likely on all paths. Furthermore, correcting setbacks in the controllable condition was as simple as eliminating one of four responses. In other situations, persistence may not be advantageous or controllable setbacks may require more complex adjustments (e.g., it may not be clear how to correct a mistake). The effect of acute stress on prefrontal function is not specific to interpretations of negative affect and might impair the ability to judge if persistence is advantageous or the ability to make complex behavioral corrections (Arnsten, 2015; Ellenbogen et al., 2002; Maren & Holmes, 2015; Otto et al., 2013; Petzold et al., 2010; Porcelli & Delgado, 2009; Putman et al., 2010; Schwabe & Wolf, 2009, 2013). An interesting direction for further research is to examine the effect of acute stress under conditions where persistence might be less advantageous or controllable setbacks require more complex adjustments.
Another effect of stress may be that it alters an individual’s interpretation of control over a setback (e.g., a stressed student focuses more on bad luck than study habits as causes of a failed exam). This possibility cannot be explored with the current paradigm because setbacks are unambiguously controllable or uncontrollable in the PAS task. Furthermore, the present study focused on acute stressors akin to temporary life stressors such as receiving a reprimand at work. A question for future work is whether more chronic types of stress impact persistence through setbacks similarly and whether increasing perceptions of control can be helpful.
An additional noteworthy issue is that participants in the current study did not receive monetary compensation based on their performance. Participants were instead instructed to try to win as many points as possible. Nonetheless, persistence in the non-stress group closely resembled behavior in similar task where participants received a monetary bonus based on their performance (Bhanji & Delgado, 2014; controllable setbacks 68.29% persistence, 95% CI = [60.98, 75.98]; uncontrollable setbacks 56.19% persistence, 95% CI = [48.18, 65.09]), as did participants’ selections of the free-choice stimulus in the preference for control measure (Leotti & Delgado, 2011; 64% preference for free-choice).
Persistence through setbacks is a complex phenomenon with multiple determining factors. The current study examines setbacks and persistence decisions over a short time scale. A real world comparison would be deciding to persist with an online course module immediately after failing an assessment. Importantly, persistence decisions can also occur over much longer time scales, such as a decision to persist with a career goal after a negative evaluation of job performance over a year. The current findings represent an important step towards understanding the factors that can influence our ability to cope with setbacks, setting up future investigations with respect to the relationship between setbacks and persistence across different time scales. Notably, the present study identifies a manner of coping with setbacks and persisting that is resistant to effects of prior stress. The significance of the findings lies in potential future applications to minimize detrimental effects of stress and to understand how stressful experiences can derail efforts to persist with life goals such as substance dependence recovery goals (Sinha, 2007) or other behavior change goals (Adam & Epel, 2007).
Supplementary Material
Acknowledgments
This research was supported by funding from the National Institute on Drug Abuse to M.R.D (DA027764) and a National Science Foundation SBE Postdoctoral Research Fellowship to J.P.B. (1305994). We thank Meg Speer for her help setting up the experimental procedures, Lauren Leotti for assistance with the choice preference task script, and Brent Hughes for helpful comments on the manuscript.
References
- Adam T, Epel E. Stress, eating and the reward system. Physiology & Behavior. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Andrews GR, Debus RL. Persistence and the causal perception of failure: Modifying cognitive attributions. Journal of Educational Psychology. 1978;70:154–166. [Google Scholar]
- Arnsten AFT. Stress weakens prefrontal networks?: molecular insults to higher cognition. Nature Neuroscience. 2015;18:1376–1385. doi: 10.1038/nn.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley MA, Fischer MJ. Why they leave: the impact of stereotype threat on the attrition of women and minorities from science, math and engineering majors. Social Psychology of Education. 2012;15:427–448. [Google Scholar]
- Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods. 2010;190:80–91. doi: 10.1016/j.jneumeth.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhanji JP, Delgado MR. Perceived control influences neural responses to setbacks and promotes persistence. Neuron. 2014;83:1369–1375. doi: 10.1016/j.neuron.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckert M, Schwieren C, Kudielka BM, Fiebach CJ. Acute stress affects risk taking but not ambiguity aversion. Frontiers in Neuroscience. 2014;8:1–11. doi: 10.3389/fnins.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS. Negative affects deriving from the behavioral approach system. Emotion. 2004;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- Delgado MR, Jou RL, Ledoux JE, Phelps EA. Avoiding negative outcomes: tracking the mechanisms of avoidance learning in humans during fear conditioning. Frontiers in Behavioral Neuroscience. 2009;3:1–9. doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:3787–3800. doi: 10.1098/rstb.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paula A, Campbell JD. Self-esteem and persistence in the face of failure. Journal of Personality and Social Psychology. 2002;83:711–724. doi: 10.1037//0022-3514.83.3.711. [DOI] [PubMed] [Google Scholar]
- DiCiccio T, Efron B. Bootstrap confidence intervals. Statistical Science. 1996;11:189–212. [Google Scholar]
- Duckworth AL, Quinn PD. Development and validation of the short grit scale (grit-s) Journal of Personality Assessment. 2009;91:166–174. doi: 10.1080/00223890802634290. [DOI] [PubMed] [Google Scholar]
- Ellenbogen MA, Schwartzman AE, Stewart J, Walker C-D. Stress and selective attention: the interplay of mood, cortisol levels, and emotional information processing. Psychophysiology. 2002;39:723–732. doi: 10.1111/1469-8986.3960723. [DOI] [PubMed] [Google Scholar]
- Folkman S, Lazarus RS. Coping as a mediator of emotion. Journal of Personality and Social Psychology. 1988;54:466–475. [PubMed] [Google Scholar]
- Grolnick WS, Ryan RM. Autonomy in children’s learning: an experimental and individual difference investigation. Journal of Personality and Social Psychology. 1987;52:890–898. doi: 10.1037//0022-3514.52.5.890. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Gorun A, Reddan MC, Ramirez F, Phelps EA. Stressor controllability modulates fear extinction in humans. Neurobiology of Learning and Memory. 2013;113:149–156. doi: 10.1016/j.nlm.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen SA, Spinhoven P, Arntz A. The effects of failing to control pain: An experimental investigation. Pain. 2004;107:227–233. doi: 10.1016/j.pain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. American Journal of Psychiatry. 2001;158:1953–1955. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- Leotti LA, Delgado MR. The inherent reward of choice. Psychological Science. 2011;22:1310–1318. doi: 10.1177/0956797611417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti LA, Iyengar SS, Ochsner KN. Born to choose: the origins and value of the need for control. Trends in Cognitive Sciences. 2010;14:457–463. doi: 10.1016/j.tics.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AH, Porcelli AJ, Delgado MR. The effects of acute stress exposure on striatal activity during Pavlovian conditioning with monetary gains and losses. Frontiers in Behavioral Neuroscience. 2014;8:1–11. doi: 10.3389/fnbeh.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R, Plessow F, Kirschbaum C, Stalder T. Classification criteria for distinguishing cortisol responders from nonresponders to psychosocial stress. Psychosomatic Medicine. 2013;75:832–840. doi: 10.1097/PSY.0000000000000002. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. NeuroImage. 2004;22:243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Otto AR, Raio CM, Chiang A, Phelps EA, Daw ND. Working-memory capacity protects model-based learning from stress. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20941–20946. doi: 10.1073/pnas.1312011110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RP, Stupnisky RH, Hall NC, Chipperfield JG, Weiner B. Bad starts and better finishes: Attributional retraining and initial performance in competitive achievement settings. Journal of Social and Clinical Psychology. 2010;29:668–700. [Google Scholar]
- Petzold A, Plessow F, Goschke T, Kirschbaum C. Stress reduces use of negative feedback in a feedback-based learning task. Behavioral Neuroscience. 2010;124:248–255. doi: 10.1037/a0018930. [DOI] [PubMed] [Google Scholar]
- Porcelli AJ, Delgado MR. Acute stress modulates risk taking in financial decision making. Psychological Science. 2009;20:278–283. doi: 10.1111/j.1467-9280.2009.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [Computer software] 2002 Retrieved from http://quantpsy.org. [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Putman P, Hermans EJ, van Honk J. Cortisol administration acutely reduces threat-selective spatial attention in healthy young men. Physiology and Behavior. 2010;99:294–300. doi: 10.1016/j.physbeh.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Raio CM, Orederu TA, Palazzolo L, Shurick AA, Phelps EA. Cognitive emotion regulation fails the stress test. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:15139–15144. doi: 10.1073/pnas.1305706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in Cognitive Sciences. 2012;16:147–156. doi: 10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends in Cognitive Sciences. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönberg T, Daw ND, Joel D, O’Doherty JP. Reinforcement learning signals in the human striatum distinguish learners from nonlearners during reward-based decision making. Journal of Neuroscience. 2007;27:12860–12867. doi: 10.1523/JNEUROSCI.2496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33:890–895. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress prompts habit behavior in humans. Journal of Neuroscience. 2009;29:7191–7198. doi: 10.1523/JNEUROSCI.0979-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Wolf OT. Stress and multiple memory systems: from “thinking” to “doing”. Trends in Cognitive Sciences. 2013;17:62–70. doi: 10.1016/j.tics.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Current Psychiatry Reports. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Tricomi E, Fiez JA. Feedback signals in the caudate reflect goal achievement on a declarative memory task. NeuroImage. 2008;41:1154–1167. doi: 10.1016/j.neuroimage.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy AS, Shallcross AJ, Mauss IB. A person-by-situation approach to emotion regulation: Cognitive reappraisal can either help or hurt, depending on the context. Psychological Science. 2013;24:2505–2514. doi: 10.1177/0956797613496434. [DOI] [PubMed] [Google Scholar]
- Weiner B. An attributional theory of achievement motivation and emotion. Psychological Review. 1985;92:548–573. [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative and Neurological Psychology. 1908;18:459–482. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.