Abstract
Objective
To determine the effects of Latanoprost on intraocular pressure (IOP) and pupil diameter (PD) in cats with inherited primary congenital glaucoma (PCG) and normal cats.
Animals Studied and Procedures
IOP and PD were measured in both eyes (OU) of 12 adult cats (6 normal, 6 PCG), 3 times per week for 3 weeks prior to; for 3 weeks during, and for 2 weeks following twice-daily treatment with 0.005% latanoprost to the right eye (OD) and vehicle to the left (control) eye (OS). IOP and PD were measured hourly, for 8h, 1 day prior to, and on the first and last days of treatment. Aqueous humor flow rate (AHF) was determined at baseline and at the end of the treatment phase in 6 normal cats.
Results
Mean IOP was significantly lower in treated vs. control eyes of PCG cats, for up to 8 h following a single latanoprost treatment and a maximal IOP reduction of 63% occurred in treated eyes at 3 h. Latanoprost acutely lowered IOP in cats with PCG, but this effect appeared to diminish over 3 weeks of treatment. AHF was modestly increased in the treated eyes of normal cats after 3 weeks of latanoprost treatment, though IOP was not significantly affected. Latanoprost caused miosis, with rebound mydriasis at 24 h post-treatment, in the treated eyes of all cats.
Conclusions
Further research is needed to determine the suitability and efficacy of latanoprost treatment for long-term IOP-lowering in cats with PCG or other forms of glaucoma.
Keywords: Glaucoma, Cat, Intraocular Pressure, Latanoprost, Pupil Diameter, Aqueous Humor Flow
Introduction
Glaucoma is a leading cause of blindness in humans and animals, including dogs and cats. In the feline population, it has been estimated that glaucoma may affect around 1% of cats older than 7 years of age.[1] In the human population, prevalence models estimated that as many as 60.5 million people were affected in 2010.[2] The disease is characterized by a degeneration of the optic nerve.[3] There are a number of risk factors associated with the development of glaucoma, however elevated intraocular pressure (IOP) appears to be the most predictive of, and the single most consistent underlying abnormality, related to glaucoma.[4] In many forms of glaucoma, aqueous humor outflow is impaired at the level of the trabecular meshwork, causing increased IOP.[5]
In cats, as in humans, glaucoma is typically insidious and gradually progressive.[6,7] In this species, glaucoma is generally associated with minimal clinical signs until the advanced stages of the disease, when pupillary dilation, mild conjunctival hyperemia, and subtle corneal edema may be seen.[1,6–9] Recently, a recessively inherited form of feline primary congenital glaucoma (PCG) has been identified and is being characterized in our laboratory as a valuable large-eyed model for glaucoma research. Although glaucoma is not rare in cats, there is currently a relative lack of published studies that address the efficacy of conventional IOP-lowering drugs in glaucomatous cats. Many glaucoma drugs approved for human use have been evaluated in normal cats but their efficacy in reducing IOP in glaucomatous cats has not been established. Topical cholinergic and adrenergic agents, such as the miotic pilocarpine, the non-selective beta blocker timolol and the alpha-2 agonist apraclonidine, have been investigated in normal cats but may be associated with local or systemic adverse effects.[10–12] Carbonic anhydrase inhibitors lower IOP in both normal and glaucomatous cats, but generally require application three times daily.[9,13–15]
Topical prostaglandin (PG) analogs, such as latanoprost, travoprost and bimatoprost, are among the most efficacious drugs currently prescribed to reduce IOP in humans, predominantly acting by increasing aqueous humor outflow via the uveoscleral route.[5,16–18] A number of studies have shown that PG analogs, including latanoprost, are effective in lowering IOP in other species, including normal and glaucomatous dogs,[19,20] normal horses,[21] and normal and glaucomatous monkeys.[22,23] However, in normal cats, latanoprost has not shown a statistically significant IOP-lowering effect.[20] The lack of efficacy of commercially available FP-prostanoid receptor-specific analogs, such as latanoprost and bimatoprost, has been attributed to species differences in prostanoid receptors in the ciliary body of the cat eye, which expresses EP and DP receptors rather than the FP receptors found in humans and monkeys.[24–28] Although latanoprost may have no effect on IOP in normal cats, the drug may have a more readily demonstrable, or different, effect in glaucomatous animals due to their different anterior segment anatomy and physiology. There have been anecdotal, unpublished reports of IOP reduction by topical PG analogs, including latanoprost, in cats with glaucoma. The purpose of this study was to determine the effect of topical 0.005% latanoprost on IOP, pupil diameter and aqueous flow in normal cats and cats with PCG.
Materials and Methods
Animals
Six normal female adult cats (Felis catus) (median age= 0.74yr, range=0.72–0.75yr) and six with PCG (median age= 1.5yr, range= 1.2–4.8yr) were housed under standard, controlled environmental conditions in a laboratory animal care facility. Animals were maintained under a consistent 12 h light: dark cycle (light phase, 6am–6pm). All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Madison and complied with the Association for Research in Vision and Ophthalmology Statement on the Use of Animals in Vision Research. Normal cats had not received any prior ocular medications; five of the six cats with PCG had not received any topical ocular medications in the four weeks prior to the study, and one of the six cats with PCG had been treated with a single drop of topical dorzolamide 19 days prior to the study. In all glaucomatous cats, prior ocular drugs were limited to topical dorzolamide 2% and tropicamide 0.5–1%. With the exception of fluorophotometry, procedures were conducted in conscious animals, lightly restrained in sternal recumbency or in a seated position.
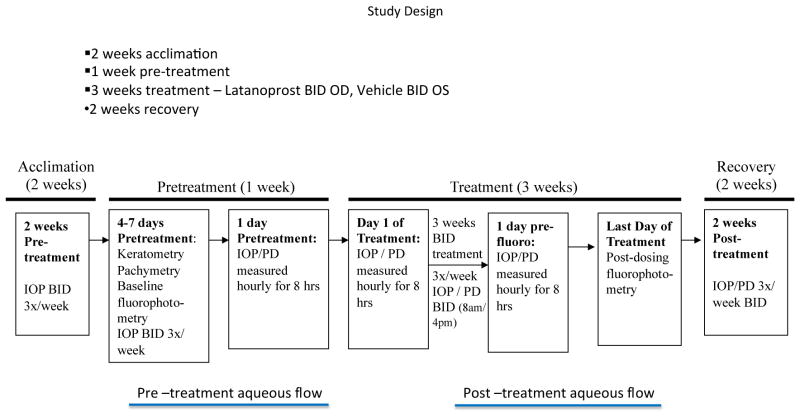
Study Design
The study was separated into three phases that were designated as “pre-treatment” (comprising a minimum of 2 weeks of acclimation to tonometry, followed by 1 week of collection of baseline data for analysis); “treatment” (3 weeks) and “recovery” (2 weeks). A schematic of the study design is shown in Figure 1.
Fig. 1.
Study Design
IOP and pupil diameter (PD) were measured in both eyes of 12 adult cats (6 normal, 6 PCG), 3 times per week for 3 weeks prior to, for 3 weeks during, and for 2 weeks after BID treatment with 0.005% latanoprost to the right eye (OD) and vehicle to the left (control) eye (OS). IOP and PD were measured hourly, for 8hrs, 1 day prior to, and on the first and last days of treatment. Aqueous humor flow rate was determined at baseline and at the end of the treatment phase in normal cats.
Evaluation of ocular irritation
To assess tolerability of topically applied latanoprost, each cat received a slit-lamp biomicroscopic examination by a board-certified veterinary ophthalmologist (GJM) one week prior to treatment, one week after initiation of treatment and one week following the last dose. Signs of ocular irritation were scored according to a modified Hackett-McDonald semi-quantitative scale.[29] The posterior segment was not evaluated during this study.
Measurement of Intraocular pressure and Pupil Diameter
Three weeks prior to treatment, each cat was acclimated to IOP measurements. Pre-treatment IOPs were obtained by a trained observer twice daily (8am and 4pm) for 3 days each week, using a TonoVet® rebound tonometer (Icare Finland Oy, Helsinki, Finland).[30] Intraocular pressure measurements at each time point represented the mean of triplicate TonoVet® readings, each of which was derived from multiple measurements with a standard deviation of ≤ 2.5 mmHg as determined by the instrument.
IOP and pupil diameter (PD) were measured in both eyes, for a total of 8 h, starting at 7am (i.e. immediately prior to the 7am treatment), then at 7:30am, 8am and then hourly until 3pm. IOP and PD were measured, as above, at baseline (one day prior to beginning treatment), on the first day of treatment, and again after 3 weeks of twice daily latanoprost treatment. Immediately following IOP measurements, maximum horizontal PD was measured in both eyes (OU) by a trained observer, using digital calipers (Fisher Science Education Traceable Digital Carbon Fiber Calipers, Fisher Scientific, Waltham, MA) held approximately 5mm from the corneal surface. All measurements were obtained under consistent illumination conditions (200–220 foot candles; Sekonic Handy Lumi model 246, Sekonic Co., Ltd, Japan) in the same room throughout each phase of the study.
During the three-week treatment phase, IOP and PD were measured 3 days a week, at least twice daily (8am and 4pm), as described above. Additional measurements were obtained at 7am and immediately prior to drug or vehicle administration during the final 2 weeks of the treatment phase in the normal cats, and for the entire 3-week treatment phase in PCG cats. During the recovery phase, IOP and PD were measured twice daily (8am and 4pm), 3 days per week for 2 weeks following the last dose of latanoprost.
Drug Administration
During the treatment phase, one drop (approximately 30μl) of 0.005% latanoprost (Xalatan, Pfizer, New York, NY) was applied topically twice daily (7am and 4pm) to the central cornea of the right eye (OD) for three weeks. An equivalent volume of vehicle, provided by the manufacturer, was administered to the left eye (OS). A frequency of twice daily treatment was chosen based on preliminary 8 hr IOP data obtained in cats and on a previous study in which IOP fluctuation was diminished by twice daily application of latanoprost in glaucomatous dogs relative to once daily application.[19]
Fluorophotometry
Baseline aqueous humor flow (AHF) was measured in both eyes of normal cats 4 – 7 days prior to initiating topical treatment with latanoprost. Measurement of AHF was also attempted in glaucomatous cats. Cats were anesthetized with ketamine hydrochloride (12.5 mg/kg, IM) and xylazine (0.5 mg/kg, IM), a drug combination that has no significant effect on IOP in cats.[31] Prior to fluorescein administration, background levels of fluorescence in the anterior chamber and cornea were measured non-invasively (Fluorotron Master, Ocumetrics, Mountain View, CA). In each subject, corneal curvature (Bausch & Lomb Keratometer), corneal thickness and anterior chamber depth were determined (Haag-Streit Optical Pachymeter attachments for Haag-Streit Slit Lamp, Koeniz, Switzerland), and maximal, horizontal limbus-to-limbus corneal diameter was measured using digital calipers. Six 10μL drops of a 5% fluorescein solution, (Akorn, Inc., Lake Forest, IL) were then applied to the axial corneal surface of both eyes. Drops were given 3 minutes apart, with the first drop administered at 0 min and the last administered at 15 min so that all drops were given over 15 min, using a positive displacement micropipette following administration of a single drop of 0.5% proparacaine hydrochloride (Bausch & Lomb, Inc., Tampa, FL) to enhance corneal penetration.[32,33]
Non-contact measurement of fluorescein concentrations in the anterior chamber was performed 12–18h after fluorescein administration. Cats were sedated with diazepam (0.1–0.2 mg/kg IV) and ketamine hydrochloride (2–5mg/kg, IV), as needed. This anesthetic regimen provided adequate short-term immobilization for the fluorophotometry scanning procedure. Prior to the first scan, the skin and fur of the forelimbs, paws and periocular region were gently washed to remove all traces of residual fluorescein. Fluorescein levels of 300–2000 ng/ml in the cornea were considered adequate to proceed with fluorophotometry and were achieved in all cats. Duplicate fluorophotometry scans were obtained every hour for 6 h. IOP was measured by rebound tonometry prior to, 3 h after the first scan, and after the last fluorophotometry scan. In normal cats, fluorophotometry was repeated following three weeks of latanoprost treatment, prior to the conclusion of the treatment phase. Keratometry, pachymetry and limbus-to-limbus corneal diameter measurements were repeated to ensure that no changes in anterior segment volumes had occurred.
Based on the rates of decline of aqueous humor fluorescein mass and dimensions of the corneal and anterior chamber compartments, aqueous humor flow was calculated mathematically, according to published methods.[34] Pre-treatment and post-treatment flow rates were then compared to establish overall change.
Data and Statistical Analysis
Within each group (normal and PCG), mean IOP and PD values at each time point, during each study phase, were compared to baseline values and values for treated eyes were compared to untreated control eyes, using repeated measures ANOVA (InStat v3.0; Graphpad Software, Inc., San Diego, CA, USA), with Tukey-Kramer Multiple Comparisons post-hoc testing where appropriate. Peak IOP and maximum fluctuation (difference between maximum and minimum IOP values) were also calculated for each week of the study in each individual cat. For all analyses, p≤0.05 was considered significant.
Results
Ocular Irritation
No significant adverse effects were identified during slit-lamp examination; only very transient signs of ocular irritation were observed immediately following topical latanoprost or vehicle application. No consistent effect on ocular irritation scores was observed due to treatment and all scores were ≤ 1, on a scale of 4 [29], in both the treated and control eyes during all phases of the study.
IOP and Pupil Diameter prior to Treatment
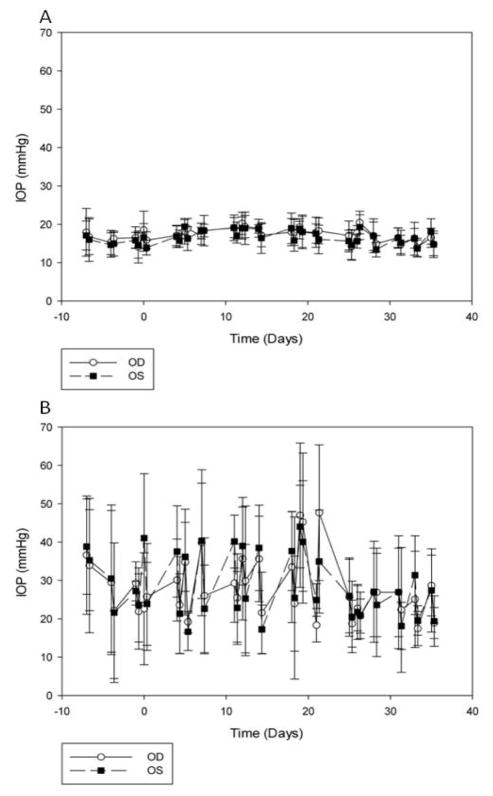
Mean IOP of acclimated glaucomatous cats over the one-week pre-treatment period was significantly higher than that of normal cats (29.2±15.2 mmHg OU vs. 15.3± 3.8 mmHg OU, respectively; p<0.001). Mean fluctuations in diurnal IOP within- and between-days were also significantly greater in cats with PCG than in normal cats (OD: 14.6±6.3 mmHg vs. 3.2±1.6 mmHg and OS: 12.5±4.5 mmHg vs. 3.0±1.9 mmHg, respectively; p≤0.0001) (Figs. 2A & B). During the pre-treatment phase of the study, one glaucomatous cat received “rescue” IOP-lowering treatment (2% Dorzolamide, Hi-Tech Pharmacal Co., Inc., Amityville, NY, USA) due to extreme elevation in IOP (>60 mmHg), however, no other ocular treatments were necessary or administered following this intervention.
Fig. 2.
Intraocular pressure (IOP) measurements in normal cats (A) and glaucomatous cats (B) throughout this study to determine effects of topical latanoprost (0.005%) on IOP. Day 0 signifies start of treatment phase, while day 21 signifies end of therapy and start of post-treatment phase. No consistent reduction in IOP was observed in treated, control or in treated vs. control eyes over time during the treatment phase in either normal (A) or glaucomatous (B) cats. Marked fluctuation in IOP was observed in glaucomatous cats throughout all phases of the study. Data are presented as mean IOP (mmHg), with error bars representing one standard deviation.
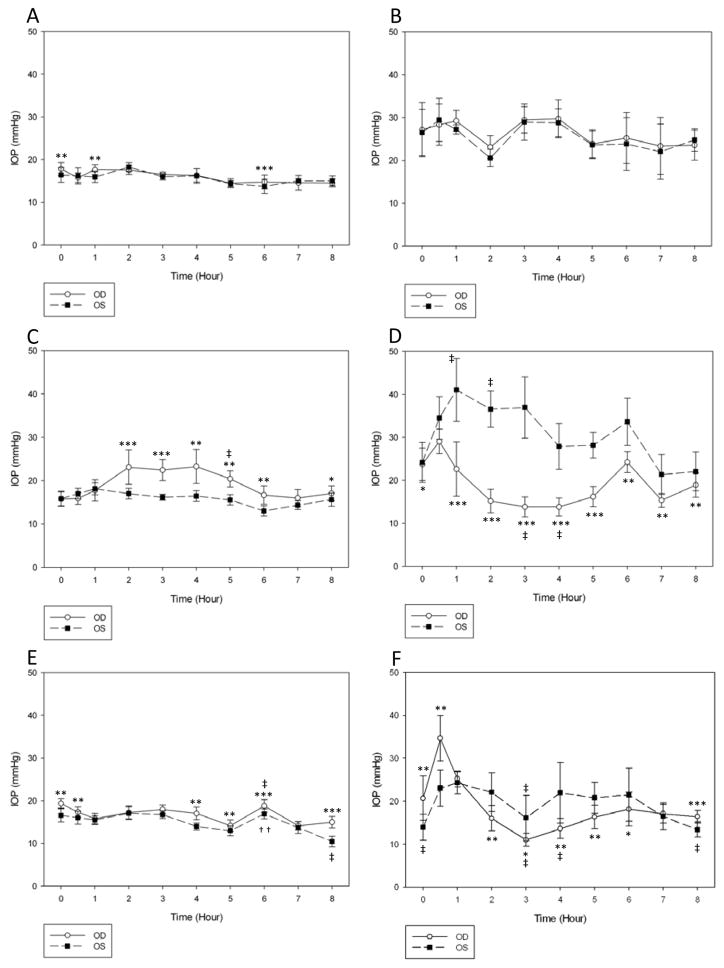
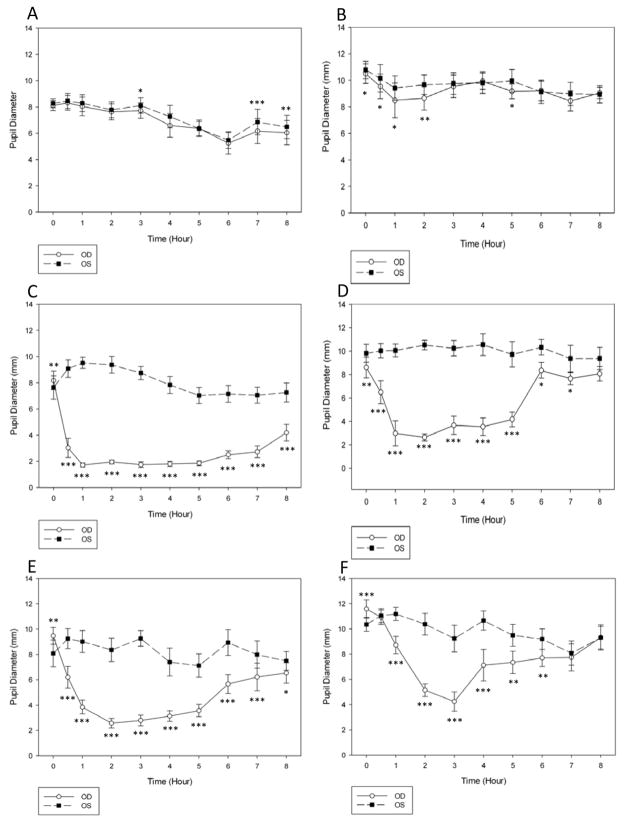
Mean PD was significantly larger in glaucomatous compared to normal cats over the one week pre-treatment period (9.4±1.9 mm OU vs. 7.2±1.9 mm OU, respectively; p<0.001). There was no significant effect of time on IOP in either group during the 8 h measurements in the pre-treatment phase. In normal cats, mean IOP OD was significantly higher than OS at time zero (8am) and at 1 h and 6 h (9am and 1pm) (Fig. 3A), but the magnitudes of the differences between the eyes were not considered clinically significant (≤ 1.7 mmHg). The right pupil was statistically smaller than the left at 3, 7 and 8 h (11am, 2pm, 3pm, respectively) (Fig. 4A), however in clinical terms there was no grossly appreciable anisocoria and the mean difference between pupil diameters was ≤ 0.7 mm. In untreated cats with PCG, there was no significant IOP difference between OD and OS (Fig. 3B). Pupil diameter in glaucomatous cats was statistically smaller in OD compared to OS at all time points up to 2 h and at 5 h (Fig.4B), but as with the normal cats there was no grossly appreciable anisocoria and differences in PD between eyes were ≤1.0 mm.
Fig. 3.
Intraocular pressure (IOP) measurements in normal cats during the pre-treatment (A), treatment (C) and post-treatment (E) phases and in glaucomatous cats during pre-treatment (B), treatment (D) and post-treatment (F) phases. Normal cats demonstrated a significant increase in mean IOP in the treated eye following drug administration, with maximal increase at 4h. Application of latanoprost 0.005% led to a significant reduction in IOP of the treated eye in glaucomatous cats, with maximal IOP reduction at 3h. Statistically significant differences are indicated by *p<0.05 latanoprost-treated eye (OD) vs. vehicle-treated control eye (OS); **p<0.01, treated vs. control; ***p <0.0001, treated vs. control; (Paired t-test); ††p<0.01, post- vs. pre-treatment value; ‡ p<0.001, post- vs. pre-treatment value (Repeated Measures ANOVA).
Fig. 4.
Pupil diameter in normal cats during the pre-treatment (A), treatment (C) and post-treatment (E) phases, and in glaucomatous cats during the pre-treatment (B), treatment (D) and post-treatment (F) phases. Application of latanoprost 0.005% led to significant miosis in the treated eyes of normal cats, with peak effect at 1h. Glaucomatous cats also demonstrated significant miosis in the treated eyes after administration, with peak effect at 2h. Statistically significant differences are indicated as follows: *p<0.05 latanoprost-treated (OD) vs. vehicle-treated control (OS); **p<0.01, treated vs. control; ***p <0.0001, treated vs. control (Paired t-test); ††p<0.01 post- vs. pre-treatment value; ‡p<0.001 post- vs. pre-treatment value (Repeated Measures ANOVA).
Influence of Topical Latanoprost Treatment on Diurnal IOP Curve and Pupil Diameter
In normal cats, during the first day of the treatment phase, the mean IOP was significantly higher in the treated compared to the control eye at several of the time points following treatment (Fig. 3C). IOP was also higher at 5 h (p≤0.001) in treated eyes compared to the same time point on the pre-treatment day. However, no significant differences in IOP were noted in either eye at any other time point relative to the same time points on the pre-treatment day. Significant miosis was observed in the treated eye at all time points, except time zero. The peak effect was noted 1 h after latanoprost treatment (Fig. 4C).
In glaucomatous cats, IOP was significantly lower in treated vs. control eyes, following a single latanoprost treatment, at all time points except time zero. Maximal IOP reduction relative to the control eye was observed 3 h after administration of latanoprost (Fig. 3D). IOP was significantly lower at 3 and 4 h after latanoprost treatment when compared to measurements obtained at the same time-points in the pre-treatment phase (mean reduction of 16 mmHg, or approximately 33%).
Pronounced miosis was observed in the treated eye compared to the control eye of glaucomatous cats from 0.5–7 h, with a peak effect 2 h after application of latanoprost (p<0.001). Grossly appreciable anisocoria persisted 8 h after latanoprost administration, but the difference between eyes was no longer consistent or statistically significant (p= 0.10) (Fig. 4D).
Effect of Topical Latanoprost on IOP following Extended Treatment
There were no statistically significant changes in weekly mean peak IOP or maximum IOP fluctuation (mean difference between maximum and minimum IOP) between treated and control eyes in the normal or the glaucomatous cats, during any week of the study. There was no tendency for IOP to be reduced over time during the treatment phase in either eye or when comparing OD vs. OS in normal or glaucomatous cats (Fig. 2A & B).
Diurnal IOP and PD after 3 weeks of Topical Latanoprost Treatment
Following 3 weeks of treatment, mean IOP was significantly higher in treated vs. control eyes of normal cats at 0, 0.5, 4–6 and 8 h, with a maximal difference between the eyes observed at 8 h (p=<0.001) (Fig. 3E). There was a significant (p≤0.001) increase in mean IOP in the treated eyes at 6 h compared to time zero. At the 7:00am time point, immediately prior to drug administration (15h following prior latanoprost administration), pupil diameter was significantly larger in treated vs. control eyes. However, administration of latanoprost still resulted in a smaller pupil diameter in treated compared to the control eyes for at least 8 h, with the peak effect noted at 2 h (Fig. 4E).
In the glaucomatous cats, mean IOP in the treated eyes was significantly higher than in control eyes immediately prior to and 30 minutes after application of latanoprost (p=0.0002 and p=0.003, respectively) with 50% higher mean IOP in treated vs. control eyes (Fig. 3F). However, there was a significantly lower mean IOP in the treated, relative to control eyes, at 2–6 h and 8 h after treatment, with a maximal IOP lowering effect observed 4 h after latanoprost application. Mean PD in the glaucomatous cats was significantly smaller in treated compared to control eyes 1–7 h after treatment with latanoprost (Fig. 4F).
Aqueous Humor Flow Rates as determined by Fluorophotometry
Mean AHF rate in untreated normal cats was 7.9±2.2μL/min, in the right eye and 7.9±1.4μL/min in the left eye (Fig. 5). The treated (right) eye had a significantly higher AHF rate (p=0.031) compared to the control (left) eye after three weeks of treatment (8.1±2.1μL/min vs. 7.1±2.0μL/min, respectively; mean difference of 1.0±0.6μl/min, or 13.4% difference in AHF). It was not possible to determine AHF rates by fluorophotometry in cats with PCG. In these cats, measurements of fluorescein in the anterior chamber were erratic and did not maintain a consistent downward slope. Variable fluctuations in fluorescence were observed in serial scans over the course of the experiment (data not shown).
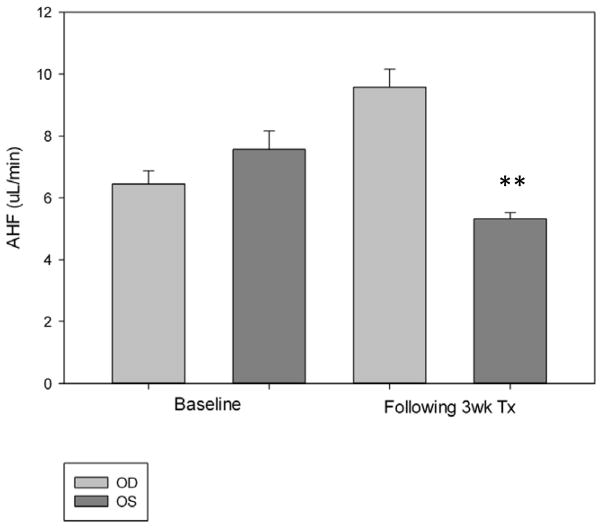
Fig. 5.
Following 3 weeks of twice daily topical application of latanoprost 0.005% to the right eye (OD), a modest increase in fluorophotometrically determined aqueous humor flow rate was observed in the treated eyes of normal cats. A modest but statistically significant decrease in aqueous humor flow rate was observed in the vehicle treated left eye (OS) over the same treatment period. Statistically significant differences are indicated as follows: **p<0.01, treated vs. control (Student’s t-test).
Discussion
In this study, 0.005% latanoprost had no consistent IOP-lowering effect in normal cats, a finding that is in line with previous studies examining the effects of commercially available prostanoids and FP receptor-specific PG analogs in cats.[20,24,27] In contrast, latanoprost treatment was associated with an acute reduction in IOP in glaucomatous cats in our study. It must be stressed that the glaucomatous cats in the present study were all affected by a single form of glaucoma, which provided us with a consistent and relatively homogeneous group of subjects. However, their response is not necessarily representative of that which may be observed in feline glaucoma patients clinically, particularly since secondary glaucoma is the most common form of the disease in cats.[9]
In humans [35] and in non-human primates,[36,37] FP receptor agonists have been found to reduce IOP predominantly by increasing outflow through the uveoscleral pathway. In the latter species, the reported time to onset of maximal IOP reduction is about 4–6 h, with the increase in uveoscleral outflow attributed to remodeling of uveal tissue extracellular matrix.[5,36] The underlying mechanism for the acute ocular hypotensive effect observed in glaucomatous cats remains unclear. Although normal cats have been shown to lack ciliary body FP receptors, and that EP rather than FP receptors mediate the IOP lowering response to prostaglandin agonists in cats, evaluation of potential differences in ocular prostaglandin receptor distribution and physiology between normal and PCG cats was not within the scope of our study. It is conceivable that the application of latanoprost acted indirectly via FP receptors in the iris to induce endogenous PGE2 synthesis.[38–40] Subsequent interaction between PGE2 and EP receptors in the feline ciliary body could then act to lower IOP.[25,41,42] Topical application of PGE2 in cats in vivo has been shown to induce a reduction in IOP as early as one hour after treatment [43], which is consistent with the timing of the response observed in our study. Normal and glaucomatous dogs show a significant ocular hypotensive response to a single application of latanoprost, with a similar time-course to that observed in the glaucomatous cats in our study.[19,20] A reduction in aqueous flow rates has been reported in dogs treated with latanoprost [44], which could explain the rapid onset and magnitude of IOP reduction observed in this species. However, no such reduction in aqueous flow was observed in the normal cats in our study, thus AHF reduction appears to be an unlikely explanation for IOP-lowering by latanoprost in cats with PCG. Baseline AHF in the normal cats in our study was comparable to, though slightly higher, than values reported in previous studies, which ranged from 5.51 ± 2.21 μL/min [45] to 6.0±1.4 μL/min.[34] Previous studies of the actions of IOP-lowering agents in other species suggest that a 30–35% reduction in aqueous flow is required for a pronounced IOP reduction [46], such as that observed acutely in our glaucomatous cats. Unfortunately, congenital anterior segment abnormalities, including mild lens zonular instability, precluded determination of aqueous humor flow rates in cats with PCG, likely due to unstable anterior segment volumes, and the potential for distribution of fluorescein into the vitreous.[47] Further evaluation of aqueous humor dynamics by other means was beyond the scope of the current study.
Due to the intense miosis observed in both normal and glaucomatous cats, no effort was made to mask the observer or randomize the eye treated. This profound reduction in pupil diameter may be attributed to the presence of FP receptors in the feline iris sphincter muscle.[25,48] It is conceivable that the miotic effect of latanoprost in cats may have increased conventional aqueous outflow in the treated eye. This hypothesis is an attractive one, as the timing of the acute reduction in IOP in cats with PCG approximately coincided with the acute decrease in pupil diameter observed in both the normal and glaucomatous cats in the treatment phase. In addition, the cholinergic miotic, pilocarpine, has been shown to lower IOP in normal cats.[10] However, in non-human primates, pupillary constriction appears to play no role in pilocarpine’s effect on outflow facility, rather the drug effect has been found to be entirely related to ciliary muscle contraction and there is no facility-relevant effect on the TM.[49] Conversely, changes in pupil diameter may have been responsible for the initial acute increase in IOP observed at 0.5 h after latanoprost administration to glaucomatous cats. However, there was no evidence that the anterior chamber depth was reduced by treatment, as might be expected in subjects with “pupil block”, based on results of slit-lamp biomicroscopy and optical pachymetry conducted prior to treatment and during the late stages of the three week treatment phase. Potential dynamic anatomic effects on the iridocorneal angle and structures of the ciliary cleft have been explored in this feline model using high-resolution ultrasonography.[50–52] In addition, studies are planned to examine the effects of topical latanoprost on tonographic aqueous outflow facility in glaucomatous cats. Enhanced outflow facility has been documented in humans following application of latanoprost [53], however, a slight enhancement in trabecular outflow is not considered to be the predominant mechanism for IOP reduction in humans and non-human primates. The magnitude of IOP reduction by twice daily application of latanoprost appeared to diminish over the 3-week treatment phase in cats with PCG. Although a relatively common phenomenon during long-term drug administration, previous studies that examined longer term-responsiveness to topical prostaglandins have provided conflicting evidence regarding the potential for tachyphylaxis.[54] However, over the 3-week treatment period of our study, the miotic effect of topical latanoprost also diminished, which supports tachyphylaxis as a mechanism for reduced efficacy in response to chronic application of the drug. An increase in episcleral venous pressure (EVP) has also been identified in dogs treated with latanoprost.[55] Non-invasive quantification of EVP prior to, and following latanoprost treatment, should be possible in normal cats, however the episcleral veins cannot be readily identified in glaucomatous cats, thus such an association may prove difficult to confirm or refute. Prior to the morning application of latanoprost, we observed significant rebound mydriasis, with reversal of anisocoria, and an increase in IOP in the treated relative to untreated eye in both the glaucomatous and normal cats. Practical considerations meant that no attempt was made to measure IOP during the nocturnal phase during this study. Nocturnal fluctuations in IOP may be extreme in glaucomatous cats [15] and arguably, are potentially very damaging to the optic nerve.[56] Marked fluctuations in IOP were noted OU in PCG cats in our study (Fig. 2B), and may contribute to the progression of the optic nerve disease in these animals.
In addition to the IOP-lowering effect in the latanoprost-treated eye of the cats with PCG during the treatment phase, there appeared to be a mild increase in IOP in the vehicle treated eye. However, there was a very wide range of IOP values within the PCG cats during this phase (range 19–60 mmHg). Although this may have represented a genuine contralateral effect, as the drug may have been absorbed systemically following topical application, this would appear unlikely as no significant change in pupil diameter was observed during the treatment phase in the vehicle-treated eye. Despite efforts to ensure that cats in the study were acclimated to topical drug administration and tonometry, we cannot exclude the possibility that stress associated with drug application during the course of the study may have been responsible for the IOP elevation observed in the untreated eyes, for the initial increase in IOP in the treated eyes of PCG and normal cats, or for the apparent reduction in responsiveness over time.
Conclusion
In summary, latanoprost effectively reduces IOP acutely in glaucomatous cats. However, this effect is substantially diminished within three weeks of twice daily treatment. Further investigations are necessary to determine appropriate frequency of application and whether latanoprost 0.005% will be an effective long-term therapy for the management of feline glaucoma. The mechanism(s) responsible for latanoprost’s unexpected and acute IOP-lowering effect observed in glaucomatous cats, the increase in IOP observed in normal cats, and the reduction in IOP-lowering efficacy observed over time in glaucomatous cats remain unclear and all warrant further investigation.
Acknowledgments
This study was supported by NIH grants K08 EY018609 (GJM) and P30 EY0016665 (PLK, JK); by the Companion Animal Fund, UW-Madison School of Veterinary Medicine (EB, GJM), by a Merial Summer Research Scholarship (JEM) and by an unrestricted award to the Department of Ophthalmology and Visual Sciences from Research to Prevent Blindness. Glaucomatous subjects were derived from a breeding colony originally maintained at Iowa State University, supported in part by the Center for Integrated Animal Genomics, Iowa State University and a Battelle Platform Project Grant from the State of Iowa.
The authors would like to thank Jackie K. Jens at Iowa State University, for her expert assistance in the management of the feline glaucoma breeding colony, and Caitlin Kuehn, Jeremy Kemmerling and Elizabeth Elsmo for their technical support in the conduct of this study.
References
- 1.Kroll MM, Miller PE, Rodan I. Intraocular pressure measurements obtained as part of a comprehensive geriatric health examination from cats seven years of age or older. Journal of the American Veterinary Medical Association. 2001;219:1406–1410. doi: 10.2460/javma.2001.219.1406. [DOI] [PubMed] [Google Scholar]
- 2.Quigley H, Broman A. The number of people with glaucoma worldwide in 2010 and 2020. British Journal of Ophthalmology. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritch R, Shields MB, Krupin T. The Glaucomas. 2. Mosby; St. Louis: 1996. [Google Scholar]
- 4.Wilensky JT. Epidemiology of open-angle glaucoma. In: Kaufman PL, Mittag TW, editors. Textbook of Ophthalmology Volume 7 (Glaucoma) Mosby; St. Louis: 1994. pp. 29–33. [Google Scholar]
- 5.Toris CB, Gabelt BT, Kaufman PL. Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Survey of Ophthalmology. 2008;53(Suppl 1):S107–S120. doi: 10.1016/j.survophthal.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blocker T, Van der Woerdt A. The feline glaucomas: 82 cases (1995–1999) Veterinary Ophthalmology. 2001;4:81–85. doi: 10.1046/j.1463-5224.2001.00169.x. [DOI] [PubMed] [Google Scholar]
- 7.Ridgway M, Brightman A. Feline Glaucoma - A retrospective study of 29 clinical cases. Journal of the American Animal Hospital Association. 1989;25:485–490. [Google Scholar]
- 8.Dietrich U. Feline Glaucomas. Clinical Techniques in Small Animal Practice. 2005;20:108–116. doi: 10.1053/j.ctsap.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 9.McLellan GJ, Miller PE. Feline glaucoma--a comprehensive review. Veterinary Ophthalmology. 2011;14(Suppl 1):15–29. doi: 10.1111/j.1463-5224.2011.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkie DA, Latimer CA. Effects of topical administration of 2. 0% percent pilocarpine on intraocular pressure and pupil size in cats. American Journal of Veterinary Research. 1991a;52:441–444. [PubMed] [Google Scholar]
- 11.Wilkie DA, Latimer CA. Effects of topical administration of timolol maleate on intraocular pressure and pupil size in cats. American Journal of Veterinary Research. 1991b;52:436–440. [PubMed] [Google Scholar]
- 12.Miller PE, Nelson MJ, Rhaesa SL. Effects of topical administration of 0.5% apraclonidine on intraocular pressure, pupil size, and heart rate in clinically normal dogs. American Journal of Veterinary Research. 1996;57:79–82. [PubMed] [Google Scholar]
- 13.Dietrich UM, Chandler MJ, Cooper T, et al. Effects of topical 2% dorzolamide hydrochloride alone and in combination with 0.5% timolol maleate on intraocular pressure in normal feline eyes. Veterinary Ophthalmology. 2007;10(Suppl 1):95–100. doi: 10.1111/j.1463-5224.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 14.Rainbow ME, Dziezyc J. Effects of twice daily application of 2% dorzolamide on intraocular pressure in normal cats. Veterinary Ophthalmology. 2003;6:147–150. doi: 10.1046/j.1463-5224.2003.00282.x. [DOI] [PubMed] [Google Scholar]
- 15.Sigle KJ, Camano-Garcia G, Carriquiry AL, et al. The Effect of Dorzolamide 2% on Circadian Intraocular Pressure in Cats with Primary Congenital Glaucoma. Veterinary Ophthalmology. 2011;14(Suppl 1):48–53. doi: 10.1111/j.1463-5224.2011.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry C, McGavin J, Culy C, et al. Latanoprost - An update of its use in glaucoma and ocular hypertension. Drugs Aging. 2003;20:597–630. doi: 10.2165/00002512-200320080-00005. [DOI] [PubMed] [Google Scholar]
- 17.Lim KS, Nau CB, O’Byrne MM, et al. Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology. 2008;115:790–795. doi: 10.1016/j.ophtha.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takagi Y, Nakajima T, Shimazaki A, et al. Pharmacological characteristics of AFP-168 (tafluprost), a new prostanoid FP receptor agonist, as an ocular hypotensive drug. Experimental Eye Research. 2004;78:767–776. doi: 10.1016/j.exer.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Gelatt KN, MacKay EO. Effect of different dose schedules of latanoprost on intraocular pressure and pupil size in the glaucomatous Beagle. Veterinary Ophthalmology. 2001;4:283–288. doi: 10.1046/j.1463-5216.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- 20.Studer ME, Martin CL, Stiles J. Effects of 0.005% latanoprost solution on intraocular pressure in healthy dogs and cats. American Journal of Veterinary Research. 2000;61:1220–1224. doi: 10.2460/ajvr.2000.61.1220. [DOI] [PubMed] [Google Scholar]
- 21.Willis AM, Diehl KA, Hoshaw-Woodard S, et al. Effects of topical administration of 0.005% latanoprost solution on eyes of clinically normal horses. American Journal of Veterinary Research. 2001;62:1945–1951. doi: 10.2460/ajvr.2001.62.1945. [DOI] [PubMed] [Google Scholar]
- 22.Gabelt BT, Hennes EA, Bendel MA, et al. Prostaglandin subtype-selective and non-selective IOP-lowering comparison in monkeys. Journal of Ocular Pharmacology and Therapeutics. 2009;25:1–8. doi: 10.1089/jop.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagliuso DJ, Wang RF, Mittag TW, et al. Additivity of bimatoprost or travoprost to latanoprost in glaucomatous monkey eyes. Archives of Ophthalmology. 2004;122:1342–1347. doi: 10.1001/archopht.122.9.1342. [DOI] [PubMed] [Google Scholar]
- 24.Bartoe JT, Davidson HJ, Horton MT, et al. The effects of bimatoprost and unoprostone isopropyl on the intraocular pressure in normal cats. Veterinary Ophthalmology. 2005;8:247–252. doi: 10.1111/j.1463-5224.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacherjee P, Williams BS, Paterson CA. Responses of intraocular pressure and the pupil of feline eyes to prostaglandin EP1 and FP receptor agonists. Investigative Ophthalmology and Visual Science. 1999;40:3047–3053. [PubMed] [Google Scholar]
- 26.Chen J, Woodward DF. Prostanoid-induced relaxation of precontracted cat ciliary muscle is mediated by EP2 and DP receptors. Investigative Ophthalmology and Visual Science. 1992;33:3195–3201. [PubMed] [Google Scholar]
- 27.Regnier A, Lemagne C, Ponchet A, et al. Ocular effects of topical 0.03% bimatoprost solution in normotensive feline eyes. Veterinary Ophthalmology. 2006;9:39–43. doi: 10.1111/j.1463-5224.2005.00435.x. [DOI] [PubMed] [Google Scholar]
- 28.Schlötzer-Schrehardt U, Zenkel M, Nüsing R. Expression and localization of FP and EP prostanoid receptor subtypes in human ocular tissues. Investigative Ophthalmology and Visual Science. 2002;43:1475–1487. [PubMed] [Google Scholar]
- 29.Hackett RB, McDonald TO. Ophthalmic toxicology and assessing ocular irritation. In: Marzulli FN, Maibach HI, editors. Dermatotoxicology. 4. Hemisphere Publishing Corp; Washington DC: 1996. pp. 749–815. [Google Scholar]
- 30.McLellan GJ, Kemmerling JP, Kiland JA. Validation of the TonoVet® rebound tonometer in normal and glaucomatous cats. Veterinary Ophthalmology. 2013;16:111–118. doi: 10.1111/j.1463-5224.2012.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ofri R, Shub N, Galin Z, et al. Effect of reproductive status on intraocular pressure in cats. American Journal of Veterinary Research. 2002;63:159–162. doi: 10.2460/ajvr.2002.63.159. [DOI] [PubMed] [Google Scholar]
- 32.Toris C, Yablonski M, Wang Y, et al. Prostaglandin A(2) increases uveoscleral outflow and trabecular outflow facility in the cat. Experimental Eye Research. 1995;61:649–657. doi: 10.1016/s0014-4835(05)80015-6. [DOI] [PubMed] [Google Scholar]
- 33.Bryant JA. Local and topical anesthetics in ophthalmolgy. Survey of Ophthalmology. 1969;13:263–283. [PubMed] [Google Scholar]
- 34.Tally DK, Bartlett JD. Topical and regional anesthesia. In: Bartlett JD, Jaanus SD, editors. Clinical Ocular Pharmacology. 3. Butterworth-Heinemann; Boston: 2007. pp. 463–477. [Google Scholar]
- 35.Giuffre G. The effects of prostaglandin F2-alpha in the human eye. Graefe’s Archive for Clinical and Experimental Ophthalmology. 1985;222:139–141. doi: 10.1007/BF02173538. [DOI] [PubMed] [Google Scholar]
- 36.Crawford K, Kaufman PL, Gabelt BT. Effects of topical PGF2-alpha on aqueous humor dynamics in cynomolgus monkeys. Current Eye Research. 1987;6:1035–1044. doi: 10.3109/02713688709034874. [DOI] [PubMed] [Google Scholar]
- 37.Gabelt BT, Kaufman PL. Prostaglandin F2α increases uveoscleral outflow in the cynomolgus monkey. Experimental Eye Research. 1989;49:389–402. doi: 10.1016/0014-4835(89)90049-3. [DOI] [PubMed] [Google Scholar]
- 38.Yousufzai SYK, Ye Z, AbdelLatif AA. Prostaglandin F-2 alpha and its analogs induce release of endogenous prostaglandins in iris and ciliary muscles isolated from cat and other mammalian species. Experimental Eye Research. 1996;63:305–310. doi: 10.1006/exer.1996.0119. [DOI] [PubMed] [Google Scholar]
- 39.Husain S, Abdel-Latif AA. Effects of prostaglandin F-2 alpha and carbachol on MAP kinases, cytosolic phospholipase A(2) and arachidonic acid release in cat iris sphincter smooth muscle cells. Experimental Eye Research. 2001;72:581–590. doi: 10.1006/exer.2001.0991. [DOI] [PubMed] [Google Scholar]
- 40.Kashiwagi K, Kanai N, Tsuchida T, et al. Comparison between isopropyl unoprostone and latanoprost by prostaglandin E-2 induction, affinity to prostaglandin transporter, and intraocular metabolism. Experimental Eye Research. 2002;74:41–49. doi: 10.1006/exer.2001.1104. [DOI] [PubMed] [Google Scholar]
- 41.Bito LZ, Srinivasan BD, Baroody RA, et al. Non-invasive observations on eyes of cats after long-term maintenance of reduced intraocular-pressure by topical application of prostaglandin-E2. Investigative Ophthalmology and Visual Science. 1983;24:376–380. [PubMed] [Google Scholar]
- 42.Bhattacherjee P, Rhodes L, Paterson CA. Prostaglandin receptors coupled to adenylyl cyclase in the iris-ciliary body of rabbits, cats and cows. Experimental Eye Research. 1993;56:327–333. doi: 10.1006/exer.1993.1042. [DOI] [PubMed] [Google Scholar]
- 43.Stern FA, Bito LZ. Comparison of the hypotensive and other ocular effects of prostaglandins E2 and F2 alpha on cat and rhesus monkey eyes. Investigative Ophthalmology and Visual Science. 1982;22:588–598. [PubMed] [Google Scholar]
- 44.Ward D. Effects of latanoprost on aqueous humor flow rate in normal dogs. Proceedings, 36th Annual Conference of the American College of Veterinary Ophthalmology; Nashville, TN. 2005. p. 15. [Google Scholar]
- 45.Crumley WR, Rankin AJ, Allbaugh RA. Evaluation of the aqueous humor flow rate in the eyes of clinically normal cats by use of fluorophotometry. American Journal of Veterinary Research. 2012;73:704–708. doi: 10.2460/ajvr.73.5.704. [DOI] [PubMed] [Google Scholar]
- 46.Millar CK, Kaufman PL. Aqueous humor: secretion and dynamics. In: Tasman W, editor. Duane’s Foundations of Clinical Ophthalmology. Lippincott-Raven; Philadelphia: 1995. pp. 1–51. [Google Scholar]
- 47.Gulati V, Toris CB. Assumption contraints of fluorophotometry in human eyes. Investigative Ophthalmology and Visual Science. 2011;52:1312–1313. doi: 10.1167/iovs.10-6865. [DOI] [PubMed] [Google Scholar]
- 48.Sharif NA, Kaddour-Djebbar I, Abdel-Latif AA. Cat iris sphincter smooth-muscle contraction: comparison of FP-class prostaglandin analog agonist activities. Journal of Ocular Pharmacology and Therapeutics. 2008;24:152–163. doi: 10.1089/jop.2007.0076. [DOI] [PubMed] [Google Scholar]
- 49.Kiland JA, Hubbard WC, Kaufman PL. Low doses of pilocarpine do not significantly increase outflow facility in the cynomolgus monkey. Experimental Eye Research. 2000;70:603–609. doi: 10.1006/exer.1999.0818. [DOI] [PubMed] [Google Scholar]
- 50.Bentley E, Miller PE, Diehl KA. Evaluation of intra- and interobserver reliability and image reproducibility to assess usefulness of high-resolution ultrasonography for measurement of anterior segment structures of canine eyes. American Journal of Veterinary Research. 2005;66:1775–1779. doi: 10.2460/ajvr.2005.66.1775. [DOI] [PubMed] [Google Scholar]
- 51.Gutierrez-Ortiz C, Teus MA, Bolivar G. Short-term effects of latanoprost on anterior chamber depth in patients with glaucoma or ocular hypertension. Investigative Ophthalmology and Visual Science. 2006;47:4856–4859. doi: 10.1167/iovs.06-0014. [DOI] [PubMed] [Google Scholar]
- 52.Delgado C, Lovstad J, Kuehn CE, et al. Effect of topical pilocarpine and latanoprost on IOP and anterior segment morphology in normal and glaucomatous cats (abstract). 43rd Transactions of the American College of Veterinary Ophthalmologists; 2012. [Google Scholar]
- 53.Bahler CK, Howell KG, Hann CR, et al. Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. American Journal of Ophthalmology. 2008;145:114–119. doi: 10.1016/j.ajo.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bito LZ, Draga A, Blanco J, et al. Long-term maintenance of reduced intraocular pressure by daily or twice daily topical application of prostaglandins to cat or rhesus monkey eyes. Investigative Ophthalmology and Visual Science. 1983;24:312–319. [PubMed] [Google Scholar]
- 55.Tsai S, Miller PE, Struble C, et al. Topical application of 0.005% latanoprost increases episcleral venous pressure in normal dogs. Veterinary Ophthalmology. 2012;15(Suppl 1):71–78. doi: 10.1111/j.1463-5224.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 56.Gao F, Miller JP, Miglior S, et al. The effect of changes in intraocular pressure on the risk of primary open-angle glaucoma in patients with ocular hypertension: an application of latent class analysis. BMC Medical Research Methodology. 2012;12:151. doi: 10.1186/1471-2288-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]