Abstract
Background
Newborn screening (NBS) for hemoglobinopathies facilitates early identification of affected individuals to ensure the prompt institution of comprehensive medical care for affected newborns in California. When linked to extensive follow-up and education, NBS has been shown to significantly reduce mortality in children with sickle cell disease. Due to changing immigration patterns from Asia and Latin America, the State of California has witnessed an increased prevalence of clinically significant hemoglobin (Hb) disorders, including those resulting from novel genotypes. In 1999, newborn screening for Hb H disorders was incorporated in the statewide hemoglobinopathy screening program.
Procedure
Primary screening for hemoglobin variants was performed using high performance liquid chromatography. Confirmatory testing on hemoglobinopathy mutations was performed by electropheresis techniques and genotyping methods.
Results
Of 530,000 newborn samples screened annually in California, 2,118 samples were referred to the Hemoglobin Reference Laboratory (HRL) for confirmatory testing between January 1, 1998 and June 30, 2006 (0.05%). Sickle cell disease was most frequently observed (1 in 6,600 births) followed by α-thalassemia (1 in 9,000 births) and β-thalassemia disease (1 in 55,000 births). The confirmatory analysis modified the initial screening in 5% of cases and revealed 25 rare or new genotypes. Diverse ethnicities were associated with hemoglobin mutations including Southeast Asian, Black, Indian/Asian, Middle Eastern, and Hispanic.
Conclusions
The California hemoglobinopathy screening program provides accurate diagnosis of hemoglobinopathies. Increasing incidence of diverse mutations require new strategies of laboratory screening, counseling, and patient management.
Keywords: anemia, newborn screening, sickle cell disease, thalassemia
INTRODUCTION
The purpose of newborn screening is to facilitate the early identification of a disease so that an intervention that alters the natural history of the disease might be instituted before significant morbidity or early mortality occurs. Newborn screening (NBS) for sickle cell disease (SCD) was implemented in California in 1990, and today all 50 U.S. States screen for SCD. Early identification of affected individuals facilitates institution of prophylactic treatment and comprehensive care prior to the development of clinical complications. In a 1988 study by Vichinsky et al. [1], the overall mortality rate in patients with sickle cell anemia diagnosed in the newborn period was 1.8%, compared to 8% mortality in a control group of patients diagnosed after 3 months of age. A mortality rate of 20% was observed in children with sickle cell anemia during the first decade of life before routine NBS and comprehensive care were the standards of care [2]. The dramatic benefit of early preventive therapy in SCD was also shown in a multicenter clinical trial: children who received prophylactic penicillin had a significantly reduced rate of infection and mortality from Streptococcous pneumoniae infections compared to children who received placebo [3].
Thalassemia is another hemoglobinopathy for which early intervention can improve outcome. In lieu of the early mortality that characterized this disorder before modern medical care, beta (β)-thalassemia major currently is characterized by long-term survival due to the availability of regular red blood cell (RBC) transfusions and effective iron-chelation therapy [4]. Early intervention can also change the course of alpha (α) thalassemia, a common hereditary condition caused by mutations or deletions of one or more of the four α-globin genes. Hemoglobin H (Hb H) disease is caused by deletion or inactivation of three α-globin genes. While historically thought to be asymptomatic, recent literature suggests that Hb H disease is not as benign a disorder as previously thought [5–8]. Significant hemolytic anemia can occur during fever, infections, and pregnancy that may necessitate the need for blood transfusions. Infection with parvovirus B19 can lead to severe aplastic crises. In addition, this condition can lead to growth retardation during childhood, and iron overload in adults regardless of previous transfusion history, leading to hepatic, cardiac, and endocrine dysfunction [6]. Hemoglobin H-Constant Spring (CS) disease, which results from the compound heterozygosity for an α-0 mutation and the nondeletional α-gene mutation Constant Spring, tends to be a more severe form of Hb H, with up to one-third of these patients being transfusion dependent [4]. The deletion of four alpha genes is known to cause the hydrops fetalis syndrome in utero with subsequent fetal demise, and there are even case reports of Hb H hydrops fetalis [7].
Given the phenotypic variations of Hb H disease, there is a need for surveillance for this condition among newborns born in communities with a high proportion of at-risk populations. Alpha thalassemia is particularly common among Southeast Asians (SEA), with a carrier frequency as high as 15% [9]. With the rise in Asian immigration to the United States and to California in particular over the past few decades, the prevalence of α-thalassemia has increased steadily [10]. As a result, NBS for α-thalassemia (Hb H disease) was initiated in California in 1999 and subsequently mandated as part of universal NBS for the state [10].
Hemoglobin E is the second most common hemoglobin variant in the world (second only to Hb S), and there is a high rate of coinheriting this variant with other mutations. In fact, up to 20% of patients heterozygous for Hb E also inherit a β-thalassemia mutation [11]. Hb E is also frequently coinherited with Hb H disease, and patients with this disease combination tend to develop severe anemia during fever episodes [12]. While until recently Hb E was observed almost exclusively in SEA, its incidence in California has also increased with changing immigration patterns [13]. Homozygous Hb EE is a clinically benign condition, while the coinheritance of Hb E and a β-thalassemia mutation results in a clinically significant disorder with a variable, but potentially severe, β-thalassemia major phenotype [9].
The Hemoglobin Reference Laboratory (HRL) at the Children’s Hospital & Research Center Oakland (CHRCO) serves as the reference lab to the California Newborn Screening for Hemoglobinopathies Follow-up Program. Potentially significant hemoglobinopathies identified by primary NBS are sent to the HRL for confirmation using second-tier diagnostic tests. In addition, samples from newborns who have received a RBC transfusion before collecting blood for NBS are sent directly to the HRL, as transfusion interferes with the protein-based assays used to detect hemoglobin mutations [14]. Specific DNA testing is performed on these samples at the HRL. Along with test results, a clinical interpretation and recommendations for follow-up of newborns with a hemoglobin disorder are sent to health care providers, so that preventative care and early education are delivered promptly.
METHODS
Primary NBS
Dried blood spot (DBS) samples from all California newborns were routinely collected and sent to one of the seven geographically distributed California State Department of Health Services (DHS) screening laboratories during the period of this study. Primary screening for hemoglobin variants was performed using high performance liquid chromatography (HPLC) (BioRad Laboratories, Hercules, CA). This technique has been previously described [15,16]. The HPLC testing in California is fully automated, with a capacity to perform hundreds of analyses daily; it is able to detect Hb F, A, S, C, D, E, and Bart’s in a 2-min run time. It is also capable of detecting hemoglobin variants at five different retention times between the known variants [17,18]. As per protocol, all newborn samples with a primary screening result indicative of a hemoglobin disorder were referred to the HRL for confirmatory testing.
Second-Tier NBS
Confirmatory testing was performed by the HRL if (1) initial screening indicated a clinically significant hemoglobinopathy, (2) a blood transfusion was administered before screening, or (3) initial screening showed four or more different Hb peptides (i.e., samples with four or more peaks on HPLC). In second-tier testing, both parent and newborn samples were analyzed. Specific methods used for confirmatory testing included thin layer isoelectric focusing (TLIF), citrate agar and cellulose acetate electrophoresis, as well as thermal HPLC to quantitate Hb A2 and Hb F. Previously described genotyping methods, including a multiplexed sequence-specific oligonucleotide (SSOP)-based linear array (RMS) and direct DNA sequencing, were used to distinguish homozygous Hb S (SCA) from Hb S/beta-thalassemia, resolve ambiguous primary screening results and identify novel Hb variants [19]. Samples showing elevated Barts hemoglobin (>25%) were tested for common deletional and non-deletional α-thalassemia mutations using a multiplexed gap-PCR assay [20,21].
RESULTS
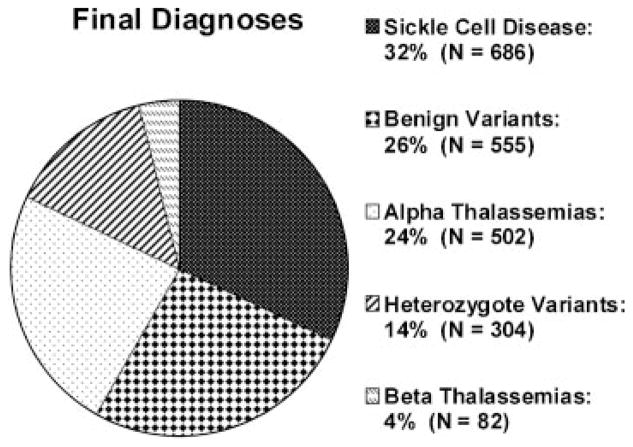
Of 530,000 newborn samples screened annually in California, 2,118 samples were referred to the HRL for confirmatory testing between January 1, 1998 and June 30, 2006 (0.05%). Prevalence data for each of the hemoglobinopathy genotypes are shown in Table I. SCD was observed most frequently (32%), followed closely by α-thalassemia conditions (24%). The prevalence of clinically significant α- and β-thalassemias combined (28%) approached that of SCD. The diagnoses established from the samples referred to the HRL are presented in Figure 1.
TABLE I.
Hemoglobin Genotypes Confirmed by the HRL (January 1998–June 2006)
| Diagnosis/genotype | No. of identified | Incidence (per 100,000 infants screened) |
|---|---|---|
| Sickle cell disease | 688 | 15.2 |
| Hb SS | 381 | 8.5 |
| Hb SC | 197 | 4.4 |
| Hb Sβ+ thal | 62 | 1.4 |
| Hb Sβ∘ thal | 35 | 0.8 |
| Hb S-HPFH | 11 | 0.2 |
| Hb S/Hb Lepore | 1 | 0.02 |
| Hb S/Hb O-Arab | 1 | 0.02 |
| Alpha thalassemia syndromes | 502 | 11.1 |
| Hb H disease | 406 | 9.0 |
| Hb H with Hb E trait | 35 | 0.8 |
| Hb H with Hb EE | 16 | 0.4 |
| Hb H-Constant Spring | 25 | 0.6 |
| Hb H with other variants | 8 | 0.2 |
| Hb H with SS | 7 | 0.2 |
| Hydrops fetalisa | 5 | 0.1 |
| Beta thalassemia syndromes | 79 | 1.8 |
| Hb β∘ | 19 | 0.4 |
| Hb Eβ∘ | 31 | 0.7 |
| Hb Cβ∘b | 7 | 0.2 |
| Hb Dβ∘b | 5 | 0.1 |
| Hb Cβ+b | 14 | 0.3 |
| Hb Eβ+b | 3 | 0.1 |
| Other mutations | 862 | 19.1 |
| Hb EE | 514 | 11.4 |
| Hb CC | 41 | 0.9 |
| Hb C-HPFH | 3 | 0.1 |
| Heterozygote variants | 304 | 6.7 |
All five cases of hydrops fetalis were homozygous for Southeast Asian (SEA) deletion;
Associated with milder disease phenotypes.
Fig. 1.
Final diagnoses of all samples forwarded to the HRL from January 1, 1998 through June 30, 2006.
The incidence rates of SCD, α-thalassemia and β-thalassemia syndromes per 100,000 California infants screened over the 8½-year period are shown in Table I. A genotype associated with a SCD phenotype was identified in 15.2 per 100,000 infants screened, with an incidence of approximately 1 in 6,600 California births. Over this same time period, Hb S trait was identified in 825 per 100,000 newborns, or 1 in 121 California births (data provided by the state, as such samples are not usually forwarded to the HRL). Collectively, heterozygous globin chain variants (Hb S, C, E, D, and rare variants) were identified in 1,340 per 100,000 newborns, or 1 in 75 California births.
α-thalassemia was identified in 11.1 per 100,000 infants screened, or roughly 1 in 9,000 California births. Hemoglobin H disease was confirmed in the majority of these cases. There were five cases of α-thalassemia major (4-gene deletions or mutations) identified, all of which were picked up on initial NBS, with Hb Barts levels 98–100%. At least two of these patients have subsequently undergone stem cell transplantation and are currently transfusion-independent. Of note, since June 2006 there have been three more cases of α-thalassemia major picked up on NBS. The diagnosis of a β-thalassemia phenotype was less frequently observed, with an incidence of 1.8 per 100,000 California infants screened, or roughly 1 in 55,000 (Table I). β-thalassemia genotypes typically associated with clinically severe phenotypes (β∘ and E/β∘) were found in 1.1 per 100,000 infants. Since the start of screening in 1990, the incidence of β-thalassemia major and Hb E/β-thalassemia has been 1.6 per 100,000 infants. All cases of Hb F only on NBS are referred for confirmatory testing. There has been one known case of β-thalassemia major missed on NBS which was later picked up by the HRL, as described below.
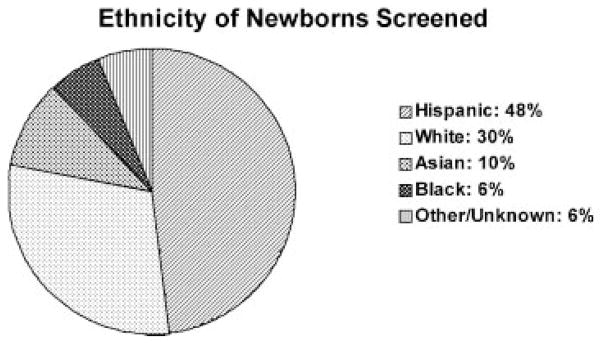
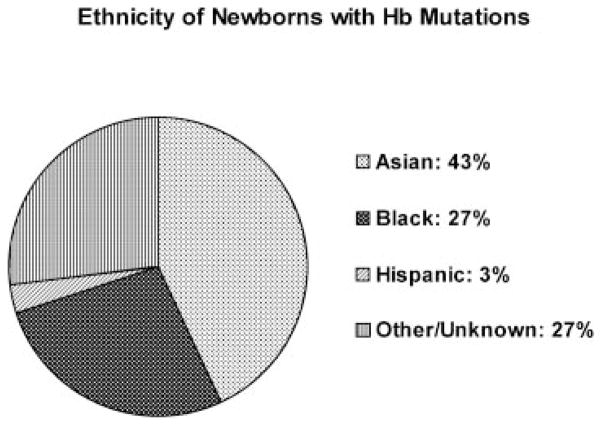
Demographic shifts within the state of California are reflected in the diverse ethnicities of the newborns screened. The ethnic groups represented in the California newborn population during a 3-year interval are depicted in Figure 2 (these are self-reported ethnicities on the NBS form). The ethnic groups associated with Hb mutations are depicted in Figure 3. As expected, the majority (93%) of sickle cell anemia cases (Hb SS) was reported in Black newborns. However, a large proportion of Hb S/β-thalassemia cases (12.5%) was observed in the Hispanic population. Most cases of α-thalassemia were reported in the SEA population (93%), whereas β-thalassemia was distributed over multiple ethnic groups.
Fig. 2.
Ethnicities of newborns screened in California during this 8-year period.
Fig. 3.
Ethnicities of newborns in California found to have hemoglobin mutations.
The diagnosis after confirmatory analysis in the HRL clarified or differed from the initial newborn screen in 113 cases (5%). In 57 cases, samples found on NBS to potentially represent significant hemoglobinopathies were found to be benign conditions. Thirty-seven cases with a benign Hb phenotype by NBS had a different benign diagnosis (n = 13) or had rare hemoglobin variants (n = 24) established by the HRL. In 18 cases, the NBS phenotype suggested potential disease, and the confirmatory diagnosis by the HRL showed a different, but still clinically significant hemoglobinopathy. The greatest potential impact occurred in two cases in which the NBS diagnosis of non-significant hemoglobin pattern was later revised to disease by the HRL. The first was a newborn with FA pattern by primary NBS, who was found to be anemic at 4 years of age. The electrophoresis showed 97% Hb F and 3% Hb A, and DNA analysis confirmed β-thalassemia major. The second case had a NBS phenotype of FAV, indicative of a potential Hb variant; however, this child received a RBC transfusion before NBS. Confirmatory testing in the HRL showed Hb SC and Hb Barts. In all, between October 1, 2004 and June 30, 2006, 563 blood samples from newborns who had a RBC transfusion were analyzed. Of these, 1 in 38 were Hb trait carriers; one was found to have Hb SC as described above.
Confirmatory testing also revealed 25 rare or previously undescribed genotypes, presented in Table II. These samples were submitted to the HRL with a diagnosis of “Hb variant,” as detected by the HPLC screen. More precise genotypes were determined by the various diagnostic techniques available in the HRL. In several cases, the variant globin found on HPLC co-migrated with a common structural variant; for example, Hb O-Arab migrates with Hb C, and Hb Lepore migrates with Hb A2. In these cases, molecular diagnostics were required to determine the genotypes. In addition, a novel alpha chain mutation, α-9 (A7) Asn-Lys substitution, was discovered. Approximately 10–15 γ variants were identified; however, as γ-globin variants are not clinically significant outside of the neonatal period, these variants were not further characterized.
TABLE II.
Rare or Previously Undescribed Genotypes Discovered on Confirmatory Testing*
| Heterozygous/homozygous alpha chain variants |
| Hb AS/G-Philadelphia |
| Hb Hasharon (4) |
| Hb Ogi/Queens |
| alpha deletion/Q-Thailand point mutation |
| Alpha 9 (A7) Asn-Lys substitution |
| Compound heterozygotes for Hb S and rare beta-chain variants: |
| Hb S/Austin |
| Hb S/Fannin-Lubock |
| Hb S/G-San Jose |
| Hb S/Hofu |
| Hb S/J-Kaohsiung |
| Hb S/N-Baltimore |
| Compound heterozygotes for beta-thalassemia and rare beta-chain variants |
| Beta thalassemia/Hb E-Saskatoon |
| Other compound heterozygous beta-chain variants |
| Hb C/G-Galveston |
| Hb E/Hope |
| Homozygous beta chain variants |
| Homozygous Hb J-Honolulu |
| Homozygous Hb Riadh |
| Heterozygous beta chain variants |
| Hb J-Honolulu |
| Hb J-Kaohsiung |
| Hb Khartoum |
| Gamma chain variantsa |
| Other variants |
| Hb Bart’s/Portlandb |
With the exception of Hb Bart’s/Portland, these rare genotypes are associated with benign phenotypes;
Gamma variants are not further characterized as they are not clinically significant;
An embryonic hemoglobin, ζ2γ2.
DISCUSSION
The initial HPLC-based NBS program in California is highly sensitive in detecting clinically significant hemoglobin disorders. Unlike other hemoglobinopathy screening programs, HPLC is exceptional in that it applies both quantitative capabilities and extreme sensitivity and specificity [16,22–24]. The rapid and efficient primary screening performed by the California NBS program is capable of identifying the vast majority of hemoglobinopathies. However, this screening technique will not detect all thalassemia and unusual Hb variants. It is important to characterize these disorders, as the phenotypes of individual genotypes can vary widely. For example, Hb EE and Hb E/β-thalassemia produce an FE result by routine NBS; Hb EE is a benign disorder and Hb E/β-thalassemia can exhibit a thalassemia major phenotype.
Confirmatory testing at the HRL is also important in confirming the diagnosis of α-thalassemia. Alpha-thalassemia major (four α-globin gene deletions; Hb Barts hydrops fetalis syndrome) is usually fatal. Prenatal diagnosis with intrauterine red cell transfusions are increasingly being utilized causing reevaluation of the approach to this disorder [7,25]. Deletional Hb H (three gene deletions) is characterized by steady-state moderate anemia with exacerbations during acute infections, hemolytic gallstones, pregnancy complications, and hemosiderosis in adulthood. Hb H-Constant Spring (two deletions and a structural mutation), the most common non-deletional α-globin mutation, is more severe and these patients often require transfusion therapy and splenectomy [5,6]. Two α-globin gene deletions cause α-thalassemia trait, with only a mild microcytic anemia in most cases. As one or two α-globin gene deletions do not generate levels of Hb Barts that exceed 25%, these cases can be missed by our HPLC protocol. However, α-thalassemia trait is extremely common in certain populations, with α-globin gene deletion carrier frequencies over 15% in certain populations [9]. This high trait frequency makes Hb H and Hb Barts disease relatively common, and the growing multicultural community in California has ushered in more α-thalassemia representation.
It is important to diagnose three α-globin gene deletions and mutations (Hb H and Hb H-CS), and also to differentiate one and two gene deletions, to make affected individuals aware of potential reproductive consequences. One and two α-globin gene deletions are associated with a benign phenotype, but the offspring of parents with such deletions may inherit three or even four gene deletions, both of which lead to more severe disease (Hb H disease and hydrops fetalis, respectively). The HRL carries out complex DNA analyses to detect and distinguish one and two α-globin gene deletions, which are tasks not accomplished by routine NBS. Hb Barts is more accurately diagnosed in the newborn period; because it is unstable, quantification of this hemoglobin after the neonatal period is unreliable. Therefore, determination of Hb H and/or DNA testing are required to make the diagnosis of Hb H disease, and to distinguish it from Hb H-CS, outside of the newborn period [10].
The success of a NBS program for any disease depends not only on the number of infants diagnosed, but on the timely fashion in which these children receive appropriate medical care. California’s NBS program for hemoglobinopathies includes a complex follow-up strategy that employs regional nurses to track positive results and ensure timely enrollment of infants into treatment systems. In a 4-year review, 97.6% of infants with positive initial results were successfully recalled for confirmatory testing. Furthermore, nearly 90% of infants diagnosed with SCD began prophylactic penicillin therapy by age 24 weeks [23].
Unfortunately, however, such timely follow-up is not always the case. Studies have found that many primary care physicians are not prepared to manage the follow-up care of children with a positive newborn screen [26]. Although most pediatricians have had a patient with a positive newborn screen, many report that they are not competent to discuss conditions included in the NBS panels [27].
This is the largest report of neonatal α-thalassemia that has been published to date, and illustrates the critical importance of a heightened awareness of this disease among the medical community. In addition, prospective studies of the natural history of Hb H disease are much needed. Linguistic isolation and socioeconomic barriers have impaired the ability to provide comprehensive care to these patients in the past, and many of these patients are not undergoing counseling [4]. Genetic counseling of α-thalassemia has become a public health emergency.
The state’s screening program and confirmatory testing at the HRL provide not only early diagnosis of hemoglobinopathies but also early intervention. However, due to the increasing incidence of these diseases in North America, new strategies are needed to ensure appropriate delivery of health care to these patients. Physicians will need to provide comprehensive education and management to this growing and diverse group of patients, and a multidisciplinary approach is necessary.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: M01-RR01271.
This work was supported in part by National Institutes of Health grant M01-RR01271. We would like to thank Erica Riray for providing assistance with editing and manuscript preparation.
References
- 1.Vichinsky E, Hurst D, Earles A, et al. Newborn screening for sickle cell disease: Effect on mortality. Pediatrics. 1988;81:749–755. [PubMed] [Google Scholar]
- 2.Rogers DW, Clarke JM, Cupidore L, et al. Early deaths in Jamaican children with sickle cell disease. Br Med J. 1978;1:1515–1516. doi: 10.1136/bmj.1.6126.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A. randomized trial. N Engl J Med. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 4.Vichinsky EP, MacKlin EA, Waye JS, et al. Changes in the epidemiology of thalassemia in North America: A new minority disease. Pediatrics. 2005;116:e818–e825. doi: 10.1542/peds.2005-0843. [DOI] [PubMed] [Google Scholar]
- 5.Chen FE, Ooi C, Ha SY, et al. Genetic and clinical features of hemoglobin H disease in Chinese patients. N Engl J Med. 2000;343:544–550. doi: 10.1056/NEJM200008243430804. [DOI] [PubMed] [Google Scholar]
- 6.Chui DH, Fucharoen S, Chan V. Hemoglobin H disease: Not necessarily a benign disorder. Blood. 2003;101:791–800. doi: 10.1182/blood-2002-07-1975. [DOI] [PubMed] [Google Scholar]
- 7.Lorey F, Charoenkwan P, Witkowska HE, et al. Hb H hydrops foetalis syndrome: A case report and review of literature. Br J Haematol. 2001;115:72–78. doi: 10.1046/j.1365-2141.2001.03080.x. [DOI] [PubMed] [Google Scholar]
- 8.Origa R, Sollaino MC, Giagu N, et al. Clinical and molecular analysis of haemoglobin H disease in Sardinia: Haematological, obstetric and cardiac aspects in patients with different genotypes. Br J Haematol. 2007;136:326–332. doi: 10.1111/j.1365-2141.2006.06423.x. [DOI] [PubMed] [Google Scholar]
- 9.Glader BE, Look KA. Hematologic disorders in children from southeast Asia. Pediatr Clin North Am. 1996;43:665–681. doi: 10.1016/s0031-3955(05)70427-2. [DOI] [PubMed] [Google Scholar]
- 10.Lorey F, Cunningham G, Vichinsky EP, et al. Universal newborn screening for Hb H disease in California. Genet Test. 2001;5:93–100. doi: 10.1089/109065701753145538. [DOI] [PubMed] [Google Scholar]
- 11.Vichinsky E. Hemoglobin E syndromes. Hematology Am Soc Hematol Educ Program. 2007;2007:79–83. doi: 10.1182/asheducation-2007.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Jetsrisuparb A, Sanchaisuriya K, Fucharoen G, et al. Development of severe anemia during fever episodes in patients with hemoglobin E trait and hemoglobin H disease combinations. J Pediatr Hematol Oncol. 2006;28:249–253. doi: 10.1097/01.mph.0000212910.99394.e0. [DOI] [PubMed] [Google Scholar]
- 13.Lorey FW, Cunningham GC, Vichinsky E, et al. Detection of Hb E/beta-thalassemia versus homozygous EE using high-performance liquid chromatography results from newborns. Biochem Med Metab Biol. 1993;49:67–73. doi: 10.1006/bmmb.1993.1007. [DOI] [PubMed] [Google Scholar]
- 14.Reed W, Lane PA, Lorey F, et al. Sickle-cell disease not identified by newborn screening because of prior transfusion. J Pediatr. 2000;136:248–250. doi: 10.1016/s0022-3476(00)70110-7. [DOI] [PubMed] [Google Scholar]
- 15.Eastman JW, Wong R, Liao CL, et al. Automated HPLC screening of newborns for sickle cell anemia and other hemoglobinopathies. Clin Chem. 1996;42:704–710. [PubMed] [Google Scholar]
- 16.Lorey F, Cunningham G, Shafer F, et al. Universal screening for hemoglobinopathies using high-performance liquid chromatography: Clinical results of 2. 2 million screens. Eur J Hum Genet. 1994;2:262–271. doi: 10.1159/000472370. [DOI] [PubMed] [Google Scholar]
- 17.Loomis SJ, Go M, Kupeli L, et al. An automated system for sickle cell screening. Am Clin Lab. 1990;9:33–40. [PubMed] [Google Scholar]
- 18.Wilson JB, Wrightstone RN, Huisman TH. Rapid cation-exchange high-performance liquid chromatographic procedure for the separation and quantitation of hemoglobins S, C, and O Arab in cord blood samples. J Lab Clin Med. 1986;108:138–141. [PubMed] [Google Scholar]
- 19.Cremonesi L, Ferrari M, Giordano PC, et al. An overview of current microarray-based human globin gene mutation detection methods. Hemoglobin. 2007;31:289–311. doi: 10.1080/03630260701459366. [DOI] [PubMed] [Google Scholar]
- 20.Eng B, Patterson M, Borys S, et al. PCR-based diagnosis of the Filipino (–(FIL)) and Thai (–(THAI)) alpha-thalassemia-1 deletions. Am J Hematol. 2000;63:54–56. doi: 10.1002/(sici)1096-8652(200001)63:1<54::aid-ajh12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Fischel-Ghodsian N, Vickers MA, Seip M, et al. Characterization of two deletions that remove the entire human zeta-alpha globin gene complex (- -THAI and - -FIL) Br J Haematol. 1988;70:233–238. doi: 10.1111/j.1365-2141.1988.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 22.Panel SCDG. Sickle Cell Disease: Screening, Diagnosis, Management, and Counseling in Newborns and Infants. Rockville: Public Health Service, U.S. Department of Health and Human Services; M.A.f.H.C.P.a.R. Clinical Practice Guideline No. 6. AHCPR Pub. No. 93 0562. Editor April 1993. [Google Scholar]
- 23.Shafer FE, Lorey F, Cunningham GC, et al. Newborn screening for sickle cell disease: 4 years of experience from California’s newborn screening program. J Pediatr Hematol Oncol. 1996;18:36–41. doi: 10.1097/00043426-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Therrell B, Pass K. Hemoglobinopathy screening laboratory techniques for newborns. In: Therrell B, editor. Laboratory methods for neonatal screening. Washington, DC: American Public Health Association; 1993. pp. 169–189. [Google Scholar]
- 25.Singer ST, Styles L, Bojanowski J, et al. Changing outcome of homozygous alpha-thalassemia: Cautious optimism. J Pediatr Hematol Oncol. 2000;22:539–542. doi: 10.1097/00043426-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Farrell MH, La Pean A, Ladouceur L. Content of communication by pediatric residents after newborn genetic screening. Pediatrics. 2005;116:1492–1498. doi: 10.1542/peds.2004-2611. [DOI] [PubMed] [Google Scholar]
- 27.Kemper AR, Uren RL, Moseley KL, et al. Primary care physicians’ attitudes regarding follow-up care for children with positive newborn screening results. Pediatrics. 2006;118:1836–1841. doi: 10.1542/peds.2006-1639. [DOI] [PubMed] [Google Scholar]