Abstract
[Purpose] This study evaluated the immediate and short-term effects of a combination of prolonged passive muscle stretching (PMS) and whole body vibration (WBV) on the spasticity, strength and balance of children and adolescents with cerebral palsy. [Subjects and Methods] A randomized two-period crossover trial was designed. Twelve subjects with cerebral palsy aged 10.6 ± 2.4 years received both PMS alone as a control group (CG) and a combination of PMS and WBV as an experimental group (EG). After random allocation to the trial schedules of either EG-CG or CG-EG, CG received prolonged PMS while standing on a tilt-table for 40 minutes/day, and EG received prolonged PMS for 30 minutes, followed by 10 minutes WBV. Both CG and EG received the treatment 5 days/week for 6 weeks. [Results] Immediately after one treatment, EG resulted in better improvement in scores on the Modified Ashworth Scale than CG. After the 6-week intervention, EG also showed significantly decreased scores on the Modified Ashworth Scale compared to CG. Both CG and EG showed significantly reduced the performance times in the five times sit to stand test, and EG also showed significantly increased scores on the pediatric balance scale. [Conclusion] This study showed that 6 weeks of combined prolonged PMS and WBV had beneficial effects on the spasticity, muscle strength and balance of children and adolescents with CP.
Key words: Modified Ashworth Scale, Five times sit to stand test, Cerebral palsy
INTRODUCTION
Muscle dysfunction of the leg is very common in children with spastic diplegia cerebral palsy (CP)1). Spasticity causes muscle stiffness and weakness, and decreases daily functional activities including standing and walking2). Passive muscle stretching is a common physical therapy for decreasing the spasticity of children and adults with CP spasticity. It has been reported that prolonged passive muscle stretching improves the range of movements and reduces spasticity3). Prolonged passive muscle stretching while standing on a tilt-table decreases the resistance to passive ankle joint movements in children with CP4). Therefore, it has been suggested as a treatment technique for children and adults with CP. Whole body vibration has also been proposed as a treatment for children and adults with spastic CP. It is simply applied while subjects stand on an oscillating platform. Some studies have shown that whole body vibration decreases the spasticity of patients with stroke, spinal cord injury and multiple sclerosis5,6,7). A whole body vibration intervention decreases spasticity and improves walking speed, muscle strength and gross motor function without any side effects in adults with CP7). Because both muscle stretching and vibration have underlying treatment efficacy in the alleviation of spasticity, and promote muscle function in adults with CP, a therapeutic program combining passive muscle stretching and whole body vibration may show better treatment effects in children and adolescents with spastic CP. However, no study has yet combined passive muscle stretching with whole body vibration for children and adolescents with spastic CP. Therefore, the aim of this study was to investigate the effect of combining passive muscle stretching and whole body vibration on the spasticity, functional strength and balance of children and adolescents with spastic CP.
SUBJECTS AND METHODS
Twelve children and adolescents with spastic CP aged 6–18 years (age, 10.58 ± 2.35 years; height, 127.25 ± 9.72 cm; body mass, 26.48 ± 7.95 kg; mean ± SD) were recruited from Khon Kaen Special School. Subjects were classified for gross motor function at levels I, II and III according to the Gross Motor Function Classification System (GMFCS), and were included in the study when their Modified Ashworth Scale (MAS) scores were greater than or equal to 1. Exclusion criteria for this study: were receiving PMS more than 3 times/week; medical treatment for spasticity such as a drugs, botulinum toxin injection, or orthopedic surgery during the last six months; medical problems such as arthritis, congenital abnormality, cardiovascular or pulmonary diseases, or neuromuscular diseases; musculoskeletal disorders; or infectious disease. This study was approved by the local Ethics Committee for Human Research of Khon Kaen University. Parents of the children and the children read and signed an informed consent form before the start of the study.
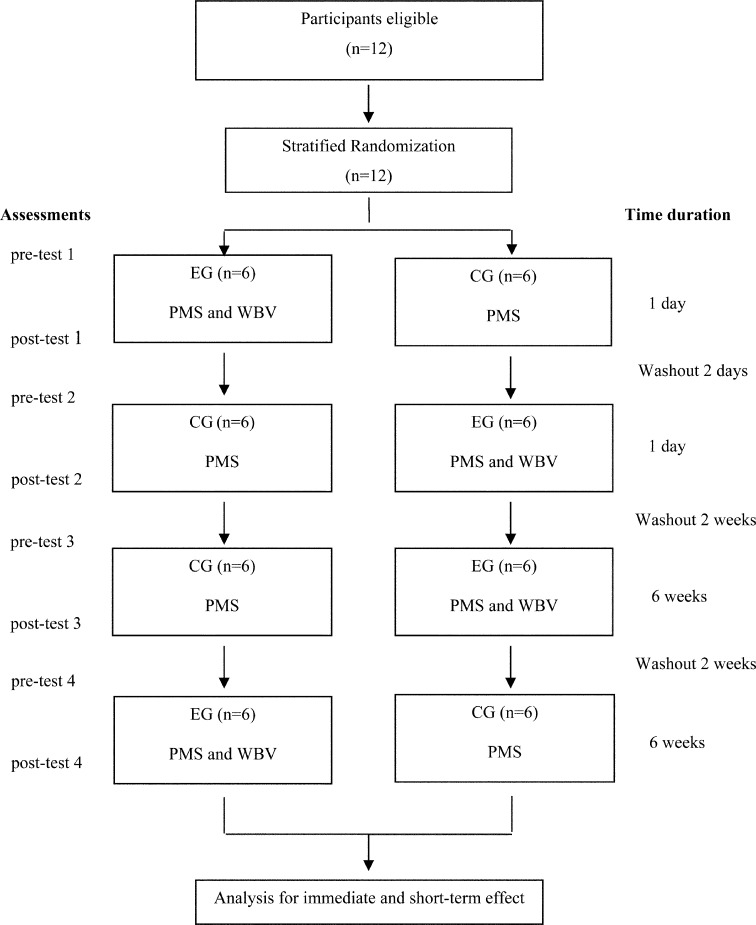
The study was designed as a two-period cross-over trial in which all subjects received both, passive muscle stretching alone as a control group (CG), and a combination of passive muscle stretching and whole body vibration as an experimental group (EG), as shown in Fig. 1. Both treatment groups were evaluated for immediate effect (one treatment) and for short-term effect (6 weeks treatment). The sample size was calculated based on a primary Modified Ashworth Scale (MAS) outcome which was determined as the predicted value (the post-test value of MAS decreased 20%). The minimum requirement of subjects was 12. Sampling randomization was stratified according to the GMFCS from levels I to III, and ages of children (6 to <10 years), and adolescents (10–19 years). After the baseline assessment of all subjects, 12 children were randomly assigned to either EG-CG or CG-EG. In EG-CG, the number of subjects with GMFCS levels I, II and III was 1, 1 and 4, respectively, and 3 of the 6 subjects were under the age of 10 while in CG-EG, the number of subjects with GMFCS levels I, II and III was 1, 2 and 3, respectively and 2 of the 6 subjects were under the age of 10. The GMFCS levels and ages were similar in both groups and each group was crossed over at the end of each phase was so that each group received the same treatment. In phase 1, subjects were evaluated for outcome variables before and immediately after a one-time treatment and in phase 2, the procedure was repeated after group crossover. All subjects rested for 2 days for a washout between phases 1 and 2. After phase 2 had finished, subjects rested for the 2-day washout period, and in phase 3, all individuals continuously received treatment for 6 weeks. After phase 3, a washout period of 2 weeks was conducted before group crossover for phase 4. Pre-and post-treatment measurements were conducted in phases 3 and 4.
Fig. 1.
The flow diagram of the randomized two-period crossover trial. CG: control group; EG: experimental group; PMS: passive muscle stretching; WBV: whole body vibration
For CG, passive muscle stretching was performed while the subjects stood on a tilt table for 40 minutes per session. The table was tilted to 70–80 degrees relative to the horizontal plane, and two straps around the chest and knees helped to support the body. For EG, a combination of passive muscle stretching and whole body vibration was conducted. Whole body vibration was applied at 20 Hz on the oscillation platform (AIKO vibrator, ETF-001CG, Thailand). After passive muscle stretching for 30 minutes, a total of 10 minutes of one-minute vibration with one minute rest was performed while the subjects stood with equal weight-bearing on both feet. Subjects stood in a standardized foot position on the platform as recommended by the manufacturer with center of the platform located between the legs placed shoulder-width apart. The amplitude of vibration becomes larger when the feet are placed further from the center line. For safety reasons, some subjects held a handle-bar during the vibration. Both the CG and EG interventions were conducted for a total of 40 minutes treatment/day, 5 days/week for 6 weeks, in addition to the regular physiotherapy program at school of 1 session/week. Before and after the single treatments and the 6-week treatments, subjects were evaluated using the Modified Ashworth Scale (MAS), the five times sit-to-stand test (FTSST) and the pediatric balance scale (PBS).
The Modified Ashworth Scale (MAS) was a primary outcome. MAS is known as an essential clinical measure of muscle spasticity in people with neurological conditions8). The hip adductor, quadriceps, hamstrings, and soleus muscles were evaluated. The topographic distribution of impairment was determined as the leg with lower or higher spasticity which represented the weaker or stronger side, respectively. MAS scores were assigned numerical values for the evaluated MAS scores (0, 1, 2, 3, 4 and 5). A value of 0 indicates no increase in muscle tone; 1 (1 point) is a slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part is moved in flexion or extension; 1+ (2 points) is a slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM (range of movement); 2 (3 points) is a more marked increase in muscle tone through most of the ROM, but the affected part is easily moved; 3 (4 points) is a considerable increase in muscle tone passivity, with movement difficult; 4 (5 points) is an affected part rigid in flexion or extension. MAS was assessed by the same evaluator throughout the experiment, and outcomes were tested for the intra-tester reliability.
The five times sit-to-stand test (FTSST) is a reliable tool for evaluating lower limb muscle strength and balance ability9). Subjects sat on a chair with their arms crossed over the chest, and they were asked to stand up and sit down again as quickly as possible five times without using arm support. The test began with the word “Go” and stopped when subjects sat after the fifth performance; the time was recorded.
The pediatric balance scale (PBS) is a modification of the Berg Balance Scale, which was developed for the evaluation of the balance ability of school-age children with mild to moderate motor impairments. PBS has been demonstrated to have good test-retest and inter-rater reliabilities10). There are 14 items and each item is scored on a five point ordinal scale from zero to four with a maximum score of 56 points. In order to demonstrate each task and give instruction, each child was allowed to practice each item once. If the child was unable to complete the task based on their inability to understand the directions, a second practice trial was allowed, and the best score was recorded11). PBS took approximately 15 minutes to conduct, and if some children needed more time or felt fatigued during the test they were allowed to rest for a few minutes.
The distribution of the data was examined using the Shapiro-Wilk’s test to examine the assumption of normality. All results were not normally distributed. Non-parametric tests were used for all outcomes. The data are presented as the median and quartiles. The differences of variables within and between the groups were assessed with the Wilcoxon Signed-Ranks test and the Mann-Whitney U-test, respectively. Significance was accepted for values of p< 0.05.
RESULTS
Tables 1 and 2 summarize the pre-and post-intervention measurement values of CG and EG. In the immediate effect of EG, MAS scores significantly decreased in all muscles of the stronger side and the soleus of the weaker side. In the immediate effect of CG, MAS scores significantly decreased in the quadriceps, hamstrings and soleus muscles of the stronger side. In the comparison of the groups, only the MAS score of the soleus of the weaker side was significantly improved by EG. After the 6-week treatment, all the evaluated items significantly improved in EG, and the MAS scores of the hamstrings and soleus of the stronger side and FTSST improved in CG. In the comparison of the groups, the MAS scores of the quadriceps and hamstrings of both sides, and soleus of the stronger side were more significantly decreased by EG than by CG. No significant differences between the groups were found for FTSST, PBS and MAS of the hip adductors.
Table 1. Pre-and post-intervention evaluation items of the control and experimental groups.
| Immediate effect | Short-term effect | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control group | Experimental group | Control group | Experimental group | |||||||
| Before | After | Before | After | Before | After | Before | After | |||
| MAS score | ||||||||||
| Stronger | ||||||||||
| Hip adductors | 2 (1:3.75) | 2 (1:2) | 2 (1:3) | 1 (1:2.75)* | 2 (1:3) | 2 (1:2.8) | 2 (1:3) | 1 (1:1)* | ||
| Quadriceps | 2.5 (2:3) | 1 (1:2)* | 2.5 (2:3) | 2 (1.25:2)* | 3 (1.3:3) | 2 (1.3:2.8) | 3 (1.3:3) | 1 (1:2)* | ||
| Hamstrings | 2 (1:3) | 1 (1:2)* | 2 (1:3) | 1 (1:1)* | 2.5 (2:3) | 2 (1.3:2.8)* | 3 (2:3) | 1 (1:1.8)** | ||
| Soleus | 2 (1.25:4.75) | 1.5 (1:3)* | 2 (1.25:4.75) | 1 (1:2)* | 3 (1.3:5) | 2 (1:4)* | 3 (2:5) | 1.5 (1:2.8)** | ||
| Weaker | ||||||||||
| Hip adductors | 2 (1:3.75) | 1 (1:3) | 2 (1:3.75) | 1 (1:3) | 1.5 (1:2.8) | 1.5 (1:2.0) | 1.5 (1:2.8) | 1 (1:1)* | ||
| Quadriceps | 2 (1:2.75) | 2 (1:2) | 2 (1:2.75) | 1 (1:2) | 2 (1:2) | 2 (1:2) | 2 (1:2) | 1 (1:1.8)* | ||
| Hamstrings | 1.5 (1:2.75) | 1 (1:2) | 1.5 (1:2.75) | 1 (1:1) | 1.5 (1:2) | 2 (1:2.8) | 1.5 (1:2) | 1 (1:1)* | ||
| Soleus | 2 (2:3.75) | 1.5 (1:3.75) | 2 (2:3.75) | 1 (1:2.5)** | 2.5 (1:4) | 2 (1:3) | 2 (1:3.8) | 1 (1:2.8)* | ||
| FTSST (s) | 16.59 (13.0:23.1) | 17.28 (10.6:22.7) | 16.93 (12.3:16.9) | 11.96 (10.9:19.9) | 13.34 (10.7:20.6) | 10.14 (9.6:12.7)* | 13.55 (11.8:21.9) | 11.36 (8.1:13.4)** | ||
| PBS (score) | 26.00 (7:42) | 25.00 (7.5:42.25) | 24.00 (8.5:42.25) | 26.50 (10:42.25) | 26.0 (7.3:45.8) | 27.5 (8.5:42.0) | 24.5 (8.5:42.0) | 31.0 (10.0:44.3)* | ||
Data are presented as median (quartile1:quartile3) for all outcomes. *p < 0.05, **p < 0.01
Table 2. Comparison of the median differences between the control and experimental groups.
| Immediate effect | Short-term effect | |||||
|---|---|---|---|---|---|---|
| Control group | Experimental group | Control group | Experimental group | |||
| MAS score | ||||||
| Stronger | ||||||
| Hip adductors | 0 (−1.0 : 0) | 0 (−1.0 : 0) | 0 (−1 : 0.75) | −0.5 (−1 : 0) | ||
| Quadriceps | −1.0 (−1.75 : 0) | −1.0 (−1 : 0) | 0 (−1 : 0) | −1.0 (−1 : −1)* | ||
| Hamstrings | −0.5 (−1.0 : 0) | −1.0 (−2.0 : 0) | −0.5 (−1 : 0) | −1.0 (−2 : −1)* | ||
| Soleus | 0 (−1.0 : 0) | −1.0 (−2.0 : 0) | 0 (−1 : 0) | −1.0 (−1.75 : −0.25)* | ||
| Weaker | ||||||
| Hip adductors | 0 (−0.75 : 0) | 0 (−1.0 : 0) | 0 (0 : 0) | 0 (−1 : 0) | ||
| Quadriceps | 0 (−0.75 : 0) | 0 (−1.75 to 0) | 0 (0 : 0) | −0.5 (−1 : 0)* | ||
| Hamstrings | 0 (−1.0 : 0) | 0 (−1.75 : 0) | 0 (0 : 0.75) | −0.5 (−1 : 0)* | ||
| Soleus | 0 (−1.0 : 0) | −1.0 (−1.75 : −0.25)* | 0 (−1 : 0) | −1.0 (−1 : 0) | ||
| FTSST (s) | −1.18 (−3.0 : −1.6) | −2.61 (−5.3 : −1.1) | −3.28 (−6.70 : −0.51) | −4.46 (−8.09 : −2.41) | ||
| PBS (score) | 0 (0 : 1) | 0 (0 : 1) | 0 (−2 : 2.75) | 2.5 (0 : 4) | ||
Data are presented as the median difference (quartile 1: quartile 3) for all outcomes. *p < 0.05
DISCUSSION
The results show the combined treatment of PMS and WBV had better effects than PMS alone on the spasticity of children and adolescents with CP. Immediately after one treatment, the combined treatment showed better improvement in the scores of the MAS than PMS alone. After the 6-week intervention, the combined treatment significantly decreased the scores of the MAS when compared to PMS alone. Both treatments significantly reduced the children’s performance times in the five times sit-to-stand test, while the combined treatment significantly increased the scores of the pediatric balance scale. Thus, the results suggest that a 6-week intervention of a combination of prolonged PMS and WBV may elicit beneficial effects for the spasticity and balance of patients with cerebral palsy.
Prolonged passive muscle stretching is a common treatment for people with spasticity CP. Sustained passive muscle stretching for a long duration improves the range of movements, and reduces the spasticity of muscles11, 12). Passive muscle stretching activates Golgi tendon organs and inhibits the excitability of alpha motor neurons. Reduced motoneuronal excitability leads to an increase in the extensibility of soft tissues13). Current evidence supports the effectiveness of passive stretching of children with spastic CP, although the reduction in spasticity may not be linked with functional activities such as walking3). A combination of exercise, massage therapy and therapeutic supports is an effective treatment for the release of muscle stiffness, decrease of pain, and improvement of physical functions14,15,16). Also, dynamic stretching exercise improves joint mobility17). Therefore, different therapeutic methods should be used to normalize muscle tone, maintain or increase soft-tissue extensibility, reduce contracture pain, and improve motor function18).
Whole body vibration improved the MAS score of the soleus on the weaker side. Oscillation of the ankle joints with a vibrator helps to induce release of a stiff ankle joint. During vibration, standing in a neutral ankle position with body weight bearing also stimulates the stretching of calf muscles. Whole body vibration decreases the spasticity of muscles and increases the gross motor function of subjects with CP7, 19). Whole body vibration improved muscle strength and power movement similar to conventional resistance training in older women20) and in adults with CP7). Whole body vibration is a mechanical stimulus characterized by an oscillatory motion. The muscle spindle senses a very small change in muscle length when a skeletal muscle perceives vibration. This information is transmitted to the fibers of group Ia or II, eventually reaching the spinal cord. In the spinal cord, the information serves as presynaptic inhibition through an interstitial cell and suppresses the alpha motor neurons21, 22). The activation of these sensory receptors results in reflexive activation of motor units similar to the tonic vibration reflex23). Whole body vibration can also improve the balance of stroke patients24, 25). Whole body vibration improved the performance of multiple sclerosis patients in the Timed Up & Go Test26). This improvement in physical balance after whole body vibration may be associated with improvement of leg muscle strength27). The activation of proprioreceptive spinal circuits can be induced by upright standing on a vibration platform. Because reflexes are related to the time-differential activation of spindles in muscles and tendons28), the improvement in balance might be positively related to muscle strength and proprioception after vibration training. Whole body vibration may allow children and adolescents with CP to walk effectively with an improvement of balance. Similar to our results, previous research has shown that whole body vibration improves the muscle tone, strength, balance and mobility of children with CP29,30,31); however, those studies used a vibration frequency with stepwise increment up to 18 Hz compared to the constant vibration frequency of 20 Hz used in the present study. Thus, future research should investigate the effect of different frequencies of vibration.
A limitation of this study was the small number of subjects and the absence of double-blinding of subjects and physiotherapists. With regard to adherence to treatment, all subjects enjoyed whole body vibration therapy and engaged in regular interaction with the physiotherapists. Therefore, whole body vibration should be added to the physiotherapy program. Although improvements in outcomes were found in this study, the optimal dose-response vibration was not clear. Thus, further studies should focus on the training protocol or whole body vibration that can provide the best results for children and adolescents with CP.
In conclusion, this present study showed that 6 weeks of combined passive muscle stretching and whole body vibration could decrease the spasticity and increase the muscle strength and balance of children and adolescents with CP. Whole body vibration could be an alternative additional treatment to passive muscle stretching for both clinical and home therapy programs for children and adolescents with CP.
Acknowledgments
The authors would like to thank all who participated in this study. The study was supported by the Research Center of the Back, Neck, Other Joint Pain and Human Performance, Faculty of Associated Medical Sciences, Khon Kaen University, Thailand. Additional thanks to Srisangwan, Khon Kaen Special School for the location and equipment that was used in this study.
REFERENCES
- 1.Sutherland DH, Davids JR: Common gait abnormalities of the knee in cerebral palsy. Clin Orthop Relat Res, 1993, (288): 139–147. [PubMed] [Google Scholar]
- 2.Flett PJ: Rehabilitation of spasticity and related problems in childhood cerebral palsy. J Paediatr Child Health, 2003, 39: 6–14. [DOI] [PubMed] [Google Scholar]
- 3.Pin T, Dyke P, Chan M: The effectiveness of passive stretching in children with cerebral palsy. Dev Med Child Neurol, 2006, 48: 855–862. [DOI] [PubMed] [Google Scholar]
- 4.Richards CL, Malouin F, Dumas F: Effects of a single session of prolonged plantarflexor stretch on muscle activations during gait in spastic cerebral palsy. Scand J Rehabil Med, 1991, 23: 103–111. [PubMed] [Google Scholar]
- 5.Sayenko DG, Masani K, Alizadeh-Meghrazi M, et al. : Acute effects of whole body vibration during passive standing on soleus H-reflex in subjects with and without spinal cord injury. Neurosci Lett, 2010, 482: 66–70. [DOI] [PubMed] [Google Scholar]
- 6.Schyns F, Paul L, Finlay K, et al. : Vibration therapy in multiple sclerosis: a pilot study exploring its effects on tone, muscle force, sensation and functional performance. Clin Rehabil, 2009, 23: 771–781. [DOI] [PubMed] [Google Scholar]
- 7.Ahlborg L, Andersson C, Julin P: Whole-body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. J Rehabil Med, 2006, 38: 302–308. [DOI] [PubMed] [Google Scholar]
- 8.Bohannon RW, Smith MB: Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther, 1987, 67: 206–207. [DOI] [PubMed] [Google Scholar]
- 9.Kumban W, Amatachaya S, Emasithi A, et al. : Five-times-sit-to-stand test in children with cerebral palsy: reliability and concurrent validity. NeuroRehabilitation, 2013, 32: 9–15. [DOI] [PubMed] [Google Scholar]
- 10.Franjoine MR, Gunther JS, Taylor MJ: Pediatric balance scale: a modified version of the berg balance scale for the school-age child with mild to moderate motor impairment. Pediatr Phys Ther, 2003, 15: 114–128. [DOI] [PubMed] [Google Scholar]
- 11.Stevenson VL: Rehabilitation in practice: spasticity management. Clin Rehabil, 2010, 24: 293–304. [DOI] [PubMed] [Google Scholar]
- 12.Tremblay F, Malouin F, Richards CL, et al. : Effects of prolonged muscle stretch on reflex and voluntary muscle activations in children with spastic cerebral palsy. Scand J Rehabil Med, 1990, 22: 171–180. [PubMed] [Google Scholar]
- 13.Wiart L, Darrah J, Kembhavi G: Stretching with children with cerebral palsy: what do we know and where are we going? Pediatr Phys Ther, 2008, 20: 173–178. [DOI] [PubMed] [Google Scholar]
- 14.Hongsuwan C, Eungpinichpong W, Chatchawan U, et al. : Effects of Thai massage on physical fitness in soccer players. J Phys Ther Sci, 2015, 27: 505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peungsuwan P, Sermcheep P, Harnmontree P, et al. : The effectiveness of Thai exercise with traditional massage on the pain, walking ability and QOL of older people with knee osteoarthritis: a randomized controlled trial in the community. J Phys Ther Sci, 2014, 26: 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prommanon B, Puntumetakul R, Puengsuwan P, et al. : Effectiveness of a back care pillow as an adjuvant physical therapy for chronic non-specific low back pain treatment: a randomized controlled trial. J Phys Ther Sci, 2015, 27: 2035–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatchawan U, Jupamatangb U, Chanchitc S, et al. : Immediate effects of dynamic sitting exercise on lower back mobility in sedentary young adults. J Phys Ther Sci, (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bovend’Eerdt TJ, Newman M, Barker K, et al. : The effects of stretching in spasticity: a systematic review. Arch Phys Med Rehabil, 2008, 89: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 19.Cheng HY, Yu YC, Wong AM, et al. : Effects of an eight-week whole body vibration on lower extremity muscle tone and function in children with cerebral palsy. Res Dev Disabil, 2015, 38: 256–261. [DOI] [PubMed] [Google Scholar]
- 20.Roelants M, Delecluse C, Goris M, et al. : Effects of 24 weeks of whole body vibration training on body composition and muscle strength in untrained females. Int J Sports Med, 2004, 25: 1–5. [DOI] [PubMed] [Google Scholar]
- 21.Gillies JD, Lance JW, Neilson PD, et al. : Presynaptic inhibition of the monosynaptic reflex by vibration. J Physiol, 1969, 205: 329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinohara M, Moritz CT, Pascoe MA, et al. : Prolonged muscle vibration increases stretch reflex amplitude, motor unit discharge rate, and force fluctuations in a hand muscle. J Appl Physiol 1985, 2005, 99: 1835–1842. [DOI] [PubMed] [Google Scholar]
- 23.Pollock RD, Woledge RC, Martin FC, et al. : Effects of whole body vibration on motor unit recruitment and threshold. J Appl Physiol 1985, 2012, 112: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi SJ, Shin WS, Oh BK, et al. : Effect of training with whole body vibration on the sitting balance of stroke patients. J Phys Ther Sci, 2014, 26: 1411–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tankisheva E, Bogaerts A, Boonen S, et al. : Effects of intensive whole-body vibration training on muscle strength and balance in adults with chronic stroke: a randomized controlled pilot study. Arch Phys Med Rehabil, 2014, 95: 439–446. [DOI] [PubMed] [Google Scholar]
- 26.Schuhfried O, Mittermaier C, Jovanovic T, et al. : Effects of whole-body vibration in patients with multiple sclerosis: a pilot study. Clin Rehabil, 2005, 19: 834–842. [DOI] [PubMed] [Google Scholar]
- 27.Lord SR, Murray SM, Chapman K, et al. : Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci, 2002, 57: M539–M543. [DOI] [PubMed] [Google Scholar]
- 28.Semler O, Fricke O, Vezyroglou K, et al. : Preliminary results on the mobility after whole body vibration in immobilized children and adolescents. J Musculoskelet Neuronal Interact, 2007, 7: 77–81. [PubMed] [Google Scholar]
- 29.El-Shamy SM: Effect of whole-body vibration on muscle strength and balance in diplegic cerebral palsy: a randomized controlled trial. Am J Phys Med Rehabil, 2014, 93: 114–121. [DOI] [PubMed] [Google Scholar]
- 30.Lee BK, Chon SC: Effect of whole body vibration training on mobility in children with cerebral palsy: a randomized controlled experimenter-blinded study. Clin Rehabil, 2013, 27: 599–607. [DOI] [PubMed] [Google Scholar]
- 31.Ruck J, Chabot G, Rauch F: Vibration treatment in cerebral palsy: a randomized controlled pilot study. J Musculoskelet Neuronal Interact, 2010, 10: 77–83. [PubMed] [Google Scholar]