Abstract
Background and Objective
The anti-oestrogen tamoxifen requires metabolic activation to endoxifen by cytochrome P450 (CYP) enzymes, predominantly CYP2D6. Potent CYP2D6-inhibiting antidepressants can seriously disrupt tamoxifen metabolism, probably influencing the efficacy of tamoxifen. For this reason, paroxetine and fluoxetine are recommended not to be used with tamoxifen in breast cancer patients. We investigated the effects of switching potent CYP2D6-inhibiting antidepressants to weak CYP2D6-inhibiting antidepressants on the plasma pharmacokinetics of tamoxifen.
Methods
Ten breast cancer patients who were treated with tamoxifen in combination with a potent CYP2D6-inhibiting antidepressant (paroxetine or fluoxetine) for at least 4 weeks were enrolled. Under close supervision by a psychiatrist, patients were switched to treatment with escitalopram or venlafaxine (weak CYP2D6-inhibiting antidepressants). Before and after the switch, pharmacokinetic blood sampling was performed over 24 h. Pharmacokinetic parameters were estimated using noncompartmental analysis. Adverse effects were recorded during the study.
Results
Endoxifen exposure was ~3-fold higher during escitalopram co-administration than during paroxetine or fluoxetine co-administration (median 387 nM·h [range 159–637 nM·h] versus 99.2 nM·h [range 70.0–210 nM·h]; P = 0.012; Wilcoxon signed-rank test). The ratio of endoxifen to N-desmethyltamoxifen and the ratio of 4-hydroxytamoxifen to tamoxifen increased by 3.3- and ~1.5-fold, reflecting increased CYP2D6 activity. Antidepressant switching did not result in psychiatric problems or antidepressant-related adverse effects.
Conclusion
In this study, switching to the weak CYP2D6 inhibitor escitalopram was safe and feasible and resulted in clinically relevant rises in endoxifen concentrations. We therefore advise switching paroxetine and fluoxetine to escitalopram in patients using tamoxifen. However, switching should always be weighed in individual patients.
Key Points
| Switching from potent cytochrome P450 (CYP) 2D6-inhibiting antidepressants to a weak CYP2D6-inhibiting antidepressant resulted in relevant rises in endoxifen systemic exposures in breast cancer patients. |
| The weak CYP2D6 inhibitor escitalopram seems to be a safe alternative in tamoxifen-treated patients requiring treatment with an antidepressant. |
| The potent CYP2D6-inhibiting antidepressants paroxetine and fluoxetine should be switched to escitalopram in tamoxifen-treated individuals. |
Introduction
Tamoxifen, a selective oestrogen receptor modulator, is the standard endocrine treatment for premenopausal women with hormone-sensitive breast cancer. In sequence with aromatase inhibitors, or as an alternative to aromatase inhibitors, tamoxifen can be given to postmenopausal women [1]. Tamoxifen reduces the 15-year risk of recurrence and breast cancer death in patients with early disease and prolongs survival in the metastatic setting. However, recurrence of disease and disease progression are observed in a substantial proportion of patients. Resistance to tamoxifen may be attributable to variability in exposure to the active metabolite [2, 3].
Tamoxifen is a pro-drug and undergoes metabolic activation to 4-hydroxytamoxifen and endoxifen. First, tamoxifen is metabolized to its primary metabolites, N-desmethyltamoxifen and 4-hydroxytamoxifen, catalysed by several cytochrome P450 (CYP) enzymes, including CYP3A, CYP2C9/19 and CYP2D6. Both primary metabolites can be metabolized to endoxifen. CYP3A and CYP2C9/19 are the main enzymes involved in the conversion of 4-hydroxytamoxifen to endoxifen, and CYP2D6 is the main enzyme for the conversion of N-desmethyltamoxifen to endoxifen [4, 5]. Endoxifen is considered to be the principal active metabolite of tamoxifen, and systemic concentrations of this metabolite probably need to exceed a threshold level for clinical efficacy in women with breast cancer [6–8].
The CYP2D6 enzyme has a key role in the metabolism of tamoxifen into endoxifen. It has been shown that patients carrying variant alleles of CYP2D6 produce little endoxifen [5, 9] and, although this has not consistently been shown, they may have a poorer clinical outcome [10–13]. CYP2D6-inhibiting medications may also interfere with tamoxifen therapy by reducing endoxifen concentrations. Selective serotonin reuptake inhibitors (SSRIs) and selective serotonin and norepinephrine reuptake inhibitors (SNRIs) are known to inhibit CYP2D6 to varying degrees. Because depressive disorder is common in breast cancer patients, but also for other indications, these antidepressant drugs are often co-prescribed in tamoxifen-treated individuals [9, 14, 15]. Paroxetine and fluoxetine are potent CYP2D6 inhibitors, which have been shown to markedly reduce endoxifen formation [7, 9] and to negatively affect the clinical outcome in women receiving tamoxifen [16, 17].
Venlafaxine and escitalopram have been proposed as safer options in patients using tamoxifen, with respect to their effects on endoxifen formation. Both drugs are weak CYP2D6 inhibitors and may reduce endoxifen concentrations only slightly [9, 14, 15]. However, an intra-patient comparison is lacking so far. Therefore, we investigated the effects of switching potent CYP2D6-inhibiting antidepressants to a weak CYP2D6-inhibiting alternative on the plasma pharmacokinetics of tamoxifen and its metabolites in breast cancer patients in a pharmacokinetic study.
Materials and Methods
Subjects
Women who were treated with 20 or 40 mg tamoxifen once daily in combination with a potent CYP2D6-inhibiting antidepressant (paroxetine or fluoxetine) for at least 4 weeks were included in the study. Other inclusion criteria were age >18 years; World Health Organization (WHO) performance score <1; and adequate haematological, renal and hepatic functions. The principal exclusion criteria were contra-indications for venlafaxine or escitalopram use, congenital long QT syndrome or suicidal ideation. Concomitant use of medications and/or supplements that could interact with tamoxifen or the antidepressant drugs was not allowed. Standard laboratory tests and an electrocardiogram were performed before the start of the study, and blood samples were obtained for CYP2D6 genotype determination. Informed consent forms were signed by all study participants before study entry, and the Erasmus MC review board approved the study protocol (Dutch Trial Registry; no. NTR3125).
Study Design
This was a prospective pharmacokinetic study designed to investigate the effects of switching from potent CYP2D6-inhibiting antidepressants (paroxetine or fluoxetine) to a weak CYP2D6 inhibitor (venlafaxine or escitalopram) on the plasma pharmacokinetics of tamoxifen and its metabolites. The study was performed between November 2011 and June 2014. Patients were asked to participate during regular visits to the outpatient clinic.
Under careful supervision by a psychiatrist (MB), patients were switched from paroxetine or fluoxetine to treatment with escitalopram or venlafaxine. The antidepressant therapy was individually adjusted, and switching strategies were supervised by the psychiatrist. Adverse effects and the use of concomitant medication were recorded by the patients during the study.
Once during concomitant use of tamoxifen and the potent CYP2D6-inhibiting antidepressant, and once during co-treatment with the weak CYP2D6 inhibitor, blood was collected for pharmacokinetic analyses of tamoxifen and its metabolites. The two periods were separated by an adequate wash-out period (30–80 days after the antidepressant switch, depending on the antidepressant). Since the switch between the antidepressants required dose tapering, the second day of blood sampling was dependent on the last day of paroxetine/fluoxetine intake.
Laboratory tests were performed on both days of blood sampling, and an additional electrocardiogram was obtained during the second sampling day, because patients were using the new antidepressant at that time.
Measurement of Tamoxifen and Its Main Metabolites in Plasma
Blood samples (4 mL; lithium-heparin) for the measurement of tamoxifen and its main metabolites were collected just before and at 0.5, 1, 1.5, 2, 4, 6, 8, 12 and 24 h after administration of tamoxifen. Plasma was isolated by centrifugation of the samples for 10 min at 2500g and was stored at −70° C until the analysis. The measurement of tamoxifen and its main metabolites in plasma was performed using a validated ultra-performance liquid chromatography (UPLC)–tandem mass spectrometry (MS/MS) assay, as described elsewhere [18].
Individual pharmacokinetic parameters, including the trough concentration (Ctrough) and maximum concentration (Cmax), were determined, and the area under the plasma concentration–time curve from time zero to 24 h (AUC0–24) was calculated by noncompartmental analysis using Phoenix WinNonlin 6.1 (Pharsight Corporation, Mountain View, CA, USA). The estimated parameters of patients who used 40 mg tamoxifen were corrected to 20 mg. The metabolic ratios were computed as AUC0–24metabolite/AUC0–24tamoxifen.
CYP2D6 Genotyping
Genomic DNA was isolated from whole blood, and genotype analyses for CYP2D6*3, *4, *6, *10, *17 and *41 were performed using TaqMan allelic discrimination assays on an ABI Prism 7000 Sequence detection system (Applied Biosystems, Foster City, CA, USA), and CYP2D6 gene deletion (*5) and duplication using a CYP2D6 TaqMan Gene Copy Number Assay.
Statistics
To detect a 25 % difference in the AUC0–24 of endoxifen between co-administration of a potent CYP2D6 inhibitor and a weak CYP2D6 inhibitor, with a two-sided 5 % significance level and power of 80 %, 13 study participants were required. This was based on a within-patient variation of 20 % in the pharmacokinetics of endoxifen.
Pharmacokinetic data are presented as medians and ranges. The differences in pharmacokinetic parameters, before and after the switch, were evaluated using Wilcoxon signed-rank tests for related samples. P values of ≤0.05 were regarded as statistically significant. Statistical tests were performed using IBM SPSS statistics, version 21 (SPSS Inc., Chicago, IL, USA).
Results
Pharmacokinetic data were available for ten patients (Table 1) [19, 20]. Because of problems with the blood sampling for pharmacokinetic analysis, only Ctrough values were available for two of these patients. Most women received adjuvant tamoxifen at a dose of 20 mg. Two women received a dose of 40 mg—one woman for metastatic disease and one because of extreme overweight.
Table 1.
Patient characteristics
| Patient | Age (years) | BMI (kg/m2) | Tamoxifen dose (mg) | Setting | Mental disordera | First antidepressant and doseb | Second antidepressant and dose | CYP2D6 genotype |
|---|---|---|---|---|---|---|---|---|
| 1 | 43 | 24.1 | 20 | Adjuvant | Depressive disorder | Paroxetine 15 mg | Escitalopram 15 mg | *1/*1 (EM) |
| 2 | 54 | 23.0 | 20 | Adjuvant | Depressive disorder | Paroxetine 60 mg | Escitalopram 20 mg | *1/*1 (EM) |
| 3 | 49 | 32.9 | 20 | Adjuvant | Depressive disorder | Paroxetine 20 mg | Citalopram 10 mgc | *1/*1 (EM) |
| 4 | 43 | 29.4 | 20 | Adjuvant | Anxiety disorder | Paroxetine 20 mg | Escitalopram 10 mg | *1/*4 (IM) |
| 5 | 55 | 33.6 | 20 | Adjuvant | Anxiety disorder | Fluoxetine 20 mg | Escitalopram 10 mg | *1/*1 (EM) |
| 6d | 48 | 45.2 | 40e | Adjuvant | Depressive disorder | Paroxetine 20 mg | Escitalopram 10 mg | *1/*1 (EM) |
| 7 | 59 | 26.4 | 20 | Adjuvant | Anxiety disorder | Paroxetine 20 mg | Escitalopram 10 mg | NA |
| 8 | 41 | 29.3 | 40 | Metastatic | Depressive disorder | Fluoxetine 30 mg | Escitalopram 10 mg | *1/*1 (EM) |
| 9 | 53 | 30.7 | 20 | Adjuvant | Anxiety disorder | Paroxetine 40 mg | Escitalopram 10 mg | *4/*41 (IM) |
| 10d | 62 | 32.0 | 20 | Adjuvant | Depressive disorder | Paroxetine 20 mg | Escitalopram 10 mg | *4/*41 (IM) |
BMI body mass index, C trough trough concentration, CYP cytochrome P450, EM extensive metabolizer, IM intermediate metabolizer, NA not available
aDiagnosed before initiation of tamoxifen therapy
bParoxetine and fluoxetine are equally potent inhibitors of CYP2D6 [19]
cOne woman received citalopram instead of escitalopram; however, the weak CYP2D6-inhibiting properties of the compounds are similar [20]
dBecause of problems with blood sampling, only C trough values were available
eBased on a high BMI
The women received antidepressants for the treatment of depressive disorder (n = 6) or anxiety disorder (n = 4), which was diagnosed before initiation of tamoxifen therapy. Eight women used paroxetine at a dose ranging from 15 to 60 mg per day; two women received fluoxetine at doses of 20 and 30 mg, respectively. Nine women were switched to escitalopram; seven patients received a dose of 10 mg per day, and two patients received higher doses of 15 and 20 mg, respectively, because of the nature of their conditions. By mistake, one woman received citalopram (a weak CYP2D6 inhibitor) at a dose of 10 mg. None of the women received venlafaxine. The ages of the study participants ranged from 41 to 62 years (median 51 years), and their body mass indices varied from 23.0 to 45.2 kg/m2 (median 30.0 kg/m2).
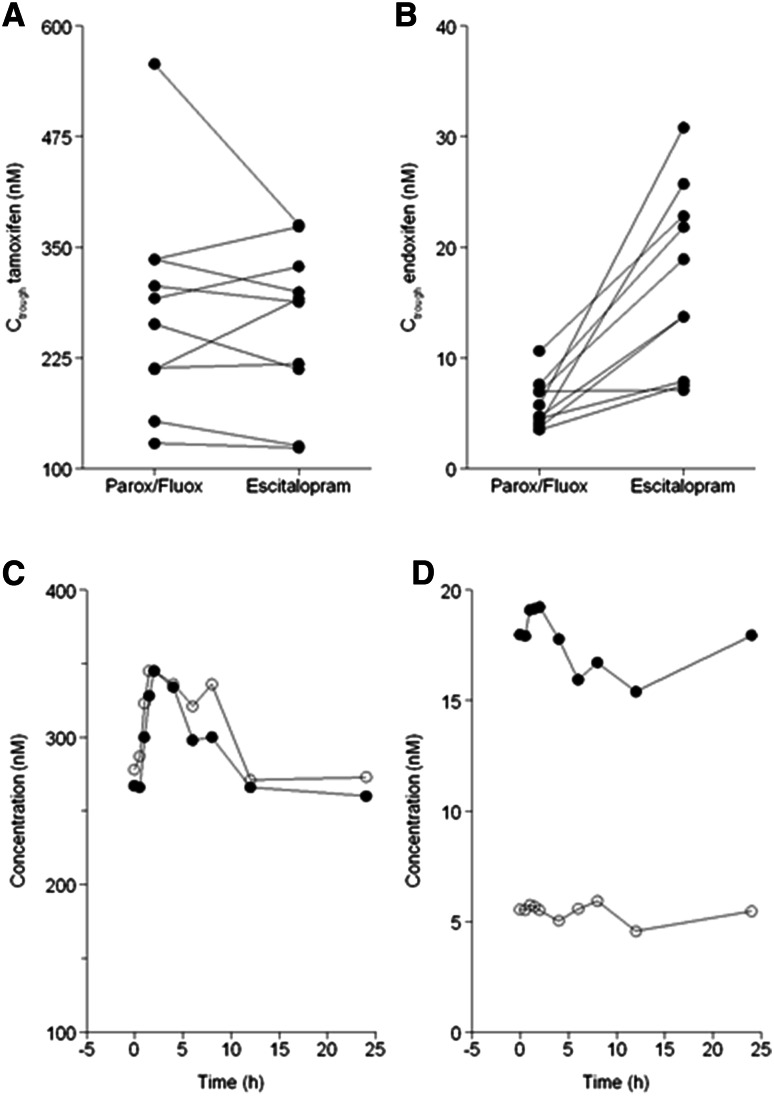
The pharmacokinetic parameters of tamoxifen and its three main metabolites during co-administration of paroxetine or fluoxetine and during escitalopram co-administration are listed in Table 2. Plasma concentration–time profiles of tamoxifen and endoxifen and individual changes in plasma exposures following the switch are shown in Fig. 1. Following the switch from a potent CYP2D6-inhibiting antidepressant to escitalopram, endoxifen plasma exposure increased markedly from 99.2 nM·h (range 70.0–210 nM·h) to 387 nM·h (range 159–637 nM·h; P = 0.012). The Ctrough and Cmax of endoxifen were also ~3-fold higher during escitalopram co-administration. The AUC0–24, Ctrough and Cmax of 4-hydroxytamoxifen increased by 34 % (P = 0.017), 40 % (P = 0.017) and 42 % (P = 0.036), respectively, after the switch. However, the pharmacokinetic parameters of tamoxifen and N-desmethyltamoxifen did not differ significantly between tamoxifen co-administration with paroxetine/fluoxetine and tamoxifen co-administration with escitalopram.
Table 2.
Effects of potent and weak cytochrome P450 (CYP) 2D6-inhibiting selective serotonin reuptake inhibitors (SSRIs) on tamoxifen pharmacokinetics
| Tamoxifen + potent CYP2D6-inhibiting SSRI | Tamoxifen + weak CYP2D6-inhibiting SSRI | Ratio of weak to potent CYP2D6-inhibiting SSRI | P valuea | |
|---|---|---|---|---|
| Tamoxifen | ||||
| C max (nM) | 369 (189–667) | 366 (177–516) | 0.97 (0.67–1.29) | 0.889 |
| C trough (nM)b | 278 (128–557) | 290 (123–375) | 0.95 (0.67–1.38) | 0.575 |
| AUC0–24 (nM·h) | 6422 (3574–12,182) | 6958 (3226–9567) | 0.98 (0.79–1.18) | 0.674 |
| ND-Tam | ||||
| C max (nM) | 528 (395–977) | 631 (365–955) | 1.09 (0.77–1.75) | 0.484 |
| C trough (nM)b | 446 (306–807) | 560 (312–704) | 0.97 (0.76–1.37) | 0.953 |
| AUC0–24 (nM·h) | 10,149 (7744–20,107) | 11,500 (7441–16,113) | 1.09 (0.75–1.27) | 0.674 |
| 4-OH-Tam | ||||
| C max (nM) | 3.46 (1.36–4.95) | 4.09 (2.42–8.25) | 1.42 (0.81–2.05) | 0.036 |
| C trough (nM)b | 2.47 (1.29–4.65) | 3.25 (1.99–6.06) | 1.40 (0.82–2.06) | 0.017 |
| AUC0–24 (nM·h) | 63.8 (27.4–98.2) | 85.8 (51.1–148) | 1.34 (0.88–1.87) | 0.017 |
| Endoxifen | ||||
| C max (nM) | 5.46 (3.86–11.1) | 23.1 (9.05–33.2) | 2.96 (1.50–7.44) | 0.012 |
| C trough (nM)b | 5.20 (3.48–10.6) | 16.3 (7.05–30.8) | 2.80 (1.02–6.33) | 0.005 |
| AUC0–24 (nM·h) | 99.2 (70.0–210) | 387 (159–637) | 2.98 (1.67–6.82) | 0.012 |
| Ratios | ||||
| Endoxifen to ND-Tam | 0.0113 (0.0065–0.014) | 0.0311 (0.018–0.057) | 3.33 (1.56–5.37) | 0.012 |
| 4-OH-Tam to tamoxifen | 0.0109 (0.0053–0.014) | 0.0149 (0.0084–0.020) | 1.51 (1.08–1.67) | 0.012 |
| Endoxifen to tamoxifen | 0.0213 (0.0057–0.029) | 0.0559 (0.034–0.10) | 2.85 (1.96–6.42) | 0.012 |
Potent CYP2D6-inhibiting SSRIs: paroxetine or fluoxetine; weak CYP2D6-inhibiting SSRIs: escitalopram (and, in one woman, citalopram)
Data are presented as median (range)
The parameters of one patient were dose corrected to 20 mg
4-OH-Tam 4-hydroxytamoxifen, AUC 0–24 area under the curve from 0 to 24 h, C max maximum concentration, C trough concentration before dosing, ND-Tam N-desmethyltamoxifen
aWilcoxon signed-rank test
b C trough data from 10 patients; the parameters of two patients were dose corrected to 20 mg
Fig. 1.
Individual changes in trough concentration (C trough) values for a tamoxifen and b endoxifen following the switch from a potent cytochrome P450 (CYP) 2D6-inhibiting antidepressant (paroxetine [Parox] or fluoxetine [Fluox]) to a weak CYP2D6-inhibiting antidepressant (escitalopram), and mean plasma concentration–time profiles for c tamoxifen and d endoxifen during concomitant use of paroxetine or fluoxetine (white circles) and during concomitant use of escitalopram (black circles)
Switching from a potent CYP2D6 inhibitor to a weak CYP2D6 inhibitor resulted in a more than 3-fold higher AUC0–24 ratio of endoxifen to N-desmethyltamoxifen and a ~1.5-fold higher ratio of 4-hydroxytamoxifen to tamoxifen ratio.
Adverse effects that were reported by the study participants included hot flashes, insomnia, nausea and joint pain. The adverse effects were mild and appeared not to be associated with antidepressant use. However, following the switch to the weak CYP2D6-inhibiting antidepressant, two individuals reported an increase in the incidence of hot flashes (up to twice as many periods of hot flashes per day), and in one woman the severity of hot flashes was increased (she suffered during a longer period from hot flashes).
Discussion
In this study, we evaluated for the first time whether switching from paroxetine or fluoxetine to escitalopram could increase endoxifen concentrations in women treated with tamoxifen. We observed that exposure to the active tamoxifen metabolites, particularly endoxifen, was considerably higher during co-administration with escitalopram than during concomitant use of paroxetine or fluoxetine. Because of the lesser degree of CYP2D6 inhibition, or no inhibition at all, during concomitant use of escitalopram, concentrations of 4-hydroxytamoxifen and endoxifen increased. This is further supported by the higher ratio of endoxifen to N-desmethyltamoxifen—and, to a lesser extent, the ratio of 4-hydroxytamoxifen to tamoxifen—during escitalopram co-administration, reflecting higher CYP2D6 activity. Although the increase in endoxifen exposure varied among the individuals, probably because of differences in CYP2D6 genotypes, even in women with the intermediate metabolizer genotype, endoxifen exposure increased following the SSRI switch.
The extremely low endoxifen concentrations during paroxetine co-administration were in line with previous findings by Stearns et al. [7], although the endoxifen concentrations they reported were slightly higher than those observed in our study. This observation is remarkable because patients in the present study received a weak CYP2D6-inhibiting antidepressant, whereas women in the previous study did not receive any CYP2D6-inhibiting medication concomitantly during the control phase [7]. This might be explained by the use of higher doses of paroxetine (>15 mg per day) in the current study, resulting in more potent CYP2D6 inhibition during paroxetine co-administration [21]. Also, we observed higher 4-hydroxytamoxifen concentrations after the switch.
Effective treatment of depression or anxiety disorders with antidepressants is vital for the disorder itself, and it may also contribute to better adherence to tamoxifen [22]. Concomitant use of potent CYP2D6-inhibiting antidepressants with tamoxifen is discouraged. Antidepressants with weak CYP2D6-inhibiting properties, such as escitalopram, have been recommended in tamoxifen-treated patients [14]. We demonstrated that during co-administration of escitalopram, women had endoxifen exposure that was similar to that observed in a genotype-matched cohort of tamoxifen-treated women not receiving CYP2D6-inhibiting co-treatment [23, 24]. No increase in the endoxifen Ctrough was observed in one woman following the switch. Besides having a CYP2D6 intermediate metabolizer genotype (CYP2D6*4/*41), this patient was using other medications. Although no interacting medications were allowed, the effects of the medications used by that patient on the pharmacokinetics of tamoxifen cannot be ruled out.
Although we found that escitalopram had little or no effect on endoxifen formation, the effect on breast cancer outcome is not completely clear. However, evidence suggests that endoxifen exposure is a predictor of tamoxifen efficacy. Madlensky et al. [8] reported a higher risk of breast cancer recurrence in patients with endoxifen concentrations below a minimal threshold level (15 nM). In our study, none of the women reached endoxifen concentrations above the proposed threshold concentration during co-treatment with the potent CYP2D6-inhibiting antidepressant. During escitalopram co-administration, five women with a CYP2D6 extensive metabolizer genotype had endoxifen concentrations above the threshold. Three women who did not reach endoxifen concentrations above the threshold level after the switch had impaired CYP2D6 metabolism according to their genotype (intermediate metabolizers; CYP2D6*4 allele). In one woman, the observed endoxifen exposure was dose corrected, because of the use of 40 mg tamoxifen instead of 20 mg. After the switch, endoxifen exposure was 2.9-fold higher in this patient, and the measured endoxifen Ctrough was well above the threshold concentration. The low endoxifen exposure in the other woman cannot be explained by her CYP2D6 genotype or other known factors. Despite 1.8-fold higher endoxifen concentrations following the switch, the endoxifen levels did not exceed the threshold concentration. Given that after the switch, women had still endoxifen concentrations below the threshold level, this indicates that therapeutic drug monitoring may be an important tool to individualize and optimize tamoxifen therapy.
Although this study was not designed to detect differences in side effects, it is interesting to note that hot flashes were reported particularly during escitalopram co-administration, which may have been due to higher endoxifen levels [25]. Few studies have investigated the relationship between endoxifen concentrations and hot flashes, and they reported contradictory results [25, 26]. This finding might also have been due to differences in the effectiveness of paroxetine/fluoxetine and escitalopram in treating hot flashes. None of the ten women had to discontinue escitalopram treatment.
Individuals were switched to escitalopram (10–20 mg/day); none of the patients received venlafaxine. Women were successfully switched, using cross-tapering, under careful supervision by an experienced psychiatrist. No antidepressant-related adverse events or psychiatric relapse were noted. However, although switching from paroxetine/fluoxetine to escitalopram was safe in this study, to ensure effective antidepressant treatment, switching should always be weighed in individual patients.
A limitation of the study might have been the small sample size; however, the results were unequivocal. Lack of adherence to tamoxifen or the antidepressant therapy might have influenced the results of the study. In addition, steady-state levels of tamoxifen metabolites were not reached in all patients, because not all women used tamoxifen for 4 months [27]. A period of 4 months to reach steady state has been suggested by Jin et al. [27]. However, steady-state levels may be reached after 2 months, on the basis of the 14-day half-life of the primary metabolite. In addition, this may have contributed to only small differences in the concentrations of tamoxifen metabolites.
Conclusion
Escitalopram seems to be a safe alternative in tamoxifen-treated patients requiring antidepressants. Clinically relevant increases in endoxifen exposure were observed following the switch to escitalopram. We strongly recommend switching paroxetine and fluoxetine to escitalopram in tamoxifen-treated breast cancer patients.
Compliance with Ethical Standards
Funding
No funding was received.
Conflict of interest
LB, MB, PdB, JR, HD, RJvA, TDdB, MHL, AJ, TvG and RM declare that there are no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Burstein HJ, Griggs JJ, Prestrud AA, Temin S. American Society of Clinical Oncology clinical practice guideline update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Oncol Pract. 2010;6:243–246. doi: 10.1200/JOP.000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Davies C, Godwin J, Gray R, Clarke M, Cutter D, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoskins JM, Carey LA, McLeod HL. CYP2D6 and tamoxifen: DNA matters in breast cancer. Nat Rev Cancer. 2009;9:576–586. doi: 10.1038/nrc2683. [DOI] [PubMed] [Google Scholar]
- 4.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 5.Murdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W, et al. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89:708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 6.Lim YC, Desta Z, Flockhart DA, Skaar TC. Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol. 2005;55:471–478. doi: 10.1007/s00280-004-0926-7. [DOI] [PubMed] [Google Scholar]
- 7.Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 8.Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89(5):718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80(1):61–74. doi: 10.1016/j.clpt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Goetz MP, Knox SK, Suman VJ, Rae JM, Safgren SL, Ames MM, et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat. 2007;101(1):113–121. doi: 10.1007/s10549-006-9428-0. [DOI] [PubMed] [Google Scholar]
- 11.Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S, et al. Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA. 2009;302(13):1429–1436. doi: 10.1001/jama.2009.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R, et al. CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the Breast International Group 1–98 Trial. J Natl Cancer Inst. 2012;104(6):441–451. doi: 10.1093/jnci/djs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rae JM, Drury S, Hayes DF, Stearns V, Thibert JN, Haynes BP, et al. CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst. 2012;104(6):452–460. doi: 10.1093/jnci/djs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zembutsu H, Sasa M, Kiyotani K, Mushiroda T, Nakamura Y. Should CYP2D6 inhibitors be administered in conjunction with tamoxifen? Expert Rev Anticancer Ther. 2011;11(2):185–193. doi: 10.1586/era.10.228. [DOI] [PubMed] [Google Scholar]
- 15.Binkhorst L, Mathijssen RH, van Herk-Sukel MP, Bannink M, Jager A, Wiemer EA, et al. Unjustified prescribing of CYP2D6 inhibiting SSRIs in women treated with tamoxifen. Breast Cancer Res Treat. 2013;139(3):923–929. doi: 10.1007/s10549-013-2585-z. [DOI] [PubMed] [Google Scholar]
- 16.Kelly CM, Juurlink DN, Gomes T, Duong-Hua M, Pritchard KI, Austin PC, et al. Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ. 2010;340:c693. doi: 10.1136/bmj.c693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aubert RE, Stanek E, Yao J, Teagarden JR, Subar M, Epstein RS, Skaar TC, Desta Z, Flockhart DA. Risk of breast cancer recurrence in women initiating tamoxifen with CYP2D6 inhibitors. J Clin Oncol 2009;27(18s):(Suppl; abstract no. CRA508).
- 18.Binkhorst L, Mathijssen RH, Ghobadi Moghaddam-Helmantel IM, de Bruijn P, van Gelder T, Wiemer EA, et al. Quantification of tamoxifen and three of its phase-I metabolites in human plasma by liquid chromatography/triple-quadrupole mass spectrometry. J Pharm Biomed Anal. 2011;56(5):1016–1023. doi: 10.1016/j.jpba.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Crewe HK, Lennard MS, Tucker GT, Woods FR, Haddock RE. The effect of selective serotonin re-uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol. 1992;34(3):262–265. doi: 10.1111/j.1365-2125.1992.tb04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Moltke LL, Greenblatt DJ, Giancarlo GM, Granda BW, Harmatz JS, Shader RI. Escitalopram (S-citalopram) and its metabolites in vitro: cytochromes mediating biotransformation, inhibitory effects, and comparison to R-citalopram. Drug Metab Dispos. 2001;29(8):1102–1109. [PubMed] [Google Scholar]
- 21.Jeppesen U, Gram LF, Vistisen K, Loft S, Poulsen HE, Brosen K. Dose-dependent inhibition of CYP1A2, CYP2C19 and CYP2D6 by citalopram, fluoxetine, fluvoxamine and paroxetine. Eur J Clin Pharmacol. 1996;51(1):73–78. doi: 10.1007/s002280050163. [DOI] [PubMed] [Google Scholar]
- 22.Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99(2):215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 23.Binkhorst L, van Gelder T, Loos WJ, de Jongh FE, Hamberg P, Moghaddam-Helmantel IM, et al. Effects of CYP induction by rifampicin on tamoxifen exposure. Clin Pharmacol Ther. 2012;92(1):62–67. doi: 10.1038/clpt.2011.372. [DOI] [PubMed] [Google Scholar]
- 24.de Graan AJ, Teunissen SF, de Vos FY, Loos WJ, van Schaik RH, de Jongh FE, et al. Dextromethorphan as a phenotyping test to predict endoxifen exposure in patients on tamoxifen treatment. J Clin Oncol. 2011;29(24):3240–3246. doi: 10.1200/JCO.2010.32.9839. [DOI] [PubMed] [Google Scholar]
- 25.Lorizio W, Wu AH, Beattie MS, Rugo H, Tchu S, Kerlikowske K, et al. Clinical and biomarker predictors of side effects from tamoxifen. Breast Cancer Res Treat. 2012;132(3):1107–1118. doi: 10.1007/s10549-011-1893-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jager NG, Koornstra RH, Vincent AD, et al. Hot flashes are not predictive for serum concentrations of tamoxifen and its metabolites. BMC Cancer. 2013;13:612. doi: 10.1186/1471-2407-13-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin Y, Desta Z, Stearns V, et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst. 2005;97:30–39. doi: 10.1093/jnci/dji005. [DOI] [PubMed] [Google Scholar]