Abstract
Background
Carbonated drinks are the second most consumed non-alcoholic beverages in the world after tea. The effects of these drinks on hard tissues and vital organs of the body have been proved beyond doubt. This study, however, explains the effect of these drinks on wound healing of oral epithelium.
Methods
Thirty-six male Wistar rats were considered for the study. A circular wound of 3.0 mm was created on the buccal mucosa of all animals and they were divided into two groups. Animals in group 1 were fed with chow pellet and water, while those in group 2 were fed with a commercially available carbonated drink instead of water. Six animals from each group were euthanized at 0, 7, and 21 days. Wound site was histologically assessed for differences in thickness and characteristics of the regenerating epithelium between two groups.
Results
There was a marked difference in the healing pattern between the two groups. Animals in group 1 showed a normal healing pattern at the end of day 21. In the group 2, the regenerated epithelium showed hyperplasia and hyperkeratosis along with acanthosis at the end of the experiment with a subsequent delayed inflammatory reaction at day 21.
Conclusion
Consumption of carbonated drinks can disrupt oral wound healing. The contents in carbonated drinks have a proinflammatory action on the soft tissue. Results suggest that epithelial changes seen in experimental group 2 could be a result of constant irritation by the acidic and fizzy nature of carbonated drinks.
Keywords: Carbonated beverages, Wounds and injuries, Dental anatomy, Epithelium, Cell biology
1. Introduction
The oral mucosa is lined by non-keratinized stratified squamous epithelium supported by a loose connective tissue,1 a barrier against exogenous substances and pathogens.2 Soft drinks are the second most consumed beverages in the world. Worldwide average consumption of carbonated drinks increased from 9.5 gallons per person per year in 1997 to 11.4 gallons per person per year in 2010.3 The oral cavity is quite often subjected to minor and major wounds on a daily basis. During healing of such wounds, the type of diet plays an imperative role.
Previous studies proved the effect of carbonated drinks on kidney,4 Liver,5 teeth,6 etc., but not enough literature is available on oral mucosa. The present study emphasizes on the histological effects of carbonated drinks on wound healing of buccal epithelium of albino rats.
2. Materials and methods
An experimental animal study was conducted at the Post Graduate Medical Institute, Lahore to observe histological changes in soft tissue on post-wound days 7 and 21. A randomly selected carbonated drink was used for this experiment. The study protocol was approved by the Advanced Studies and Research Board of University of Health Sciences, Lahore and Ethical Committee of PGMI, Lahore. Thirty-six healthy male adult albino Wistar rats, weighing between 180 and 250 g, were procured from NIH, Islamabad. Animals were tagged and were housed in cages with wire bar lids to hold the water bottle and feed, to prevent contamination with urine and feces. Bedding was placed directly into the cage to allow the absorption of urine. They were kept in a well-ventilated room at ambient temperature of 28.0 ± 2.0 °C and humidity (60 ± 10%) under 12-h light/dark cycles and well provided with food and water ad libitum. All animals were consistently checked for signs of pre- and post-procedure infections.
All animals used in this study were handled as per the international, natural, and institutional guidelines for the care and use of laboratory animals in biomedical research as promulgated by the National Research Council.7 The rats were divided into two equal groups by using a random number generator (Table 1). Animals in groups 1 and 2, after induction of soft tissue wound, were given ad libitum access to water and carbonated drink, respectively.
Table 1.
Detail of animal groups.
| Groups | Experimental day | Number of animals | Remarks |
|---|---|---|---|
| Experimental group 1 | Day 0 | 6 | Food with water |
| Day 7 | 6 | ||
| Day 21 | 6 | ||
| Experimental group 2 | Day 0 | 6 | Food with carbonated drink |
| Day 7 | 6 | ||
| Day 21 | 6 |
On day 0, a uniform piece of tissue was removed from the left buccal mucosa of rats using a disposable punch biopsy tool of 3.0 mm circumference8 after anesthetization with ketamine (100 mg/kg body weight) and xylazine (10 mg/kg body weight) by an intraperitoneal injection.9 The cut was made deep to the level of the dermis. The wound was left open for healing and the animal was returned to its cage to recover from anesthesia. None of the animals showed signs of post-procedural infection. On day 0, 7, and 21, six animals (n = 6) from each group were placed in a carbon dioxide plus chloroform chamber and euthanized under deep anesthesia.7 The whole left cheek was dissected out and washed with saline for further treatment. The tissue was processed and Hematoxylin and Eosin stain was used for routine histological study of the buccal epithelium. Data were analyzed by using two-paired student's t-test for quantitative differences between experimental group 1 and experimental group 2 at the 5% level of significance. A p-value of equal to or less than 0.05 was considered statistically significant.
3. Results
In both groups, the normal (baseline) epithelium at day 0 was stratified squamous keratinized, with four basic layers. It consisted of a darkly stained layer of tall columnar cells known as stratum basale (basal layer), over which there were 7–8 strata of polyhedral cells forming the stratum spinosum (prickle layer). On top of these were 3–5 layers of flat cells forming stratum granulosum (granular layer). These layers were covered by a fine layer of anucleated corneocytes forming the stratum corneum (cornified layer). Rete ridges extending down toward the connective tissue had a usual conical shape. The epithelial thickness was measured to be 295 ± 5.00 μm. The lamina propria displayed fine network of collagen bundles with fibroblasts and blood vessels (Fig. 1).
Fig. 1.

Section of left buccal mucosa of rat showing normal epithelium with stratum basale (red arrow), stratum spinosum (blue arrow), stratum granulosum (green arrow), and stratum corneum (yellow arrow). The lamina propria consists of blood vessels (BV), fibroblasts (F), and collagen fibers (C). H & E stain: photomicrograph approx. 200×.
On post-wound day 7, there was a prominent increase in the thickness of epithelium in both groups. In group 1, the stratified squamous epithelium of mean thickness of 153.33 ± 4.08 μm (Table 2) was present. The epithelium consisted of a single layer of large columnar cells constituting stratum basale; 4–5 layers of polyhedral cells forming stratum spinosum and 2–3 layers of flat cells with keratohyalin granules forming stratum granulosum were present on top (Fig. 2).
Table 2.
Comparison of thickness of epithelium (μm) between groups 1 and 2 on Day 7 and Day 21.
| Experimental day | Experimental group 1 (in μm) (mean ± S.D.) | Experimental group 2 (in μm) (mean ± S.D.) | Number of animals in each group (N) | p value |
|---|---|---|---|---|
| Day 0 | 295 ± 5.00 | 295 ± 5.00 | 6 | >0.00 |
| Day 7 | 153.33 ± 4.08 | 56.67 ± 4.07 | 6 | <0.001 |
| Day 21 | 285.0 ± 4.47 | 401.67 ± 7.53 | 6 | <0.001 |
Fig. 2.
Section of left buccal mucosa of group 1 on post-wound day 7 showing epithelium with stratum basale (red arrow), stratum spinosum (blue arrow), and stratum granulosum (green arrow). The lamina propria contains fibroblasts (F), collagen fibers (C), blood vessels (BV), and inflammatory cells (L) forming the granulation tissue. H & E stain: photomicrograph approx. 200×.
In group 2, however, the stratified squamous epithelium was about the mean thickness of 56.67 ± 4.07 μm (Table 2). The difference in values between two groups was statistically significant (p < 0.005). The epithelium possessed a single layer of columnar cells constituting the stratum basale, 2–3 layers of polyhedral cells forming stratum spinosum and only 1–2 layer thick stratum granulosum made of flat cells containing keratohyalin granules as the outermost layer (Fig. 3).
Fig. 3.
Section of left buccal mucosa of group 2 on post-wound day 7 showing epithelium with stratum basale (red arrow), stratum spinosum (blue arrow), and stratum granulosum (green arrow). The lamina propria consists of inflammatory cells (IL) and collagen fibers (C). H & E stain: photomicrograph approx. 200×.
On post-wound day 21, there was complete regeneration of buccal epithelium in group 1 with normal stratification of cells. A layer of tall columnar cells formed the stratum basale. About 6–8 layers of polyhedral cells were seen forming statum spinosum over the basal layer. Stratum granulosum was 3–4 layers thick with a fine keratin layer as the outermost layer of the epithelium. The thickness of the epithelium was 285.0 ± 4.47 μm (Table 2) (Fig. 4).
Fig. 4.
Section of left buccal mucosa of group 1d on post-wound day 21 showing stratified squamous epithelium keratinized with stratum basale (red arrow), stratum spinosum (blue arrow), stratum granulosum (green arrow), and stratum corneum (yellow arrow). The connective tissue shows collagen fiber bundles (C) and blood vessels (BV). H & E stain: photomicrograph approx. 200×.
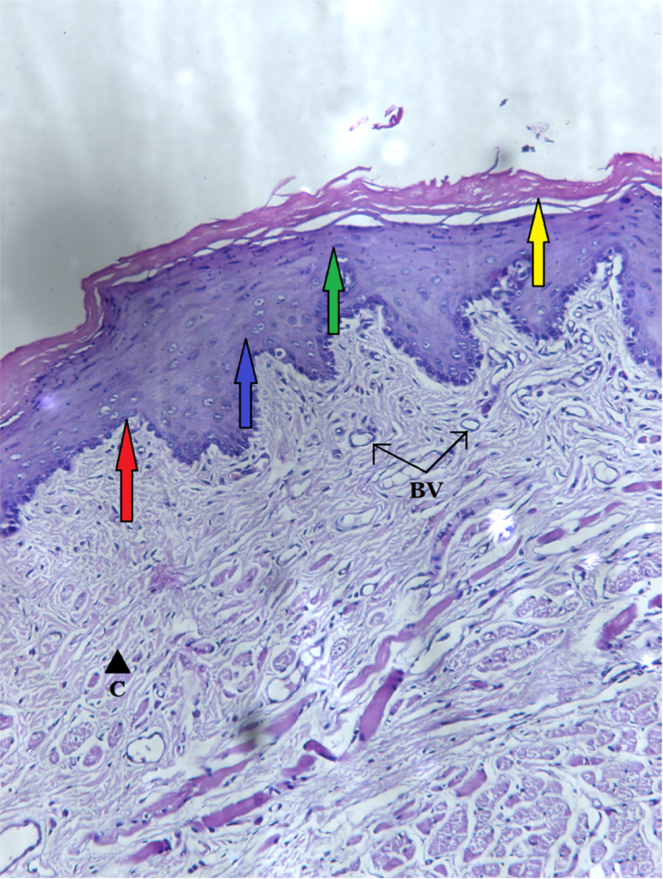
In contrast to this, group 2 epithelium was extremely thick, 401.67 ± 7.53 μm (Table 2). There were 12–15 layers forming stratum spinosum and 5–7 layers of flat cells forming stratum granulosum over it. The epithelium showed hyperkeratosis and acanthosis. Stratum granulosum was prominent with abundant keratohyalin granules. Rete ridges were irregular, bulbous, and extended up to the cornium layer in some places. The difference in values in the two groups was statistically significant (p < 0.005) (Fig. 5).
Fig. 5.
Section of left buccal mucosa of group 2d on post-wound day 21 showing acanthotic epithelium with stratum basale (red arrow), hypertrophic stratum spinosum (blue arrow), stratum granulosum (green arrow) with prominent keratohyaline granules (K), and hyperkeratotic stratum corneum (yellow arrow). Irregular rete ridges (R) are present. The lamina propria consists of fibroblasts (F), collagen bundles (C), blood vessels (BV), and inflammatory cells (IL). H & E stain: photomicrograph approx. 200×.
4. Discussion
On day 7, there was considerable stratification of the buccal epithelium in experimental groups 1 and 2; stratum basale, spinosum and granulosum were easily appreciated. Although the epithelium of group 2 had not reached its full thickness yet, there was no sign of keratinization in any of the animals. Doyle et al.10 conducted a study to assess the effects of energy beverages on mesenchymal cells. Their results revealed a decrease in lamellipodia formation and a decreased proliferative activity of fibroblasts leading to delay in wound closure. A study conducted by Suragimath et al.11 supported the delay in connective tissue fibroblast proliferation. Since the optimal pH required for viability and activity of the fibroblasts has been reported to be 7.2–7.5, a pH below 3.0 created by carbonated drinks would hinder fibroblast proliferation.12 Thus, a delay in connective tissue formation leads to the delay in epithelial proliferation and stratification.
On day 21, complete regeneration of the epithelium was visible in group 1, with a normal amount of keratin production. All four layers of stratified squamous epithelium were appreciated. The thickness of the regenerating epithelium in group 1 was measured to be approximately 285 μm. A study was done by Prestin et al.13 to measure the thickness of human buccal epithelium by optical coherence tomography. According to their study, the thickness of human buccal epithelium was 294 μm, which suggests that rat and human buccal epithelium thickness are comparable to each other and that future studies performed on rat buccal mucosa will be of value to researchers.
However, hyperkeratosis was seen in group 2, along with mild acanthosis, a very prominent stratum granulosum. Rete ridges were seen to reach up to the corneum. Various irritants such as foods, chemicals, friction, tobacco, arsenic, thermal/mechanical injury, metals, spices, and oral care products have been documented to cause irritant reactions in oral mucosa, particularly if used under exaggerated exposure conditions.14 These epithelial changes could be manifested as simple diseases like contact stomatitis, hyperkeratotic, or dysplastic changes to drastic changes causing premalignant lesions. Carbonated drinks can therefore be considered as constant irritants to the oral environment.
A study done by Sirsat and Kandarkar15 concurred with the idea that a diet containing irritants could cause epithelial changes in the mucosa. In their study, they observed the histological changes in rat buccal mucosa treated with calcium hydroxide. The results revealed drastic epithelial changes such as hyperplasia, hyperkeratosis, acanthosis, prominent stratum granulosum, and hyalinization of subepithelial connective tissue with continuous application of calcium hydroxide. Interestingly, few of these results coincide with the findings of the present study suggesting that carbonated drinks could be placed in the category of low-grade oral irritants.
Conflicts of interest
The authors have none to declare.
Acknowledgements
I am extremely grateful to Dr. Ambreen Imran (histopathologists) and Dr. Maryam Ashraf (anatomist) for their significant contribution regarding the results of this study. I am very thankful to Mr. Shahid, the animal handler at PGMI, Lahore animal house for his services. All financial arrangements were made by the corresponding author.
References
- 1.Nanci A. 8th edition. Elsevier; Mosby, Missouri: 2013. Ten Cate's Oral Histology: Development, Structure and Function. [Google Scholar]
- 2.Liu J., Mao J.J., Chen L. Epithelial–mesenchymal interactions as a working concept for oral mucosa regeneration. Tissue Eng Part B Rev. 2011;17(1):25–31. doi: 10.1089/ten.teb.2010.0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basu S., McKee M., Galea G., Stuckler D. Relationship of soft drink consumption to global overweight, obesity, and diabetes: a cross-national analysis of 75 countries. Am J Public Health. 2012;103(11):2071–2077. doi: 10.2105/AJPH.2012.300974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adjene J.O., Ezeoke J.C., Nwose E.U. Histological effects of chronic consumption of soda pop drinks on kidney of adult Wister rats. N Am J Med Sci. 2010;2(5):215–217. doi: 10.4297/najms.2010.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assy N., Nasser G., Kamayse I. Soft drink consumption linked with fatty liver disease in the absence of traditional risk factors. Can J Gastroenterol. 2008;22(10):811–816. doi: 10.1155/2008/810961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim S., Sohn W., Burt B.A. Cariogenicity of soft drinks, milk and fruit juice in low-income African-American children. J Am Dent Assoc. 2008;139(7):959–967. doi: 10.14219/jada.archive.2008.0283. [DOI] [PubMed] [Google Scholar]
- 7.[NRC] National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals . 8th edition. National Academies Press (US); Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. Available from: http://www.ncbi.nlm.nih.gov/books/NBK54050/ [Google Scholar]
- 8.Logan R.M., Goss A.N. Biopsy of the oral mucosa and use of histopathology services. Aust Dent J. 2010;1:9–13. doi: 10.1111/j.1834-7819.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 9.Fish R.E., Brown M.J., Danneman P.J., Karas A.Z. 2nd edition. Academic press; London, England: 2011. Anesthesia and Analgesia in Laboratory Animals. [Google Scholar]
- 10.Doyle W., Shide E., Thapa S., Chandrasekaran V. The effects of energy beverages on cultured cells. Food Chem Toxicol. 2012;50(10):3759–3768. doi: 10.1016/j.fct.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Suragimath G., Krishnaprasad K.R., Moogla S., Sridhara S.U., Raju S. Effect of carbonated drink on excisional palatal wound healing: a study on Wistar rats. Indian J Res Dent. 2010;21(3):330–333. doi: 10.4103/0970-9290.70789. [DOI] [PubMed] [Google Scholar]
- 12.Lengheden A., Jansson L. pH effects on experimental wound healing of human fibroblasts in vitro. Eur J Oral Sci. 1995;103(3):148–155. doi: 10.1111/j.1600-0722.1995.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 13.Prestin S., Rothschild S.I., Betz C.S., Kraft M. Measurement of epithelial thickness within the oral cavity using optical coherence tomography. Head Neck. 2012;34(12):1777–1781. doi: 10.1002/hed.22007. [DOI] [PubMed] [Google Scholar]
- 14.Davis C.C., Squier C.A., Lilly G.E. Irritant contact stomatitis: a review of the condition. J Periodontol. 1998;69(6):620–631. doi: 10.1902/jop.1998.69.6.620. [DOI] [PubMed] [Google Scholar]
- 15.Sirsat S.M., Kandarkar S.V. Histological changes in the oral mucosa of the Wistar rat treated with commercial lime (calcium hydroxide) – an optical and submicroscopic study. Br J Cancer. 1968;22(2):303–315. doi: 10.1038/bjc.1968.38. [DOI] [PMC free article] [PubMed] [Google Scholar]