Abstract
Aims
The present study investigated the surface properties and murine pre-osteoblast cell (MC3T3-E1) responses of phosphoric acid (H3PO4) treated commercially pure titanium.
Methods
Titanium discs were treated with various concentration of H3PO4 (5%, 10%, and 20%; v/v) at 90 °C for 30 min. Surface properties were evaluated by profilometer, contact angle meter, and scanning electron microscopy (SEM) with energy dispersive X-rays. MC3T3-E1 attachment and spreading were evaluated by SEM and phalloidin immunohistochemistry staining.
Results
Surface roughness and wettability were not statistically difference among all experimental and control groups. Phosphate and oxygen were detected on H3PO4 treated surfaces. At 20 min, cell attachment was significantly higher in 10% and 20% H3PO4 treated groups compared to the control. Cells exhibited orientated-cytoskeleton fibers on 20% H3PO4 modified titanium surface. Though, there was no difference in cell spreading stage among all treatment groups.
Conclusion
H3PO4 treatment on titanium may influence early cell response, particularly on attachment and spreading.
Keywords: Titanium, Osteoblast, Surface modification, Cell attachment, Cell spreading
1. Introduction
Dental implant is utilized for tooth replacement therapy. It improves patient satisfactory and chewing ability.1 Common materials used in dental implant are titanium and its alloys, since they have an excellent biocompatibility. Osseointegration, an important requirement for the success in dental implant treatment, depends on several osteoblasts/material interactions including osteoblastic cell attachment/adhesion, osteoblastic cell proliferation, and matrix mineralization at the material interface.2, 3
Numerous studies have demonstrated that titanium surface modification improves osseointegration.4, 5, 6, 7, 8, 9, 10 The exemplifications of surface modification are surface roughness alteration, hydroxyapatite coating, protein immobilization, growth factor release, and ions immobilization.4, 5, 6, 7, 8, 9, 10 Acid etching is one of the approaches utilized to improve surface characteristics. Among various acid solutions, phosphoric acid (H3PO4) has been used, as it could improve physical surface properties.11 In addition, H3PO4 treated surface could release phosphate ions, which may further influence osteoblastic cell behaviors.11 The surface treatment with phosphate incorporation is of interest as it is a potential technique for achieving preferable physical and biological properties.12, 13 However, the methods normally employed for H3PO4 treatment are either used high temperature and/or pressure treatment. Therefore, the aim of this study was to investigate the effect of low-temperature H3PO4 modified titanium surface on surface characteristics and early murine osteoblastic cell responses.
2. Materials and methods
2.1. Titanium disc preparation
Commercial pure titanium discs, size 10 mm in diameter and 3 mm in thickness (cpTi grade 2, KVM Heating Element, Thailand), were polished using 800 and 1200 grit SiC paper in a polishing machine (NANO 2000 grinder-polisher, PACE Technologies, USA) for 15 and 30 min, respectively. After polishing, all specimens were cleaned by ultrasonic cleaning machine with deionized water, rinsed with acetone, 70% ethanol, and deionized water, respectively. Titanium discs were immersed in deionized water (control), 5% H3PO4, 10% H3PO4, or 20% H3PO4 at 90 °C for 30 min. Discs were rinsed with deionized water and dried at room temperature.
2.2. Surface characterization
Surface roughness was measured using a surface roughness tester (Talyscan 150, England). Average surface roughness (Ra) and the root mean square roughness (Rq) measurements were randomly taken at three different locations on each disk under the following condition: measurement speed at 1500 μm/s, one way direction measurement. Surface morphology was observed using scanning electron microscopy (SEM; JSM-5410LV, JEOL, Tokyo, Japan). The surface chemical composition was analyzed by energy dispersive X-rays (EDX) (JSM-5410LV, JEOL, Tokyo, Japan). Surface wettability was determined by measuring the contact angles with one drop (5 μl) of deionized water using a contact meter (DSA 10 MK2, Kruss, Hamburg, Germany).
2.3. Cell culture
MC3T3-E1 cells, a mouse calvarial-derived osteoblast-like cell line, were grown in alpha Minimal Essential Medium (alpha-MEM) containing 10% fetal bovine serum, 1% antibiotic and antimycotic and 1% l-glutamine (Gibco, USA). Cells were maintained in humidified atmosphere and 5% CO2, at 37 °C. The medium was changed every 2 days. Titanium specimens were sterilized by UV light for 30 min. Cells were seeded on titanium discs at a density of 10,000 cells/disc. After incubated for 20 min, 1 h, 6 h, and 24 h, cells were fixed in 3% glutaraldehyde for further analyses.
For cell attachment assay, cells were stained with DAPI (1:1,000 dilution) to visualize nuclei. Five different areas of each specimen were randomly captured by using fluorescence microscope under 100× magnification. Cell number was evaluated using Analyse Particle command in ImageJ software. All analyzed pictures were set at the same threshold. For morphological analysis, the specimens were permeabilized with 0.1% Triton™ X-100. Subsequently, the cells were stained with rhodamine-phalloidin (Invitrogen, USA) at 1:100 dilution in 10% horse serum. F-actin orientation was evaluated by fluorescence microscope. Some specimens were dehydrated using an ascending series of alcohol and hexamethyldisilazane. After critical point drying and gold coating, cell morphologies on the various titanium specimens were observed using SEM.
2.4. Cell spreading analysis
Cell spreading was determined from five different fields from SEM micrographs (350× magnification) in each specimen. The criteria for staging of cell spreading were modified from Lumbikanonda and Sammons14 as follows: stage 1, round cells with few filopodia; stage 2, numerous focal cytoplasmic extensions from cells; stage 3, cells with circumferential cytoplasmic spreading; and stage 4, polygonal full spreading and flattening of cells. The number of cells at each stage was expressed as percentage of cell spreading stage.
2.5. Statistical analyses
Data were expressed as box plot with whisker bar. Statistical analyses were performed using Mann–Whitney U test (SPSS Software, Chicago, IL, USA) to compare with the control condition. The statistical significance was considered when p < 0.05.
3. Results
3.1. Surface characterization
Surface morphology of titanium discs was evaluated using SEM (Fig. 1). It was noted that 20% H3PO4 treated group exhibited irregularity of surface morphology. However, no statistically difference was found in the surface roughness parameters (Ra and Rq values) in any H3PO4 treated groups comparing with the control (Table 1). Surface chemical compositions were determined by EDX analysis (Table 2). The control titanium surface chemistry consisted of Ti, nitrogen (N) and carbon (C). As expected, the H3PO4 modified titanium surfaces had phosphorus (P) and oxygen (O) on the surface. P and O composition of the surface increased, corresponding to the H3PO4 concentration. The statistical significance was noted in H3PO4 treated surfaces as compared to the control. Further, the surface hydrophilicity was determined by measuring the contact angle of a water-drop on the specimens’ surfaces (Table 3). Although, the median value of contact angle increased corresponding to the concentration of H3PO4, there was no significant difference compared to the control.
Fig. 1.
Representative SEM micrographs illustrated surface morphology of the control (A), 5% (B), 10% (C), and 20% (D) H3PO4 treated titanium surfaces (2,000X magnification).
Table 1.
Surface roughness parameters.
| Parameters | Control | 5% H3PO4 | 10% H3PO4 | 20% H3PO4 |
|---|---|---|---|---|
| Ra (μm) | 0.01907 (0.01831–0.02080) |
0.01659 (0.01604–0.02074) |
0.02566 (0.02188–0.03237) |
0.01901 (0.01825–0.02460) |
| Rq (μm) | 0.02474 (0.02357–0.02761) |
0.02192 (0.02125–0.02745) |
0.03485 (0.02902–0.04219) |
0.02514 (0.02428–0.03283) |
Data presented as median (minimum–maximum).
Table 2.
Surface chemical composition.
| Element (%) | Control | 5% H3PO4 | 10% H3PO4 | 20% H3PO4 |
|---|---|---|---|---|
| Ti | 86.41 (85.33–87.26) |
74.09 (70.39–77.55) |
71.89 (59.68–75.00) |
71.03 (66.52–73.59) |
| N | 9.73 (9.38–10.47) |
9.10 (8.47–9.20) |
8.89 (8.31–9.59) |
6.31 (5.74–8.66) |
| C | 3.65 (3.36–4.21) |
3.69 (3.43–4.09) |
3.75 (3.42–4.32) |
3.16 (2.71–3.88) |
| P | 0.00 (0.00–0.00) |
0.03* (0.00–0.06) |
0.16* (0.07–0.42) |
0.31* (0.21–0.42) |
| O | 0.00 (0.00–0.00) |
13.40* (9.31–16.66) |
15.18* (12.41–26.90) |
18.62* (16.33–21.61) |
Data presented as median (minimum–maximum). Asterisks indicated statistical significance compared to the control.
Table 3.
Contact angle.
| Group | Contact angle (degree) |
|---|---|
| Control | 84.41 (55.00–90.00) |
| 5% H3PO4 | 82.00 (32.10–178.10) |
| 10% H3PO4 | 87.73 (76.86–111.83) |
| 20% H3PO4 | 88.43 (74.23–110.20) |
Data presented as median (minimum–maximum).
3.2. Cell attachment and spreading
Cell attachment was evaluated on different modified titanium surfaces after seeding for 20 min and 1 h (Fig. 2). The amount of attached cells increased corresponding to the incubated time. Further, the 10% and 20% H3PO4 treated surface promoted significant higher cell attachment at 20 min comparing to the control (Fig. 2). Though, no statistical significance was noted at 1 h.
Fig. 2.
Graphs showed the number of cells per filed normalized to the control at 20 min (A) and 1 h (B). Asterisks indicated the statistical significant difference compared to the control.
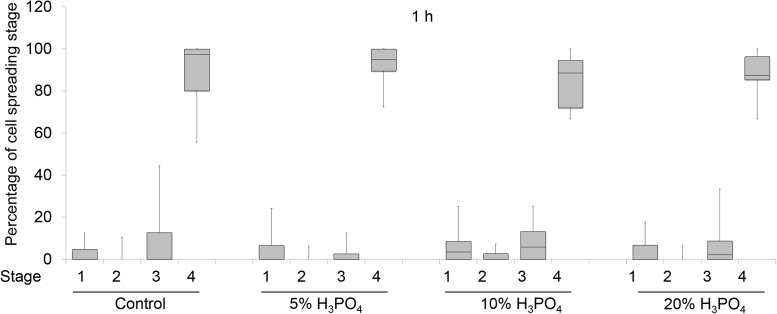
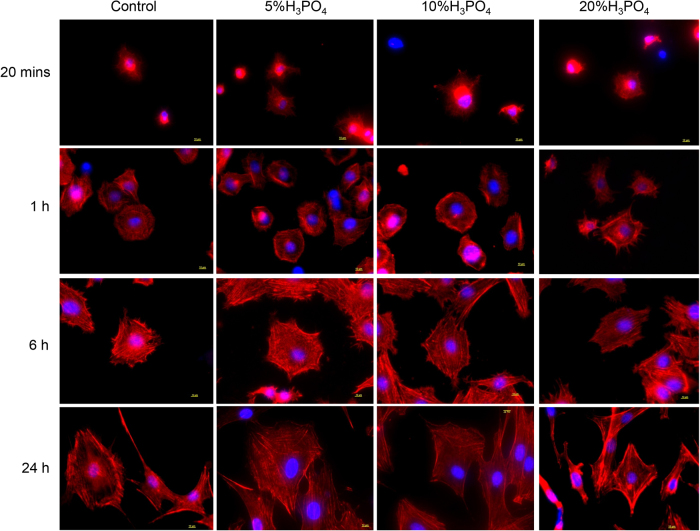
Cell spreading was further examined using SEM analysis (Fig. 3, Fig. 4). At 20 min, a round shape cell attachment was obviously noted on all treated surfaces. Cells on H3PO4 modified surfaces demonstrated small and thin filopodia. In contrast, the lamellopodia was noted in cells on the control surface. At 1 h, cells morphology was changed into polygonal and cells were larger and flatter than those at 20 min. Cells on 20% H3PO4 modified titanium surface displayed flatter characteristic than those on other surfaces (Fig. 4). To evaluate cell spreading in semi-quantitative manner, cell spreading was categorized into 4 spreading stages.14 Image analysis demonstrated that there was no significant difference in the percentage of cell spreading stage between H3PO4 treated surfaces and the control (Fig. 5). At 6 h, cells attached on all difference surfaces appeared more spread and flatten than 1 h (Fig. 3, Fig. 4). Also, some intercellular contacts were also evidently seen in all groups. At 24 h, most of cells appeared flat, fully spread on the surface, with some interspersed round and loosely attached cells, which attached over the first cell layer. No difference in cell morphology was obviously detectable among all surfaces.
Fig. 3.
Representative SEM micrographs illustrated cell morphology on different titanium surface modifications (350× magnification).
Fig. 4.
Representative SEM micrographs illustrated cell morphology on different titanium surface modifications (2000× magnification).
Fig. 5.
Graphs showed the percentage of cell spreading stages as evaluated by SEM at 1 h after cells seeding on modified surfaces.
To study the cytoskeletal organization of adhered cells on modified titanium surfaces, F-actin was immunostained using rhodamine-phalloidin (Fig. 6). At 20 min, no obvious organization of actin filament was noted. At 1 h, the dense staining of actin was observed. The accumulation of stress fibers around the edge of cells was seen in H3PO4 treated groups. After 6 h and 24 h, the actin filaments were organized with frequent cortical filament bundles at cell periphery. There was no obvious difference in actin orientation among groups.
Fig. 6.
Representative images illustrated the F-actin orientation at different time points after cells seeding on different surface modifications. Bars indicated 10 μm.
4. Discussion
There are many titanium surface modification techniques aiming to improve osseointregration. Acid etching is usually employed with the wide variety of acid types i.e. H3PO4, nitric acid (HNO3), hydrochloric acid (HCl), and sulfuric acid (H2SO4).15, 16, 17 The acid etching methods generally aimed to alter surface chemistry and micro-roughness. Among these acids, H3PO4 is of interest due to not only the potential change in physical characteristics of titanium surface influences cell response but also the phosphate remaining on the surface may influence cell behaviors. It has previously been shown that phosphate ions promoted osteogenic differentiation of various cell types, for example osteoblasts, cementoblasts, and periodontal ligament cells.18, 19, 20, 21
In the present study, titanium surfaces were modified with different H3PO4 concentration using low temperature treatment. No significant difference of surface roughness was noted. Corresponding to previous study, there is no difference in average surface roughness (Ra) values among titanium surfaces treated with different H3PO4 concentration. Though, the different osteoblastic cell response was noted among these H3PO4 treated condition.11, 22 H3PO4 treatment was shown to enhance surface wettability.11, 22 The higher concentration of H3PO4 produced higher hydrophilic surface.11, 22 On the contrary, we did not note the marked difference of surface hydrophilicity among conditions employing in the present study. Several reasons could be addressed for these discrepancies. First, the low temperature heat treatment (90 °C) was utilized in the present study, while other study employed high temperature for acid treatment (180 °C). By using high temperature treatment, higher H3PO4 concentration resulted in the higher surface roughness and wettability.11 The limitations of hydrothermal methods are the high cost of technology equipment, the complication procedure, and the difficulty for management. Thus, the low temperature heat treatment for acid etching might be an alternative procedure to modify titanium surfaces. In the present study, the lower temperature method resulted in the change of surface morphology as evaluated by SEM at high H3PO4 concentration.
As described above, low temperature treatment of high H3PO4 concentration may alter titanium surface morphology without significant difference in surface roughness parameters. This may be due to the limitation of equipment's sensitivity. The sensitivity of the surface roughness tester can detect the roughness only in micrometer levels but the effect of the high concentration of phosphoric acid modified surface may produce roughness in nanometer levels. Osteoblast cells over the different roughness at nanometer levels surface exhibited the difference in cell response.23 The result from this study suggested that the difference in cell response probably due to the different roughness in nanometer levels. Though, further investigation should be performed to confirm this hypothesis.
The influence of surface hydrophilicity on cell behaviors is still controversy. It has been shown that hydrophilic surfaces have been shown to promote cell attachment, adhesion, and bone apposition.11, 22, 24, 25, 26 On the contrary, it has also shown that the decreased surface wettability promoted protein absorption and cell attachment.27 Another study demonstrated that alkaline phosphatase immobilization on titanium surface led to the alteration in water contact angle but not cell behaviors.28 In the present study, we noted the increase of surface wettability corresponding to the concentration of H3PO4, but it did not reach the significant difference. Though, early cell attachment was markedly noted on 10% and 20% H3PO4 treated surface. Thus, the surface wettability may not be a major parameter controlling cell response in this particular setting.
It was noted in this study that cell attachment was higher in H3PO4 modified titanium surface. Several parameters may contribute in this response, including the phosphate ions presenting on the surface and potential surface nano-roughness. Particularly on surface chemistry, it has been shown that the initial cell adhesion onto the surfaces involved in many kinds of biological and chemical interactions relating to calcium, phosphorus, carbon, oxygen and nitrogen elements.29 This result may imply that the more phosphorus found the better early cell attachment. Though, the number of attached cells was similar at 1 h. There are several studies demonstrating the correlation of cell attachment and osteogenic differentiation ability.30, 31 Thus, the significant increase of cell attachment on H3PO4 treated titanium surface at early time point may contribute to later cell differentiation, though, the evidence was not strong to conclude this hypothesis. More investigations are indeed necessitated.
Previous study showed that hydrothermal treatment of H3PO4 modified titanium surface resulted in the improvement of cell adhesion and spreading.11 In the present study, cell spreading was not obviously different among examined groups as evaluated by SEM. Though, the F-actin organization on cell periphery was observed at early time point of 20% H3PO4 modified titanium surface. It has been reported that the modified surface promoting early cell spreading led to the enhancement of osteogenic differentiation as determined by the increase of alkaline phosphatase enzymatic activity and osteocalcin expression.32
In conclusion, the results from this study demonstrate that high concentration of H3PO4 modified titanium surface may improve early cell attachment. However, further adjustment and modification are necessitated. The evaluation of cell differentiation ability was also needed.
Conflicts of interest
The authors have none to declare.
Acknowledgements
The present study was supported by the Ratchadapiseksomphot Endowment Fund of Chulalongkorn University (RES560530156-HR) and Dental Research Project Fund (3200502#/2013), Faculty of Dentistry, Chulalongkorn University. PP was supported by the Research Chair Grant 2012, the National Science and Technology Development Agency (NSTDA).
References
- 1.Zancope K., Abrao G.M., Karam F.K., Neves F.D. Placement of a distal implant to convert a mandibular removable Kennedy class I to an implant-supported partial removable Class III dental prosthesis: a systematic review. J Prosthet Dent. 2015;113:528–533. doi: 10.1016/j.prosdent.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Branemark R., Branemark P.I., Rydevik B., Myers R.R. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. 2001;38:175–181. [PubMed] [Google Scholar]
- 3.Cooper L.F., Masuda T., Yliheikkila P.K., Felton D.A. Generalizations regarding the process and phenomenon of osseointegration. Part II: In vitro studies. Int J Oral Maxillofac Implants. 1998;13:163–174. [PubMed] [Google Scholar]
- 4.Ostman P.O., Hellman M., Sennerby L. Direct implant loading in the edentulous maxilla using a bone density-adapted surgical protocol and primary implant stability criteria for inclusion. Clin Implant Dent Relat Res. 2005;7(suppl 1):S60–S69. doi: 10.1111/j.1708-8208.2005.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 5.Webster T.J., Ergun C., Doremus R.H., Siegel R.W., Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000;21:1803–1810. doi: 10.1016/s0142-9612(00)00075-2. [DOI] [PubMed] [Google Scholar]
- 6.Buser D., Schenk R.K., Steinemann S., Fiorellini J.P., Fox C.H., Stich H. Influence of surface characteristics on bone integration of titanium implants: a histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25:889–902. doi: 10.1002/jbm.820250708. [DOI] [PubMed] [Google Scholar]
- 7.Schneider G.B., Perinpanayagam H., Clegg M. Implant surface roughness affects osteoblast gene expression. J Dent Res. 2003;82:372–376. doi: 10.1177/154405910308200509. [DOI] [PubMed] [Google Scholar]
- 8.Zinger O., Zhao G., Schwartz Z. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials. 2005;26:1837–1847. doi: 10.1016/j.biomaterials.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., Huse R.O., de Groot K., Buser D., Hunziker E.B. Delivery mode and efficacy of BMP-2 in association with implants. J Dent Res. 2007;86:84–89. doi: 10.1177/154405910708600114. [DOI] [PubMed] [Google Scholar]
- 10.Mendonca G., Mendonca D.B., Aragao F.J., Cooper L.F. Advancing dental implant surface technology – from micron- to nanotopography. Biomaterials. 2008;29:3822–3835. doi: 10.1016/j.biomaterials.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Park J.W., Kim Y.J., Jang J.H., Kwon T.G., Bae Y.C., Suh J.Y. Effects of phosphoric acid treatment of titanium surfaces on surface properties, osteoblast response and removal of torque forces. Acta Biomater. 2010;6:1661–1670. doi: 10.1016/j.actbio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Sailaja G.S., Ramesh P., Varma H.K. Ultrastructural evaluation of in vitro mineralized calcium phosphate phase on surface phosphorylated poly(hydroxy ethyl methacrylate-co-methyl methacrylate) J Mater Sci Mater Med. 2010;21:1183–1193. doi: 10.1007/s10856-010-3987-7. [DOI] [PubMed] [Google Scholar]
- 13.Sailaja G.S., Sreenivasan K., Yokogawa Y., Kumary T.V., Varma H.K. Bioinspired mineralization and cell adhesion on surface functionalized poly(vinyl alcohol) films. Acta Biomater. 2009;5:1647–1655. doi: 10.1016/j.actbio.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Lumbikanonda N., Sammons R. Bone cell attachment to dental implants of different surface characteristics. Int J Oral Maxillofac Implants. 2001;16:627–636. [PubMed] [Google Scholar]
- 15.Egoshi T., Taira Y., Soeno K., Sawase T. Effects of sandblasting, H2SO4/HCl etching, and phosphate primer application on bond strength of veneering resin composite to commercially pure titanium grade 4. Dent Mater J. 2013;32:219–227. doi: 10.4012/dmj.2012-261. [DOI] [PubMed] [Google Scholar]
- 16.Faverani L.P., Assuncao W.G., de Carvalho P.S. Effects of dextrose and lipopolysaccharide on the corrosion behavior of a Ti-6Al-4V alloy with a smooth surface or treated with double-acid-etching. PLOS ONE. 2014;9:e93377. doi: 10.1371/journal.pone.0093377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maekawa K., Yoshida Y., Mine A., van Meerbeek B., Suzuki K., Kuboki T. Effect of polyphosphoric acid pre-treatment of titanium on attachment, proliferation, and differentiation of osteoblast-like cells (MC3T3-E1) Clin Oral Implants Res. 2008;19:320–325. doi: 10.1111/j.1600-0501.2007.01477.x. [DOI] [PubMed] [Google Scholar]
- 18.An S., Ling J., Gao Y., Xiao Y. Effects of varied ionic calcium and phosphate on the proliferation, osteogenic differentiation and mineralization of human periodontal ligament cells in vitro. J Periodontal Res. 2012;47:374–382. doi: 10.1111/j.1600-0765.2011.01443.x. [DOI] [PubMed] [Google Scholar]
- 19.Beck G.R., Jr. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem. 2003;90:234–243. doi: 10.1002/jcb.10622. [DOI] [PubMed] [Google Scholar]
- 20.Beck G.R., Jr., Moran E., Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res. 2003;288:288–300. doi: 10.1016/s0014-4827(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 21.Foster B.L., Nociti F.H., Jr., Swanson E.C. Regulation of cementoblast gene expression by inorganic phosphate in vitro. Calcif Tissue Int. 2006;78:103–112. doi: 10.1007/s00223-005-0184-7. [DOI] [PubMed] [Google Scholar]
- 22.Park J.W., Kim Y.J., Jang J.H. Enhanced osteoblast response to hydrophilic strontium and/or phosphate ions-incorporated titanium oxide surfaces. Clin Oral Implants Res. 2010;21:398–408. doi: 10.1111/j.1600-0501.2009.01863.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.W., Lee K.B., Jeon H.S., Park H.K. Effects of surface nano-topography on human osteoblast filopodia. Anal Sci. 2011;27:369. doi: 10.2116/analsci.27.369. [DOI] [PubMed] [Google Scholar]
- 24.Shibata Y., Hosaka M., Kawai H., Miyazaki T. Glow discharge plasma treatment of titanium plates enhances adhesion of osteoblast-like cells to the plates through the integrin-mediated mechanism. Int J Oral Maxillofac Implants. 2002;17:771–777. [PubMed] [Google Scholar]
- 25.Shibata Y., Miyazaki T. Anode glow discharge plasma treatment enhances calcium phosphate adsorption onto titanium plates. J Dent Res. 2002;81:841–844. doi: 10.1177/154405910208101209. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto H., Shibata Y., Miyazaki T. Anode glow discharge plasma treatment of titanium plates facilitates adsorption of extracellular matrix proteins to the plates. J Dent Res. 2005;84:668–671. doi: 10.1177/154405910508400717. [DOI] [PubMed] [Google Scholar]
- 27.Bumgardner J.D., Wiser R., Elder S.H., Jouett R., Yang Y., Ong J.L. Contact angle, protein adsorption and osteoblast precursor cell attachment to chitosan coatings bonded to titanium. J Biomater Sci Polym Ed. 2003;14:1401–1409. doi: 10.1163/156856203322599734. [DOI] [PubMed] [Google Scholar]
- 28.Nijhuis A.W., van den Beucken J.J., Jansen J.A., Leeuwenburgh S.C. In vitro response to alkaline phosphatase coatings immobilized onto titanium implants using electrospray deposition or polydopamine-assisted deposition. J Biomed Mater Res A. 2014;102:1102–1109. doi: 10.1002/jbm.a.34776. [DOI] [PubMed] [Google Scholar]
- 29.Feng B., Weng J., Yang B.C., Qu S.X., Zhang X.D. Characterization of titanium surfaces with calcium and phosphate and osteoblast adhesion. Biomaterials. 2004;25:3421–3428. doi: 10.1016/j.biomaterials.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 30.Fraioli R., Rechenmacher F., Neubauer S. Mimicking bone extracellular matrix: Integrin-binding peptidomimetics enhance osteoblast-like cells adhesion, proliferation, and differentiation on titanium. Colloids Surf B. 2015;128:191–200. doi: 10.1016/j.colsurfb.2014.12.057. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q., Limthongkul W., Sidhu G. Covalent attachment of P15 peptide to titanium surfaces enhances cell attachment, spreading, and osteogenic gene expression. J Orthop Res. 2012;30:1626–1633. doi: 10.1002/jor.22116. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida E., Yoshimura Y., Uo M., Yoshinari M., Hayakawa T. Influence of nanometer smoothness and fibronectin immobilization of titanium surface on MC3T3-E1 cell behavior. J Biomed Mater Res A. 2012;100:1556–1564. doi: 10.1002/jbm.a.34084. [DOI] [PubMed] [Google Scholar]