Abstract
Background
GLUTs are a family of proteins that mediate glucose transport through the membrane, expressed in head and neck squamous cell carcinoma. GLUT-1 positivity in malignant cells indicates increased proliferative activity, energy requirements, aggressive behaviour and poor radiation response.
Aim
To observe the expression of GLUT-1 protein in oral squamous cell carcinoma in tobacco and non-tobacco users and to correlate the expression with histopathological grading and pathological staging.
Methods
50 cases (25 tobacco and 25 non-tobacco) of oral squamous cell carcinoma, selected during period of August 2014 to July 2015. Histopathological grading, TNM and staging were done. Immunohistochemical staining was performed using standard protocol for paraffin embedded sections. Analysis was performed on SPSS software (Windows version 17.0).
Results
Significant association of GLUT-1 expression was found with history of tobacco (p < 0.001), Bryne's grade (p < 0.001), tumour size (p = 0.001), nodal metastasis (p = 0.022) and stage (p < 0.001). Higher GLUT-1 expression in stage II, stage III and stage IV was found as compared to stage I. GLUT-1 immunoexpression also shows progressive switch from membranous to cytoplasmic to combined location correlating with histopathologic grade and pTNM stage.
Conclusion
GLUT-1 expression correlates significantly with histological grade and pTNM staging of oral squamous cell carcinoma. It also significantly correlates with tobacco addiction. Thus, GLUT-1 expression may serve as a biomarker for patients of oral squamous cell carcinoma.
Keywords: GLUT-1, Oral squamous cell carcinoma, Tobacco, Non-tobacco
1. Introduction
Oral cancer ranks the eighth position in the cancer incidence ranking worldwide, with epidemiologic variations between different geographic regions. In India, it ranks third most common malignancy, which accounts for more than 30% of all cancers reported in the country.1, 2 Squamous cell carcinoma (SCC) is the most common clinical entity accounting for more than 90% of all oral malignancies.3 In Indian subcontinent, main cause of oral cancer is chewing tobacco and tobacco related products, which is the most common exogenous cause of human cancer. Nicotine, which is a major component of tobacco, is not a carcinogen by itself but has been implicated in progression of cancer and metastasis.4, 5, 6 The pathophysiological effects of nicotine and its derivatives are believed to be mediated by nicotine acetylcholine receptors, which increase oxidative stress and activate NF-Kb and multiple signalling pathways that regulate the progression, growth and metastasis of tumours.7, 8
Oral squamous cell carcinoma (OSCC) is a locally aggressive neoplasm with rapid progression and significantly reduced oxygen concentration.9 Under sustained hypoxic conditions, some tumour cells can survive, and they adapt themselves, via hypoxia induced cellular changes, which can lead to more aggressive phenotype, which results in invasion and metastasis.10
GLUTs are a family of proteins that mediate glucose transport through the membrane without depending on energy.11 GLUT-1 is the most widely expressed and dominant member of the family expressed in SCC head and neck.12 GLUT-1 positivity in malignant cells indicates increased proliferative activity, energy requirements and aggressive behaviour.13 The ability of nicotine to drive acquisition of malignant phenotype characterized by increased risk of metastasis is mostly similar for hypoxia.14 Pre-treatment GLUT-1 expression in the tumour is a marker of radio-resistance in OSCC, with high expression being associated with poor radiation response and shorter survival.15 The presence of hypoxia in tumours is leading to resistance to radiotherapy and chemotherapy and is associated with an increased potential for metastasis. Therefore, pre-treatment characterization of tumour hypoxia may be useful for predicting the prognosis, and this approach may help in establishing a risk adapted treatment strategy.16 This study was conducted to observe the expression of GLUT-1 protein in OSCC in tobacco and non-tobacco users using immunohistochemistry and to find the correlation of the expression of this protein with clinico-pathological factors, histopathological grading and staging.
2. Materials and methods
This prospective study was conducted in the Post Graduate Department of Pathology, King George Medical University; Lucknow; during period of August 2014–July 2015. Sample for this study was comprised of 50 cases of oral squamous cell carcinoma patients with age range of 28–78 years (mean age of 47.74 ± 11.55 yrs). Out of these 50 samples, 30 were taken by wide local excision with radical neck dissection, and 20 were tissue biopsies taken from lip, buccal mucosa, anterior part of tongue, hard palate, floor of mouth and gingiva. The sample was further categorized into two groups each having 25 patients with tobacco addiction and non-tobacco addiction respectively. Out of these 25 patients with tobacco addiction, 13 patients had additional alcohol consumption also. The relevant clinical and demographic details were obtained. pTNM staging according to AJCC (American Joint Committee on Cancer) and histopathological grading by Bryne's Invasive Tumor Front Grading system (1989 and 1992) was done on H & E stained sections (Fig. 1). 3–4 micron thin sections were obtained from formalin fixed embedded tissue blocks. All the study sections were stained by standard immunohistochemistry methods using primary anti-glucose transporter GLUT-1 antibody rabbit polyclonal antibody; Clone ab128033; supplied by abcam England with dilution factor 1:100. GLUT-1 immuno-staining was graded as membranous, cytoplasmic and combination of both on the basis of intra cellular localization in >50% of positive tumour cells (Fig. 2). The quantitative scoring was calculated as percentage of cells showing GLUT-1 positivity in 10 HPF in well stained area of tumour. We regarded GLUT-1 expression in endothelial cells as positive internal control.
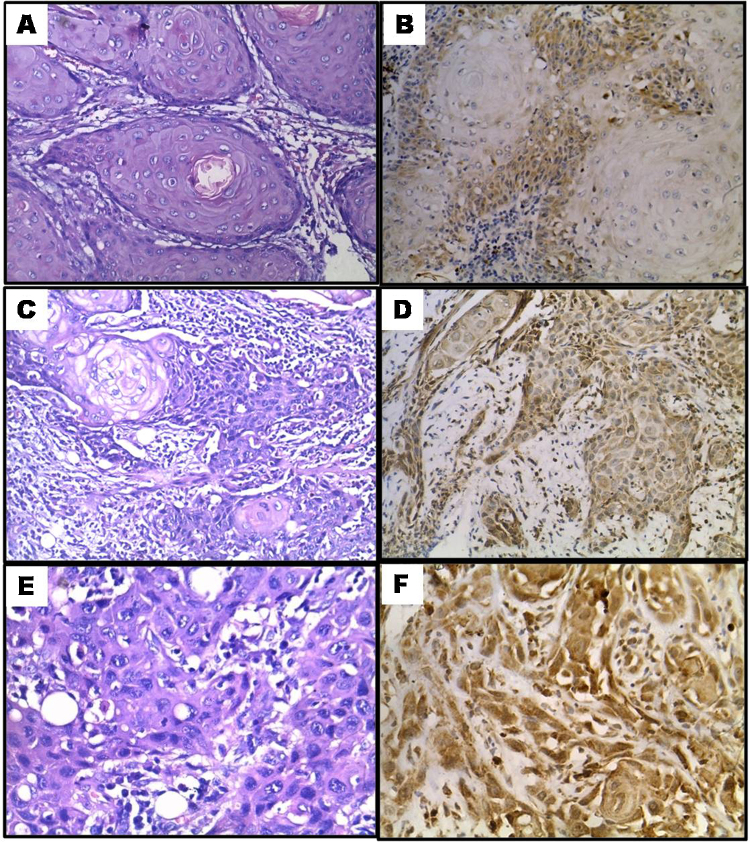
Fig. 1.
Bryne's grade I tumour (A) H&E 400×, (B) mild (prostromal) GLUT-1 immunoexpression 200×. Bryne grade II tumour (C) H&E 200×, (D) Moderate GLUT-1 immunoexpression 200×. Bryne's grade III tumour (E) H&E 400×, (F) high GLUT-1 immunoexpression 200×.
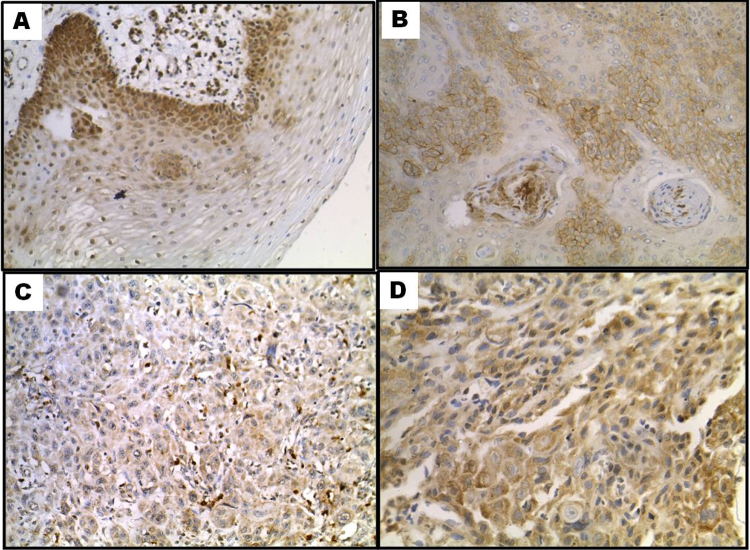
Fig. 2.
GLUT-1 immunoexpression (A) basal layer of normal epithelium 200×, (B) membranous pattern 200×, (C) cytoplasmic pattern 200×, (D) combined membranous and cytoplasmic pattern 200×.
2.1. Statistical analysis
Data analysis was performed using SPSS software (window version 17.0). Data were summarized as mean ± SD (standard deviation) for each group. Groups were compared by student's t-test, one way analysis of variance (ANOVA), Tukey post hoc test and Chi-square test.
3. Results
The basic characteristics of OSCC patients are summarized in Table 1. Most patients were above 40 years and predominantly males (74.0%). In tobacco group 48.0% patients had habit of tobacco and 52.0% had habit of tobacco with alcohol. Tongue (42.0%) was the most common and lip and floor of the mouth was least (2%) common site. Most patients were in stage II (53%) and most common tumour sizes were T2 (76.7%). Nodal metastasis was present in 33.0% of cases and distant metastasis was absent.
Table 1.
Oral squamous cell carcinoma- age, sex and distribution of patients with various variables.
| Variables | Number of patients (n) and (%) |
|---|---|
| Age (years) | |
| <40 | 12 (24.0%) |
| >40 | 38 (76.0%) |
| Sex | |
| Male | 37 (74.0%) |
| Female | 13 (26.0%) |
| History of tobacco | |
| Absent | 25 (50.0%) |
| Present | 25 (50.0%) |
| History of alcohol consumption with tobacco | |
| Absent | 12 (48.0%) |
| Present | 13 (52.0%) |
| Tumour site | |
| Lip | 1 (2.0%) |
| Buccal mucosa | 18 (36.0%) |
| Alveolus & gingiva | 6 (12.0%) |
| Hard palate | 3 (6.0%) |
| Floor of mouth | 1 (2.0%) |
| Tongue | 21 (42.0%) |
| Bryne's histopathologic grade | |
| Grade I | 20 (40.0%) |
| Grade II | 28 (56.0%) |
| Grade III | 2 (4.0%) |
| Specimen type | |
| Biopsy | 20 (40.0%) |
| Wide local excision with lymph node dissection | 30 (60.0%) |
| Tumour size | |
| T1 | 5 (16.7%) |
| T2 | 23 (76.7%) |
| T3 | 2 (6.7%) |
| Nodal metastasis | |
| N0 | 20 (66.7%) |
| N1 | 4 (13.3%) |
| N2 | 6 (20.0%) |
| Distant metastasis | |
| Absent | 50 (100%) |
| TNM stage | |
| I | 3 (10.0%) |
| II | 16 (53.3%) |
| III | 5 (16.7%) |
| IV | 6 (20.0%) |
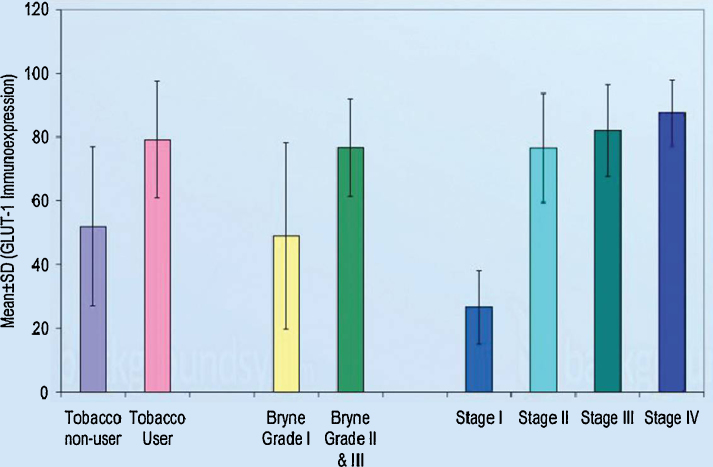
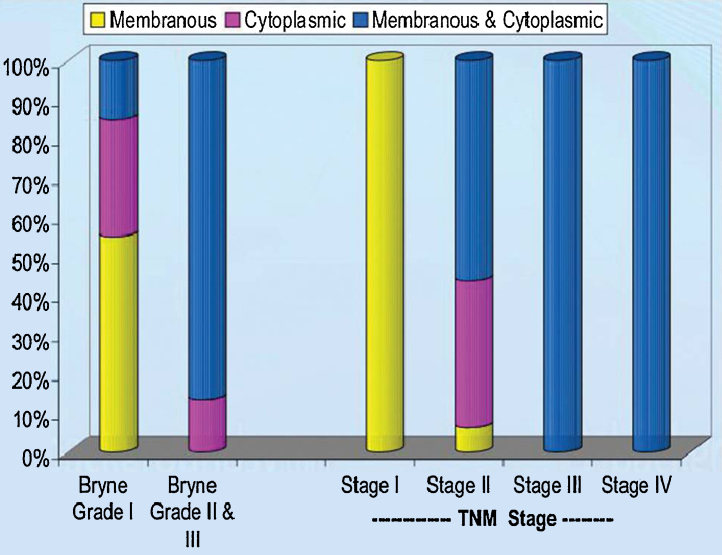
Imunnohistochemistry findings of OSCC patients are summarized in Table 2, Table 3 and Bar diagram 1, Bar diagram 2. All cases showed positive staining with GLUT-1 antibody. The GLUT-1 positivity ranged from 15% to 95% (mean 65.60 ± 25.67%). The intracellular location of GLUT-1 expression was 22.0% membranous, 20.0% cytoplasmic and 58.0% combined membranous and cytoplasmic (Fig. 2).
Table 2.
A % positivity (mean ± SD) of GLUT-1 immunoexpression in oral squamous cell carcinoma.
| Variables | Number of patients (n) | % positivity (mean ± SD) | t/F value | p value |
|---|---|---|---|---|
| History of tobacco | ||||
| Absent | 25 | 52.00 ± 24.96 | 4.39 | <0.001 |
| Present | 25 | 79.20 ± 18.35 | ||
| History of alcohol consumption with tobacco | ||||
| Absent | 12 | 80.42 ± 15.59 | 0.31 | 0.758 |
| Present | 13 | 78.08 ± 21.17 | ||
| Bryne's grade | ||||
| I | 20 | 49.00 ± 29.29 | 4.37 | <0.001 |
| II/III | 30 | 76.67 ± 15.33 | ||
| Tumour size | ||||
| T1 | 5 | 47.00 ± 29.07 | 3.65 | 0.001 |
| T2/T3 | 25 | 80.20 ± 16.17 | ||
| Nodal metastasis | ||||
| No | 20 | 68.25 ± 24.08 | 2.42 | 0.022 |
| N1/N2 | 10 | 87.50 ± 9.20 | ||
| Stage | ||||
| I | 3 | 26.67 ± 11.55 | 11.84 | <0.001 |
| II | 16 | 76.56 ± 17.10*** | ||
| III | 5 | 82.00 ± 14.40*** | ||
| IV | 6 | 87.50 ± 10.37*** | ||
p < 0.05, statistically significant, t = Student's t test, F = ANOVA.
Table 3.
Intracellular location of GLUT-1 immunoexpresssion in Oral squamous cell carcinoma.
| Variables | Number of patients | GLUT-1 expression in intracellular location |
χ2 value | p value | ||
|---|---|---|---|---|---|---|
| Membranous n (%) |
Cytoplasmic n (%) |
Membranous and cytoplasmic n (%) |
||||
| History of tobacco | ||||||
| Absent | 25 | 9 (36.0) | 5 (20.0) | 11 (44.0) | 6.142 | 0.046 |
| Present | 25 | 2 (8.0) | 5 (20) | 18 (72.0) | ||
| Bryne's grade | ||||||
| I | 20 | 11 (55.0) | 6 (30.0) | 3 (15.0) | 28.79 | <0.001 |
| II/III | 30 | 0 (0.0) | 4 (13.3) | 26 (86.7) | ||
| Stage | ||||||
| I | 3 | 3 (100.0) | 0 (0.0) | 0 (0.0) | 28.31 | <0.001 |
| II | 16 | 1 (6.3) | 6 (37.5) | 9 (56.3) | ||
| III | 5 | 0 (0.0) | 0 (0.0) | 5 (100.0) | ||
| IV | 6 | 0 (0.0) | 0 (0.0) | 6 (100.0) | ||
Bar diagram 1.
Oral squamous cell carcinoma % of GLUT-1 immunoexpresssion.
Bar diagram 2.
Oral squamous cell carcinoma intracellular location of GLUT-1 immunoexpresssion.
On comparing, t/F test showed significant association of GLUT-1 expression with history of tobacco (p < 0.001), Bryne's grade (p < 0.001), tumour size (p = 0.001), nodal metastasis (p = 0.022) and stage (p < 0.001). Further, Tukey revealed significantly (p < 0.001) higher GLUT-1 expression in stage II, stage III and stage IV as compared to stage I (Fig. 1). On correlating, χ2 test revealed significant association of location with Bryne's grade (p < 0.001), tumour size (p = 0.003), nodal metastasis (p = 0.024) and tumour stage (p < 0.001) of the patients.
4. Discussion
Immunohistochemical expression of GLUT-1 was reported to be associated with a poor prognosis for patients with OSCC. GLUT-1 is the crucial target gene for hypoxia inducible factor (HIF) that mediates glucose influx into cells under certain conditions that have higher metabolic requirements such as cell division, malignant transformation and nutrient depletion. Thus, deregulated expression of GLUT-1 has been well documented in association with malignancy.12, 17, 18, 19 Angadi et al.20 and Rudlowski et al.21 found that GLUT-1 immuno-expression in normal oral mucosa is membranous in basal layer which progressively reduced in suprabasal layers. This suggests that proliferative activity is associated with glucose transport from the basement membrane. GLUT-1 expression increases with the severity of oral epithelial dysplasia.20, 21 It was intense and extended in entire epithelium including granular and corneal layers in severe dysplasia. This association with grade of dysplasia has been linked to the low or absent glycogen content of the cells in dysplastic areas of epithelium.21, 22
In our study 76% cases were above the age of 40 years and males were predominant (74%). Similar findings were observed by Malhotra et al.,23 Shyam Sunder et al.24 and Doshi Neena et al.25 Tobacco addiction group consisted 92.30% males and only 7.70% females. High proportion of cases among males may be due to tobacco consumption in them. Most common tumour site in our study was tongue (42%), followed by buccal mucosa (36%), whereas least common site was lip (2%) and floor of mouth (2%). In meta analysis, Bhawna and Newell found that buccal mucosa is the most common site followed by tongue, and least common site was lip.26 In our study, out of 50 cases, 56% cases were in Bryne's grade II and in 30 cases (WLE with lymph node dissection) 53.3% were in pTNM stage II. 76.7% tumour cases were in T2 stage, 33.3% were in nodal metastasis, and distant metastasis was not evident.
Statistically significant correlation (p < 0.001) between Bryne's histopathological grading of tumours and percentage of cells showing GLUT-1 immunoexpression was found in this study, which was consistent with the findings of Angadi et al.20 We also observed that Bryne's Grade I tumours show 55% membranous, 30% cytoplasmic and 15% combined membranous and cytoplasmic positivity. In contrast Bryne's Grade II and III tumours showed 13.3% cytoplasmic and 86.7% combined cytoplasmic and membranous positivity. This showed that as the Bryne's grade increases, the location of the intracellular GLUT-1 expression changes significantly (p < 0.001) from membranous to combined cytoplasmic and membranous positivity. Similar findings were also observed by Vasconcelos et al. in their study.27 Angadi et al.20 reported that location of GLUT-1 showed a progressive switch from a membranous to cytoplasmic location then to a combination of both as the grade of OSCC increase. Whereas Jyotsna et al.28 observed GLUT-1 expression predominantly membranous in all grades of OSCC. Chul-Hwan Km et al.22, Yoon Seok Choi et al.16 and Mei Tian et al.29 found no correlation between GLUT-1 expression and histological differentiation.
Anti-GLUT-1 antibody usually recognizes membrane bound proteins on epithelial cells. GLUT-1 induction following hypoxia involves a succession of changes to its intrinsic activity, kinetics and expression. There is unmasking of glucose transporter protein in the cell membrane to facilitate more glucose to cell. Further stimulation results in translocation of existing glucose transporter from cytoplasmic vesicle to plasma membrane and eventual increase in synthesis of GLUT-1mRNA. Airley et al.30 suggested that these changes lead to combined membranous and cytoplasmic expression because of co-localization of GLUT-1 with golgi bodies.
The presence of glycogen is related to cellular maturation of squamous epithelium and disappears with loss of differentiation during neoplastic transformation. In well differentiated tumours, increased accumulation of glycogen in keratin pearls has inversely correlated with GLUT-1 immunoexpression, suggesting that differentiation and mature cells present in keratinized regions lack GLUT-1 expression. In poorly differentiated tumours, it has been suggested that hypoxia driven GLUT-1 stimulation creates an antistromal staining pattern in area devoid of squamous differentiation/keratinization.20, 21, 31 Reisser et al. have demonstrated GLUT-1 staining in outer layers of tumour nests, attributed to the role of this protein in cellular differentiation suggesting that it might be able to predict to the degree of histologic differentiation.32
In this study, tobacco addiction group showed more percentages of cells displaying GLUT-1 immunostaining (79.2%) in comparison to non-tobacco group (52%) which was statistically significant (p < 0.001). In non-tobacco group membranous and combined immunoexpression was in 36% and 44% cases respectively. While in tobacco group predominant pattern of GLUT-1 expression was combined (72%). However, these findings were not statistically significant. No significant association of additional alcohol consumption and GLUT-1 expression was observed in our study. Ayala et al.31 found significant association with alcohol consumption (p = 0.004).
In our follow-up of 6 months to 1 year, 4 patients died due to disease, 12 were not in follow-up, while 34 were alive.
5. Conclusion
GLUT-1 expression correlates significantly with histological (Bryne's) grade and pTNM staging of oral squamous cell carcinoma. With advancing stage and grade of tumour, intracellular location of GLUT-1 expression shift from membranous to combined. It also significantly correlates with tobacco addiction; however addition of alcohol over tobacco showed no statistical correlation. Thus, GLUT-1 expression may serve as a biomarker for patients of oral squamous cell carcinoma.
To validate the findings of this study, further study is needed in future comprising of large sample size.
Conflicts of interest
The authors have none to declare.
References
- 1.Elango J.K., Gangadharan P., Sumithra S., Kuriakose M.A. Trends of head and neck cancers in urban and rural India. Asian Pac J Cancer Prev. 2006;7(1):108–112. [PubMed] [Google Scholar]
- 2.Sankaranarayanan R., Ramadas K., Thomas G. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365(9475):1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 3.Bagan J., Sarrion G., Jimenez Y. Oral cancer: clinical features. Oral Oncol. 2010;46(6):414–417. doi: 10.1016/j.oraloncology.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta P., Rastogi S., Pillai S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116(8):2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dasgupta P., Rizwani W., Pillai S. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124(1):36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshino I., Kometani T., Shoji F. Induction of epithelial-mesenchymal transition-related genes by benzo-pyrene in lung cancer cells. Cancer. 2007;110(2):369–374. doi: 10.1002/cncr.22728. [DOI] [PubMed] [Google Scholar]
- 7.Egleton R.D., Brown K.C., Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29(3):151–158. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Yildiz D. Nicotine, its metabolism and an overview of its biological effects. Toxicon. 2004;43(6):619–632. doi: 10.1016/j.toxicon.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Ryu M.H., Park H.M., Chung J. Hypoxia-inducible factor-1alpha mediates oral squamous cell carcinoma invasion via upregulation of alpha5 integrin and fibronectin. Biochem Biophys Res Commun. 2010;393:11–15. doi: 10.1016/j.bbrc.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 10.Hockel M., Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 11.Parente P., Coli A., Massi G. Immunohistochemical expression of the glucose transporters Glut-1 and Glut-3 in human malignant melanomas and benign melanocytic lesions. J Exp Clin Cancer Res. 2008;27:34. doi: 10.1186/1756-9966-27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunkel M., Reichert T.E., Benz P. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97(4):1015–1024. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum M.J., Haspel H.C., Rosen O.M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci USA. 1986;83:5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo L., Li L., Wang W. Mitochondrial reactive oxygen species mediates nicotine-induced hypoxia-inducible factor-1α expression in human non-small cell lung cancer cells. Biochim Biophys Acta. 2012;1822(6):852–861. doi: 10.1016/j.bbadis.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Kunkel M., Moergel M., Stockinger M. Overexpression of GLUT-1 is associated with resistance to radiotherapy and adverse prognosis in squamous cell carcinoma of the oral cavity. Oral Oncol. 2007;43(8):796–803. doi: 10.1016/j.oraloncology.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Choi Y.S., Kim S.J., Kim D.S. Glucose transporter-1 expression in squamous cell carcinoma of the tongue. Cancer Res Treat. 2007;39(3):109–115. doi: 10.4143/crt.2007.39.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohba S., Fujii H., Ito S. Overexpression of GLUT-1 in the invasion front is associated with depth of oral squamous cell carcinoma and prognosis. J Oral Pathol Med. 2010;39:74–78. doi: 10.1111/j.1600-0714.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 18.Eckert A.W., Lautner M.H., Schütze A. Coexpression of hypoxia-inducible factor-1α and glucose transporter-1 is associated with poor prognosis in oral squamous cell carcinoma patients. Histopathology. 2011;58:1136–1147. doi: 10.1111/j.1365-2559.2011.03806.x. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho K.C., Cunha I.W., Rocha R.M. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics (Sao Paulo) 2011;66(6):965–972. doi: 10.1590/S1807-59322011000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angadi V.C., Angadi P.V. GLUT-1 immunoexpression in oral epithelial dysplasia, oral squamous cell carcinoma, and verrucous carcinoma. J Oral Sci. 2015;57(2):115–122. doi: 10.2334/josnusd.57.115. [DOI] [PubMed] [Google Scholar]
- 21.Rudlowski C., Becker A.J., Schroder W. GLUT1 messenger RNA and protein induction relates to the malignant transformation of cervical cancer. Am J Clin Pathol. 2003;120:691–698. doi: 10.1309/4KYN-QM58-62JW-2GD7. [DOI] [PubMed] [Google Scholar]
- 22.Kim C.H., Kim M.Y. Correlation between glucose transporter type-1 expression and 18F-FDG uptake on PET in oral cancer. J Korean Assoc Oral Maxillofac Surg. 2012;38:212–220. [Google Scholar]
- 23.Malhotra A., Borle R., Bhola N. Demographic, histopathological patterns and clinical profile of oral squamous cell carcinoma (OSCC) at a tertiary level referral Hospital in Vidarbha (Central India): a 7-year retrospective study. J Dental Med Sci. 2014;13(11):53–56. [Google Scholar]
- 24.Syam Sundar B., Nageswara rao R., Faheem M.K. Epidemiological and clinico pathological study of oral cancers in a tertiary care hospital. Int J Biol Med Res. 2012;3(4):2376–2380. [Google Scholar]
- 25.Doshi N.P., Shah S.A., Patel K.B. Histological grading of oral cancer: a comparison of different systems and their relation to lymph node metastasis. Natl J Commun Med. 2011;2(1):136–142. [Google Scholar]
- 26.Gupta B., Johnson N.W. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PLOS ONE. 2014;9(11):e113385. doi: 10.1371/journal.pone.0113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasconcelos M.G., Vasconcelos R.G., Pereira de Oliveira D.H. Distribution of hypoxia-inducible factor-1α and glucose transporter-1 in human tongue cancers. J Oral Maxillofac Surg. 2015;73(9):1753–1760. doi: 10.1016/j.joms.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 28.Harshani J.M., Yeluri S., Guttikonda V.R. Glut-1 as a prognostic biomarker in oral squamous cell carcinoma. J Oral Maxillofac Pathol. 2014;18:372–378. doi: 10.4103/0973-029X.151318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian M., Zhang H., Nakasone Y. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging. 2004;31(1):5–12. doi: 10.1007/s00259-003-1316-9. [DOI] [PubMed] [Google Scholar]
- 30.Airley R., Loncaster J., Davidson S. Glucose transporter Glut-1 expression correlates with tumor hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin Cancer Res. 2001;7:928–934. [PubMed] [Google Scholar]
- 31.Ayala F.R., Rocha R.M., Carvalho K.C. GLUT1 and GLUT3 as potential prognostic markers for oral squamous cell carcinoma. Molecules. 2010;15(4):2374–2387. doi: 10.3390/molecules15042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reisser C., Eichhorn K., Herold-Mende C. Glucose uptake in malignant tumors of the head and neck. HNO. 1999;47(8):712–717. doi: 10.1007/s001060050450. [DOI] [PubMed] [Google Scholar]