Highlights
-
•
We report a rare case of adenoma that developed after sigmoidocolocystoplasty.
-
•
There were 11 cases of oncogenesis after bladder augmentation in the literature, including our case.
-
•
We recommend routine surveillance cytoscopy and cytology for patients after bladder augmentation, although this remains controversial.
Keywords: Tubular adenoma, Bladder augmentation, Malignancy, Neurogenic bladder
Abstract
Introduction
We encountered a rare case of tubular adenoma developing after bladder augmentation. We here report our case as well as summarize reports in the literature on adenomas developing after bladder augmentation.
Presentation of case
A 23-year-old man came to our hospital for routine surveillance cystoscopy. He was born with a lipomyelomeningocele and neurogenic bladder with low bladder compliance, and hence his bladder was routinely emptied by clean intermittent catheterization. He was also treated with anticholinergic agents. However, because the patient’s neurogenic bladder was unstable, he underwent sigmoidocolocystoplasty when he was 8-years old. After the bladder augmentation, he was examined annually by surveillance cystoscopy.
On cystoscopy, a 5-mm pedunculated polyp was found on the front side of the sigmoid colon cap. Therefore, we performed snare polypectomy together with electrocoagulation under cystoscopy. The patient’s final diagnosis was tubular adenoma (mild atypia) with no malignancy, as assessed by histopathology. There has been no evidence of recurrence after the polypectomy on routine surveillance cystoscopy.
Discussion
To the best of our knowledge, there have been 11 cases of adenoma occurring after bladder augmentation reported in the literature, including our present case. There are several carcinogenic pathways associated with colorectal oncogenesis. Adenomas that are larger than 1.0 cm in diameter with a marked villous component have a high risk of oncogenesis.
Conclusion
We believe that the early detection of carcinoma or adenoma and their treatment at an early stage is crucial. Therefore, we recommend routine surveillance cystoscopy for patients after bladder augmentation.
1. Introduction
This work has been reported in line with the CARE criteria [1]. Enterocystoplasty (ECP) using the colon, ileum, or stomach has become an accepted reconstructive option for patients with intractable incontinence and poor bladder compliance owing to various neurogenic and non-neurogenic disorders [2].
However, recently, there have been an increasing number of reports of benign and malignant tumors developing in the neobladder of post-ECP patients, particularly around the line of anastomosis between the colon cap and the native bladder remnant, which are detected on long-term follow-up [2]. We previously reported that 55 cases of malignancy occurring after ECP have been published in the literature [3]. Although N-nitrosamines were reported to be associated with carcinogenesis following bladder augmentation, the mechanisms involved remained unclear [4]. There are several carcinogenic pathways associated with colorectal oncogenesis [5]. Adenomas that are greater than 1.0 cm in diameter, and with a marked villous component have a high risk of developing into cancer [5]. Therefore, the early detection of adenomas is important as it will enable early treatment, and therefore the prevention of malignant changes.
We routinely perform annual surveillance cystoscopy in patients who have undergone bladder augmentation in our departments. One such patient was found to have developed tubular adenoma at the patch site of the sigmoid colon cap. The incidence of adenoma after ECP is rare. To the best of our knowledge, there are 11 cases of adenoma reported in the literature, including our present case [4], [6], [7], [8], [9], [10], [11], [12], [13], [14]. Here we report our rare case and review the literature on cases of benign tumors that occurred after ECP.
2. Case report
A 23-year-old man came to our hospital for routine surveillance cystoscopy. He had no symptoms, such as upper tract infection, flank pain, and hematuria. He was born with a lipomyelomeningocele and neurogenic bladder with low bladder compliance. He had been undergoing clean intermittent catheterization and taking anti-cholinergic agents since he was 3-years old. However, his neurogenic bladder was unstable, and his bladder capacity decreased and his bladder compliance worsened. He hence underwent sigmoidocolocystoplasty when he was 8-years old. His postoperative course was stable, and his bladder capacity increased and his urinary continence improved. The patient has been undergoing annual routine surveillance cystoscopy from 1 year after the bladder augmentation. The patient has no family history of bladder and colorectal disease.
There were and no abnormalities on laboratory data before cystoscopy. Tumor markers, such as α-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 19–9 were not increased.
Upon cystoscopy, there were no abnormalities around the anastomosis between the native bladder and sigmoid colon. However, a 5-mm pedunculated polyp was found on the front side of the sigmoid colon cap, and hence we performed a biopsy (Fig. 1). On histopathology, the polyp was identified as tubular adenoma (Fig. 2). Therefore, we performed snare polypectomy together with electrocoagulation under cystoscopy (Fig. 3). The patient's postoperative course was uneventful and his bladder function was maintained. His bladder was drained for 24 h. His final diagnosis was tubular adenoma (mild atypia) and no malignancy was found on histopathology (Fig. 4a,b). We performed routine annual surveillance cystoscopy from 1 year after the polypectomy, because we believe that the early diagnosis of adenoma will decrease the risk of malignancy development. During the subsequent 5 years, there has been no evidence of recurrence.
Fig. 1.
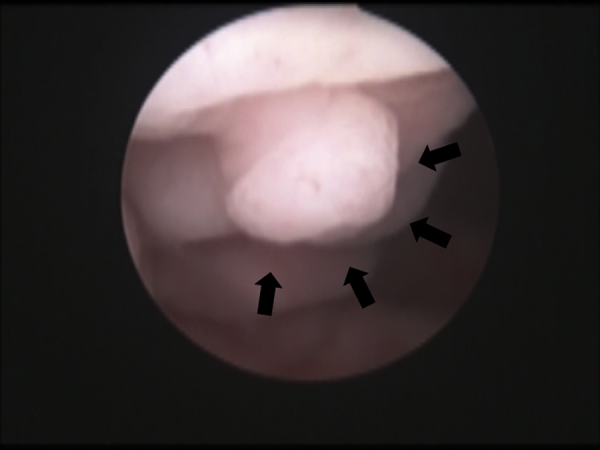
Cystoscopic findings before transurethral resection.
An image of the patient’s bladder. A 5-mm pedunculated polyp was found on the front side of the colon cap. There were no abnormalities in other areas of the bladder, including in the anastomosis between the colon cap and the native bladder (arrows).
Fig. 2.
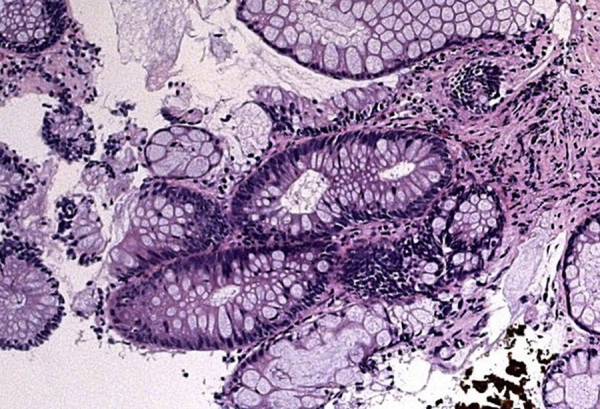
Histopathological analysis of the tumor on biopsy.
Hematoxylin and eosin staining of the tumor specimen (× 40). The tumor was contained within the glandular component. No malignant changes were detected. The diagnosis was tubular adenoma.
Fig. 3.

Cystoscopic findings at the site of transurethral resection.
Image of the site of resection after snare polypectomy together with electrocoagulation. No perforations or bleeding was detected.
Fig. 4.
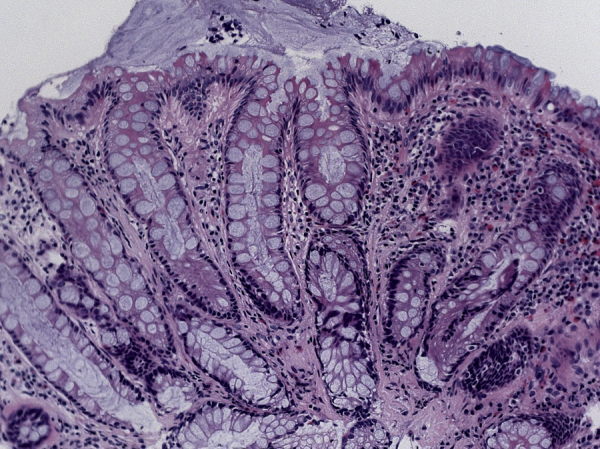
Histopathological analysis of the tumor on transurethral resection.
A high magnification image of the tumor (× 400) showing the presence of glandular epithelium. No malignant changes were detected.
3. Discussion
Recent reports have suggested an increased incidence of malignancy after ECP [2], [15], [16], [17], [18]. However, the incidence of adenoma is rare. To the best of our knowledge, there have been 11 cases of adenoma occurring after bladder augmentation reported in the literature, including our present case [4], [6], [7], [8], [9], [10], [11], [12], [13], [14] (Table 1). The sex distribution of the patients was 7 men (64%) and 4 women (36%). The age at ECP ranged from 5 to 50 years (mean: 27.1 years). The original underlying disease was urinary tuberculosis in 5 cases (45%), neurogenic bladder in 3 cases (27%), rhabdomyosarcoma in 1 case (9%), post bladder surgery complications in 1 case (9%), and detrusor instability in 1 case (9%). Symptoms included hematuria in 6 (55%), lumbar pain and oliguria in 1 (9%), frequent urination in 1 (9%), recurrent pyelonephritis in 1 (9%), and no symptoms (detected on a routine examination) in 1 (9%). Bladder augmentation involved the colon in 7 cases (64%), small bowel in 3 cases (27%), and stomach in 1 case (9%). Histopathology confirmed tubulovillous adenoma in 4 cases (36%), villous adenoma in 3 cases (27%), tubular adenoma in 3 cases (27%), and adenocarcinoma in the tubulovillous adenoma in 1 case (9%). Adenoma arose from the anastomosis in 4 cases (36%), intestinal cap in 6 cases (55%), and native bladder in 1 case (9%). Treatment was transurethral resection in 7 cases (64%), cystectomy and ECP using other segments of the intestine in 2 cases (18%), and partial resection in 2 cases (18%). There were 2 recurrent cases in the literature [9], [13].
Table 1.
Summary of our case and a literature review of cases of adenoma occurring after bladder augmentation.
| Authors | Sex | Age at ECP (yrs) | Original disease | Segment for ECP | Duration from ECP (yrs) |
Symptoms | Site of tumor | Pathology | Size (mm) |
Treatment | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| King et al. [6] | M | 20 | Tb | Ileum | 30 | Dysuria | Ileum | TVA | 75 | TUR | (−) |
| Gepi-Attee et al. [7] | M | 28 | Tb | Colon | 25 | Frequent urination | Colon | VA | 60 | Ileocystoplasty | (−) |
| Gousse et al. [8] | F | 77 | DI | Colon | 5 | Hematuria | Colon | TVA | 35 | Ileocystoplasty | (−) |
| Yip et al. [9] | F | 14 | Tb | Colon | 24 | Hematuria | Colon | VA | ND | TUR | (+) |
| Yamada et al. [10] | F | 18 | Tb | Ileum | 44 | Hematuria | Anastomosis | TVA | ND | TUR | (−) |
| Armah et al. [11] | M | 5 | RMS | Ileum | 34 | Hematuria | Anastomosis | TA | 50 | TUR | (−) |
| Elphick et al. [4] | M | 50 | NB | Colon | 12 | Hematuria | Colon | TA | 25 | TUR | (−) |
| Husillos Alonso et al. [12] | M | 42 | Tb | Colon | 24 | Lumbar pain Oliguria |
Anastomosis | VA | ND | TUR | (−) |
| Rubino et al. [13] | M | ND | MBS | Colon | ND | Pyelonephritis | Anastomosis | TVA | 40 | Resection | (+) |
| Lin et al. [14] | F | 9 | NB | Stomach | 9 | Hematuria | Native bladder | Adenocarcinoma in adenoma | 18 | Resection | (−) |
| Our case | M | 8 | NB | Colon | 15 | Routine surveillance | Colon | TA | 5 | TUR | (−) |
ECP: enterocystoplasty, M: male patient, F: female patient, ND: not described, Tb: tuberculosis, DI: detrusor instability, RMS: rhabdomyosarcoma, NB: neurogenic bladder, MBS: multiple bladder surgery, TVA: tubulovillous adenoma, VA: villous adenoma, TA: tubular adenoma, TUR: transurethral resection.
The mechanism of colorectal cancer occurrence has been analyzed in detail. There are at least 4 types of carcinogenic pathways that are associated with colorectal oncogenesis, namely, the adenoma-carcinoma sequence type, the de novo type, the hereditary non-polyposis colorectal cancer type, and the colitic cancer type [5]. Among these carcinogenic pathways, the de novo type and the adenoma-carcinoma sequence type are thought to be particularly important.
In the de novo type pathway, colorectal cancer develops directly in the normal colorectal mucosa without adenoma. In the 1980s, several Japanese researchers reported that they detected a flat type of carcinoma with a diameter of less than 10 mm arising de novo, which tended to reach the deeper layers at an earlier stage than polypoid-type carcinoma in adenoma [5].
In the adenoma-carcinoma sequence pathway, an adenoma initially forms and increases in size, and then a carcinoma forms in the adenoma. This type of carcinogenesis is associated with several genes, such as the adenomatous polyposis coli gene, K-ras gene, p53 gene, and deleted in colorectal carcinoma gene. Adenomas are well-demarcated lumps of epithelial tumor cells, which can be classified into the following 3 major histological types: tubular, villous, and tubulovillous. The mechanism of the adenoma–carcinoma sequence in colonic segments transposed to the urinary tract has not been established, but may be associated with chronic infection and inflammation, possibly via the reduction of urinary nitrites to nitrates by bacteria, accompanied with the formation of N-nitrosamines, which are potent carcinogens [4]. Adenomas that are larger than 1.0 cm in diameter with a marked villous component have the risk of developing into cancer [5]. Therefore, we believe that the early diagnosis of adenoma should decrease the risk of malignancy development.
The use of routine surveillance cystoscopy and cytology is controversial [19]. Higuchi et al. [20] reported that no tumors developed in their patients during a median surveillance time of 15 years after ECP. Furthermore, they reported that they discontinued performing annual surveillance endoscopy and cytology because of the low incidence of malignancy, the lack of proven benefit, and for cost containment. Hamid et al. [21] also reported that it is not necessary to perform annual check cystoscopies in patients with augmented bladders at least for the first 15 years after surgery. However, they recommended that if the patient develops hematuria or other symptoms of concern, including suprapubic pain or recurrent unexplained urinary tract infections, a full evaluation including cystoscopy should be performed. Soergel et al. and Biers et al. [16], [17] recommended that routine cystoscopic surveillance of all patients with a history of bladder augmentation should be performed from 10 years after the initial surgery. On the other hand, Lin et al. [14] recommended that long-term active surveillance is necessary for all patients who undergo gastrocystoplasty, because they encountered an extremely rare case of a tubulovillous adenoma that transformed into adenocarcinoma in the native bladder segment of an augmented bladder 9 years after surgery. We also perform annual cystoscopic evaluations and cytological examinations in patients after bladder augmentation in our departments. We encountered 1 case of sarcoma occurring 13 years after bladder augmentation [3], and 1 case of adenoma occurring 15 years after bladder augmentation. Most cases of malignancy occur more than 10 years after bladder augmentation. However, some cases occur within 10 years after bladder augmentation [3]. We cannot predict the time of occurrence of malignancy. Therefore, we believe that the early detection of carcinoma or adenoma should lead to their successful treatment. We also recommend routine annual routine cystoscopy from 1year after bladder augmentation, because the bladder mucosa can be directly examined by this technique.
4. Conclusions
We reported a rare case of adenoma developing after sigmoidocolocystoplasty. We recommend performing routine annual surveillance in patients with bladder augmentation for the early detection of tumors.
Conflict of interest
None.
Funding
No funding was received for this study.
Ethical approval
This is a case report and hence ethical approval has not been received.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Authors contribution
Yutaka Hayashi was the attending physician and wrote the paper.
Satoko Shiyanagi treated the patient.
Itsuro Nagae treated the patient.
Tetsuo Ishizaki treated the patient.
Kazuhiko Kasuya treated the patient.
Kenji Katsumata treated the patient.
AtsuyukiYamataka supervised the patient’s treatment.
Akihiko Tsuchidasupervised writing of this paper and treated the patient.
Guarantor
Yutaka Hayashiaccepts full responsibility for this case report, and can be contacted via e-mail at rimpoo@tokyo-med.ac.jp.
Acknowledgement
The authors are indebted to Dr. Helena A. Popiel of the Department of International Medical Communications of Tokyo Medical University for the editorial review of this manuscript.
References
- 1.Gagnier J.J., Kienle G., Altman D.G., Moher D., Sox H., Riley D., CARE Group The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013 doi: 10.1186/1752-1947-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayashi Y., Yamataka A., Kaneyama K., Kato Y., Lane G.J., Miyano T. Review of 86 patients with myelodysplasia and neurogenic bladder who underwent sigmoidocolocystoplasty and were followed more than 10 years. J. Urol. 2006;176:1806–1809. doi: 10.1016/j.juro.2006.03.123. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi Y., Shiyanagi S., Okawada M., Koga H., Fujimura J., Nagae I., Tsuchida A., Yamataka A. Undifferentiated sarcoma developing 14 years after colocystoplasty: our experience and literature review. J. Pediatr. Surg. Case Rep. 2015;3:385–388. [Google Scholar]
- 4.Elphick D.A., Tophill P.R., Suvarna K., Riley S.A. Flat adenomas in a colonic bladder augmentation patch: cystoscopic removal using an endoscopic mucosal resection technique. Urology. 2008;72(230):e1–e3. doi: 10.1016/j.urology.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka T. Colorectal carcinogenesis: review of human and experimental animal studies. J. Carcinog. 2009;8:5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King P.H., Osborn D.S., Mackay E.H. Tubulovillous adenoma arising 30 years after ileocystoplasty. J. Clin. Pathol. 1992;45:928–929. doi: 10.1136/jcp.45.10.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gepi-Attee S., Ganabathi K., Abrams P.H., MacIver A.G. Villous adenoma in augmentation colocystoplasty: a case report and discussion of the pathogenesis. J. Urol. 1992;147:128–130. doi: 10.1016/s0022-5347(17)37157-4. [DOI] [PubMed] [Google Scholar]
- 8.Gousse A.E., Safir M.H., Cortina G., Safman K., Raz S. Tubulovillous adenoma in the cecal segment after cecocystoplasty. J. Urol. 1998;160:490–491. [PubMed] [Google Scholar]
- 9.Yip S.K., Wong M.P., Cheung M.C., Li J.H. Mucinous adenocarcinoma of renal pelvis and villous adenoma of bladder after a caecal augmentation of bladder. Aust. N. Z. J. Surg. 1999;69:247–248. doi: 10.1046/j.1440-1622.1999.01540.x. [DOI] [PubMed] [Google Scholar]
- 10.Yamada Y., Fujimura T., Takahashi S., Takeuchi T., Takazawa Y., Kitamura T. Tubulovillous adenoma developing after urinary reconstruction using ileal segments. Int. J. Urol. 2006;13:1134–1135. doi: 10.1111/j.1442-2042.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- 11.Armah H.B., Krasinskas A.M., Parwani A.V. Tubular adenoma with high-grade dysplasia in the ileal segment 34 years after augmentation ileocystoplasty: report of a first case. Diagn. Pathol. 2007;13(2):29. doi: 10.1186/1746-1596-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husillos Alonso A., Subira Rios D., Molina Escudero R., Hernandez Fernandez C. Villous adenoma in augmentation colocystoplasty asociated to infiltrating urotelial cancer in bladder remanent. Arch. Esp. Urol. 2010;63:876–879. [PubMed] [Google Scholar]
- 13.Rubino B., Dorin R., Naemi K., Skinner E.C. A 37-year-old man with a history of bladder augmentation presented with gross hematuria, weight loss and flank pain. Urology. 2012;79:256–259. doi: 10.1016/j.urology.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Lin T.P., Chen M., Hsu J.M., Sheu J.C. Adenocarcinoma arising from tubulovillous adenoma in a native bladder following gastrocystoplasty. Pediatr. Surg. Int. 2014;30:123–126. doi: 10.1007/s00383-013-3414-5. [DOI] [PubMed] [Google Scholar]
- 15.Vemulakonda V.M., Lendvay T.S., Shnorhavorian M., Joyner B.D., Kaplan H., Mitchell M.E. Metastatic adenocarcinoma after augmentation gastrocystoplasty. J. Urol. 2008;179:1094–1097. doi: 10.1016/j.juro.2007.10.089. [DOI] [PubMed] [Google Scholar]
- 16.Soergel T.M., Cain M.P., Misseri R., Gardner T.A., Koch M.O., Rink R.C. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J. Urol. 2004;172:1649–1652. doi: 10.1097/01.ju.0000140194.87974.56. [DOI] [PubMed] [Google Scholar]
- 17.Vajda P., Kaiser L., Magyarlaki T., Farkas A., Vastyan A.M., Pinter A.B. Histological findings after colocystoplasty and gastrocystoplasty. J. Urol. 2002;168:698–701. [PubMed] [Google Scholar]
- 18.Filmer R.B., Spencer J.R. Malignancies in bladder augmentations and intestinal conduit. J. Urol. 1990;143:671–678. doi: 10.1016/s0022-5347(17)40055-3. [DOI] [PubMed] [Google Scholar]
- 19.Biers S.M., Venn S.N., Greenwell T.J. The past present and future of augmentation cystoplasty. Br. J. Urol. Int. 2012;109:1280–1293. doi: 10.1111/j.1464-410X.2011.10650.x. [DOI] [PubMed] [Google Scholar]
- 20.Higuchi T.T., Fox J.A., Husmann D.A. Annual endoscopy and urine cytology for surveillance of bladder tumors after enterocystoplasty for congenital bladder anomalies. J. Urol. 2011;186:1791–1795. doi: 10.1016/j.juro.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Hamid R., Greenwell T.J., Nethercliffe J.M., Freeman A., Venn S.N., Woodhouse C.R.J. Routine surveillance cystoscopy for patients with augmentation and substitution cystoplasty for benign urological conditions: is it necessary? Br. J. Urol. Int. 2009;104:392–395. doi: 10.1111/j.1464-410X.2009.08401.x. [DOI] [PubMed] [Google Scholar]


