Abstract
The aim of this study was to present a model that uses multi-parametric quantitative MRI to estimate the presence of myelin and edema in the brain. The model relates simultaneous measurement of R1 and R2 relaxation rates and proton density to four partial volume compartments, consisting of myelin partial volume, cellular partial volume, free water partial volume, and excess parenchymal water partial volume. The model parameters were obtained using spatially normalized brain images of a group of 20 healthy controls. The pathological brain was modeled in terms of the reduction of myelin content and presence of excess parenchymal water, which indicates the degree of edema. The method was tested on spatially normalized brain images of a group of 20 age-matched multiple sclerosis (MS) patients. Clear differences were observed with respect to the healthy controls: the MS group had a 79 mL smaller brain volume (1069 vs. 1148 mL), a 38 mL smaller myelin volume (119 vs. 157 mL), and a 21 mL larger excess parenchymal water volume (78 vs. 57 mL). Template regions of interest of various brain structures indicated that the myelin partial volume in the MS group was 1.6 ± 1.5% lower for gray matter (GM) structures and 2.8 ± 1.0% lower for white matter (WM) structures. The excess parenchymal water partial volume was 9 ± 10% larger for GM and 5 ± 2% larger for WM. Manually placed ROIs indicated that the results using the template ROIs may have suffered from loss of anatomical detail due to the spatial normalization process. Examples of the application of the method on high-resolution images are provided for three individual subjects: a 45-year-old healthy subject, a 72-year-old healthy subject, and a 45-year-old MS patient. The observed results agreed with the expected behavior considering both age and disease. In conclusion, the proposed model may provide clinically important parameters, such as the total brain volume, degree of myelination, and degree of edema, based on a single qMRI acquisition with a clinically acceptable scan time.
Keywords: quantitative magnetic resonance imaging, brain tissue modeling, myelin, edema, T1 relaxation, T2 relaxation, proton density
Introduction
Myelin is crucial for efficient signal transmission over long ranges in the nervous system because it increases the speed at which the impulses propagate along the axons. Axons are coated piecewise by multiple layers of phospholipid membranes (“sheaths”) with embedded proteins produced by oligodendrocytes and Schwann cells in the central and peripheral nervous systems, respectively. Degradation of myelin impairs the signal transmission, and the nerve may eventually wither, leading to brain atrophy and brain dysfunction. Knowledge of myelin content supports the investigation of early brain development (1, 2). Accurate myelin measurements are valuable in studies of neurodegenerative diseases, such as multiple sclerosis (MS) (3, 4) and dementia (5–7). Thus, measurements and monitoring of myelin content would provide important information for the diagnosis and prognosis in patients with suspected myelin degradation.
One established MRI method for myelin detection is based on the measurement of the multi-exponential transverse T2 relaxation time using a Carr–Purcell–Meiboom–Gill (CPMG) sequence (8–10). The short-time component of the observed T2 relaxation represents the presence of water trapped between the myelin sheaths, termed myelin water (MyW), whereas the medium-time T2 relaxation component is attributed to the intra- and extracellular water. Commonly, the myelin water fraction (MWF), corresponding to the ratio of both components, is calculated. The proportionality of MWF with the myelin content has been verified in vitro and by histopathology (11, 12). More recently, an alternative approach called mcDESPOT was developed (13). This method consists of a combination of spoiled gradient echo (SPGR) and balanced steady-state free precession (bSSFP) acquisitions at multiple flip angles, resulting in the measurement of MyW and intra- and extracellular water pools. In particular, the mcDESPOT method has been applied to myelin development in children (14).
Limitations of the two described methods are mainly practical. Due to the very short myelin T2 relaxation time (10–15 ms), the multi-exponent T2 measurement mainly depends on the amplitude of the first echo signal, and mcDESPOT is highly sensitive to the accuracy of the applied flip angle, making the measurements demanding in terms of both SNR and time as well as highly dependent on corrections for B1 field and RF pulse profile effects. The underlying models of both approaches are considerably different, resulting in widespread estimations of the myelin content.
Here, we propose a model to estimate the presence of myelin and edema in the brain based on multi-parametric quantitative MRI (qMRI), where the longitudinal relaxation rate R1, transverse relaxation rate R2, and proton density PD are determined simultaneously in one acquisition. It was previously reported that pathological processes, such as axonal damage, gliosis, inflammation, and edema are related to changes in the values of R1, R2, and PD (15–19). Currently, multi-parametric MR quantification of R1, R2, and PD can be achieved at high resolution within a 6–8 min scan time (20), which would make such an approach attractive for routine clinical use. The aim of this study was to present a model that relates the appearance of a qMRI-derived R1–R2–PD data structure to the myelin partial volume of the brain. The model parameters were derived based on data from Ref. (21), where brain images of a group of healthy controls were spatially normalized and averaged to characterize the healthy brain. The second aim of this study was to explore the possibilities of the model to detect both the differences in myelin content and the presence of edema in the pathological brain. Examples of the application of the method are provided for a group of MS patients and three individual subjects.
Materials and Methods
The Relaxation Model
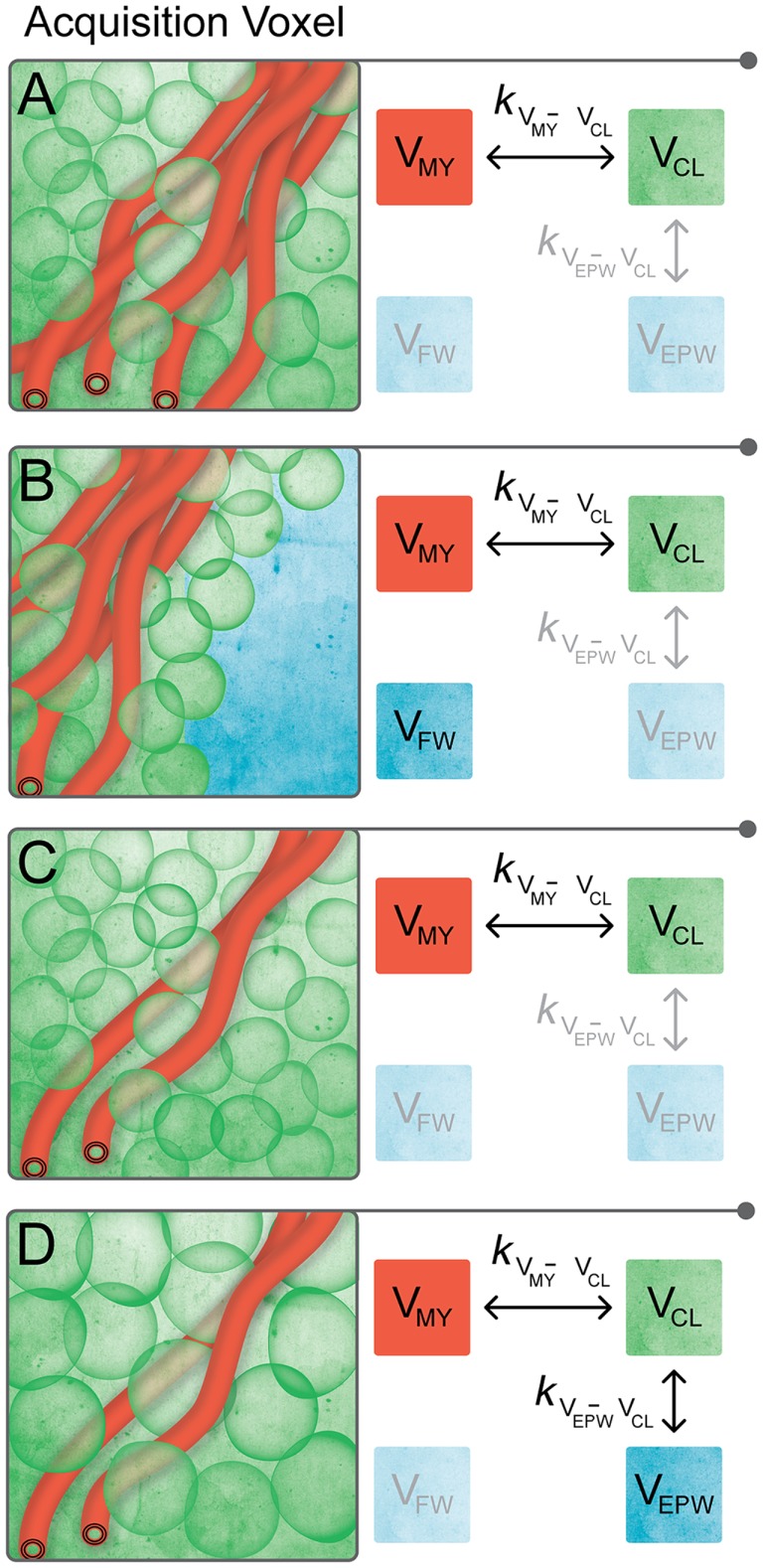
The proposed model for the observed R1, R2, and PD values of the brain is visualized in Figure 1: each MRI acquisition voxel is composed of four partial volume compartments: the myelin partial volume VMY, cellular partial volume VCL, free water partial volume VFW, and excess parenchymal water partial volume VEPW. The content in each partial volume compartment can range from 0 to 100%, where the sum of the four compartments is 100%. Each partial volume compartment has its own relaxation properties (R1,MY, R2,MY, PDMY, R1,CL, R2,CL, PDCL, R1,FW, R2,FW, PDFW, R1,EPW, R2,EPW, PDEPW), without further detailed knowledge of the multitude of interacting pools within each of the compartments. Using this approach, each partial volume compartment can be described by its R1–R2–PD values, its fraction of the acquisition voxel and the magnetization exchange with other partial volume compartments. The total acquisition voxel exhibits R1–R2–PD values that reflect the effective, combined relaxation behavior of all four compartments. An MR quantification sequence measures the effective R1–R2–PD values of acquisition voxels in the total imaging volume, which can provide input to the model.
Figure 1.
Proposed compartmental exchange system for modeling brain parenchyma. Each MRI acquisition voxel is composed of four partial volume compartments, where each partial volume can range from 0 to 100%, and where the sum is 100%. A compartment is grayed out when its partial volume is equal to 0. (A) Normal brain parenchyma consists of myelin partial volume VMY and cellular partial volume VCL. Between VMY and VCL, there is a magnetization net exchange rate kVMY-VCL. (B) At the interface of brain parenchyma with the surrounding bulk CSF, an acquisition voxel contains a mixture of VMY and VCL (i.e., brain parenchyma) and free water partial volume VFW. There is no magnetization exchange between VFW and the other partial volumes. (C) In pathological brain parenchyma, myelin loss may occur, resulting in a relative decrease in VMY. The relative amount of VCL in the acquisition voxel increases to maintain 100% tissue, resulting in a decrease in the total brain volume. (D) Alternatively, there can be edema in pathological brain parenchyma, included in the model by the presence of the non-zero excess parenchymal water partial volume VEPW. No distinction can be made between excess parenchymal water and the already present parenchymal water of the VCL, making the exchange rate kVEPW-VCL infinitely high. The combination of VCL and VEPW effectively dilutes the myelin content, resulting in a relative decrease in VMY per acquisition voxel and an increase in the total brain volume.
The VMY contains the thin layers of MyW and myelin sheets that are closely packed around the axons. The close proximity of MyW to the surrounding structure results in a very fast relaxation of this compartment. The VCL consists of intra- and extracellular (interstitial) water, axonal water, and all cellular macromolecules, not being related to myelin. The presence of the macromolecules results in a medium-time relaxation of VCL, which is slower than VMY, but longer than VFW. Between VMY and VCL, there is a magnetization exchange rate kVMY-VCL. In the model, acquisition voxels in the normal brain parenchyma contain a mixture of VMY and VCL, where voxels in gray matter (GM) have a low VMY and voxels in white matter (WM) have a high VMY (see Figure 1A). The two compartments VMY and VCL are an approximation of the four-pool model (22), where VMY contains MyW and myelin semi-solids and VCL contains intracellular and extracellular water and non-myelin semi-solids pools, albeit with less degrees of freedom.
The brain is surrounded by cerebrospinal fluid (CSF), making it necessary to add a free water partial volume VFW to the model, as also pointed out in Ref. (23). Because bulk CSF is physically separated from the brain parenchyma except for the interface, there is no magnetization exchange between VFW and any other compartment (i.e. “free”). Hence, at the border of the brain, acquisition voxels contain a mixture of VMY and VCL (brain parenchyma) and VFW (CSF), see Figure 1B.
In the pathological brain, two distinct processes are modeled: compared with the normal brain, there can be myelin loss, resulting in a relative decrease in VMY. To maintain 100% tissue, the relative amount of VCL in an acquisition voxel will increase. Therefore, the loss of myelin results in a compaction of the brain and, thus, a decrease in the total brain volume (Figure 1C). The second process is the occurrence of edema, modeled as the presence of excess parenchymal water partial volume VEPW, which adds water to VCL. No distinction can be made between excess parenchymal water and the already present parenchymal water of VCL and, therefore, the exchange rate kVEPW-VCL is infinitely high. Modeling two separate partial volume compartments with an infinite exchange is a mathematical approach to acquire knowledge on the degree of edema without knowledge of the exact internal composition of VCL. The cellular swelling due to a non-zero VEPW effectively dilutes the myelin present in the acquisition voxel, resulting in a relative decrease in VMY. In this case, the total brain volume increases (Figure 1D).
Bloch Simulation
A numerical simulation of coupled Bloch equations of the four partial volume compartments was performed using 150 identical magnetization elements i, spread equidistantly over a distance of 15 mm in the acquisition slice direction, where each element had a distance from the center of the slice. Each of the 150 elements consisted of the same partial volume distribution of interacting VMY, VCL, VFW, and VEPW with normalized magnetization vectors MMY, MCL, MFW, and MEPW, respectively. The evolution of each magnetization was calculated in small time-steps t, where each sequential magnetization of each element i was calculated from the original magnetization Mi,n using:
| (1) |
RRF is the rotation matrix for the applied slice-selective RF pulses. The envelope of the RF pulses was approximated by a series of block pulses with constant amplitudes over the time interval t. The rotation flip angle α, achieved in time t over the x- or y-axis, is equal to 2πγt, where B1 is the amplitude of the RF pulse at that particular time interval, and γ is the gyromagnetic ratio. RGR is the rotation matrix for the applied slice-selective gradients. The rotation flip angle ω, achieved in time t over the z-axis, is equal to 2πγ, where G is the gradient strength and is the distance from the center of the slice.
RR1 is the relaxation matrix for the elements for the longitudinal relaxation rate R1. RR1 only acts on the component of each according to:
| (2) |
where = exp(−tR1,MY), = exp(−tR1,CL), = exp(−tR1,FW), = exp(−tR1,EPW) and = exp(−tkMY-CL). The exchange rate is the combined forward and backward exchange rate between VMY and VCL. The exchange rate between VEPW and VCL is infinitely high. The scaling factors SMY = VCL * PDCL/(VMY * PDMY + VCL * PDCL), = VMY * PDMY/ (VMY * PDMY + VCL * PDCL), = VEPW * PDEPW/(VEPW * PDEPW + VCL * PDCL) and SEPW = VCL * PDCL/(VEPW * PDEPW + VCL * PDCL) are required to take the relative volumes of PD in each compartment into account.
RR2 is the relaxation matrix for the elements for the transverse relaxation rate R2. RR2 only acts on the component of each according to:
| (3) |
where = exp(−tR2,MY), = exp(−tR2,CL), = exp(−tR2,FW), = exp(−).
MR Quantification Sequence
The presented Bloch equations form a general description of the magnetization evolution for each acquisition voxel and only have meaning when applied to an actual MRI sequence. The specifics of this MRI sequence, with the applied RF pulses, gradients, and timings, dictate the observable signal behavior. The MRI quantification method employed was a multi-echo, multi-delay saturation recovery spin echo sequence (QRAPMASTER) as described previously (20). It was a multi-slice sequence where slice-selective saturation pulses were interleaved with a CPMG acquisition of 5 echoes at 14-ms multiples. The saturation pulse acted on slice n, whereas the subsequent acquisition acted on slice m. By a fixed shift between slices n and m, an effective delay time TD was created between the saturation and acquisition of each particular slice. The sequence was repeated four times where the shift between n and m, and hence the saturation delay, was changed. The result of the sequence was a matrix of 20 images at five different echo times TE and at four different saturation delay times TD. The applied slice-selective RF pulse profiles and amplitudes, gradient strengths, and timings were extracted from the scanner. The repetition time TR was 2950 ms with 30 slices of 4-mm thickness with an in-plane resolution of 1 mm. The saturation pulse had a flip angle of 120° over the x-axis followed by a delay of 100, 400, 1380, and 2860 ms, corresponding to a shift between n and m of 1, 4, 14, and 29 slices, respectively. The excitation pulse had a flip angle of 90° over the x-axis, followed by refocusing pulses of 180° over the y-axis. The refocusing pulses were straddled by spoiler gradients. The scan time was 8:21 min on a Philips Achieva 1.5T (Philips Healthcare, Best, The Netherlands).
Application of the Bloch Simulation on the Quantification Sequence
The RF pulses, gradients, and timings of the quantification sequence were implemented as a script into the model calculations. The product of all matrices in Eq. 1 does not commute (AB ≠ BA) and, therefore, Eq. 1 is only valid if time-steps are chosen such that the relaxation rates cause a near-zero change of magnetization per time step. Typical relaxation in the brain occurs in the order of millisecond. Therefore, we choose time steps t of 1 μs, which is three orders of magnitude smaller, but still results in a reasonable calculation time. The observable signal intensity I at each combination of TE and TD was calculated as the product of the total proton density for each partial volume (V * PD) and the xy-component of the magnetization of these spins, summed over all elements i:
| (4) |
In this way, the Block simulation also produced 20 images with different TE and TD, identical to the in vivo quantification sequence.
Subjects
MR quantification was performed on two groups of subjects, one with 20 patients diagnosed with Clinically Definite Multiple Sclerosis (5 males and 15 females; mean age of 47 ± 12 years). The mean extended disability status scale [EDSS (24)] of the MS group was 3.6 ± 2.2, and the mean disease duration was 15 ± 10 years. The second group consisted of age- and gender-matched healthy controls (5 males and 15 females; mean age of 47 ± 11 years). Three female participants were used as individual examples: one healthy subject of 45 years old, one healthy subject of 72 years old, and a secondary progressive MS patient of 45 years old (EDSS of 3.5; disease duration of 17 years). The study was approved by the regional ethical review board and written informed consent was obtained from all participants (full name of the board: “Regionala etikprövningsnämnden i Linköping”; registered under number Dnr. M88-07).
Image Post-Processing
R1, R2, and PD maps were retrieved from both the simulated and in vivo acquired images using SyMRI 7.0 (SyntheticMR, Linköping, Sweden). In summary, a least squares fit was performed as a function of the different TE and TD times according to:
| (5) |
where α is the excitation flip angle, θ is the saturation flip angle, and B1 is the amplitude of the B1 field. A is an overall scaling factor that considers the coil sensitivity, RF chain amplification, and voxel volume (20). This equation explicitly has two mono-exponential functions, in R1 and R2, and hence it will reflect the dominant component of the relaxation behavior.
For spatial normalization of the in vivo brain data, the R1, R2, and PD maps were used to synthesize a stack of T2-weighted images with TE = 100 ms and TR = 4500 ms. The synthetic T2-weighted images were smoothed with an 8-mm Gaussian kernel and used as source images to calculate the transformation matrix to a standard stereotactic space in Montreal Neurological Institute (MNI) coordinates (26). The images were then transformed to match the size and position of a standard template using a 12-parameter (translation, rotation, shear, zoom) affine regularization and non-linear deformations by a linear combination of three-dimensional discrete cosine basis functions. The same transformation matrix was then applied to the R1, R2, and PD maps. The resulting data were re-gridded to 2 mm × 2 mm × 2 mm to obtain an isotropic dataset. All of the subjects were averaged to obtain the mean R1–R2–PD values of the MS and control group. Finally, the mean R1, R2, and PD values were used as coordinates in a R1–R2–PD multi-parametric space, as presented in Ref. (21). The 2D histograms of the entire brain were created with 200 bins for R1 on a scale of 0–2 s−1, 200 bins for R2 on a scale of 0–15 s−1, and 200 bins for PD on a scale of 50–100%.
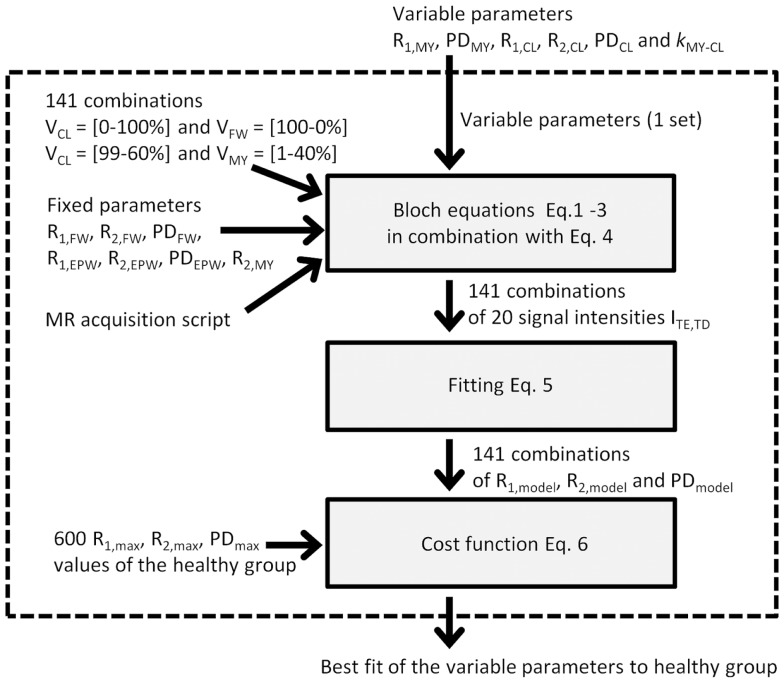
Determining the Model Parameters
The procedure to determine the model parameters is schematically depicted in Figure 2. In the model, the relaxation parameters for water, both for VFW and VEPW, were fixed to literature values for CSF at R1 = 0.24 s−1, R2 = 0.87 s−1, and PD = 100% (20). Additionally, the R2 relaxation for VMY was fixed to a reported value, at R2,MY = 77 s−1 (corresponding to T2,MY = 13 ms) (22). Therefore, only six remaining model parameters, R1,MY, PDMY, R1,CL, R2,CL, PDCL, and kMY-CL, were allowed to vary. The six model parameters were given a random value under the restriction that R1,FW < R1,CL < R1,MY and R2,FW < R2,CL < R2,MY. For each set of variable parameters, the magnetization evolution was calculated for all combinations of VMY and VCL and for all combinations of VCL and VFW, using steps of 1% partial volume. Since the maximum amount is 100%, a setting of for example 20% VFW requires a setting of 80% VCL, hence producing 101 combinations of VFW and VCL. VMY was restricted to a maximum of 40%, since no higher values were expected to occur in the brain and we wanted to avoid values that could not be evaluated. This produced 40 combinations of VMY and VCL, making a total of 141 combinations. The magnetization evolution was calculated using Eqs 1–3, resulting in the signal intensities at five different echo times TE and four different saturation delay times TD for each partial volume combination (Eq. 4). The sets of 20 values were then fitted using Eq. 5, resulting in 141 , , and values for each specific set of variable parameters.
Figure 2.
Schematic depiction of the procedure to optimize the variable parameters: One set of variable parameters is chosen and evaluated within the dotted box. Evaluation is performed by running the Bloch equations of the simulated MR acquisition on 141 combinations of VMY, VCL, and VFW. This provides 20 signal intensities at various echo times and saturation delays times. The 20 signal intensities are fitted, resulting in an R1, R2, and PD value of the model. The model values are then compared to the observed R1, R2, and PD values of the healthy group using the maximum values in the 2D histograms. A cost function provides a measure for closeness of the model R1, R2, and PD values to the observed R1, R2, and PD values. The evaluation is performed for many sets of variable parameters, resulting in the best fit.
To evaluate how close these 141 R1–R2–PD values mimicked the observed data structure in the 2D histograms of the healthy control group, the maximum values in the histogram for each bin in R1 were determined, and the corresponding R2 and PD values were recorded. This procedure was repeated for R2 and PD. Because the 2D histograms had 200 × 200 bins, this procedure provided 600 , , and values to define the characteristic data structure of the healthy group. From these 600 combinations, 141 were selected that were closest to the 141 model combinations.
Finally, a cost function was set up to evaluate the difference between the , , and values for each parameter setting with the selected , , and values of the 2D histograms of the in vivo spatially normalized data:
| (6) |
To ensure that R1, R2, and PD had the same weight in the cost function, the square of the residuals was normalized using the variance σ2 of R1, R2, and PD (27).
The entire procedure was repeated, where each of the variable parameters was varied individually, with increasingly smaller steps until the minimum residual was found. To avoid convergence to a local minimum, this procedure was repeated 100 times, after which the lowest residual was regarded as the global minimum.
The confidence interval of the optimized parameters was calculated using the finite sample confidence intervals in the maximum likelihood (25). According to this approach, the confidence region is found by varying a single parameter and minimizing all others such that the cost function remains under the value of χ2(a, df), where a corresponds to the confidence level and df is the number of degrees of freedom. Using a = 0.05 and df = 5, the χ2(a, df) function becomes 9.488. The Bloch simulation and minimization procedure was implemented in an in-house developed IDL program (ITT visual information solutions, Boulder, CO, USA).
Calculation of Total Volumes and Regions of Interest
Segmentation of the intracranial volume (ICV) was performed using an automatic procedure in SyMRI 7.0. The total myelin volume (MYV), cellular volume (CV), free water volume (FWV), and excess parenchymal water volume (EPWV) were calculated by summing all partial volumes within the ICV. The brain parenchymal volume (BPV) was defined as the ICV minus the total FWV. The brain parenchymal fraction (BPF) corresponds to BPV divided by ICV. The myelin fraction (MYF) was calculated as the total MYV divided by the BPV. Also, the cellular water fraction (CF) and excess parenchymal water fraction (EPWF) were calculated in a similar manner as the total CV divided by the BPV and total EPWV divided by the BPV, respectively.
The MWF can be derived from the model parameters because the MyW corresponds to the PDMY in the VMY, and the intra- and extracellular water corresponds to the sum of PDCL and PDEPW in the VCL and VEPW, such that MWF for each acquisition voxel can be calculated as MWF = (VMY * PDMY)/(VCL * PDCL + VEPW * PDEPW). Additionally, the total aqueous content of the tissue can be calculated, corresponding to the sum of the MyW, cellular water, free water, and excess parenchymal water, VMY * PDMY + VCL * PDCL + VFW * PDFW + VEPW * PDEPW. The total non-aqueous content then corresponds to 100% minus the aqueous content.
To define regions of interest for the spatially normalized brain images, the cropped ROI templates, based on the Wake Forrest University (WFU) PickAtlas, were taken [Ref. (21)]. To verify that the standard ROIs in spatially normalized, averaged brain images provide similar results as spatially non-normalized, separate brain images, 3 mm × 3 mm ROIs were manually placed in a subset of brain structures in all participants of Ref. (21). This was also done for the three example subjects. In the MS cases, areas with MS lesions were avoided.
Results
Optimizing the Model Parameters to the Healthy Brain
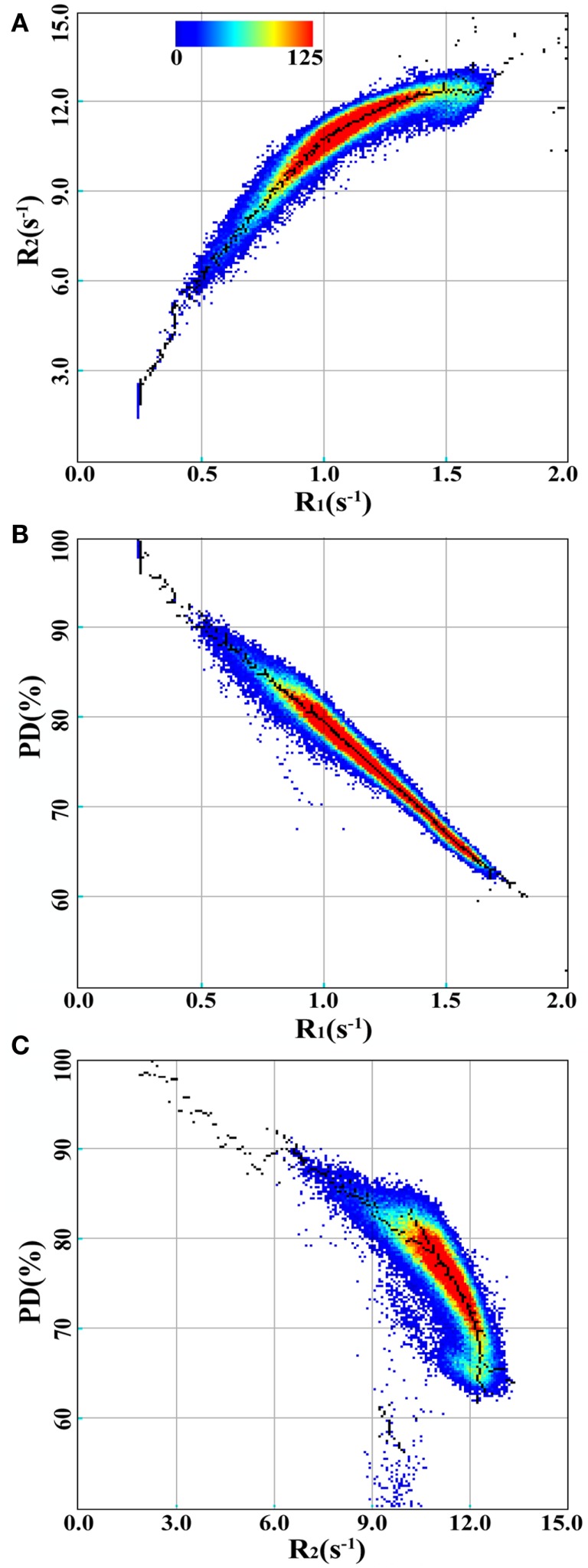
In Figure 3, the R1, R2, and PD values for the spatially normalized brains of the group of controls are shown as 2D histograms of R1 and R2, R1 and PD, and R2 and PD. The color scale indicates the number of voxels for each coordinate in the histogram. The black dots are placed at the maximum values of the histograms in each direction, generating the 600 maxima defining the structure in the R1–R2–PD space.
Figure 3.
2D-histograms of R1, R2, and PD values for the spatially normalized brain images of the group of control subjects. The 2D-histograms of R1 and R2, R1 and PD, and R2 and PD are shown in (A–C), respectively. The color scale indicates the number of voxels for each coordinate. The black dots are placed at the maximum values of the 2D-histograms in each direction.
Using these 600 maxima, the six variables in the model were optimized to find the minimum value of the cost function (See Figure 2). The values of the parameters at the minimum residual (3.446) are given in Table 1. Each parameter was varied individually while re-optimizing all others such that the cost function remained below 9.488, resulting in the determination of the SDs of the parameters, as also listed in Table 1.
Table 1.
The parameter values of the model; on the left the fixed parameters (see MATERIALS AND METHODS); on the right, the optimized parameters where the cost function was minimized for the brain data of the control group (n = 20).
| Fixed parameters | Optimized parameters |
|---|---|
| R2,MY = 77 s−1 | R1,MY = 16.6 ± 13.2 s−1 |
| R1,FW = R1,EPW = 0.24 s−1 | PDMY = 42 ± 33% |
| R2,FW = R2,EPW = 0.87 s−1 | kVMY-VCL = 6.7 ± 5.2 s−1 |
| PDFW = PDEPW = 100% | R1,CL = 0.78 ± 0.13 s−1 |
| kVEPW-VCL = ∞s−1 | R2,CL = 10.3 ± 0.6 s−1 |
| PDCL = 85 ± 5% |
The SD of the latter values is given for a significance level of a = 0.05.
Behavior of the Model for the Pathological Brain
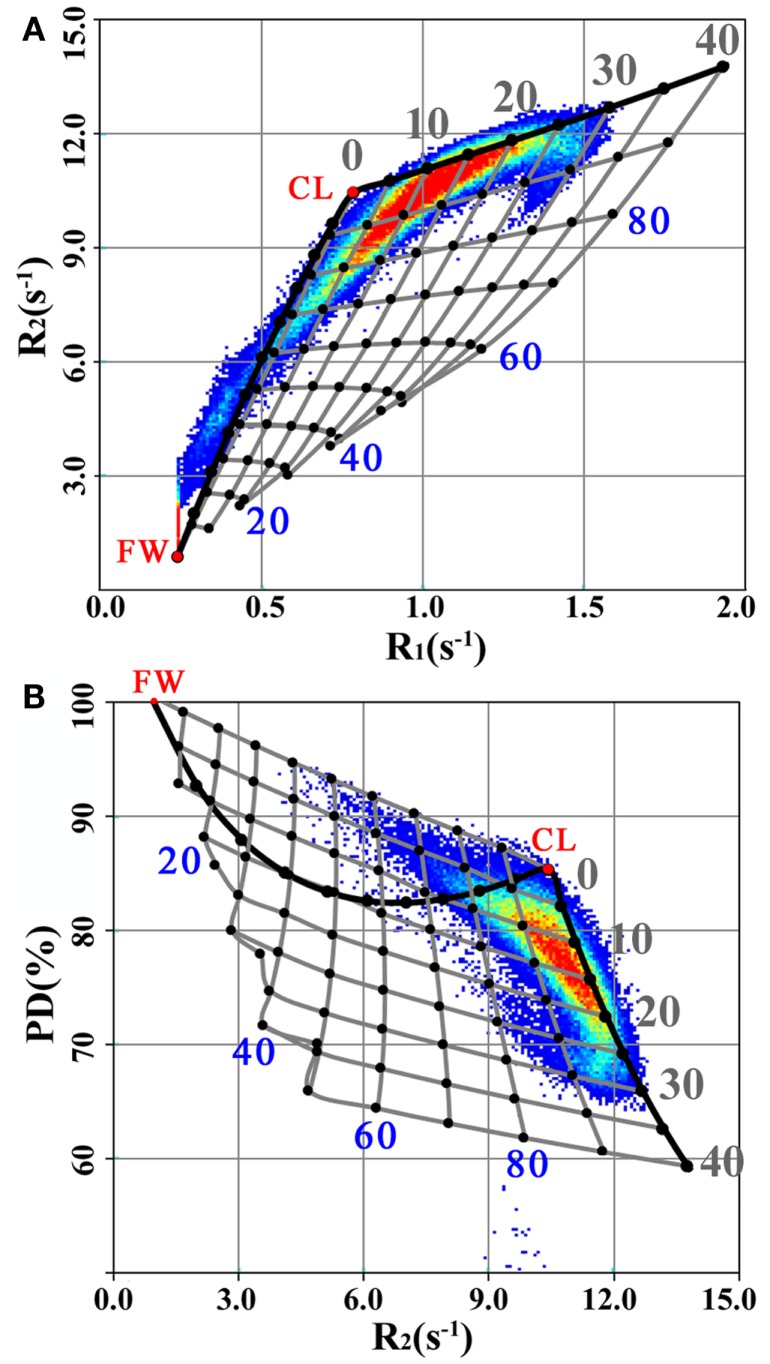
The mean values in Table 1 provide the relaxation parameters for the four partial volumes for the healthy brain. According to the model, all observed R1, R2, and PD values in the healthy brain can be reproduced by combinations of VFW, VCL, and VMY using these characteristics. This is indicated as the thick black curve in Figure 4 showing the transition from 100% VFW at (R1, R2, PD) = (0.24 s−1, 0.87 s−1, 100%) to 100% VCL at (R1, R2, PD) = (0.78 s−1, 10.3 s−1, 85%), continuing toward 100% VMY at (R1, R2, PD) = (16.6 s−1, 77 s−1, 42%). In the Figure, the positions of 100% VFW and 100% VCL are indicated at the red dots labeled by “FW” and “CL,” respectively. The 100% VMY position is outside the range of the plot, the grid is clipped at 40% VMY.
Figure 4.
R1, R2, and PD values for the spatially normalized brain images of the group of MS patients, plotted in the same manner as Figure 3 for the R1–R2 (A) and R2–PD (B) projections. Additionally, the thick black line indicates the transition from 100% VFW (the red dot at “FW”) to 100% VCL (the red dot at “CL”) until 40% VMY, using the model parameter settings for the healthy controls (Table 1). The grid of gray lines indicates the expected changes in R1, R2, and PD values for the pathological brain under myelin loss (Figure 1C) and under the presence of excess parenchymal water (Figure 1D). The cross points of the grid are placed at each 5% change in VMY and each 10% change in VEPW. The VMY partial volume is indicated by the gray numbers 0–40%. The VEPW partial volume is indicated by the blue numbers 20–80%.
For the pathological brain, two processes can occur in the model: (1) a decrease in VMY and (2) the presence of non-zero VEPW. In Figure 4 a grid is displayed, indicating steps of possible combinations of 5% difference of VMY and 10% difference of VEPW. This grid spans a curved surface in the R1–R2–PD space. In the background of Figure 4 the data for the spatially normalized brain for the MS group were plotted. It can be seen that the MS data values are shifted toward lower R1 and R2 and higher PD relative to the black curve, which was optimized using the healthy data values.
Modeling the Spatially Normalized Brain Images
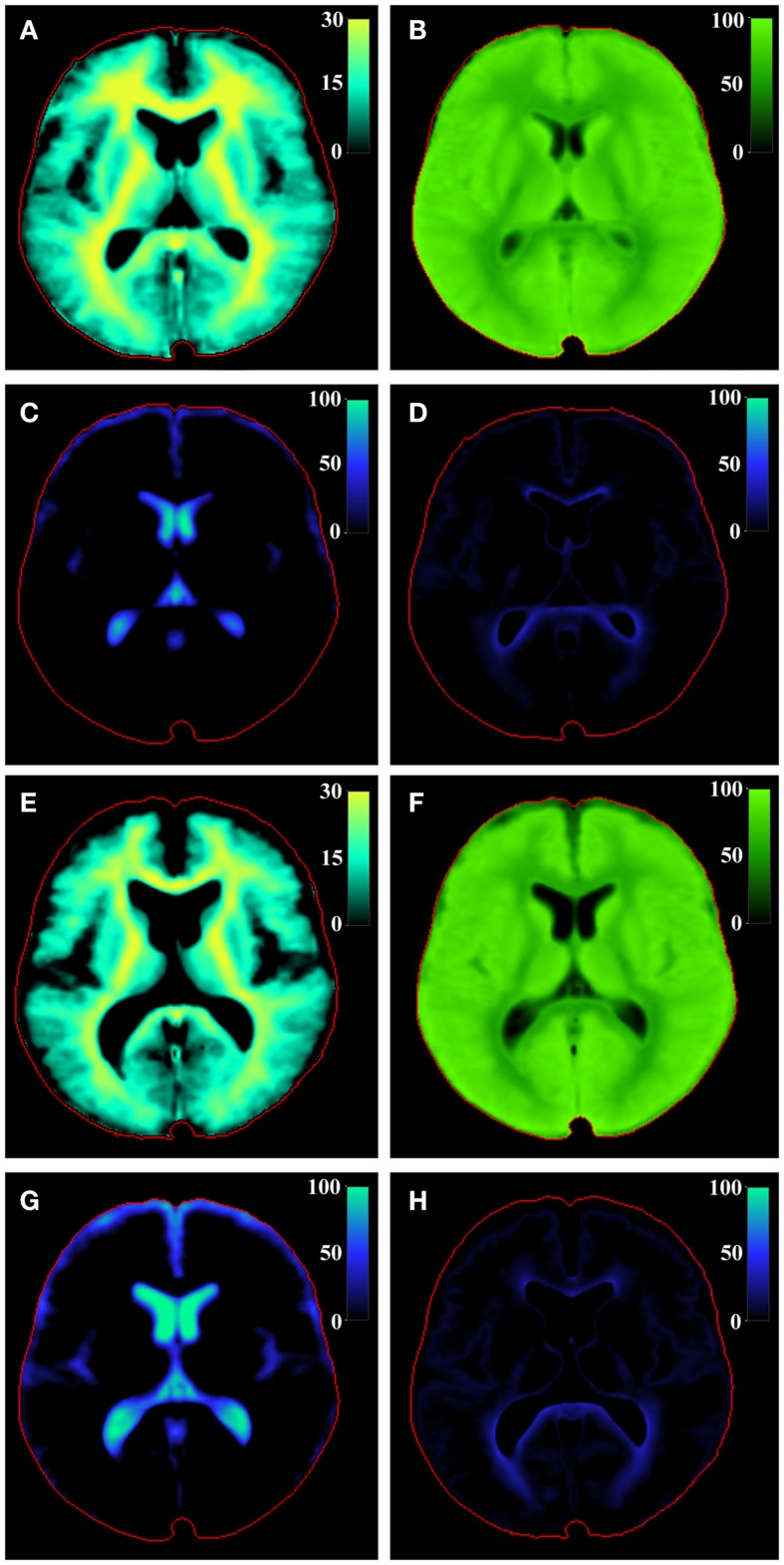
The grid in Figure 4 was used to relate the R1, R2, and PD values of the spatially normalized brain data to combinations of VMY, VCL, VFW, and VEPW. The result is shown in Figure 5 for the spatially normalized brain images of the control and MS groups. The VMY is substantially higher for the controls than for the MS group. The total MYVs were 157 and 119 mL, respectively, a difference of 38 mL. Also, the total FWV was visibly lower, at 65 mL for the control group versus 144 mL for the MS group, a difference of 79 mL. The ICV of the spatially normalized datasets was 1213 mL for both groups, resulting in brain volumes of 1148 and 1069 mL, corresponding to a BPF of 94.6 and 88.1%, respectively. All volumes and volume fractions in relation to brain volume are provided in Table 2.
Figure 5.
Model calculation of (A) VMY, (B) VCL, (C) VFW, and (D) VEPW of the central slice of the brain of the spatially normalized group of healthy controls and of the spatially normalized group of MS patients [(E–H), respectively]. The red line indicates the intracranial volume. Note that VMY is scaled to 30%, whereas the other partial volumes are scaled to 100%.
Table 2.
The total volumes and volume fractions for the spatially normalized healthy control group and spatially normalized MS group of Figures 4 and 5 as well as for the three individual subjects of Figure 6.
| MYV (mL) | CV (mL) | FWV (mL) | EPWV (mL) | BPV (mL) | ICV (mL) | MYF (%) | CF (%) | EPWF (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 157 | 934 | 65 | 57 | 1148 | 1213 | 13.7 | 81.4 | 5.0 |
| MS | 119 | 872 | 144 | 78 | 1069 | 1213 | 11.1 | 81.6 | 7.3 |
| 45 year | 155 | 911 | 117 | 24 | 1090 | 1207 | 14.2 | 83.6 | 2.2 |
| 72 year | 142 | 944 | 308 | 41 | 1127 | 1435 | 12.6 | 83.7 | 3.7 |
| 45 year-MS | 119 | 875 | 204 | 37 | 1031 | 1234 | 11.5 | 84.9 | 3.6 |
Listed are the total myelin volume (MYV), cellular volume (CV), free water volume (FWV), excess parenchymal water volume (EPWV), total brain volume (BPV), and intracranial volume (ICV). The volume components that constitute the brain were normalized on BPV, resulting in the myelin fraction (MYF), cellular fraction (CF), and excess parenchymal water fraction (EPWF) of the brain.
The observed R1, R2, and PD values in the standard WFU PickAtlas ROIs of separate brain structures were used to calculate the local mean VMY, VCL, and VEPW of the spatially normalized control group and spatially normalized MS group (see Table 3). For the healthy group, VMY for the GM structures was in the range of 8–15% (average 14 ± 3%), whereas that for WM structures was 18–27% (average 23 ± 3%). For the MS group, VMY was 1–4% lower, with most of the difference in the WM structures; the average was 13 ± 5% for GM structures (difference: 1.6 ± 1.5%) and 20 ± 3% for WM structures (difference: 2.8 ± 1.0%). The mean VCL was 0–10% lower in the MS group. VEPW was higher in the MS group, with a difference of 9 ± 10% and 5 ± 2%, respectively, compared to the healthy group. Large differences were observed for the caudate nucleus, for which the MS group had a 28% lower VCL and 31% higher VEPW compared with the healthy group. For completeness, also the MWF was derived from the model, which was 8.3 ± 2.9% for GM structures and 14.4 ± 2.3% for WM structures for the healthy group and 7.2 ± 3.0% and 11.9 ± 2.3%, respectively, for the MS group, a difference of 1.2 ± 0.9% and 2.5 ± 0.7%, respectively. The MWF values show the same trend as VMY but are substantially lower, 43% on average.
Table 3.
The mean myelin partial volume VMY, cellular partial volume VCL, and the excess parenchymal water partial volume VEPW of various brain structures, estimated as a percentage of the acquisition voxel volume.
| Healthy controls |
Multiple sclerosis patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| VMY (%) | VCL (%) | VEPW (%) | MWF (%) | VMY (%) | VCL (%) | VEPW (%) | MWF (%) | |
| Insula | 8 | 75 | 17 | 4 | 8 | 66 | 26 | 4 |
| Cingulate cortex | 12 | 81 | 7 | 7 | 8 | 78 | 14 | 4 |
| Caudate nucleus | 9 | 87 | 4 | 5 | 6 | 59 | 35 | 3 |
| Cortical gray matter | 15 | 74 | 11 | 9 | 14 | 66 | 20 | 8 |
| Pons | 18 | 69 | 13 | 11 | 17 | 60 | 23 | 10 |
| Putamen | 15 | 85 | 0 | 9 | 15 | 85 | 0 | 9 |
| Mid brain | 19 | 81 | 0 | 12 | 18 | 79 | 3 | 11 |
| Thalamus | 19 | 81 | 0 | 12 | 16 | 84 | 0 | 9 |
| Occipital white matter | 18 | 82 | 0 | 11 | 15 | 83 | 2 | 9 |
| Frontal white matter | 21 | 77 | 2 | 14 | 19 | 73 | 8 | 11 |
| Parietal white matter | 21 | 77 | 2 | 14 | 19 | 73 | 8 | 11 |
| Sub-lobar white matter | 25 | 66 | 9 | 16 | 21 | 65 | 14 | 13 |
| White matter | 23 | 75 | 2 | 15 | 19 | 73 | 8 | 11 |
| Corpus callosum | 27 | 60 | 13 | 18 | 25 | 55 | 20 | 16 |
The values were calculated using the proposed model and the reported relaxation rates R1 and R2 and proton density PD in the WFU Pickatlas ROIs of the spatially normalized, averaged group of healthy controls and the spatially normalized, averaged group of multiple sclerosis patients from Ref. (21) (Table 2, cropped ROI templates). Added are the expected myelin water fraction MWF values, calculated as PDMY/(PDCL + PDEPW).
For comparison, ROIs were manually placed in a subset of all brain structures for all participants in the study, using the original, spatially non-normalized brain images (Table 4). The differences between GM and WM structures are far more extreme in this case. For example, for the healthy group, the VMY for cortical GM decreases from 15% for the standard ROI to 2% for the manually placed ROI, whereas for the corpus callosum VMY increases from 27 to 41%. Most of the VEPW values decrease, except for the occipital WM (9%). For the manual ROIs, no significant differences were observed for the GM structures between the MS patients and the control group. For WM, however, VMY was 3% lower for occipital WM (p = 0.04), 2% lower for frontal WM (p = 0.04), and 5% lower for corpus callosum (p = 0.02).
Table 4.
The mean myelin partial volume VMY, cellular partial volume VCL, and the excess parenchymal water partial volume VEPW of various brain structures, estimated as a percentage of the acquisition voxel volume.
| Healthy controls |
Multiple sclerosis patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| VMY (%) | VCL (%) | VEPW (%) | MWF (%) | VMY (%) | VCL (%) | VEPW (%) | MWF (%) | |
| Cingulate cortex | 2 | 96 | 2 | 1 | 2 | 95 | 3 | 1 |
| Caudate nucleus | 8 | 92 | 0 | 4 | 9 | 91 | 0 | 5 |
| Cortical gray matter | 2 | 95 | 3 | 1 | 2 | 95 | 3 | 1 |
| Putamen | 11 | 89 | 0 | 6 | 10 | 90 | 0 | 5 |
| Thalamus | 19 | 81 | 0 | 12 | 15 | 84 | 1 | 9 |
| Occipital white matter | 34 | 57 | 9 | 25 | 31 | 61 | 8 | 22 |
| Frontal white matter | 36 | 62 | 2 | 28 | 34 | 64 | 2 | 25 |
| Corpus callosum | 41 | 56 | 3 | 35 | 36 | 60 | 4 | 29 |
The values were calculated using the proposed model and the relaxation rates R1 and R2 and proton density PD in manually placed ROIs in all participants of Ref. (21). Added are the expected myelin water fraction MWF values, calculated as PDMY/(PDCL + PDEPW).
Modeling the High-Resolution Brain Images
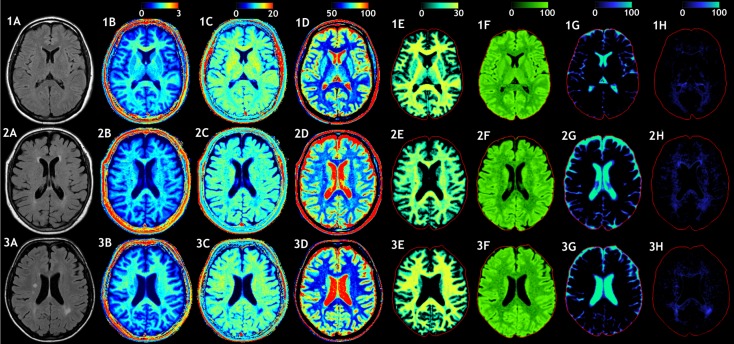
In Figure 6, the model was applied on high-resolution image datasets of a middle-aged (45 years) and elderly control subject (72 years) and an MS patient (45 year-MS), in combination with a conventional FLAIR image (A). The R1, R2, and PD maps (B–D) demonstrate that the 72 year (row 2) had generally lower R1 and R2 values and higher PD values throughout the brain than the 45 year (row 1). For the 45 year-MS (row 3), the R1, R2, and PD values were similar to those for the 45 year, but much lower in the areas where the MS lesions were located. Figure 6E presents the estimated VMY, with a high VMY in the WM (33%, see Table 5) and low VMY in the GM (4%) for the 45 year. The 72 year showed less myelin throughout the brain than the 45 year, with an average VMY of 26% in the WM. Only the corpus callosum showed higher values (33%). The estimated total MYVs were 155 mL for the 45 year, 142 mL for the 72 year and 119 mL for the 45 year-MS, corresponding to a MYF of 14.2, 12.6 and 11.5%, respectively (see Table 2). The cellular fractions (Figure 6F) were 83.7, 83.7, and 84.9%, respectively. Figure 6G presents VFW, highlighting the ventricular system and periphery of the brain. Using the ICV and FWV of the subjects, the BPV can be calculated, which was 1090 mL for the 45 year, 1127 mL for the 72 year, and 1031 mL for the 45 year-MS. Correspondingly, the BPF was 90.3, 78.5, and 83.5%, respectively.
Figure 6.
Examples of the model calculation on an axial slice of the brain. (row 1) Healthy subject, female 45 years old, (row 2) elderly control subject, female 72 years old, and (row 3) patient, female, 45 years old, diagnosed with secondary progressive MS. (A) A conventional FLAIR image of the same slice is added as a visual reference. (B) The measured R1 relaxation rate is shown on a scale of 0–3 s−1, (C) the R2 relaxation rate is shown on a scale of 0–20 s−1, and (D) the proton density PD is shown on a scale of 50–100%, where 100% corresponds to pure water at 37°C. (E) Using the R1, R2, and PD values in combination with the look-up grid of Figure 4 the myelin partial volume VMY was calculated, as shown on a scale of 0–30%, (F) the cellular partial volume VCL, (G) free water partial volume VFW, and (H) excess parenchymal water partial volume VEPW were all calculated all on a scale 0–100%. The red intracranial cavity outline is displayed in all tissue images for visual guidance.
Table 5.
The mean myelin partial volume VMY, cellular partial volume VCL, the excess parenchymal water partial volume VEPW, and myelin water fraction MWF of various brain structures, estimated as a percentage of the acquisition voxel volume for the three example subjects.
| 45 years |
72 years |
45 year-MS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VMY (%) | VCL (%) | VEPW (%) | MWF (%) | VMY (%) | VCL (%) | VEPW (%) | MWF (%) | VMY (%) | VCL (%) | VEPW (%) | MWF (%) | |
| Insula | 4 | 95 | 1 | 2 | 3 | 91 | 6 | 2 | 7 | 92 | 1 | 4 |
| Cingulate cortex | 4 | 95 | 1 | 2 | 6 | 91 | 3 | 3 | 2 | 93 | 5 | 1 |
| Caudate nucleus | 13 | 87 | 0 | 7 | 9 | 91 | 0 | 5 | 10 | 90 | 0 | 5 |
| Cortical gray mater | 3 | 94 | 3 | 2 | 7 | 91 | 2 | 4 | 4 | 93 | 3 | 2 |
| Pons | 23 | 76 | 1 | 15 | 22 | 76 | 2 | 14 | 22 | 78 | 0 | 14 |
| Putamen | 11 | 89 | 0 | 6 | 9 | 91 | 0 | 5 | 12 | 88 | 0 | 7 |
| Mid brain | 19 | 81 | 0 | 12 | 18 | 79 | 3 | 11 | 21 | 78 | 1 | 13 |
| Thalamus | 19 | 81 | 0 | 12 | 20 | 79 | 1 | 12 | 21 | 79 | 0 | 13 |
| Occipital white mat | 31 | 58 | 11 | 22 | 27 | 57 | 16 | 18 | 32 | 56 | 12 | 23 |
| Frontal white mater | 35 | 60 | 5 | 26 | 25 | 61 | 14 | 16 | 36 | 62 | 2 | 27 |
| Parietal white mater | 35 | 61 | 4 | 26 | 26 | 70 | 4 | 17 | 35 | 64 | 1 | 27 |
| Sub-lobar white mat | 32 | 63 | 5 | 23 | 21 | 75 | 4 | 13 | 30 | 70 | 0 | 21 |
| White matter | 33 | 59 | 8 | 24 | 26 | 72 | 12 | 15 | 32 | 61 | 7 | 24 |
| Corpus callosum | 31 | 63 | 6 | 22 | 33 | 60 | 7 | 24 | 33 | 54 | 13 | 24 |
The 45 year exhibited a small amount of VEPW (Figure 6H), mainly around the occipital horns of the lateral ventricles, with a maximum of 11% in the occipital WM. The 72 year had elevated VEPW in the complete periventricular region, with values of up to 16% partial volume. The 45 year-MS showed moderate VEPW values at the periventricular area and 12% in the occipital WM. At the location of MS lesions, however, high VEPW values, up to approximately 50% were observed. The VEPW volumes were 24 mL for the 45 y, 41 mL for the 72 y, and 37 mL for the 45 year-MS, corresponding to an EPWF of 2.2, 3.5, and 3.6%, respectively.
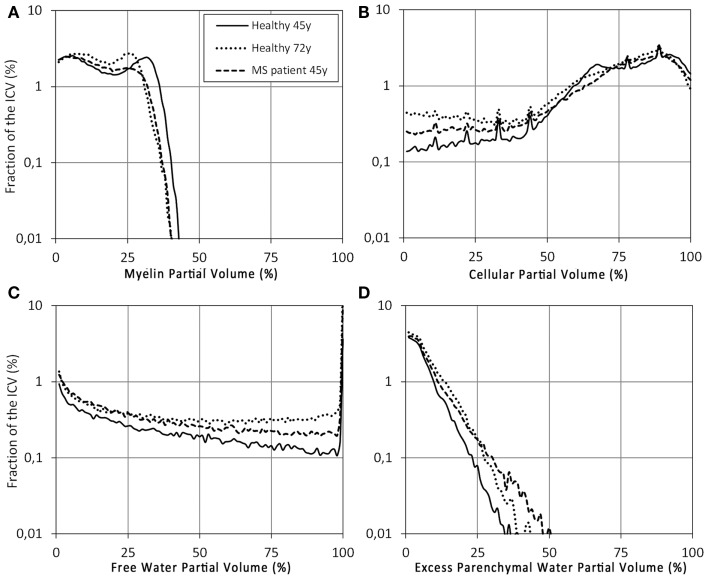
The histograms of VMY, VCL, VFW, and VEPW are shown in Figure 7 to assess the distribution of the partial volumes of the three subjects. The histograms contain 100 bins from 0 to 100% partial volume and are plotted as a percentage of the ICV volume to compensate for the difference in subject head size. The 45 year exhibited two peaks in the VMY histogram at 5 and 32% VMY. For the 72 year, the peak VMY values occurred at 25%. The 45 year-MS did not have a clear peak at higher VMY values. The VCL values peaked at 68 and 92% for the 45 year, but only one peak was observed for both the 72 and 45 year-MS at 89%. VFW values were generally low (<0.5%) in the complete range but exhibited a sharp peak at 100% VFW, with a maximum of 3.7% for the 45 year, 23.3% for the 72 year, and 11.9% for the 45 year-MS. VEPW was observed in all three subjects, but the values were lowest for the 45 year.
Figure 7.
Histograms of the (A) VMY, (B) VCL, (C) VFW, and (D) VEPW partial volume distributions of the control subject (solid line), elderly control subject (dotted line), and MS patient (dashed line) from Figure 6. The x-axis was divided into 100 bins of 1% partial volume over the range 0–100%. The scaling on the y-axis is logarithmic, as a percentage of the ICV.
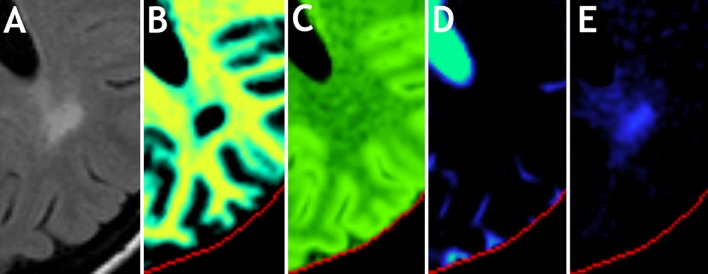
The area with the lesion of the MS patient, posterior to the left lateral ventricle, was zoomed out and displayed in Figure 8, showing a FLAIR image together with VMY, VCL, VFW, and VEPW, taken from Figures 6A,E–H. At the location of the FLAIR hyper-intensity, the VMY was equal to 0, whereas the VEPW values were up to 55% partial volume. The diffuse hyper-intensity, located between the lesion and lateral ventricle, exhibited VMY values of 15–20% and VEPW values of 25–30% partial volume. Elevated VEPW values were observed in a large area around the lesion. The VCL varied only slightly, ranging between 45% at the lesion and 55% at the diffusely hyper-intense area.
Figure 8.
Zoomed part on an MS lesion of the patient in Figure 6, row 3. Shown are (A) the conventional FLAIR image, (B) myelin partial volume VMY, (C) cellular partial volume VCL, (D) free water partial volume VFW, and (E) excess parenchymal water partial volume VEPW. Color scaling is identical to Figure 6.
Using the four partial volumes, the total aqueous content of the brain can be derived. The sum of all PD contributions of VMY, VCL, VFW, and VEPW is shown in Figure 9A for the 45 year-MS, for the same slice as Figures 6 and 8. The centers of the MS lesions exhibit a total aqueous content of 85–95%, consisting entirely of the PD component of VCL and VEPW. Normal appearing WM for this patient showed not only a total aqueous content approximately 70%, consisting mainly of the PD component of VMY and VCL but also a minor contribution of VEPW in the order of 5%. Normal appearing GM shows a total aqueous content of approximately 85%, consisting largely of the PD component of VCL, but with a small contribution of VMY, up to 5%. The remaining non-aqueous content is shown in Figure 9B.
Figure 9.
Calculated total aqueous content (A), corresponding to the sum of myelin water, cellular water, free water and excess parenchymal water, and the remaining, total non-aqueous content (B) of the 45 year-MS patient. The same slice and zoomed part are displayed as in Figures 6 and 8.
Discussion
In the present study, the R1, R2, and PD values, as measured in the brain using a fast multi-parametric qMRI sequence, were modeled by four partial volume compartments per acquisition voxel, (1) the myelin partial volume VMY, (2) cellular partial volume VCL, (3) free water partial volume VFW, and (4) excess parenchymal water partial volume VEPW. The major advantage of this model is that it produces an estimate of three clinically important parameters, the total brain volume, the degree of myelination of the brain parenchyma, and the degree of edema of the brain parenchyma, based on a single, relatively short acquisition.
For a complex organ, such as the brain, with an abundance of magnetically interacting microscopic substructures, MR signal relaxation will behave as a multitude of exponentials. Multi-component measurements, such as the multi-exponential T2 relaxation and mcDESPOT approaches, typically regularize relaxation signals to force the solution into a fast component attributed to MyW, a medium-time component attributed to intra- and extracellular water and occasionally in a long-time component attributed to CSF. Attempts to experimentally resolve the fast component, however, are very challenging. The qMRI sequence employed in this work cannot resolve the fast signal component, but can accurately measure the medium-time relaxation component (28). The estimation of myelin partial volume of our model is, therefore, based on the shift of this medium-time component due to magnetization exchange between MyW and surrounding intra- and extracellular water. Such a shift is observable both in the R1 and R2 relaxation rates, thus, building a specific pattern in the R1–R2–PD space, as visualized in Figure 3 for a group of healthy controls and in Figure 4 for a group of MS patients. Therefore, the model relies on a combined R1–R2–PD measurement as a single component/multi-parametric quantification strategy, in contrast to the multi-component/single parametric quantification methods, such as the multi-component T2 relaxation. The observed values for brain parenchyma of R1 in the range of 0.9–1.9 s−1 (T1 = 530–1100 ms) and R2 in the range of 10.5–13 s-1 (T2 = 75–95 ms) corresponded well with previously reported values for GM and WM (29, 30), where other qMRI methods were used. Also, the measured PD corresponds well to the reported values with GM structures of 80–86% and WM of 74–76% (31, 32).
The determined optimal parameter values for the partial volume compartments are listed in Table 1. The result of the optimization provides three specific coordinates in the R1–R2–PD space, for pure VFW [set by literature values to (R1, R2, PD) = (0.24 s−1, 0.87 s−1, 100%)], pure VCL [estimated at (0.78 s−1, 10.3 s−1, 85%)] and pure VMY [estimated at (16.6 s−1, 77 s−1, 42%)]. The characteristics of the VCL are close to those of cortical GM (20, 29, 30). The characteristics of the VMY are within the range of previous reported values (11, 22). Using the model, the possible value combinations of R1, R2, and PD in the healthy brain were visualized by the solid black curve through the R1–R2–PD space, as plotted in Figure 4. The difference between the healthy brain and pathological brain was described using two components: (1) the variation of the VMY, indicating myelin loss, and (2) the presence of VEPW, indicating the presence of edema. These two components expanded the (healthy) curve to a curved surface grid, as shown in Figure 4. Each observed value combination of R1, R2, and PD in acquisition voxels of a pathological brain is regarded as a combination of the VMY, VCL, VFW, and VEPW partial volume compartments. As shown in Figure 5, substantial differences were observed between the spatially normalized control group and spatially normalized MS group in all partial volumes. The MS group had a smaller VMY and VCL (a difference of 3.1 and 5.1% of the ICV, respectively) and larger VFW and VEPW (a difference of 6.5 and 1.7% of the ICV, respectively). Consequently, the average brain volume of the MS group was smaller than that of the control group (88.1% versus 94.6% of the ICV), the degree of myelination in the brain was lower (11.1% versus 13.7% of the BPV) and the degree of edema in the brain was higher (7.3% versus 5.0% of the BPV). This result is congruent with knowledge concerning the disease progression of MS (3–5). The relative CV in the brain was virtually identical (81.6 and 81.4%), as can be expected in a model where edema is described by a separate class of excess parenchymal water, which is an addition of water to the normal cellular partial volume. The values in Table 3 for the various brain structures confirm the image shown in Figure 5.
The model was tested on three individual subjects as examples for high-resolution imaging. This can by no means be representative for entire groups of subjects and, hence, is purely used as example of the application of the model. Inclusion of larger groups to assess statistical differences with different age groups and diseases will be performed in future work. Clear differences were observed among the three subjects. Compared with the healthy controls, the VMY partial volume was lower for both the elderly subject and MS patient (Figure 6). Additionally, the MS patient showed strong local decreases at the location of MS lesions. Similar to the spatially normalized brains of Figure 5, the cellular fraction of the brain was virtually identical for all subjects. The VFW clearly highlights the CSF in the ventricular system and brain periphery, making it possible to calculate the brain volume of the subjects. The elderly subject had the smallest brain, with a BPF of 78.5%, compared with the 90.3% for the healthy 45 year and 83.5% for the MS patient. Simultaneously, the MS patient had the lowest myelination, with a MYF of 11.5%, compared with 14.2% for the healthy 45 year and 12.6% for the 72 year. In Figure 7 the cause of the reduction can be attributed to a substantial loss of high VMY values for both the MS patient and 72 year. The EPWF was substantially higher for the 72 year and the 45 year-MS compared with the healthy 45 year. These findings are consistent with general myelin loss and edema during aging and MS disease progression.
The behavior of the partial volume components around the MS lesion of the 45 year-MS, displayed in the zoomed sections shown in Figure 8, is particularly interesting. The hyper-intensity on the FLAIR image has diffuse edges, making it difficult to estimate the exact volume of the lesion. However, on the VMY image, a clear center, where the myelin has completely vanished, can be observed. At the same location, there is an elevation of the VEPW, but this area is larger and decreases toward 0 outwards. On a FLAIR image, no distinction can be made between edema and myelin loss because both processes result in a hyper-intense signal. Using the model, on the other hand, the partial volume images indicate a demyelinated center within a larger area of edema. This example suggests that the model can distinguish between myelin loss and the presence of excess water in edema.
An interesting derivate of the model is the total aqueous content and the corresponding, remaining non-aqueous content. The used sequence cannot resolve the short R2 relaxation component and, therefore, the observed PD value will correspond to the visible PD of the medium and long-time components. Using the observed shift in R1 and R2 the model can predict the presence of the myelin component and, therefore, the true PD value as would be measured at an echo time of 0. The non-aqueous content (Figure 9B) can be attributed to the presence of macromolecules in the brain. From the results, it can be derived that the macromolecular content for the 45 year-MS in the MS lesions was 15–5%, of normal appearing WM approximately 30%, and of normal appearing GM approximately 15%. These results are very similar to the reported values of Mezer et al. (33) and Abbas et al. (34). Our intention is to validate our results further on larger groups of MS patients in future work. Within the possible restrictions of ethical permission, the actual myelin content must be validated by histopathology in combination with the selective staining of individual tissue components.
In Table 2 the MWF is also listed, as directly derived from the model PD values. The definitions of VMY and MWF are not identical; VMY is the estimated MYF of an acquisition voxel based on the effective relaxation properties of that voxel, whereas MWF corresponds to the ratio of observable short-time relaxation (myelin) and medium-time relaxation (cellular) water content. The calculated MWF values are considerably lower than VMY (43% on average). The cause is that MyW only covers a fraction of the total MYV, which also includes the (non-observable) myelin semi-solids. An issue reported by Zhang et al. (35), however, may cause a difference between our observed MWF and the reported MWF values: Using the multi-echo T2 relaxation in combination with the NNLS method, the magnetization exchange, responsible for the shift of the medium-time component, is ignored. Such an exchange not only results in a shift of the medium-time component, but is also responsible for a simultaneous decrease in the short-time component. This will lead to a lower observed value for MWF. Studies measuring MWF using multi-exponential T2 relaxation indeed reported lower values than our estimated MWF values, such as 7.0–10.1% in WM, 3.6–5.6% in the putamen, and 4.5–4.7% in the thalamus (8, 10, 36–38), compared with our values of 15, 9, and 12%, respectively (Table 3). By contrast, the mcDESPOT approach does account for magnetization exchange and consequently exhibits considerably higher values of MWF. For example, the observed MWF values were as high as 28–30% for WM, 11–13% for the putamen, and 14–15% for the thalamus (13), which are more in the range of our estimated VMY values. In our opinion, this discrepancy is a highly interesting field that must be explored and further understood. A thorough validation study on patients and healthy controls using our method will be the subject of future research.
A limitation of our approach is that the model had to be grossly simplified in order to provide any reasonable results. Each compartment can have very different behavior throughout the brain and with various diseases. Magnetic interaction was reduced to two exchange rates and a number of parameters were fixed to reasonable, but unvalidated values. Adding more degrees of freedom, however, would make it impossible for the model to converge to a solution. The used spatial normalization process resulted in a low resolution of the brain images. This inevitably led to the loss of anatomical detail and smearing of tissue characteristics, which can explain the differences between the values of Table 3 and Tables 4 and 5. For example, voxels that are partially filled with bulk CSF at the periphery of the brain may be seen at low resolution as brain tissue with VEPW. Indeed, the spatially normalized brains of the controls in Figure 5 had a relatively high amount of VEPW at 57 mL, whereas all three individual examples in Figure 6 had much lower values. The estimated VEPW for healthy controls in Table 2 was high for the insula, cortical GM, pons, and corpus callosum; the caudate nucleus showed an extreme value for the MS group. All of these structures interface with bulk CSF and, hence, the VEPW likely is lower in reality. Also, GM and WM structures may be blended at this resolution. For the spatially normalized brain images of the healthy controls cortical GM had 15% and WM had 23% VMY, whereas the differences between GM and WM for all subjects in Ref. (21), and for the three example subjects, were much more extreme, 2–7% for GM and 26–41% for WM. In Figure 7, VMY peaks can be observed at 5 and 32% for the 45 year (6A), and VCL peaks can be observed at 68 and 92% (6B), which are likely to be centered at GM and WM. A higher resolution of the spatial normalization procedure would likely change the values of Table 3 and make them more similar to those of Tables 4 and 5. Future work will focus on high-resolution spatial normalization in combination with a better definition for the regions of interest to improve the distinction between the various brain structures. Standard, template ROIs have an advantage over (time-consuming and user-dependent) manually placed ROIs, but our data show that the loss of anatomic detail has a large effect on the results.
Another limitation of our method is that the measured VMY properties in Table 1 have large SDs. This is a result of the relatively shallow minimum in the optimization, where a change in one parameter can be compensated for by a change in other parameters. Therefore, our model cannot accurately determine the characteristics of pure VMY. The effect of parameter changes in VMY on the calculated grid in Figure 4, however, is relatively small. Brain parenchyma typically has <30% VMY, and substantial changes near 100% VMY only have a small effect at lower values. For example, when perturbing R1,MY and PDMY by one SD, the grid points of VMY in Figure 4 changed by <5%, indicating that our model is relatively robust for practical purposes.
All parameters of the model were adapted to 1.5 T spatially normalized data. Because relaxation rates change with field strength, the modeled grid from Figure 4 must be re-optimized for other field strengths. Furthermore, it is important to realize that the partial volumes are measured by observation of magnetic properties of the brain. The fast-relaxing, non-observable MyW has a magnetization exchange with the surrounding cellular water, resulting in an increase in the effective relaxation rate of cellular water in the vicinity. This effect will decrease with distance and, thus, cannot define a hard boundary. Therefore, myelin partial volume in the acquisition voxel reflects the extent of the effect of magnetization exchange in space rather than defining a physical boundary of the myelin sheets. Using this argument, it is not likely that the measured total MYV using this model is identical to the total MYV, which could be measured by summing the volumes of all myelin sheets under a microscope.
For quantitative monitoring of patients in clinical routine, we consider it important that not only the brain volume is monitored. Although this is an important clinical measure, it is only a volumetric measure. It does not reveal any pathological changes in the tissue composition of the brain. Neurological degeneration is related to differences in R1, R2, and PD and may be characterized by the observation of changes in these values. In this work, an attempt was made to capture the change in quantitative values in a clinically realistic context using the MYF, which is an indirect measure of the myelination degree of the brain, and the EPWF, which is an indirect measure of edema in the brain. Therefore, we believe that BPF, MYF, and EPWF are complementary measures to monitor the quantity and quality of the patient’s brain in relation to intervention or progress of disease or aging.
In conclusion, a model is presented in which each MRI acquisition voxel in the brain is composed of a myelin partial volume, a cellular partial volume, a free water partial volume, and an excess parenchymal water partial volume. The magnetization vector evolution during an MRI quantification sequence was simulated for all partial volume distributions. The parameters of the model were obtained using spatially normalized brain data of a group of healthy control subjects. The differences for a pathological brain were described with myelin loss and the presence of excess parenchymal water. Application of the model showed clear differences between the control group and a spatially normalized MS group, as well as among three individual examples of high-resolution imaging of a healthy middle-aged subject, an elderly control subject, and an MS patient. Using this model, clinically important information, such as the brain volume, degree of myelination, and degree of edema, may be estimated based on an acquisition with a clinically acceptable scan time.
Author Contributions
MW, ME, and AT contributed to the design, the acquisition, analysis, and interpretation of data for the work; MW, ME, AT, and PL all contributed to writing, reviewing and given final approval of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The Linköping University and County Council Östergötland are acknowledged for funding this work.
References
- 1.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke (2007) 38:724–30. 10.1161/01.STR.0000254729.27386.05 [DOI] [PubMed] [Google Scholar]
- 2.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci (2008) 31:361–70. 10.1016/j.tins.2008.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller DH, Barkhof F, Frank JA, Parker GJ, Thompson AJ. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain (2002) 125:1676–95. 10.1093/brain/awf177 [DOI] [PubMed] [Google Scholar]
- 4.Bakshi R, Thompson AJ, Rocca MA, Pelletier D, Dousset V, Barkhof F, et al. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol (2008) 7:615–25. 10.1016/S1474-4422(08)70137-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinman JD, Abraham CR. What’s behind the decline? The role of white matter in brain aging. Neurochem Res (2007) 32:2023–31. 10.1007/s11064-007-9341-x [DOI] [PubMed] [Google Scholar]
- 6.Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol (2002) 31:581–93. 10.1023/A:1024157522651 [DOI] [PubMed] [Google Scholar]
- 7.Matsusue E, Sugihara S, Fujii S, Ohama E, Kinoshita T, Ogawa T. White matter changes in elderly people: MR-pathologic correlations. Magn Reson Med Sci (2006) 5:99–104. 10.2463/mrms.5.99 [DOI] [PubMed] [Google Scholar]
- 8.Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DK, Paty DW. In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med (1997) 37:34–43. 10.1002/mrm.1910370107 [DOI] [PubMed] [Google Scholar]
- 9.Webb S, Munro CA, Midha R, Stanisz GJ. Is multicomponent T2 a good measure of myelin content in peripheral nerve? Magn Reson Med (2003) 49:638–45. 10.1002/mrm.10411 [DOI] [PubMed] [Google Scholar]
- 10.MacKay A, Laule C, Vavasour I, Bjarnason T, Kolind S, Mädler B. Insights into brain microstructure from the T2 distribution. Magn Reson Imaging (2006) 24:515–25. 10.1016/j.mri.2005.12.037 [DOI] [PubMed] [Google Scholar]
- 11.Bjarnason TA, Vavasour IM, Chia CL, MacKay AL. Characterization of the NMR behaviour of white matter in bovine brain. Magn Reson Med (2005) 54:1072–81. 10.1002/mrm.20680 [DOI] [PubMed] [Google Scholar]
- 12.Laule C, Leung E, Lis DK, Traboulsee AL, Paty DW, MacKay AL, et al. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler (2006) 12:747–53. 10.1177/1352458506070928 [DOI] [PubMed] [Google Scholar]
- 13.Deoni SC, Rutt BK, Arun T, Pierpaoli C, Jones DK. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn Reson Med (2008) 60:1372–87. 10.1002/mrm.21704 [DOI] [PubMed] [Google Scholar]
- 14.Deoni SC, Dean DC, III, O’Muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage (2012) 63:1038–53. 10.1016/j.neuroimage.2012.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar R, Delshad S, Woo MA, Macey PM, Harper RM. Age-related regional brain T2-relaxation changes in healthy adults. J Magn Reson Imaging (2012) 35:300–8. 10.1002/jmri.22831 [DOI] [PubMed] [Google Scholar]
- 16.Neema M, Stankiewicz J, Arora A, Dandamudi VS, Batt CE, Guss ZD, et al. T1- and T2-based MRI measures of diffuse gray matter and white matter damage in patients with multiple sclerosis. J Neuroimaging (2007) 17:16S–21S. 10.1111/j.1552-6569.2007.00131.x [DOI] [PubMed] [Google Scholar]
- 17.Oh J, Cha S, Aiken AH, Han ET, Crane JC, Stainsby JA, et al. Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging (2005) 21:701–8. 10.1002/jmri.20335 [DOI] [PubMed] [Google Scholar]
- 18.Larsson HB, Frederiksen J, Petersen J, Nordenbo A, Zeeberg I, Henriksen O, et al. Assessment of demyelineation, edema and gliosis by in-vivo determination of T1 and T2 in the brain of patients with acute attack of multiple sclerosis. Magn Reson Med (1989) 11:337–8. 10.1002/mrm.1910110308 [DOI] [PubMed] [Google Scholar]
- 19.Vymazal J, Righini A, Brooks RA, Canesi M, Mariani C, Leonardi M, et al. T1 and T2 in the brain of healthy subjects, patients with Parkinson’s disease and patients with multiple system athrophy: relation to iron content. Radiology (1999) 211:489–95. 10.1148/radiology.211.2.r99ma53489 [DOI] [PubMed] [Google Scholar]
- 20.Warntjes JBM, Dahlqvist Leinhard O, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: optimization for clinical usage. Magn Reson Med (2008) 60:320–9. 10.1002/mrm.21635 [DOI] [PubMed] [Google Scholar]
- 21.Engström M, Warntjes JBM, Tisell A, Landtblom AM, Lundberg P. Multi-parametric representation of voxel-based quantitative magnetic resonance imaging. PLoS One (2014) 9:e111688. 10.1371/journal.pone.0111688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levesque R, Pike GB. Characterizing healthy and diseased white matter using quantitative magnetisation transfer and multicomponent T2 relaxometry: a unified view via a four-pool model. Magn Reson Med (2009) 62:1487–96. 10.1002/mrm.22131 [DOI] [PubMed] [Google Scholar]
- 23.Deoni SCL, Matthews L, Kolind SH. One component? Two components? Three? The effect of including a nonexchanging “free” water component in multi-component driven equilibrium single pulse observation of T1 and T2. Magn Reson Med (2013) 70:147–54. 10.1002/mrm.24429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology (1983) 33:1444–52. 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 25.Meeker W, Escobar L. Teaching about approximate confidence regions based on maximum likelihood estimation. Am Stat (1995) 49:48–53. 10.1080/00031305.1995.10476112 [DOI] [Google Scholar]
- 26.Warntjes JB, Engström M, Tisell A, Lundberg P. Brain characterization using normalised quantitative magnetic resonance imaging. PLoS One (2013) 8:e70864. 10.1371/journal.pone.0070864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cedersund G, Roll J. Systems biology: model based evaluation and comparison of potential explanations for given biological data. FEBS J (2009) 276:903–22. 10.1111/j.1742-4658.2008.06845.x [DOI] [PubMed] [Google Scholar]
- 28.Krauss W, Gunnarsson M, Andersson T, Thunberg P. Accuracy and reproducibility of a quantitative magnetic resonance imaging method for concurrent measurements of tissue relaxation times and proton density. Magn Reson Imaging (2015) 33:584–91. 10.1016/j.mri.2015.02.013 [DOI] [PubMed] [Google Scholar]
- 29.Deoni SCL, Rutt BK, Peters TM. High resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med (2005) 53:237–41. 10.1002/mrm.20314 [DOI] [PubMed] [Google Scholar]
- 30.Deichmann R. Fast high-resolution T1 mapping of the human brain. Magn Reson Med (2005) 54:20–7. 10.1002/mrm.20552 [DOI] [PubMed] [Google Scholar]
- 31.Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. 1: compartments and water. J Magn Reson B (1993) 102:1–8. 10.1006/jmrb.1993.1055 [DOI] [Google Scholar]
- 32.Neeb H, Ermer V, Stocker T, Shah NJ. Fast quantitative mapping of absolute water content with full brain coverage. Neuroimage (2008) 42:1094–109. 10.1016/j.neuroimage.2008.03.060 [DOI] [PubMed] [Google Scholar]
- 33.Mezer A, Yeatman JD, Stikov N, Kay KN, Cho NJ, Dougherty RF, et al. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat Med (2013) 19:1667–72. 10.1038/nm.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbas Z, Gras V, Möllenhoff K, Oros-Peusquens A-M, Shah NJ. Quantitative water content mapping at clinically relevant field strengths: a comparative study at 1.5 T and 3 T. Neuroimage (2015) 106:404–13. 10.1016/j.neuroimage.2014.11.017 [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, Kolind SH, Laule C, MacKay AL. Comparison of myelin water fraction from multiecho T2 decay curve and steady-state methods. Magn Reson Med (2015) 73:223–32. 10.1002/mrm.25125 [DOI] [PubMed] [Google Scholar]
- 36.Oh J, Han ET, Pelletier D, Nelson SJ. Measurement of in vivo multi-component T2 relaxation times for brain tissue using multi-slice T2 prep at 1.5 and 3 T. Magn Reson Imaging (2006) 24:33–43. 10.1016/j.mri.2005.10.016 [DOI] [PubMed] [Google Scholar]
- 37.Levesque IR, Chia CL, Pike GB. Reproducibility of in vivo magnetic resonance imaging-based measurement of myelin water. J Magn Reson Imaging (2010) 32:60–8. 10.1002/jmri.22170 [DOI] [PubMed] [Google Scholar]
- 38.Laule C, Vavasour IM, Moore GR, Oger J, Li DK, Paty DW, et al. Water content and myelin water fraction in multiple sclerosis. A T2 relaxation study. J Neurol (2004) 251:284–93. 10.1007/s00415-004-0306-6 [DOI] [PubMed] [Google Scholar]