Abstract
Herbal medicine is an ancient practice that has been gaining acceptance of the medical class through scientific studies that prove its effectiveness. However, its use should still be cautious. Medicinal plants have potential toxic effects not yet discovered, and may have unproven interactions with other medications. The use of drugs during pregnancy is still very dangerous and vigorously studied; however, there are few studies of herbal medicines in pregnant women. Existing studies prioritize on teratogenic or abortifacient effects. The aim of this study was to analyze the toxic effects of Mikania glomerata Sprengel administration, popularly known as “guaco” during the gestational period of hypertensive rats. For this experimental groups consisting of pregnant Wistar rats received treatments with guaco extract (1 to 2 mL). In order to analyze the possible toxic effects of guaco during pregnancy, weight gain of rats was assessed during pregnancy; reproductive performance of rats, morphological parameters, and fetal placental histology were compared. Although some parameters presented significant differences, we can conclude that changes prioritized by literature, such as toxicity, vasodilation and hypotension, have not been caused by guaco. The only fetal changes observed were due to the maternal hypertension. Some studies have reported vasodilator and hypotensive effects of guaco. However, only a few studies exist, and its actual effects remain unknown. Specific studies should be developed with higher doses of guaco for a definitive conclusion of its toxic and non-toxic effects.
Keywords: Guaco, Hypertensive pregnant rats, Mikania glomerata, Perinatal toxicity
Introduction
Phytotherapic plants surpassed many barriers and obstacles in the present day. Many phytotherapic plants are sold in farmer’s markets and fairs, they are present in some backyards, and are used as a therapeutic resource that is accessible to the population (Amorozo and Gely, 1988; Prance, 1992).
Pharmacological experiments provide a high level of medical acceptance. This acceptance is extremely important, because investing in drugs synthesized from natural products could be a center of growth for the chemical and pharmacological industry in Brazil, due to the country’s great biodiversity of plants and medicinal raw materials (Cechinel-Filho and Yunes, 1998; Britto et al., 2007).
Any medication used during pregnancy, including the use of medicinal plants, should always have its cost-effectiveness and benefit-harm considered in every situation. The scarcity of data of the use of medication during pregnancy makes it even more critical (Ferro, 1991).
Currently, other properties of the guaco extract were recognized, such as antimicrobial, anti-inflammatory, analgesic (Ferro, 1991), spasmodic, vasodilator, anti-ulcer, central nervous system depressant, anti-venom serum, anti-stress, insecticide, molluscicide, fungicide, anticoagulant and allergenic (Barreto and Hiruma-Lima, 2002). These effects are due to a component present in the leaves of Mikania glomerata Sprengel called coumarin (Ruppelt et al., 1991). Coumarin is a volatile active ingredient found in several other plant species (Pereira et al., 1994).
Mikania glomerata promotes relaxation of airways’ smooth muscles, bronchi mainly, by improving the fluidization of exudates by tracheobronchial cough reflex, and exerts considerable diaphoretic effect on febrile cases (Oliveira et al., 1984). The influence of the aqueous extract of guaco on acute inflammatory response was verified, showing that guaco inhibits the mobilization of leukocytes from the bone marrow into the blood (Vieira et al., 2008).
By relaxing the smooth muscles, guaco could promote a decrease in blood pressure in patients with hypertension during pregnancy (Assis et al., 2008; Ferrão et al., 2006; Almeida et al., 2008). Maternal hypertension may cause decreased placental blood flow. This condition results in low fetal and placental weight (Karlsson et al., 1982; Kingdom and Kaufmann, 1999; Lieb et al., 1981; Rudge et al., 1999; Wallace et al., 1999; Wigglesworth, 1964). It is suggested that, in hypertension, the oxygen deficiency is also decreased. This confirms that the oxygen saturation in the umbilical arteries and veins of the fetus of pregnant women with pre-eclampsia is decreased, leading to the conceptual commitment.
During hypertension, an accumulation of fibrin in the intima of the vessels occurs, which can lead to its occlusion. The hyalinization and necrosis of the vessel wall deposits, coupled to macrophages, are present in two maternal conditions: high blood pressure associated with vasculitis or deciduous bacterial infection (Benirschke and Kaufmann, 2000; Janthanaphan et al., 2006; Huppertz, 2008). Administration of guaco, with its active principle coumarin, can change this modification in the hypertensive placenta.
The pregnant rat is an important animal model for studies of reproductive toxicity. Until now, little information exists about the development of the placenta of pregnant rats (de Rijk et al., 2002). Analysis of placentas submitted to other substances such as herbs, vitamins, minerals and bacteria have been reported (Hall, 1973; Lewis et al., 2001; Kosif et al., 2008; Graber et al., 1971).
In this project, the effects of guaco were tested through macroscopic and histological examination of the placentas taken from normotensive and hypertensive rats, treated with guaco during pregnancy.
Materials and Methods
All the experiments were performed according to the standards established by the Brazilian Society of Laboratory Animal Science (SBCAL).
Normotense female and male Wistar rats, as well as spontaneously hypertensive female and male Wistar rats were used for the experiment, with a total of 15 females and 2 males. The spontaneously hypertensive rats presented tail blood pressure higher than 160 mmHg, and the females presented tail blood pressure higher than 150 mmHg, weighing 180-250 g. All the animals were kept in cages with water and food ad libitum, in an environment with controlled temperature and lighting (12 hours with light and 12 hours without light). The pregnancy was confirmed by the presence of spermatozoids in the vaginal lavage, which indicated the first day of pregnancy.
Dry extract of Mikania glomerata (guaco) resuspended in milli-Q water (20% solution) was administered by gavage to female spontaneously hypertensive rats, which received a volume of 1 mL (corresponding to 0.2 g of the extract guaco) or 2 mL (corresponding to 0.4 g of extract guaco) on the first, fifth, tenth and fifteenth day of gestation, at a fixed time. Control group of rats (normotensive and spontaneously hypertensive groups) received 1 mL of saline solution, with the same pattern of administration. Before the administration by gavage, the pregnant rats fasted for 3 hours, and the weighing of each animal was performed.
After 18 days of pregnancy, cesarean sections were performed. The ovaries and corpora lutea were carefully dissected and preserved, the fetuses were removed with their placentas. After macroscopic inspection, the fetuses were euthanized by halothane inhalation, weighted and fixed in Bouin solution for 48 hours, and later substituted with 70% alcohol. The placentas were kept in formaldehyde 10%.
During the macroscopic inspection, the following parameters were evaluated: number of corpora lutea and implants, number of living fetuses, weight of the fetuses and corresponding placenta. Anatomic measurements were performed using a caliper: anteroposterior skull, lateral-lateral skull, anteroposterior thorax, lateral-lateral thorax, skull-tail and tail.
The fertility rate of rats was assessed by the following equations:
Pre-implantation loss = (number of corpora lutea – number of deployments)/number of corpora lutea.
Post-implantation loss = (number of deployments – number of living fetuses)/number of deployments.
Index vitality was obtained using the following equation:
Number of living and lifeless ____ 100 %
Number of living fetuses ________ x %
For the placentas, macroscopic observation was performed. For histological evaluation, a minimum of six areas of the placenta were separated. Histological cuts were made using the paraffin inclusion protocol and rotary microtome, with 3 microns, and hematoxylin and eosin staining. A total of 168 slides were confectioned and visualized, considering the following patterns: measurement of the placenta fragment on the slide, measurement of the thickness of a vessel, presence of congestion under chorionic membrane, fibrin deposit, edema, and inflammation.
Results
Assessment of pregnancy
The rat’s pregnancy was confirmed with the presence of sperm in the smear from the vaginal lavage of normotensive and/or hypertensive rats.
Weight gain of pregnant rats
Throughout the gestational period of rats, animal’s weight was also held on the 1st, 5th, 10th, 15th and 18th day of gestation. It is observed that, in all protocols performed with hypertensive rats, weight gain was lower when compared to normotensive control group, suggesting that hypertension compromises the weight gain of pregnant rats (data not shown).
Evaluation of reproductive capacity
All cesarean sections were performed on the 18th day of gestation. The following parameters were observed: quantity and vitality of fetuses, presence or absence of fetal resorption, number of corpora lutea present in each ovary (Table 1).
Table 1.
Morphological parameters and reproductive analysis (n=3-5).
| Parameters | Treatments/Groups | |||
|---|---|---|---|---|
| Normotensive rats | Spontaneously hypertensive rats | Spontaneously hypertensive + guaco (1mL) | Spontaneously hypertensive + guaco (2mL) | |
| Number of fetuses | 56 | 26 | 28 | 36 |
| Number of corpora lutea | 58 | 39 | 40 | 50 |
| Pre-implantation loss (%) | 0.03 ± 0.02 | 0.30 ± 0.10 | 0.30 ± 0.20 | 0.30 ± 0.07 |
| Post-implantation loss (%) | 0.02 ± 0.02 | 0.05 ± 0.05 | - | 0.05 ± 0.05 |
| Index vitality (%) | 100 | 100 | 100 | 100 |
During the evaluation of fetal vitality, it was observed that the fetuses of all groups were alive, i.e. vitality equal to 100%. It was noticed that all groups showed pre-implantation loss, or found a greater number of corpora lutea than fetuses, but were not significantly different from the control groups of both normotensive rats and hypertensive rats, a fact physiologically normal in this experimental design. As for post-implantation losses, it is noteworthy that there was no significant difference between the experimental groups, although fetal resorption observed in control and treated groups.
Figure 1 shows the average weights of fetuses and placentas from different experimental groups, after the sacrifice of pregnant rats on day 18 of gestation. It is observed that fetuses and placentas with significantly lower weights predominated in the groups of hypertensive rats, this result was expected since this group had less weight gain during pregnancy; also note that the results were independent of the administration of guaco extract or saline solution, since there were no significant difference between spontaneously hypertensive group (saline solution) with spontaneously hypertensive + guaco (1 ml or 2 mL) group, due to the effect of hypertension.
Fig. 1.
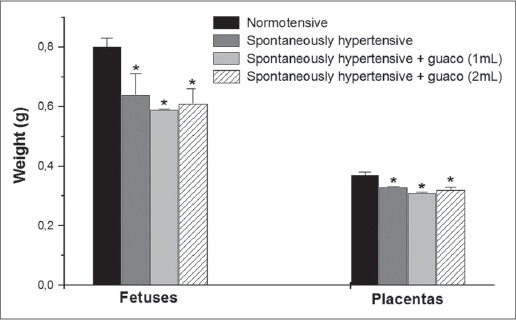
Fetuses and placentas’ weights (n=26-56).
Macroscopic analysis
All fetuses underwent fixation technique in Bouin’s solution and then the macroscopic analysis was performed. All fetuses from hypertensive groups of rats (spontaneously hypertensive rats received saline solution, spontaneously hypertensive rats treated with 1 - 2 mL of guaco) presented measurements of external morphological parameters (antero-posterior and latero-lateral of cranium; antero-posterior and latero-lateral of thorax; cranium-caudal and tail) significantly lower when compared with the normotensive rats (control group), which was also expected since these fetuses were significantly lower when weighted at the time of cesarean section, effect also attributed to hypertension, as there was no significant difference between the groups treated with guaco (1 mL or 2 mL) (Fig. 2).
Fig. 2.
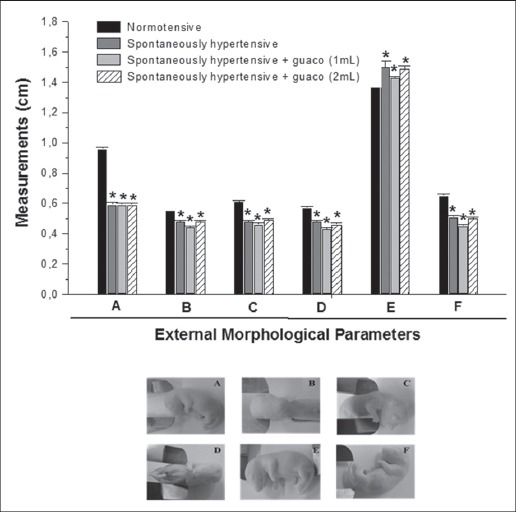
External morphological parameters (cm) of fetuses of rats exposed to the following treatments: normotensive rats (n=56), spontaneous hypertensive rats (n = 26); spontaneously hypertensive rats treated with 1mL of guaco (n=28); spontaneously hypertensive rats treated with 2mL of guaco (n=36): antero-posterior (A) and latero-lateral (B) of cranium; antero-posterior (C) and latero-lateral (D) of thorax; cranium-caudal (E) and tail (F). Cesarean sections were performed on the 18th day of gestation. *p<0.05 when compared to the control group.
Microscopic Analysis
In the normotensive rats control group, fragments of placentas averaged 0.30 cm, the largest fragment 0.60 cm and smaller 0.10 cm. The thickness of the vessels ranged from 0.01 to 0.05 mm, their average was 0.03 mm, for a total of 80 blades. Areas of congestion were found in 72 cases (90%), foci of hemorrhage in 61 cases (76.25%), inflammation in only one slide. The presence of fibrin was observed in seven placentas, and no areas with edema were observed in this group (Table 2).
Table 2.
Histological placental parameters analyzed during different treatments.
| Average Vessel Thickness (mm) | Congestion/Hemorrhage (%) | Fibrin (%) | Inflammation (%) | Total placentas (n) | |
|---|---|---|---|---|---|
| Normotensive | 0.03 | 83.7 | 8 | 1.25 | 80 |
| Spontaneously hypertensive | 0.02 | 100 | 0 | 2.2 | 45 |
| Spontaneously hypertensive + guaco (1mL) | 0.03 | 33.3 | 0 | 5.5 | 18 |
| Spontaneously hypertensive + guaco (2mL) | 0.02 | 84 | 0 | 4 | 25 |
In the group of spontaneously hypertensive rats (with saline solution), 45 slides were analyzed. Fragments measurement averaged 0.30 cm, the largest fragment of 0.4 cm and 0.2 cm from the smaller. The thickness of the vessels ranged from 0.02 to 0.04 mm, the average being 0,0266 mm. Congestion areas were observed in all cases, bleeding was found (86.66%), acute inflammation was observed in only one slide, and there no cases of edema and/or fibrin were observed in this group (Table 2).
In the spontaneously hypertensive rats group treated with 1 mL of guaco, consisting of 18 placentae, fragments averaged 0.3 cm, being the smallest in size of 0.2 cm. The thickness of the vessels ranged from 0.03 to 0.04 mm and average was 0.031 mm. Congestion was observed in 16 cases, corresponding to 88.80%. Bleeding was found in seven placentae (43.75%). Acute inflammation was observed in only one placenta, and no cases of edema and/or fibrin were observed in this group (Table 2).
In the spontaneously hypertensive rats group treated with 2 mL of guaco, 25 slides were analyzed. Fragments averaged 0.3 cm, the largest of 0.5 and smallest of 0.2 cm. Vessel thickness ranged from 0.01 to 0.04 mm and the mean value was 0.026 mm. Congestion was observed in 21 rats (84%). Inflammation was observed in only one placenta, and no cases of edema and/or fibrin were observed in this group (Table 2).
Foci of thrombosis were also found in a placenta in the hypertensive control group, suggesting the progression of maternal hypertension. Foci of lymphocytic infiltrate were observed in all groups of rats, which corroborate the findings of inflammation of some placentas, since lymphocytes are markers of inflammation (Fig. 3). Results are summarized in Table 2.
Fig. 3.
Foci of lymphocytic infiltrate (H&E, 40x).
Discussion
Toxicology is a science which has greatly evolved, emerging reproductive toxicology, which studies the actions of toxic substances on various phases of the reproductive and developmental process (Gerenutti et al., 1992). Numerous plants are used as an alternative treatment for various diseases, but without proper evaluation of the toxicity of its components (Fraser and Nora, 1986). Most fetal changes are mainly due to maternal exposure to chemical agents (Gerenutti et al., 2006), which indicates the importance of this study to assess potential toxicities of guaco extract when administered during pregnancy in hypertensive rats.
The rat is an important animal model in reproductive toxicity testing. However, histopathological knowledge about development of the placenta, placental abnormalities, or inadequacies of the fetus and placenta is scarce and incomplete. An understanding of the development of normal placenta is required, in order to recognize the possible abnormalities, and there are few studies on morphometric changes during development of normal and hypertensive placenta.
Currently there are only two studies that reported the pregnant rat maternal blood parameters (Janthanaphan et al., 2006; Huppertz, 2008). Requirements related to maternal toxicity, required for current guidelines for registration of drugs, are limited mainly to food consumption and drug use (Hall, 1973). Drugs of herbal origin are not yet included in studies of maternal toxicity.
The use of spontaneously hypertensive rats is justified based on the results obtained in the literature, suggesting that guaco induces smooth muscle relaxing effect, vasodilator, and hypotensive effects that could promote reduction of blood pressure in hypertensive pregnant women, which have hypotensive reduced ability (Leite et al., 1992; Santos et al., 1996), indicating this plant as a promising pharmacological tool in complementary therapy for pregnancy-related hypertension.
Maternal hypertension may cause decreased placental blood flow, reducing the transfer of specific nutrients such as glucose and amino acids. This condition results in low fetal and placental weight (Karlsson et al., 1982; Kingdom and Kaufmann, 1999; Lieb et al., 1981; Rudge et al., 1999; Wallace et al., 1999; Wigglesworth, 1964), data corroborated when pregnant hypertensive rats, treated or not with guaco extract, exhibited reduced weight of fetuses and placentas. According to some authors, in cases of hypertension there is a decreased placental perfusion (Wallace et al., 1999). In the opinion of others, this decrease is caused by changes in placental spiral arterioles, which leads to insufficient placental flow and, consequently, the intrauterine growth retardation (Low and Galbraith, 1974; Kingdom and Kaufmann, 1999; Scott and Jordan, 1972). Small placentas are associated with factors such as low pre-pregnancy weight, high levels of maternal hemoglobin during pregnancy, hypertension, among others (Naeye, 1987).
In hypertension, the ability to vasodilate is reduced. The guaco extract has the active ingredient coumarin, which triggers various biological effects, including vasodilation - an important change to reduce the effects of hypertension on offspring (Schenkel et al., 2001). This effect was not observed in this study with the concentrations studied, since the restriction of the blood supply was maintained during pregnancy, as confirmed by the decrease in weight of fetuses and placentas when compared to normotensive rats. The methodology used in this study was similar to other studies that have observed acute toxicity (Pérez-Guerrero et al., 2001) and reproductive capacity (Gerenutti et al., 2006).
The pre-implantation and post-implantation of blastocysts undergo differential physiological effects under the actions of chemicals, serving to distinguish embryotoxicity and direct toxic effects on uterine function. The rate of pre-implantation losses establishes the relationship between two variables: number of corpora lutea and number of implantation (Ford, 1982). The results of this study showed no change in number of deployments in relation to number of corpora lutea. In relation to post-implantation losses, the finding of resorptions present in both control and hypertensive groups can be justified, since the placenta is exposed to the same influences of intrauterine environment, and other countless extra-uterine aggressions of various kinds (Beebe et al., 1996; Albuquerque, 2009).
In fetuses subjected to macroscopic analysis, anatomical abnormalities were not seen in any of the experimental groups (Damasceno et al., 2008). But the measures of external morphological parameters (anteroposterior and lateral-lateral skull, anteroposterior and lateral-lateral thorax, cranial-caudal and tail) were significantly lower when compared with the normotensive control group, which was also proved, since these fetuses were significantly lower when weighted at the time of cesarean section, effect attributed to hypertension.
In conclusion, the results obtained in this study show that the Mikania glomerata extract did not show the possibility of teratogenicity, and also did not determine control over the vasoconstrictor effect in hypertensive rats, with the studied concentrations. The histological analysis concluded that no significant alterations between the analyzed groups occurred; all the groups presented congestion, whereas inflammation, edema and fibrin deposits were found in an insignificant number of placentas, independent of control or treated group. The weight reduction of the hypertensive rats, fetuses and their respective placentas were determined by hypertension, not by the administration of the plant extract, data confirmed when compared to the spontaneously hypertensive rats (saline solution).
Therefore, new complementary studies about the plant extract are necessary, in order to determine the toxic and vasodilatation potential, which will indicate a safe use for pregnant women, who use the commercialized Mikania glomerata extract as an alternative treatment for superior airway problems, especially to fluidize tracheobronchial exudates and for its significant effect of relaxing the smooth muscles of the bronchi.
Acknowledgments
The authors thank PIBIC/CNPq and CEPE/CNPq for financial support.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Albuquerque L.B.L. Estudos in vitro e in vivo de Plathymenia reticulata Benth [dissertação] São Paulo: Programa de Pós-Graduação em Ciências Farmacêuticas, Universidade de Sorocaba; 2009. [Google Scholar]
- Almeida M.F.B, Guinsburg R, Martinez F.E, Procianoy R.S, Leone C.R, Marba S.T.M, Rugolo L.M.S.S, Luz J.H, Lopes J.M.A. Perinatal factors associated with early deaths of preterm infants born in Brazilian Network on Neonatal Research centers. Jornal de Pediatria da Sociedade Brasileira de Pediatria. 2008;84(4):300–307. doi: 10.2223/JPED.1787. [DOI] [PubMed] [Google Scholar]
- Amorozo M.C.M, Gely A. Uso de Plantas Medicinais por Caboclos do Baixo Amazonas. Vol. 4. Barcarena: PA: Museu Paraense Emílio Goeldi; 1988. p. 47. [Google Scholar]
- Assis T.R, Viana F.P, Rassi S. Estudo dos Principais Fatores de Risco Maternos nas Síndromes Hipertensivas da Gestação. GO, Brasil: Universidade Federal de Goiás1, Universidade Católica de Goiás2, Goiânia; 2008. [Google Scholar]
- Barreto T.E, Hiruma-Lima C.A. Potencial farmacológico de um Fragmento de Mata de Galeria, Fisionomia do Bioma Cerrado, denominado Mata do Butignoli – Botucatu – SP. Botucatu: Estudo monográfico –Instituto de Biociências, Universidade Estadual Paulista; 2002. [Google Scholar]
- Beebe L.A, Cowan L.D, Altshuler G. The epidemiology of placental features:associations with gestacional age and neonatal outcome. Obstet. Gynecol. 1996;87:771–778. doi: 10.1016/0029-7844(95)00483-1. [DOI] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P. Pathology of the human placenta. 4th ed. New York: Springer; 2000. [Google Scholar]
- Britto V.L.M.Q, Resende R.F, Gouveia N.M, Amaral F.C, Teixeira E.H, Pereira W.F, Espíndola F.S. Plantas medicinais e fitoterápicos no contexto da academia, governo e organizações da sociedade civil:exemplo de iniciativas populares no município de Uberlândia-MG. Rev. Ed. Popular, Uberlândia. 2007;6:93–101. [Google Scholar]
- Cechinel-Filho V, Yunes R.A. Estratégias para a obtenção de compostos farmacologicamente ativos a partir de plantas medicinais:conceitos sobre modificação estrutural para otimização da atividade. Química Nova. São Paulo. 1998;21(1):99–105. [Google Scholar]
- Damasceno D.C, Kempinas W.G, Volpato G.T, Consoni M, Rudge M.V.C, Paumgartten F.J.R. Anomalias Congênitas –Estudos Experimentais. Belo Horizonte-MG: Editora Coopmed; 2008. pp. 1–99. [Google Scholar]
- de Rijk E.P.C.T, Esch E.V, Flik G. Pregnancy Dating in the Rat: Placental Morphology and Maternal Blood Parameters. Toxicol. Pathol. 2002;30(2):271–282. doi: 10.1080/019262302753559614. [DOI] [PubMed] [Google Scholar]
- Ferrão M.H.L, Pereira A.C.L, Gersgorin H.C.T.S, Paula T.A.A, Corrêa R.R.M, Castro E.C.C. Efetividade do tratamento de gestantes hipertensas. MG, Brasil: Departamento de Ciências Biológicas da Universidade Federal do Triângulo Mineiro; 2006. [Google Scholar]
- Ferro V.O. Aspectos Farmacognósticos de Mikania smilacina DC. Curso de Pós-Graduação em Ciências Farmacêuticas da USP, Tese de Doutorado. 1991 [Google Scholar]
- Ford W.C. The effect of deoxy-6-fluoroglucose on the fertility of male rats and mice. Contraception. 1982;25(5):535–545. doi: 10.1016/0010-7824(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Fraser F.C, Nora J.J. Genética Humana. 2nd Ed. Guanabara: Rio de Janeiro; 1986. [Google Scholar]
- Gerenutti M, Del Fiol F, Groppo F.C. Performance reprodutiva de ratas grávidas e efeitos embriotóxicos da ciprofloxacina. Pharmazie. 2006;61(1):79–80. [PubMed] [Google Scholar]
- Gerenutti M, Spinosa H.deS, Bernardi M.M. Effects of bracken fern (Pteridium aquilinum L Kuhn) feeding during the development of female rats and their offspring. Vet. Hum. Toxicol. 1992;34(4):307–310. [PubMed] [Google Scholar]
- Graber S.E, Scheffel U, Hodkinson B, McIntyre P.A. Placental transport of vitamin B12 in the pregnant rat. J. Clin. Invest. 1971;50(5):1000–1004. doi: 10.1172/JCI106569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G.A. Changes in the Rat Placenta Following Inoculation with Salmonella Dublin. Am. J. Pathol. 1973;72(1):103–118. [PMC free article] [PubMed] [Google Scholar]
- Huppertz B. Placental origins of preeclampsia: challenging the current hypothesis. Hypertension. 2008;51(4):970–975. doi: 10.1161/HYPERTENSIONAHA.107.107607. [DOI] [PubMed] [Google Scholar]
- Janthanaphan M, Kor-anantakul O, Geater A. Placental weight and its ratio to birth weight in normal pregnancy at Songkhlanagarind Hospital. J. Med. Assoc. Thai. 2006;89(2):130–137. [PubMed] [Google Scholar]
- Karlsson K, Ljungblad U, Lundgren Y. Blood flow of the reproductive system in renal hypertensive rats during pregnancy. Am. J. Obstet. Gynecol. 1982;142:1039–1044. doi: 10.1016/0002-9378(82)90790-6. [DOI] [PubMed] [Google Scholar]
- Kingdom J.C, Kaufmann P. Oxigen and placental vascular development. Adv. Exp. Med. Biol. 1999;474:259–275. doi: 10.1007/978-1-4615-4711-2_20. [DOI] [PubMed] [Google Scholar]
- Kosif R, Akta G, Öztekin A. Microscopic Examination of Placente of Rats Prenatally Exposed to Aloe barbadensis: A Preliminary Study. Int. J. Morphol. 2008;26(2):275–281. [Google Scholar]
- Leite M.G.R, Silva M.A.M, Lino C.S, Viana G.S.B, Matos F.J.A. Atividade broncodilatadora em Mikania glomerata Justicia pectoralis e Torresca cearensis. Anais do XII Simpósio de Plantas Medicinais do Brasil. 1992;1:21. [Google Scholar]
- Lewis R.M, Doherty C.B, James L.A, Burton G.J, Hales C.N. Effects of Maternal Iron Restriction on Placental Vascularization in the Rat. Placenta. 2001;22(6):534–539. doi: 10.1053/plac.2001.0679. [DOI] [PubMed] [Google Scholar]
- Lieb S.M, Zugaib M, Nuwayhid B, Tabsh K, Erkkola R, Ushioda E, Brinkmann C.R, III, Assali N.S. Nitroprusside induced hemodynamic alterations in normotensive and hypertensive pregnant sheep. Am. J. Obstet. Gynecol. 1981;139:925–931. doi: 10.1016/0002-9378(81)90960-1. [DOI] [PubMed] [Google Scholar]
- Low J.A, Galbraith R.S. Pregnancy characteristics of intrauterine growth retardation. Obstet. Gynecol. 1974;44:122–126. [PubMed] [Google Scholar]
- Naeye R.L. Functionally important disorders of the placenta, umbilical cord, and fetal membranes. Hum. Pathol. 1987;18:680–691. doi: 10.1016/s0046-8177(87)80239-3. [DOI] [PubMed] [Google Scholar]
- Oliveira F, Alvarenga M.A, Akisue G, Akisue M.K. Isolamento e identificação de componentes químicos de Mikania glomerata Sprengel e de Mikania laevigata Schultz Bip. ex Baker. Rev. Farm. Bioq USP, São Paulo. 1984;20(2):169–183. [Google Scholar]
- Pereira N.A, Pereira B.M, do Nascimento M.C, Parente J.P, Mors W.B. Pharmacological screening of plants recommended by folk medicine as anti-snake venom; IV Protection against jararaca venom by isolated constituents. Planta Med. 1994;60:99–100. [PubMed] [Google Scholar]
- Pérez-Guerrero C, Herrera M.D, Ortiz R, Alvarez de Sotomayor M, Fernández M.A. A pharmacological study of Cecropia obtusifolia Bertol aqueous extract. J. Ethnopharmacol. 2001;76:279–284. doi: 10.1016/s0378-8741(01)00253-7. [DOI] [PubMed] [Google Scholar]
- Prance G.T. Out of the Amazon. London: HMSO; 1992. p. 83. [Google Scholar]
- Rudge M.V, Gomes C.M, Calderon Ide M, Ramos M.D, Abbade J.F, de Oliveira M.G, da Silva M.G. Study of the evolution of the placenta and fetal pancreas in the pathophysiology of intrauterine growth retardation due to restricted maternal diet. São Paulo Med. J. 1999;117:49–56. doi: 10.1590/s1516-31801999000200002. [DOI] [PubMed] [Google Scholar]
- Ruppelt B.M, Pereira E.F, Gonçalves L.C, Pereira N.A. Pharmacological screening of plants recommended by folk medicine as anti-snake venom I. Analgesic and anti-inflammatory activities. Memórias do Instituto Oswaldo Cruz. 1991;86(Suppl. II):203–205. doi: 10.1590/s0074-02761991000600046. [DOI] [PubMed] [Google Scholar]
- Santos T.C, Tomassini C.B, Sanchez E, Cabral L.M. Anais do XII Simpósio de Plantas Medicinais do Brasil. 1996:149. [Google Scholar]
- Schenkel E.P, Zaninnin M, Mentz L.A, Bordignon S.A.L, Irgang B. Plantas Tóxicas. In: Simões C.M.O, Schenkel E.P, Gossmann G, Mello J.C.P, Mentz L.A, Petrovick P.R, editors. Farmacognosia:da planta ao medicamento. Vol. 1. Porto Alegre: UFSC/UFRGS; 2001. pp. 755–788. [Google Scholar]
- Scott J.M, Jordan J.M. Placental insufficiency and small-for-dates baby. Am. J. Obstet. Gynecol. 1972;113:823–832. doi: 10.1016/0002-9378(72)90565-0. [DOI] [PubMed] [Google Scholar]
- Vieira R.B, Macedo S.M.D, Schwanz M. Inibição do recrutamento celular pelo extrato aquoso de Mikania laevigata Schultz Bip. ex. Baker e cumarina em ratos submetidos àperitonite. URI –Campus de Erechim: Departamento de Ciências da Saúde, Curso de Farmácia, Universidade Regional Integrada do Alto Uruguai e das Missões; 2008. [Google Scholar]
- Wallace J.M, Bourke D.A, Aitken P.P. Nutrition and fetal growth:paradoxical effects in the overnourished adolescent sheep. J. Reprod. Fertil. Suppl. 1999;54:385–399. [PubMed] [Google Scholar]
- Wigglesworth J.S. Experimental growth retardation in fetal rat. J. Pathol. Bacteriol. 1964;88:1–13. [PubMed] [Google Scholar]


