Highlights
-
•
Pancreatic undifferentiated carcinomas with OGCs are very rare neoplasms that may have an atypical clinical presentation.
-
•
Less than a hundred cases have been described until today.
-
•
Early diagnosis and aggressive surgical treatment is the only way to prolong survival.
Abbreviations: OGCs, osteoclast-like giant cells; UC-OGCs, undifferentiated carcinomas of the pancreas with OGCs; UC-PGCs, undifferentiated carcinomas of the pancreas with pleomorphic/sarcomatoid giant cells; WHO, World Health Organization; CT, computed tomography; MRI, magnetic resonance imaging; TBL, total bilirubin; ALP, alkaline phosphatase; γ-GT, gamma glutamyltranspeptidase; CEA, carcinoembryonic antigen; CA 19-9, cancer antigen 19-9; FNA, fine needle aspiration
Keywords: Osteoclast giant cell tumors, Pancreatic tumor, Undifferentiated carcinoma
Abstract
Introduction
Undifferentiated head of pancreas carcinoma with osteoclast-like giant cells (UC-OGC) is a rare neoplasm, with less than a hundred cases reported. We present such a case, in which the UC-OGC presented atypically as a cystic lesion following acute pancreatitis and led to late diagnosis.
Presentation of case
A 75-year-old female patient, who had suffered acute pancreatitis three years ago, was referred with a diagnosis of osteoclast-like giant cell (OGC) tumor of the head of pancreas. She had suffered acute pancreatitis three years ago. Two years ago she developed abdominal pain, steatorrhea and weight loss. Abdominal computed tomography imaging showed a cystic mass in the head of the pancreas (maximum diameter 4 cm). The initial diagnosis was pancreatic pseudocyst; however as the mass gradually increased in size and the patient continued to be symptomatic, a CT-guided biopsy was performed. Histological examination revealed an OGC pancreatic tumor. In laparotomy a large (9 cm) encapsulated heterogeneous mass was found with partial involvement of the common hepatic artery. Pancreaticoduodenectomy was performed and the involved part of the common hepatic artery was replaced with a homologous graft from the major saphenous vein. Post-operative course was uneventful. Histology revealed an undifferentiated pancreatic adenocarcinoma with OGCs. She survived 10 months after the operation.
Discussion
Pancreatic undifferentiated carcinomas with OGCs are very rare neoplasms and can present with an atypical clinical picture.
Conclusions
A symptomatic cystic lesion of the pancreas, which is growing in size, should be investigated promptly in order to exclude the presence of malignancy.
1. Introduction
Undifferentiated carcinomas of the pancreas are very rare, non-endocrine pancreatic neoplasms of high malignancy. Despite their histological differences, the current WHO classification places these tumors in a single category under the name of ‘undifferentiated carcinoma of the pancreas’ [1]. The presence of osteoclast-like giant cells (OGCs), which is suggested to be non-neoplastic reaction, distinguishes these carcinomas from the plain undifferentiated carcinomas. Prognosis is poor with most patients surviving less than a year, as a result of late diagnosis and tumor aggressiveness [2].
We present a case of undifferentiated head of pancreas carcinoma with OGCs of a very unusual clinical presentation, that of a symptomatic cystic lesion following acute pancreatitis.
2. Presentation of case
The work has been reported in line with the CARE criteria [3].
A 75-year-old female patient with a medical history of hypertension was referred to our unit with the diagnosis of a giant cell tumor at the head of pancreas. Three years ago she presented to her local hospital with acute idiopathic pancreatitis. Receiving conservative treatment, she was asymptomatic for the subsequent 12 months, before she started suffering from abdominal discomfort, steatorrhea and weight loss. Computed tomography (CT) abdominal scan and magnetic resonance imaging (MRI) showed a cystic mass (max. diameter 4 cm) in the head of pancreas, which was thought to be a pancreatic pseudo-cyst, but due to increasing size and ensuing of symptoms, a CT-guided biopsy was performed. Histopathology showed the presence of multinuclear OGCs and she was referred to our center.
Our CT scan showed a 9 cm large head of pancreas mass, with solid and cystic components, in close relation (but no invasion) to the mesenteric vessels and the common hepatic artery (Fig. 1). The main pancreatic (but not the common bile duct) was dilated; the body and tail of pancreas were atrophic with no evidence of acute inflammation or lymphadenopathy. Chest CT scan was normal. Preoperative blood tests (TBL, ALP, γ-GT, CEA) were normal, except for CA 19-9 at 110 U/ml (normal values: 0–37).
Fig. 1.
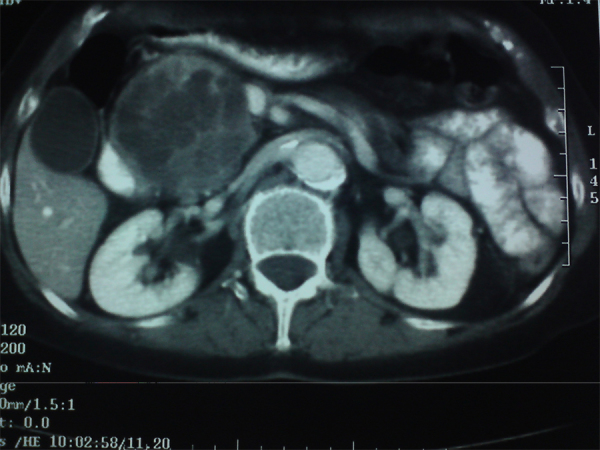
Abdominal computed tomography scan showing a 9 cm large head of pancreas mass, with solid and cystic components, in close relation to the mesenteric vessels and the common hepatic artery, but with no clear evidence of vascular invasion. The pancreatic duct is dilated and the rest of the pancreas is atrophic.
At theater a large encapsulated mass was discovered, in direct contact with the common hepatic artery and with the superior mesenteric vein. During dissection of the tumor from the common hepatic artery, it was realized that the common hepatic artery was invaded by the tumor. Although it was not clear if a resection would be beneficial for the patient it was decided the resection to go ahead as the common hepatic artery was partially injured during the dissection and a reconstruction was needed. For this reason a Whipple procedure was performed with excision of the involved part of the common hepatic artery and blood flow was restored with a 3 cm long venous graft from the left major saphenous vein. Postoperative course was uneventful. The patient denied having chemotherapy. Follow-up at 3 and 6 months found her in good health, but unfortunately she developed local recurrence and died 10 months after surgery.
Pathology revealed a round, circumscribed neoplastic mass measuring 8.5 cm in maximum dimension, extending to the ampulla of Vater. Microscopic examination showed a malignant neoplasm composed of undifferentiated cells with great pleomorphism and nuclear atypia, as well as of scattered OGCs (Fig. 2). Moreover, a minor well-differentiated adenocarcinoma component was also seen. Immunohistochemically, the great majority of undifferentiated neoplastic cells expressed vimentin and at least some of them were stained for cytokeratin and p53. OGCs reacted strongly for CD68, KP1 and PGM1, and were negative for all other markers. None of the seven peripancreatic lymph nodes found showed evidence of metastatic disease.
Fig. 2.
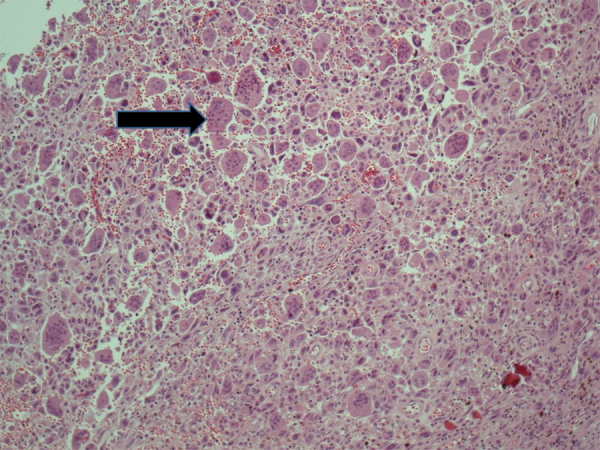
Tumor histology: undifferentiated cells with great pleomorphism and nuclear atypia, as well as of scattered osteoclast-like giant cells (black arrow).
3. Discussion
Undifferentiated carcinomas of the pancreas with OGCs (UC-OGCs) are very rare, representing less than 1% of all pancreatic malignancies [4], [5], [6]. They were first described by Rosai [7] and are characterized by the presence of undifferentiated, pleomorphic, mononuclear cells and multinucleated OGCs [4]. ‘Pancreatic osteoclast-like giant cell tumors’ is a term widely used to refer to these neoplasms, however a more appropriate one would be UC-OGCs, which describes better their biological behavior [1]. Most of them are located in the body or tail of the pancreas and usually have atypical clinical presentation [8]. Nodal involvement is uncommon and metastases are slow, however most tumors invade adjacent structures [2], [8]. Only few cases report early diagnosis with tumor diameter less than 3 cm [9], [10]. Imaging techniques may identify a large cystic mass with hemorrhage and necrosis, which sometimes may be mistaken for pancreatic pseudocyst, as in our case.
Patients with anaplastic (undifferentiated) pancreatic cancers live less than those with pancreatic ductal adenocarcinoma [11], [12]. UC-OGCs generally have an overall poor prognosis; median survival is 11 months, dropping to 6.5 months in cases of non-operable tumors [2], [4], [8]. However, the interval between diagnosis and death may vary significantly (from 4 months to 10 years) [13], [14]. In the review by Kobayashi et al. some favorable prognostic factors were identified: younger age at time of diagnosis, female gender, smaller lesions and absence of lymph node involvement [13]. UC-OGCs seem to have a clearly better outcome than tumors with pleomorphic/sarcomatoid giant cells (UC-PGCs) or other variables [11], [12]. However, there is still controversy regarding the prognosis of UC-OGCs when compared to ductal adenocarcinoma [6], [11], [15].
Histogenesis of UC-OGCs is another issue of controversy. Studies on light and electron microscopy, on immunophenotype of the tumor cells and molecular biology analysis, have been used to support a mesenchymal or an epithelial origin of the tumor [16], [17]. Some authors contributed the idea that it arises from a precursor pluripotent cell that may differentiate to distinctive phenotypes [2], [16], while others consider it carcinosarcoma-like neoplasm that consists of both epithelial and histiocytic-mesenchymal component [7], [17]. Acinar cell origin or derivation from a mucinous cystic neoplasm or from ductal cells have also been discussed [4], [5]. Adequate sampling of the neoplasm will reveal at least a minor component of atypical neoplastic glands [10]. Furthermore, in some cases it has been shown that OGCs tumor co-exists with pancreatic intraepithelial neoplasia (PanIN 2 and 3) [4], [10]. These findings strongly support a possible epithelial origin of the neoplasm. However, the main question remains, whether the undifferentiated mononuclear cells and the OGCs are of non-neoplastic mesenchymal, neoplastic mesenchymal or epithelial origin.
The role of adjuvant treatment, either chemotherapy or radiotherapy, has not yet been clearly established, in part due to the rarity of this pathological entity. There are reports on the use of chemotherapeutic agents (such as cis-platin, paclitaxel and gemcitabine) with favorable outcome, given the epithelial origin of the tumor [9], [18], [19]. On the other hand, radiotherapy has been applied on the basis of radiosensitivity of giant-cell tumors of the bones [4] or as a means of eliminating any remnant cancer cells in the tumor bed after positive-margin excision [15], sometimes with satisfactory results [20]. However, due to the limited experience in the treatment of such neoplasms, no safe conclusions can be drawn [21].
In our patient, the characteristic morphology of the tumor, its co-existence with a small area of well differentiated pancreatic adenocarcinoma, as well as the immunophenotype of both undifferentiated pleomorphic cells and OGCs, support the diagnosis of a UC-OGCs. The unique characteristics in our case is the history of acute pancreatitis 3 years earlier which was misleading, the presence of the symptomatic cystic lesion post-pancreatitis which was miss-interpreted as pancreatic pseudo-cyst and the evidence of chronic pancreatitis in serial CT with severe parenchymal atrophy and dilation of the main pancreatic duct. This finding of chronic pancreatitis has not been reported before. It could be attributed to tumor pressure effects.
4. Conclusion
These rare pancreatic tumors present with atypical symptoms, can mimic a pseudocyst and usually are diagnosed in late stage with poor prognosis. A high index of suspicion is required in order the prognosis to be improved. The learning point from our study is that when a symptomatic pancreatic cystic lesion, which is increasing in size, is seen on follow up scans then we should proceed to further investigation of the lesion, preferably with endoscopic ultrasound and FNA. This will lead to early diagnosis and effective treatment in case of malignancy.
Conflict of interest
None declared.
Consent
Written informed consent was obtained from the patient’s next of kin (daughter) prior to publication.
Funding
None.
Ethical approval
Not applicable.
Author contribution
Georgios Κ. Georgiou, Michalis Fatouros and Georgios Glantzounis performed the operation. Athina Tsili performed the imaging studies. Ephimia Balasi and Vasiliki Siozopoulou performed the pathological analysis and wrote the corresponding part in the text. Georgios Κ. Georgiou wrote the manuscript, while Michalis Fatouros and Georgios Glantzounis critically revised it.
Guarantor
Georgios Glantzounis, MD, PhD, FEBS Associate Professor of Surgery, Department of Surgery University, Hospital of Ioannina, Tel: +30 2651099887, +30 6984189292 Fax: +30 2651099890 E-mail: gglantzounis@uoi.gr gglantzounis@gmail.com.
References
- 1.Bosman F.T., Carneiro F., Hruban R.H., Theise N.D. WHO classification of tumours of the digestive system. In: Bosman F.T., editor. IARC Press; France, Lyon: 2010. p. 2010. [Google Scholar]
- 2.Jo S. Huge undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. World J. Gastroenterol. 2014;20:2725–2730. doi: 10.3748/wjg.v20.i10.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gagnier J.J., Kienle G., Altman D.G., Moher D., Sox H., Riley D. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 2013;2013 doi: 10.1186/1752-1947-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhal A., Shrago S.S., Li S.F., Huang Y., Kohli V. Giant cell tumor of the pancreas: a pathological diagnosis with poor prognosis. Hepatobiliary Pancreat. Dis. Int. 2010;9:433–437. [PubMed] [Google Scholar]
- 5.Manduch M., Dexter D.F., Jalink D.W., Vanner S.J., Hurlbut D.J. Undifferentiated pancreatic carcinoma with osteoclast-like giant cells: report of a case with osteochondroid differentiation. Pathol. Res. Pract. 2009;205:353–359. doi: 10.1016/j.prp.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Maksymov V., Khalifa M.A., Bussey A., Carter B., Hogan M. Undifferentiated (anaplastic) carcinoma of the pancreas with osteoclast-like giant cells showing various degree of pancreas duct involvement. A case report and literature review. JOP. 2011;12:170–176. [PubMed] [Google Scholar]
- 7.Rosai J. Carcinoma of pancreas simulating giant cell tumor of bone. Electron-microscopic evidence of its acinar cell origin. Cancer. 1968;22:333–344. doi: 10.1002/1097-0142(196808)22:2<333::aid-cncr2820220210>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Shiozawa M., Imada T., Ishiwa N., Rino Y., Hasuo K., Takanashi Y., Nakatani Y., Inayama Y. Osteoclast-like giant cell tumor of the pancreas. Int. J. Clin. Oncol. 2002;7:376–380. doi: 10.1007/s101470200059. [DOI] [PubMed] [Google Scholar]
- 9.Bauditz J., Rudolph B., Wermke W. Osteoclast-like giant cell tumors of the pancreas and liver. World J. Gastroenterol. 2006;12:7878–7883. doi: 10.3748/wjg.v12.i48.7878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergmann F., Esposito I., Michalski C.W., Herpel E., Friess H., Schirmacher P. Early undifferentiated pancreatic carcinoma with osteoclastlike giant cells: direct evidence for ductal evolution. Am. J. Surg. Pathol. 2007;31:1919–1925. doi: 10.1097/PAS.0b013e318067bca8. [DOI] [PubMed] [Google Scholar]
- 11.Strobel O., Hartwig W., Bergmann F., Hinz U., Hackert T., Grenacher L., Schneider L., Fritz S., Gaida M.M., Buchler M.W., Werner J. Anaplastic pancreatic cancer: presentation, surgical management, and outcome. Surgery. 2011;149:200–208. doi: 10.1016/j.surg.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Clark C.J., Graham R.P., Arun J.S., Harmsen W.S., Reid-Lombardo K.M. Clinical outcomes for anaplastic pancreatic cancer: a population-based study. J. Am. Coll. Surg. 2012;215:627–634. doi: 10.1016/j.jamcollsurg.2012.06.418. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi S., Nakano H., Ooike N., Oohashi M., Koizumi S., Otsubo T. Long-term survivor of a resected undifferentiated pancreatic carcinoma with osteoclast-like giant cells who underwent a second curative resection: a case report and review of the literature. Oncol. Lett. 2014;8:1499–1504. doi: 10.3892/ol.2014.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao H.Q., Yang Y.M., Zhuang Y., Liu P. Locally advanced undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. World J. Gastroenterol. 2015;21:694–698. doi: 10.3748/wjg.v21.i2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Temesgen W.M., Wachtel M., Dissanaike S. Osteoclastic giant cell tumor of the pancreas. Int. J. Surg. Case Rep. 2014;5:175–179. doi: 10.1016/j.ijscr.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukas Z., Dvorak K., Kroupova I., Valaskova I., Habanec B. Immunohistochemical and genetic analysis of osteoclastic giant cell tumor of the pancreas. Pancreas. 2006;32:325–329. doi: 10.1097/01.mpa.0000202951.10612.fa. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe M., Miura H., Inoue H., Uzuki M., Noda Y., Fujita N., Yamazaki T., Sawai T. Mixed osteoclastic/pleomorphic-type giant cell tumor of the pancreas with ductal adenocarcinoma: histochemical and immunohistochemical study with review of the literature. Pancreas. 1997;15:201–208. doi: 10.1097/00006676-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Yoshioka M., Uchinami H., Watanabe G., Takahashi T., Nakagawa Y., Andoh H., Yoshioka T., Nanjo H., Yamamoto Y. Effective use of gemcitabine in the treatment of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas with portal vein tumor thrombus. Intern. Med. 2012;51:2145–2150. doi: 10.2169/internalmedicine.51.7670. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzawa G., Shirabe K., Gion T., Tsujita E., Ooya M., Kajiyama K., Nagaie T. Surgically resected undifferentiated carcinoma with osteoclast-like giant cells of the periampullary region involving the orifice of the papilla of Vater: report of a case. Surg. Today. 2010;40:376–379. doi: 10.1007/s00595-009-4078-6. [DOI] [PubMed] [Google Scholar]
- 20.Drexler L.J., Mitre R.J., Madan E., Zidar B.L. Pancreatic giant cell carcinoma of the osteoclastic type: response to 5-fluorouracil and radiation. Am. J. Gastroenterol. 1986;81:1093–1097. [PubMed] [Google Scholar]
- 21.Moore J.C., Bentz J.S., Hilden K., Adler D.G. Osteoclastic and pleomorphic giant cell tumors of the pancreas: a review of clinical, endoscopic, and pathologic features. World J. Gastrointest. Endosc. 2010;2:15–19. doi: 10.4253/wjge.v2.i1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]


