Abstract
An adequate chest drainage system aims to drain fluid and air and restore the negative pleural pressure facilitating lung expansion. In thoracic surgery the post-operative use of the conventional underwater seal chest drainage system fulfills these requirements, however they allow great variability amongst practices. In addition they do not offer accurate data and they are often inconvenient to both patients and hospital staff. This article aims to simplify the myths surrounding the management of chest drains following chest surgery, review current experience and explore the advantages of modern digital chest drain systems and address their disease-specific use.
Keywords: Chest tube, intercostal drain (ICD), digital devices, air leak, pleural effusion
Introduction
The pleural cavity is an air-tight closed space that contains a small amount of pleural fluid. The pressure within this closed chamber is sub-atmospheric and is variable during the breathing cycle; increases during expiration and decreases in inspiration. The pleural fluid ensures lung coupling to the chest wall and acts as a lubricant. It is under a constant dynamic equilibrium of production and re-absorption.
The aims for an adequate chest drainage system are to drain fluid and air, prevent these from returning back into the pleural cavity, and restore the negative pleural pressure facilitating lung expansion. In thoracic surgery the post-operative use of the conventional underwater seal chest drainage system fulfills these requirements, however they allow great variability amongst practices. In addition they do not offer accurate data and they are often inconvenient to both patients and hospital staff.
This article aims to simplify the myths surrounding the management of chest drains following chest surgery, review current experience and advantages of modern digital chest drain systems and address their disease-specific use.
Historical background
In 1965 Hughes advocated the use of closed tube thoracostomies with under water seal system for evaluation and treatment of haemothorax particularly in war victims with massive haemothorax. The one-bottle system was the first to be introduced where the bottle collected the fluid and at the same time sealed the air leaks (Figure 1). A two-bottle system then became available to drain significant quantity of fluid where greater pressure is needed to drain air. Two years later Deknatel introduced the first integrated disposable chest drainage unit based on a three-bottle system (Figure 2). The measurement of air leak was subjective and scaled from 1 (low) to 7 (high). This system had a unique calibrated manometer that measured the amount of negative pressure within the pleural cavity (Chamber F). In the absence of air leak the water level moved with respiration reflecting normal intrapleural pressure changes. The system had a wet-suction mechanism where suction is regulated by adjusting the height of a column of water in the suction control chamber. By then tube thoracostomy had been accepted as the standard of care in patients who had their pleural cavity breached either electively or as a result of trauma.
Figure 1.
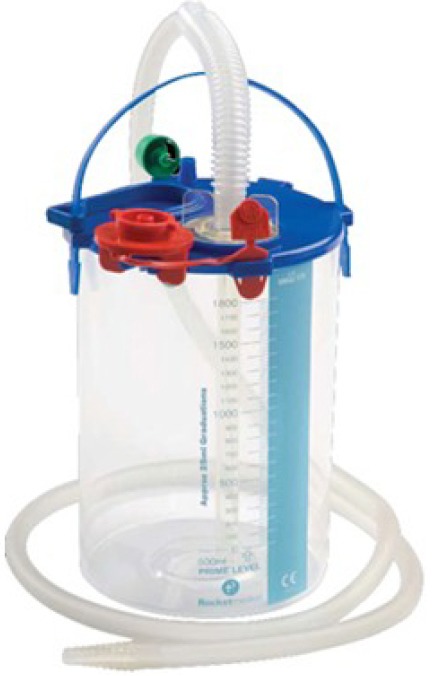
One bottle system (20 cm in diameter) with sterile water filled to the 0 mark to provide a water seal and a low resistance one-way valve. The distal end of the drainage tube is immersed 2 cm below water. This depth determines the hydrostatic pressure needed to overcome expiration. The collection chamber should ideally be placed at least 100 cm below the chest to prevent the chamber fluid getting sucked back during inspiration.
Figure 2.
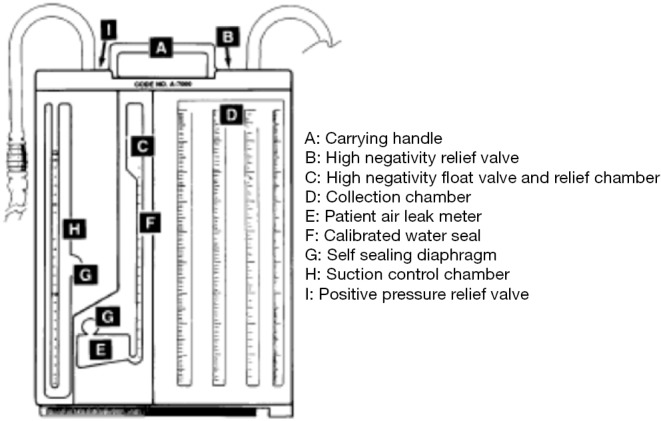
An integrated single unit based three-bottle system. The patient tubing connects the drainage unit directly to the chest tube. Any drainage from the chest flows into chamber D. The collection chamber is calibrated and has a write-on surface to allow for easy measurement and recording of the time, date, and amount of drainage. Chamber F is responsible for measuring the negative pressure within the pleural cavity.
The next step in chest drainage system evolution was to replace the wet-suction mechanism of the three-bottle systems by a dry-suction mechanism where the degree of suction was regulated by a self-compensating regulatory dial rather than the column of water in the wet-suction. The unique design of the dry-suction control immediately responded to either changes in patient pressure (patient air leak) or changes in suction pressure (surge/decrease at the suction source). This has shown to have many advantages such as achievement of higher suction pressure levels, easy set-up, quiet operation with no continuous bubbling, and no fluid evaporation eliminating the risk of suction variability.
A major disadvantage of the traditional systems was impaired patient mobility and comfort and the potential risk of infection when disconnecting the device to mobilise. Furthermore, the wall suction is variable and extremely unreliable and none of these systems had the means to objectively and accurately record the amount of air leak. As thoracic medicine has advanced over the last two decades these became significant issues. The invention of the electronic chest drainage systems addressed all such inefficiencies and standardised the postoperative management of chest tubes.
Digital drainage systems (DDS): principles of operation
All DDS are portable and powered by a rechargeable battery with a sufficiently long run time. They have alarms for various situations, including but not limited to tube occlusion, disconnection and suction failure. Being a completely closed system, the fluid has no contact with the outside environment, and provides improved bio-safety for the health care team and patients themselves. Furthermore these devices eliminate inter-observer variability with objective measurement of air leaks recorded in the system (mL/min) and displayed on a screen.
The most important advantage though is the ability to apply regulated pressure in the pleural space independent of patient, tube and device position. Thopaz® (Medela, Switzerland) DDS is an example of those devices. It constantly maintains a regulated suction pressure preset by the user. In case of an air leak the device remains active, and produces additional suction effect to maintain the desired negative intrapleural pressure initially preset by the user. This is achieved using an accurate sensor that collects data against changes of the intrapleural pressure to the required preset value. The data then is translated into a recorded air leak through a complex mathematical algorithm. The amount of liquid is measured directly into a graded container. The most up to date systems have the ability to record fluid drainage on a graph, thus allowing full data capture of chest tube drainage. Both these features allow medical personnel to take safe and confident decisions based on data and not on snapshot observations only as with the traditional systems.
Post-operative use of DDS
The first study to evaluate the role of the DDS was reported in 2006 (1). The investigators used the AIRFIX sensor device connected to the thoracic drainage system to measure air leaks. They concluded that air leaks of 20 mL/min or less permitted drain removal without the risk of pneumothorax or re-insertion.
Similar studies using the world’s first DDS, the DigiVentV® (Millicore, Sweden), showed the effectiveness of measuring air leaks eliminating the inter-observer subjective decision as to when a chest tube can be safely removed (2,3).
The first randomised comparative trial between the conventional drainage systems and DDS was performed on 100 patients undergoing elective pulmonary resections (4). The results showed a significant reduction in chest tube duration (3.1 vs. 3.9 days) and length of stay (3.3 vs. 4.0 days) with the DDS.
Similarly, three other single centre prospective randomised trials confirmed reduction in both chest tube duration and length of hospital stay with the DDS (5-7).
In 2014, we conducted the first multicentre international randomised controlled trial comparing two systems: conventional versus Thopaz® DDS (8). The group concluded that DDS was associated with significant reduction in air leak duration, duration of chest tube placement and post-operative length of stay (1.0 vs. 2.2 days, 3.6 vs. 4.7 days, and 4.6 vs. 5.6 days, respectively). In addition and similar to other investigators, DDS have proven to be user friendly and popular, both by medical and nursing staff and patients (4,8,9).
Use of DDS in the non-surgical setting
In the management of pneumothorax with air leak Jablonski et al randomised 60 patients to the use of the conventional drainage system (n=30) with the DDS (n=30) for persistent air leak (10). The group concluded reduced duration of the drainage, the length of hospital stay and overall hospitalisation cost with the latter system. DDS have the ability to predict quickly the likelihood of spontaneous resolution of the pneumothorax by interpreting the recorded graph of air leak. This process differentiates confidently those who would need urgent surgery compared with their counterparts in whom the pneumothorax will resolve with simple drainage.
Case presentation
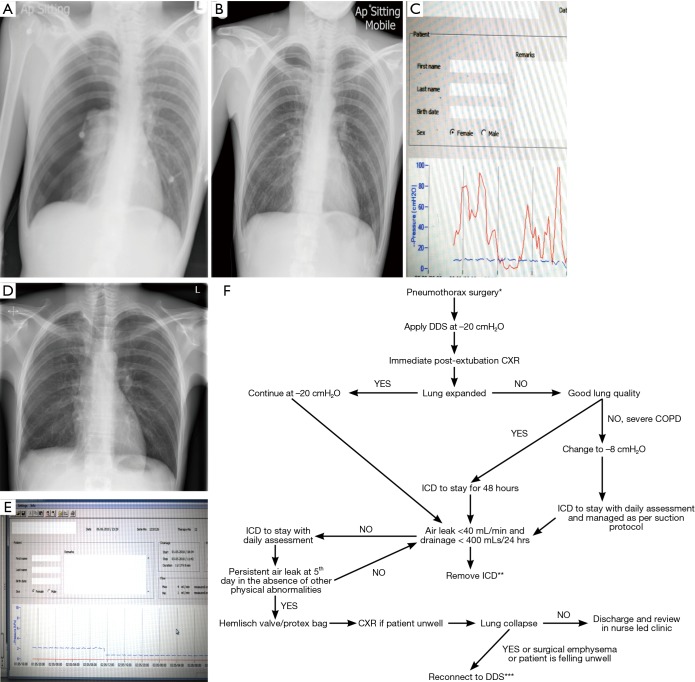
A young, fit male is admitted through the Respiratory Department with his first episode of primary spontaneous pneumothorax. A chest tube was inserted and connected to the traditional system. He spends 5 days in the medical ward with no resolution of his pneumothorax when subsequently referred for a surgical opinion. His chest drain is connected to a DDS which revealed a constant air leak for 24 hours with no signs of improvement (Figure 3). He receives eventually VATS and pleurodesis with resolution of the pneumothorax. He was discharged home within 48 hours from surgery. This case illustrates clearly the prognostic value of a modern DDS allowing early referral and management with overall reduction in hospital stay. Figure 3F represents a flowchart with recommendations of chest tube management following pneumothorax surgery connected with DDS.
Figure 3.
An example of a pneumothorax patient managed initially with a traditional chest drain. (A) Right pneumothorax; (B) chest drain in situ connected to a traditional one-bottle system; (C) after 5 days, connection to DDS showed persistent airleak for 24 hours with the unlikely hood of resolving; (D) post-VATS pleurodesis CXR with no evidence of pneumothorax; (E) 48 hours of flat line on DDS before drain removal; (F) flow chart with recommendations of chest tube management following pneumothorax surgery connected with DDS. *, although the management in general does not differ between primary and secondary pneumothorax it is imperative to adjust the suction depending on the quality of the underlying lung parenchyma; **, in cases of significant emphysema, drains should be removed when there is no excessive swing on the tubing; ***, the vast majority of patients will experience significant lung collapse when there is a large air leak on the background of severe emphysema. Suction should be low and never exceed −8 cmH2O although lower settings might be necessary in the presence of large leak. An individual approach is necessary to achieve best balance between adequate suction without promoting an air leak. DDS, Digital Drain System. VATS, video assisted thoracoscopic surgery; ICD, intercostal drain; CXR, chest X-rays.
The use of DDS has also been advocated in the management of persistent spontaneous secondary pneumothorax in patients with underlying interstitial lung disease (11).
Cost effectiveness of DDS
Technological advances are often credited by default with an overall increase of medical care costs. There is through enough medical evidence to confirm that early adoption of modern technology in the postoperative management of patients has both direct and indirect cost benefits to health care systems.
Several authors have confirmed reduced length of stay (3,4), the ability therefore to cycle more patients through the same bed space (6,7) and also complete or follow up treatment safely at domiciliary level when patients are discharged with chest tubes in situ connected to a DDS (12).
Furthermore, data on postoperative pleural air and fluid drainage, recorded on a DDS, becomes a ‘visual’ adjunct in the hands of experienced clinicians obviating the need for postoperative chest films with further cost savings.
Interpretation of digital data and personal experience
Despite its benefits, the use of DDS remains somewhat guarded to a number of thoracic surgical and respiratory medical units. We believe this is multi factorial and is a result of:
Restrictive adoption of new technology by several hospitals classed as expensive and not justified;
Presence of comfortable and conservative practices;
Concerns with risks associated with adoption of new and potentially complex perceived technology;
Lack of adequate knowledge and standardised training in managing chest tubes with modern electronic devices.
As anticipated, learning curves vary between different units depending on the current protocols being used and the engagement of clinicians, and nursing teams. In our experience, dated March 2008, our learning curve sloped down for the first 50 patients before reaching a plateau and took another 50 patients to become fully confident of how to use the Thopaz® electronic device (13). Our learning curve was complemented by multiple visits and training sessions from the industry before we confidently acquired enough skills to integrate the DDS into our chest tube management protocols. We respected the opinion of our nursing staff and patients before applying a standard protocol. Other investigators used 40 patients before gaining confidence in managing patients after lobectomy (13).
In comparison with the old traditional systems we asked similar questions on the feasibility of the electronic devices. Most were answered as we have gained the experience and the understanding of the device. Some, however, need further refinements:
(I) Do we need to apply suction postoperatively? If so, do we apply the same value of suction in all types of procedures and in all patients?
It is common practice to apply suction of –20 cmH2O to chest tubes directly after pulmonary resections to enhance pleural apposition. Suction causes a significant reduction in the differential pleural pressure (maximum minus minimum intrapleural pressure) hence it plays a role in decreasing the patient’s effort in breathing after lung resection. This reduction is more significant in patients undergoing upper lobectomies as compared to lower lobectomies (14). The role of pleural manometry alone cannot be considered a useful tool in perioperative care after pulmonary lobectomy.
Suctioning has been questioned by five randomized trials that compared the role of suction versus plain under water seal after lung resection. The results were inconsistent and an optimal algorithm towards the application of suction was not developed. Three of the studies favored post-operative suction over plain under water seal, however, there were no statistically significant differences between the groups concerning the duration of air leak and the number of cases with persistent air leak (15-17). The other two studies favored the plain under water seal protocol claiming reduced air leak duration with the traditional system (18,19). The general consensus however was that applying suction to emphysematous lungs carries risk of prolonged air leak (PAL) and if necessary should not exceed −10 cmH2O.
This philosophy does not apply to modern systems. In DDS, the provided suction is consistent, accurate and regulated with the device having the ability to keep it at desired levels regardless of patient, tube or canister position. Continuous digital air leak assessment reduces the degree of variability, and allows safe and confident decisions for tube removal (20).
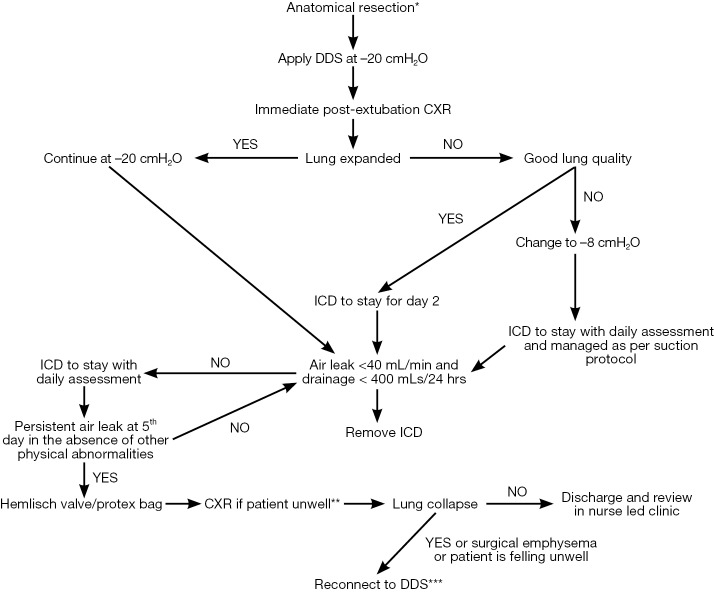
Our recommendations are based on individual subject’s lung quality and post-resection space observed on immediate post-operative chest X-rays (CXR). Figure 4 is a flowchart of how to manage chest drains following lung resections. In patients with uncomplicated resections and good lung compliance and quality our standard protocol is to offer continuous suction at −20 cmH2O. Although this value is arbitrary, it offers a standardized protocol to the Unit and provides a ‘common’ understanding.
Figure 4.
Flow chart with recommendations of chest tube management following lung resections connected with DDS. *, the use of DDS with suction is not recommended following pneumonectomy; **, it is not necessary to perform routine chest films before and after drain removal; ***, in the presence of moderate to a large air leak (>500 mL/min) we recommend a trial of Heimlich valve on the 5th or 6th postoperative day. If clinically significant lung collapses surgical emphysema develops the drain should be reconnected to a suction device at low settings (−8 cmH2O). ICD, intercostal drain; DDS, digital drain system.
What we have frequently witnessed is the recording of an air leak when suction is applied immediately after closure of skin in the operating room and while patients are still ventilated. One should resist in re-opening the chest urgently when large leaks are recorded as these settle when the patient is extubated, unless the Anesthetist is losing large amounts of tidal volume. A routine film in the recovery confirms the appropriate chest tube position and lung re-inflation.
We do not generally recommend DDS in the management of post pneumonectomy spaces. Although the value of −8 cmH2O is considered as physiological and coincides with the physiological negative pressure of the underwater seal, the device will continue to operate to achieve the desirable negative pressure. What we are not yet confident is how the individual space will react and remodel with the application of this physiological negative pressure set within seconds.
In fragile lungs, suction is not recommended and a small to moderate basal space is acceptable especially with lower lobectomies since suction for a post-lower lobectomy space will not exert significant benefit (14). However, in the presence of moderate to large apical space and in the absence of air leak suction should not exceed −8 cmH2O as higher suction can disrupt the visceral pleura of a fragile lung and lead to the development of delayed air leak. The reader should be reminded of the physiology of gas absorption from the pleural space: given sufficient time the air will get absorbed naturally and the space will resolve following discharge of the patient with no further sequelae. Hence the modern Thoracic surgeon needs to overcome the obsession of nil-space presence, following a lung resection, before a patient is allowed to be discharged home. In the event of a persistent air leak (5th postoperative day) patients should be allowed to go home on a Heimlich valve. Several surgeons would request a chest film to confirm or exclude a pneumothorax when patients are transitioned from a hospital device to a Heimlich valve. Unfortunately, in the presence of a pneumothorax many patients are fined with prolonged hospital stay. Thus, our recommendations are that patients should be allowed home, even in the presence of a space as long as this does not have clinical significance. For added safety these can be reviewed on a weekly basis at a nurse lead clinic until drain removal (21).
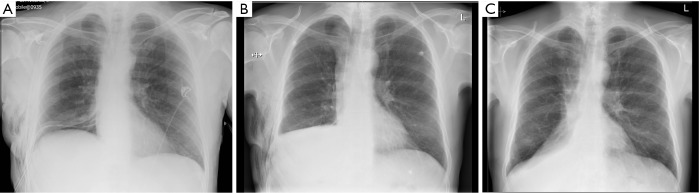
We have termed this phenomenon ‘permissive pneumothorax’. It develops due to the presence of a transient insignificant micro-leak. This reduces the negative intrapleural pressure, drops the pressure gradient between the lung and pleural space and allows the leaking alveoli to heal with subsequent absorption of gases from the pleural space. In summary, the pneumothorax facilitates treatment. Had these patients remained with a chest tube on suction with no lung opposition to the chest wall, the leak would have persevered and the patients would have remained longer in hospital. It is therefore not necessary to request routine chest films following lung resections and drain removal, unless patients experience new signs or symptoms (Figure 5).
Figure 5.
(A) Post lobectomy CXR with ICD in situ connected to DDS; (B) the drain was removed based on absence of air leak and drainage of less than 400 mL/24 hours. Post-drain removal CXR revealed pneumothorax. Patient was discharged as remained asymptomatic; (C) at 6 weeks follow-up CXR revealed no pneumothorax. DDS, digital drain system; ICD, intercostal drain; CXR, chest X-rays.
In restrictive lungs re-expansion can only be achieved by suction. However, suction should be applied gradually starting from −5 cmH2O reaching −20 cmH2O over 24–48 hours. This is important to avoid lung tearing.
(II) Is there a value below which, the measurement is not classed as an air leak or is not clinically significant to keep a tube in the chest and a patient in Hospital? What is the most appropriate time to remove safely a drain?
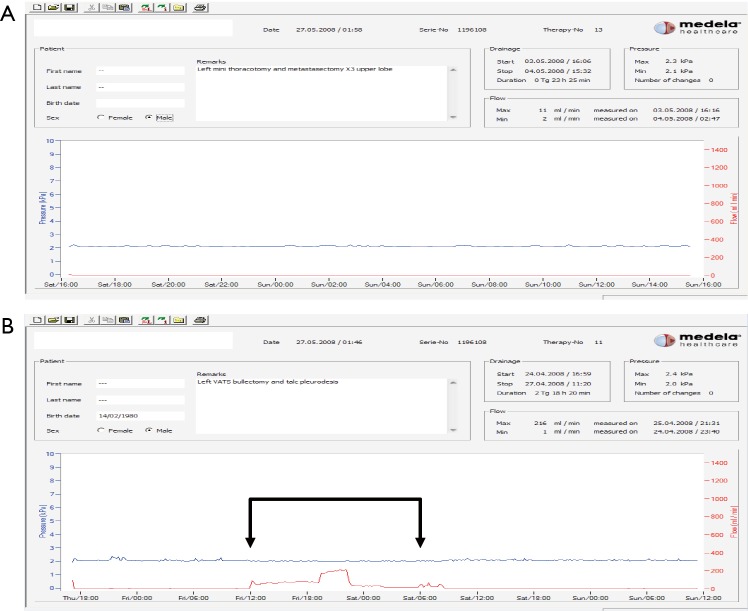
The amount of air leak in relation to suction determines when a chest tube can be removed. Based on our early observations we recommend an air leak/flow of less than 40 mL/min for at least 6 hours before a drain is removed. However, the decision should be confirmed by the presence of a ‘quiet’ graph recorded on the device with no significant oscillations (Figure 6).
Figure 6.
Examples of what happens to the pressures and air leak in relation to suction. (A) Straightforward case with no air leak; (B) footage from DDS showing initial absence of air leak. Then air leak was evident on the footage for 18 hours (marked with arrowed bracket). The use of the traditional underwater seal would have led to early removal of the drain with possible clinical implications. After 26 hours of continuous recording, no air-leak was identified and the drain was removed. Thus, decisions of chest drain management rely on footage and not a snapshot. DDS, digital drain system.
(III) What is the relevance of fluid swinging in the connecting tube in the absence of air leak recording?
This is a phenomenon witnessed by most Thoracic Surgeons. It is believed that the swinging in the tube, in the absence of air leak, is due to physiological fluctuations in the intrapleural pressure. Brunelli et al. used differential pleural pressure measurements in conjunction with the amount of air leak identified at the 6th post-operative hour to independently predict the risk of PAL (Table 1) (22).
Table 1. The independent role of differential pleural pressure and air-flow predicts the risk of prolonged air leak (PAL), defined as air leak longer than 7 days. Adapted from (22).
| Differential pleural pressure & flow | Risk of PAL |
|---|---|
| ΔP ≤10 mmHg & flow ≤50 mL/min | 4% |
| ΔP >10 mmHg & flow ≤50 mL/min | 15% |
| ΔP ≤10 mmHg & flow >50 mL/min | 36% |
| ΔP >10 mmHg & flow >50 mL/min | 52% |
Unfortunately the current technology does not allow prospective measurement of differential pleural pressures and these data can only be retrieved retrospectively after device disconnection making them clinically irrelevant in day to day practice.
However, we can confidently state that in subjects with good lung quality and in the absence of air leak (flat line) for at least 24 hours, the presence of a swing should not hinder chest tube removal. From our experience we have not witnessed tube re-insertion or re-admission with pneumothorax in this group of patients (Figure 7).
Figure 7.

Day 4 post lobectomy patient has a large swing as presented by the fluid column in the drain whilst on suction. No air leak is recorded on the DDS. ICD was removed with no re-insertion or post removal pneumothorax (23). DDS, digital drain system. ICD, intercostal drain. Available online: http://www.asvide.com/articles/809
In patients with severe COPD and evidence of hyperinflated, emphysematous lungs, the swing has to be interpreted cautiously. Removal of the chest drain while connected on a digital device could produce a sudden and large fluctuation of the intrapleural pressure leading to an air leak and a clinically significant pneumothorax for these patients who have minimal or absent respiratory reserves. We would therefore recommend a transition to Heimlich valve and 24-hour observation until chest drain removal.
(IV) What is the maximum amount of pleural fluid recorded that justifies removal of a chest tube and over what period?
Our group has reported that fluid drainage of equal or less than 400 mL/24 hour warrants safe chest tube removal (24). In a similar way, other investigators have recommended that fluid drainage as high as 500 mL per day is safe for drain removal (25). Nevertheless, patients with COPD, over 70 years undergoing lower lobectomies have an increased risk in developing large effusions on day 2 postoperatively. It is therefore advisable that in this group of patients a more conservative approach is adopted with a recommendation to remove the chest tube after the second postoperative day.
Although no robust data are yet available, we are confident that future decisions will not be based anymore on 24-hour collective drainage but rather a trend recorded on a graph. This will trim further chest tube duration and enhance fast track surgery attitudes around the globe.
Future directions
There is no doubt that postoperative chest tube management should be practiced in the name of good judgement. Many of the decisions taken by junior members are based on mentors’ recommendations and quite often are not evidence based. The adoption of digital devices in a modern practice allows data collection. These can be reviewed retrospectively and be utilised in correlation with clinical observations to allow safe and confident decisions for patient care. Moreover, such data will not only become part of patient’s medical record but can be potentially viewed on mobile devices with wireless technology and provide a future pre-programmed platform with individual settings for individual patients and thoracic pathology.
Conclusions
The introduction of the electronic chest drainage systems has a major contribution to the advancement of thoracic surgery as it has facilitated standardised management of chest tubes. There is ample evidence to confirm its cost effectiveness and safety. However, data interpretation has to be exercised with wisdom and in correlation with clinical observations. Every time the device is interrogated we need to go through a safety checklist to avoid potential complications and inconvenience. Furthermore, it is important though that the surgeon continues to exercise good judgement and always be reminded that technology should facilitate treatment and not influence it.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Mr. Papagiannopoulos have lectured for Medela but no funds or grants have been received for these activities. The other author has no conflicts of interest to declare.
References
- 1.Anegg U, Lindenmann J, Matzi V, et al. AIRFIX: the first digital postoperative chest tube airflowmetry - a novel method to quantify air leakage after lung resection. Eur J Cardiothorac Surg 2006;29:867-72. [DOI] [PubMed] [Google Scholar]
- 2.Dernevik L, Belboul A, Rådberg G. Initial experience with the world’s first digital drainage system. The benefits of recording air leaks with graphic representation. Eur J Cardiothorac Surg 2007;31:209-13. [DOI] [PubMed] [Google Scholar]
- 3.Varela G, Jiménez MF, Novoa NM, et al. Postoperative chest tube management: measuring air leak using an electronic device decreases variability in the clinical practice. Eur J Cardiothorac Surg 2009;35:28-31. [DOI] [PubMed] [Google Scholar]
- 4.Cerfolio RJ, Bryant AS. The benefits of continuous and digital air leak assessment after elective pulmonary resection: a prospective study. Ann Thorac Surg 2008;86:396-401. [DOI] [PubMed] [Google Scholar]
- 5.Bertolaccini L, Rizzardi G, Filice MJ, et al. ‘Six sigma approach’ - an objective strategy in digital assessment of postoperative air leaks: a prospective randomised study. Eur J Cardiothorac Surg 2011;39:e128-32. [DOI] [PubMed] [Google Scholar]
- 6.Filosso PL, Ruffini E, Solidoro P, et al. Digital air leak monitoring after lobectomy for primary lung cancer in patients with moderate COPD: can a fast-tracking algorithm reduce postoperative costs and complications? J Cardiovasc Surg (Torino) 2010;51:429-33. [PubMed] [Google Scholar]
- 7.Brunelli A, Salati M, Refai M, et al. Evaluation of a new chest tube removal protocol using digital air leak monitoring after lobectomy: a prospective randomised trials. Eur J Cardiothorac Surg 2010;37:56-60. [DOI] [PubMed] [Google Scholar]
- 8.Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. [DOI] [PubMed] [Google Scholar]
- 9.Rathinam S, Bradley A, Cantlin T, et al. Thopaz Portable Suction Systems in Thoracic Surgery: an end user assessment and feedback in a tertiary unit. J Cardiothorac Surg 2011;6:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jablonski S, Brocki M, Wawrzycki M, et al. Efficacy assessment of the drainage with permanent airflow measurement in the treatment of pneumothorax with air leak. Thorac Cardiovasc Surg 2014;62:509-15. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins WS, Hall DP, Dhaliwal K, et al. The use of a portable digital thoracic suction Thopaz drainage system for the management of a persistent spontaneous secondary pneumothorax in a patient with underlying interstitial lung disease. BMJ Case Rep 2012;2012. pii: bcr0220125881. [DOI] [PMC free article] [PubMed]
- 12.Southey D, Pullinger D, Loggos D, et al. Discharge of thoracic patients on portable digital suction: Is it cost-effective? Asian Cardiovasc Thorac Ann 2015:23:832-8. [DOI] [PubMed] [Google Scholar]
- 13.Pompili C, Brunelli A, Salati M, et al. Impact of the learning curve in the use of a novel electronic chest drainage system after pulmonary lobectomy: a case-matched analysis on the duration of chest tube usage. Interact Cardiovasc Thorac Surg 2011;13:490-3; discussion 493. [DOI] [PubMed] [Google Scholar]
- 14.Varela G, Brunelli A, Jiménez MF, et al. Chest drainage suction decreases differential pleural pressure after upper lobectomy and has no effect after lower lobectomy. Eur J Cardiothorac Surg 2010;37:531-4. [DOI] [PubMed] [Google Scholar]
- 15.Brunelli A, Monteverde M, Borri A, et al. Comparison of water seal and suction after pulmonary lobectomy: a prospective, randomized trial. Ann Thorac Surg 2004;77:1932-7; discussion 1937. [DOI] [PubMed]
- 16.Alphonso N, Tan C, Utley M, et al. A prospective randomized controlled trial of suction versus non-suction to the under-water seal drains following lung resection. Eur J Cardiothorac Surg 2005;27:391-4. [DOI] [PubMed] [Google Scholar]
- 17.Brunelli A, Sabbatini A, Xiume’ F, et al. Alternate suction reduces prolonged air leak after pulmonary lobectomy: a randomized comparison versus water seal. Ann Thorac Surg 2005;80:1052-5. [DOI] [PubMed] [Google Scholar]
- 18.Cerfolio RJ, Bass C, Katholi CR. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg 2001;71:1613-7. [DOI] [PubMed] [Google Scholar]
- 19.Marshall MB, Deeb ME, Bleier JI, et al. Suction vs water seal after pulmonary resection: a randomized prospective study. Chest 2002;121:831-5. [DOI] [PubMed] [Google Scholar]
- 20.Bertolaccini L, Rizzardi G, Terzi A. A golden key can open any door of new protocol: the use of continuous digital measurement for postoperative air leak. Interact Cardiovasc Thorac Surg 2011;12:31. [DOI] [PubMed] [Google Scholar]
- 21.Williams S, Williams J, Tcherveniakov P, et al. Impact of a thoracic nurse-led chest drain clinic on patient satisfaction. Interact Cardiovasc Thorac Surg 2012;14:729-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunelli A, Cassivi SD, Salati M, et al. Digital measurements of air leak flow and intrapleural pressures in the immediate postoperative period predict risk of prolonged air leak after pulmonary lobectomy. Eur J Cardiothorac Surg 2011;39:584-8. [DOI] [PubMed] [Google Scholar]
- 23.George RS, Papagiannopoulos K. Day 4 post lobectomy patient has a large swing as presented by the fluid column in the drain whilst on suction. No air leak is recorded on the DDS. ICD was removed with no re-insertion or post removal pneumothorax. Asvide 2016;3:058. Available online: http://www.asvide.com/articles/809
- 24.Hristova R, Pompili C, Begum S, et al. An aggregate score to predict the risk of large pleural effusion after pulmonary lobectomy†. Eur J Cardiothorac Surg 2015;48:72-6. [DOI] [PubMed] [Google Scholar]
- 25.Bjerregaard LS, Jensen K, Petersen RH, et al. Early chest tube removal after video-assisted thoracic surgery lobectomy with serous fluid production up to 500 ml/day. Eur J Cardiothorac Surg 2014;45:241-6. [DOI] [PubMed] [Google Scholar]