Abstract
The paradigm for postoperative care for thoracic surgical patients in the United States has shifted with efforts to reduce hospital length of stay and improve quality of life. The increasing usage of minimally invasive techniques in thoracic surgery has been an important part of this. In this review we will examine our standard practices as well as the evidence behind both general contemporary postoperative care principles and those specific to certain operations.
Keywords: Thoracic surgery, postoperative care, fast track, protocol
Introduction
The paradigm for postoperative care for thoracic surgical patients in the United States has shifted with efforts to reduce hospital length of stay and improve quality of life. The increasing usage of minimally invasive techniques in thoracic surgery has been an important part of this. In this review we will examine our standard practices as well as the evidence behind both general contemporary postoperative care principles and those specific to certain operations.
General principles
There are multiple goals of postoperative care for the patient, which can be viewed from the perspective of both the patient and the hospital/health care delivery system. If viewed broadly, these goals can include:
Minimize recovery time;
Decrease the physical impact of the operation on the patient;
Minimize complications from the operation;
Evaluate/treat complications that do occur.
Concepts such as “recovery time” can encompass a number of different metrics, such as length of postoperative hospital stay, return to normal activities of daily living, and return to work. The goals of postoperative care can conflict with each other. For instance, decreasing the length of hospital stay could theoretically come at the cost of increased rates of readmission or emergency room visits.
Perioperative measures
The postoperative care of patients truly begins preoperatively, as interventions delivered before and during surgery can impact the postoperative morbidity experienced by patients.
Pulmonary rehabilitation
Enrollment in a pulmonary rehabilitation program preoperatively consisting of breathing exercises/training, working on effective coughing, and developing muscle strength and stamina can be beneficial in patients with borderline respiratory function for lung resection. Patients with non-small cell lung cancer (NSCLC) who underwent a preoperative pulmonary rehabilitation program for 6 days a week for 4 weeks showed significant increases in PaO2 (60±10 vs. 82±12 mmHg, P=0.02), VO2max (12.9±1.8 vs. 19.2±2.1 mL/kg/min, P=0.0001) and forced expiratory volume in 1 second (FEV1) (1.14±0.7 vs. 1.65±0.8 L, P=0.02) (1). These improvements can translate into decreased length of stay, fewer days with a chest tube, and lower rates of prolonged chest tube drainage (2). Other studies have corroborated the beneficial effects of pulmonary rehabilitation in patients undergoing lung resection (3).
Preoperative pulmonary rehabilitation has also shown to be useful for decreasing respiratory complications in patients undergoing esophagectomy (4). Interestingly, these studies evaluated all candidates for esophagectomy, not only those with compromised pulmonary function (5). We refer patients for pulmonary rehabilitation who have limited exercise capacity and/or borderline lung function for resection.
Smoking cessation
Preoperative smoking cessation has been shown to decrease overall complication rates in a wide variety of surgeries (6). The impact of smoking cessation of pulmonary complications after thoracic surgery is less well defined. Certain studies have shown benefit while others have not (7,8). Although non-smokers have decreased pulmonary complications compared to smokers, the risk of the latter is mitigated slowly by smoking cessation (9). However, for lung cancer specifically, smoking cessation is associated with decreased risk of all-cause mortality and recurrence, as well as development of a second primary tumor (10). Furthermore, the longer a patient has quit smoking preoperatively, the more likely they are to remain nonsmoking postoperatively (11). For these reasons, we recommend that all patients be counseled on smoking cessation prior to resection. In general we defer on operating on patients who have not made meaningful progress in terms of smoking cessation preoperatively.
Intraoperative measures
Attention to intraoperative details can lead to increased efficiency and decreased resource utilization combined with better postoperative outcomes.
Monitoring devices
The use of extraneous invasive monitoring devices adds time, cost, and the potential for complications to the process of thoracic surgery (12). We have eliminated the usage of arterial lines in the majority of our cases, reserving them for situations in which we expect significant hemodynamic derangement or otherwise have a specific indication for them (e.g., severe pulmonary hypertension or cardiac dysfunction). We have similarly reduced our use of central venous catheters. We reserve Foley catheter use for patient who receive epidural catheters and/or are expected to be under anesthesia for 4 or more hours. We have found that our perioperative morbidity and mortality has not increased in spite of using fewer monitoring devices on increasingly complex and older patients (unpublished data). We routinely use noninvasive blood pressure monitoring, continuous ECG monitoring, pulse oximetry, and capnography.
Analgesia
We generally omit the use of epidural catheters in patients. Epidural catheter use can be associated with increased time, urinary retention, hypotension, and fluid administration. Increasing evidence supports the usage of paravertebral blockade as an alternative to epidurals. One meta-analysis of 18 trials showed that paravertebral blockade was equivalent to epidural analgesia in terms of early (48 hours and less) pain scores and narcotic usage, and better than epidural analgesia in terms of side effects such as urinary retention, nausea/vomiting, hypotension, and failed block (13). Liposomal bupivacaine, which is a formulation of bupivacaine that allows the continuous release of the anesthetic from liposomal vesicles over a 96-hour period, has also shown to be comparable in terms of efficacy to epidural analgesia during thoracic surgery (14). We generally perform a bupivacaine intercostal nerve block, which is similar to paravertebral nerve block (although it does not allow for continuous infusion postoperatively) on patients undergoing both open and minimally invasive thoracic operations. We also give pre-emptive local analgesia at the site of incisions prior to incision.
Airway management
We generally favor double lumen endotracheal tubes over single lumen tubes for airway management during thoracic operations. The placement of a double lumen tube allows for selective ventilation of the non-operative lung and we believe are more stable than using bronchial blockers, which are also more difficult to reposition once dislodged. However in patients with small tracheas or difficult airways, thoracoscopy can be done with the use of a single lumen tube with a bronchial blocker, or if a blocker cannot be reliably placed, intermittent periods of apnea (15). This can be especially useful in short cases such as pleural effusion drainage, pleural biopsy, and pleurodesis. However, for performance of anatomic lung resections, esophagectomy, decortications, and more prolonged cases, selective lung ventilation is critical. An easy, reproducible method to ensure efficient, timely placement of the double lumen endotracheal tube is described. The left-sided double lumen endotracheal tube of the appropriate size is used to intubate the trachea. A pediatric fiberoptic bronchoscope is placed through the bronchial lumen of the tube and driven down into the left lower lobe bronchus. The endotracheal tube is then slid over the bronchoscope, which facilitates passage of the bronchial lumen of the tube into the left lower lobe bronchus. The bronchoscope is then reinserted via the tracheal lumen and correct positioning of the tube is confirmed.
Positioning
Decreasing the time from entry to the operating room to incision decreases room utilization and costs, not to mention expediting the performance of cases by the surgeon during the day. Streamlining the process of positioning the patient is part of this efficiency. After proper placement of the double lumen tube is confirmed, the patient is turned on their side. Neither a bean bag or axillary roll is used, which minimizes equipment that needs to be procured before/during the case. The bed is then flexed, and the patients’ arms are folded in front of them and positioned on the bed. An arm board is not used. Foam padding and blankets are used for the arms and head, and pillows are used between the legs, to ensure that the vulnerable spots on the patients’ extremities are protected and nerve injury avoided. The patient is secured in the lateral decubitus position with straps over the thighs and tape holding the hip and arms/head in place. A lower body warming blanket is placed. Tube positioning is rechecked and patient tolerance for single lung ventilation is confirmed before draping. If a robotic case is being performed, monitoring lines and the ventilation tubing are consolidated into a single bundle with the assistance of towels and tape to avoid interfering with surgeon and assistant movement around the patient, and the bed is turned in the room as needed for docking of the robot. Photographs depicting patient positioning are shown in Figures 1,2,3.
Figure 1.
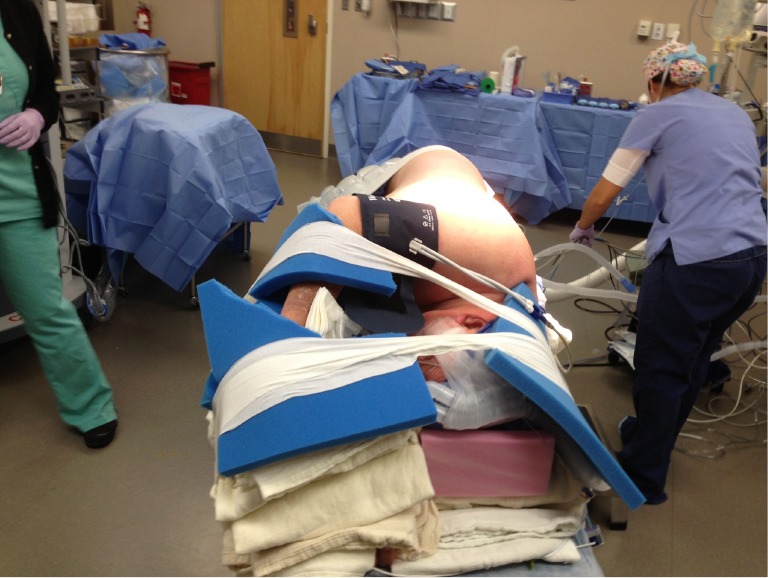
Lateral decubitus patient positioning.
Figure 2.
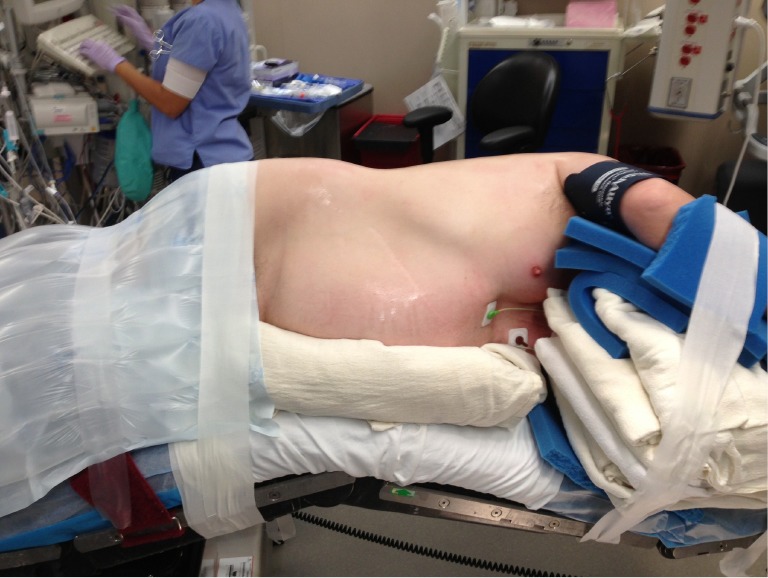
Anterior view of lateral decubitus positioning.
Figure 3.
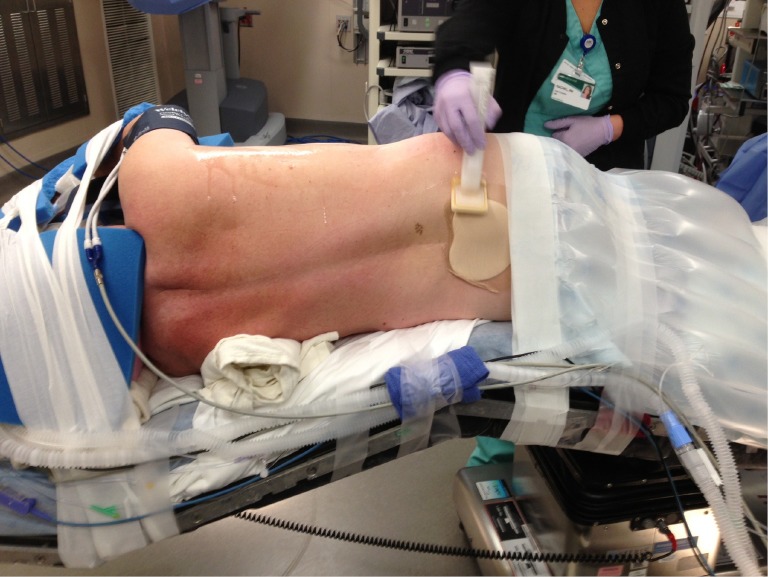
Posterior view of lateral decubitus positioning.
Fluid/blood administration
A higher rate of fluid administration during thoracic surgery has been associated with an increased risk of acute lung injury and acute respiratory distress syndrome (ARDS) (16,17). An increased amount of fluid given intraoperatively (more than 6 mL/kg/hour) has been linked with an increase in cumulative postoperative pulmonary complications (defined as ARDS, need for intubation or bronchoscopy, prolonged air leak, atelectasis, pneumonia, and failure of lung to expand) after anatomic lung resection (18). Intraoperative fluid balance was also associated with an increased risk of postoperative acute exacerbation of idiopathic pulmonary fibrosis in patients with the disease undergoing anatomic lung resection (19). We collaborate with our anesthesiologists to consciously restrict the amount of fluid given during operations.
Ventilation strategies
Hyperventilation of the non-operative lung during thoracic surgery can decrease the amount of working space on the operative side. Furthermore, one randomized trial has shown that a protective lung ventilation strategy (FiO2 of 0.5, tidal volume of 6 mL/kg, positive end-expiratory pressure of 5 cmH2O, and pressure control ventilation) decreased pulmonary complications after lung cancer resection compared to conventional ventilation [FiO2 1.0, tidal volume 10 mL/kg, positive end expiratory pressure (PEEP) of zero, and volume control ventilation] (20). Shorter duration of single-lung ventilation also seems to be beneficial in terms of reducing the rise in inflammatory mediators once the patient is returned to dual lung ventilation (21). Given the evidence that preoperative chemotherapy is associated with impairments in lung function and postoperative pulmonary complications, attention to oxygen concentration during subsequent lung surgery is important especially in these patients (22,23). We advocate minimizing the amount of FiO2 and tidal volume utilized during single-lung ventilation for thoracic operations.
Postoperative measures
Fast-track protocols
The consistent management of postoperative care after pulmonary resection has been shown to reduce cost and length of stay (24,25). We emphasize avoidance of the intensive care unit (ICU), use of oral pain medications rather than intravenous pain medications or epidural catheters, early ambulation, minimizing postoperative intravenous fluids, and early feeding (jejunostomy tube feeding in esophagectomy patients on postoperative day 1). Our standard practice has translated to a median hospital length of stay of 2 days for robotic pulmonary lobectomy and 7 days for both robotic and open esophagectomy (26-28). In spite of our protocols, risk factors such as increased age (>70 years), high body mass index (BMI >35 kg/m2), impaired FEV1 (<45%) and smoking history have been associated with increased likelihood of failure to “fast-track,” or to have prolonged length of stay and/or complications (29). Specific interventions, such as avoiding epidural usage in patients >70 years and increasing assistance for ambulation and respiratory treatments for patients with obesity and poor pulmonary function, decreased the rate of morbidity in these populations (29).
Pain control
We employ multimodality therapy to optimize pain relief after thoracic operations. In addition to narcotics, we utilize lidocaine transdermal patches, acetaminophen, and ibuprofen for patients. In general patients who undergo minimally invasive thoracic surgery with video-assisted thoracoscopic surgery (VATS), laparoscopy, or robotic assistance do not require patient-controlled intravenous narcotic analgesia (PCA). Occasionally patients who are either tolerant to narcotics or otherwise have poorly controlled pain require PCA usage. Breakthrough pain medication is made available for patients not on a PCA.
Postoperative care: lung resection
Chest tube management
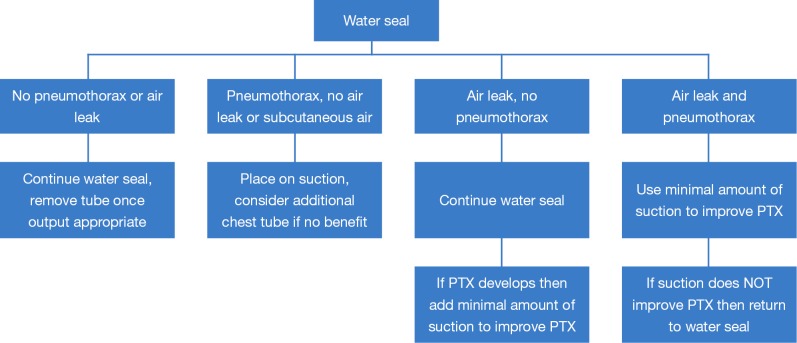
The early removal of chest tubes, beyond facilitating earlier discharge, has been associated with more rapid recovery of parameters such 6-minute walk distance (30). Furthermore, chest tube removal at volumes of up to 500 mL of drainage per day has been shown to be safe (31). We generally remove chest tubes when daily output is less than this and no air leak is present, and have had only a 0.55% readmission rate for symptomatic recurrent effusion (32). The use of suction (as opposed to water seal) appears to prolong the duration of air leak (33,34). At our institution, patients are placed on water seal by postoperative day 1 following lung resection. Our algorithm for chest tube management in patients undergoing lung resection is shown in Figure 4. Those with significant pneumothoraces when placed on water seal may require the temporary use of suction. Pleurodesis with either talc or doxycycline is considered if a patient has a persistent air leak at 5–7 days (35). If the air leak persists, however, the chest tube can still eventually be removed; we have shown that 100% of patient discharged with an air leak after lung resection can have their chest tubes safely removed at a median of 16.5 days after an operation, even if a pneumothorax and/or air leak is still present (36). Patients who are discharged home with a chest tube are kept on oral antibiotics until removal. In terms of method of removal, chest tubes that are removed on end expiration had fewer pneumothoraces after removal than those removed at full inspiration (37). This is our preferred technique.
Figure 4.
Algorithm for management of air leaks following pulmonary lobectomy. If air leak is greater than 750 mL/min on Thopaz on POD#2 then prepare for outpatient Thopaz usage. Place on Thopaz then discharge home. Remove chest tube when leak <20 mL/min for 2 days. After 3 weeks, can remove chest tube even if air leak persists if pneumothorax does not increase and no subcutaneous air is present (can perform clamp trial if desired). PTX, pneumothorax; POD, postoperative day.
Digital air leak devices
The use of digital air leak devices allows for the quantification of air leaks present and the discharge of patients who require some degree of suction to maintain lung inflation. They have been shown to decrease the time to chest tube removal and duration of hospital length of stay (38,39). We generally convert patients with an air leak from standard chest tube drainage device to Thopaz digital thoracic drainage system (Medela Healthcare U.S.; McHenry, IL, USA) on postoperative day 1 if a significant air leak is present on exam. Patients can be safely discharged home on the device, with few device-related malfunctions and/or readmissions (unpublished data).
Postoperative care: esophagectomy
Nasogastric (NG) tube
We do not use NG tubes routinely after esophagogastrectomy. Although evidence exists that NG tube decompression with a “sump” type tube decreases tracheal acid exposure and respiratory complications after esophagectomy, this data was derived from patients undergoing esophagectomy via a left thoracoabdominal approach, which is a fairly morbid incision (40). Furthermore, the presence of a NG tube following esophagectomy does not appear to decrease the risk of anastomotic leak, or the incidence of nausea, vomiting, and/or distention after esophagectomy (41). We believe that the disadvantages of NG tubes including discomfort and decreased mobility outweigh any perceived benefit in the typical patient.
Fast track
Patients undergoing esophagectomy at our hospital generally go to the post-anesthesia recovery room and then to a “step-down” floor with continuous telemetry following the operation. Tube feeding via jejunostomy tube placed at the time of the operation is initiated on postoperative day 1. Nutritional support is quickly increased over the next 24–48 hours as long as the patient does not develop significant distention or ileus. The chest tube is removed once nutritional support is at goal and no chylothorax or obvious clinical leak is demonstrated. A clinical speech evaluation is generally performed on postoperative day 4 or 5, followed by a barium swallow if there is no bedside evidence of aspiration on the speech evaluation. Patients are discharged home on full liquids or a soft diet and full nutritional support at a median of 8 days after the robotic-assisted Ivor Lewis esophagectomy (manuscript in press). The “fast-track” protocol for patients undergoing esophagectomy at our institution is shown in Table 1 (28).
Table 1. Fast-track protocol for patients undergoing robotic Ivor Lewis esophagectomy.
| Day of surgery |
| Patient sent to floor |
| Nasogastric tube placed to suction |
| Monitor chest tube output and urinary outputs q4 h |
| Continuous monitoring of heart rhythm and pulse oximeter |
| POD1 |
| Jejunal tube feedings at full concentration, starting at 20 mL/h |
| Ambulate patient four times per day; physical therapy every day |
| Chest tube to suction |
| Strict aspiration precautions; head of bed at 30°; nasogastric tube suction |
| POD2 |
| Continue to ambulate patient a minimum of four times per day until discharge |
| Increase rate of jejunal tube feedings, 10 mL/4 h |
| Consult nutritional therapist |
| Continue aspiration precautions; physical therapy |
| Remove Foley catheter |
| POD3 |
| Increase rate of jejunal tube feedings until achieving goal rate |
| Remove chest tube if no chyle and drainage <450 mL/d |
| Consult speech pathologist |
| Continue aspiration precautions; physical therapy |
| POD4 |
| Gastrografin swallow (or on POD5 if POD4 is a Sunday) |
| Continue jejunal tube feeds |
| If swallow shows no leak, advance patient to a full liquid diet and skip clear liquids |
| Continue aspiration precautions; patient warned not to eat while drowsy or to lie recumbent within 3 h of eating |
| Continue physical therapy |
| Education on chewing and swallowing |
| POD5 |
| Advance to soft diet as tolerated |
| Start compressing jejunal feedings by increasing rate and turning off for 4 h/d |
| Continue aspiration precautions; physical therapy |
| POD6 |
| Nutrition education provided by dietician |
| Set up home jejunal tube feedings |
| Start to compress jejunal tube feedings 7 pm to 7 am |
| Continue aspiration precautions; physical therapy |
| POD7 |
| Discharge home on soft diet with continued aspiration precautions and compressed tube feedings at night only |
POD, postoperative day.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Dr. Cerfolio disclosures: Intuitive Surgical, Ethicon, Covidien, Bovie, CSAT, KCI, Community Health Systems, Myriad.
References
- 1.Divisi D, Di Francesco C, Di Leonardo G, et al. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg 2013;43:293-6. [DOI] [PubMed] [Google Scholar]
- 2.Benzo R, Wigle D, Novotny P, et al. Preoperative pulmonary rehabilitation before lung cancer resection: results from two randomized studies. Lung Cancer 2011;74:441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagarajan K, Bennett A, Agostini P, et al. Is preoperative physiotherapy/pulmonary rehabilitation beneficial in lung resection patients? Interact Cardiovasc Thorac Surg 2011;13:300-2. [DOI] [PubMed] [Google Scholar]
- 4.van Adrichem EJ, Meulenbroek RL, Plukker JT, et al. Comparison of two preoperative inspiratory muscle training programs to prevent pulmonary complications in patients undergoing esophagectomy: a randomized controlled pilot study. Ann Surg Oncol 2014;21:2353-60. [DOI] [PubMed] [Google Scholar]
- 5.Yamana I, Takeno S, Hashimoto T, et al. Randomized Controlled Study to Evaluate the Efficacy of a Preoperative Respiratory Rehabilitation Program to Prevent Postoperative Pulmonary Complications after Esophagectomy. Dig Surg 2015;32:331-7. [DOI] [PubMed] [Google Scholar]
- 6.Lindström D, Sadr Azodi O, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg 2008;248:739-45. [DOI] [PubMed] [Google Scholar]
- 7.Barrera R, Shi W, Amar D, et al. Smoking and timing of cessation: impact on pulmonary complications after thoracotomy. Chest 2005;127:1977-83. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa M, Tanaka H, Tsukuma H, et al. Relationship between the duration of the preoperative smoke-free period and the incidence of postoperative pulmonary complications after pulmonary surgery. Chest 2001;120:705-10. [DOI] [PubMed] [Google Scholar]
- 9.Mason DP, Subramanian S, Nowicki ER, et al. Impact of smoking cessation before resection of lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database study. Ann Thorac Surg 2009;88:362-70; discussion 370-1. [DOI] [PubMed] [Google Scholar]
- 10.Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ 2010;340:b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dresler CM, Bailey M, Roper CR, et al. Smoking cessation and lung cancer resection. Chest 1996;110:1199-202. [DOI] [PubMed] [Google Scholar]
- 12.Brodsky JB. What intraoperative monitoring makes sense? Chest 1999;115:101S-105S. [DOI] [PubMed] [Google Scholar]
- 13.Ding X, Jin S, Niu X, et al. A comparison of the analgesia efficacy and side effects of paravertebral compared with epidural blockade for thoracotomy: an updated meta-analysis. PLoS One 2014;9:e96233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice DC, Cata JP, Mena GE, et al. Posterior Intercostal Nerve Block With Liposomal Bupivacaine: An Alternative to Thoracic Epidural Analgesia. Ann Thorac Surg 2015;99:1953-60. [DOI] [PubMed] [Google Scholar]
- 15.Cerfolio RJ, Bryant AS, Sheils TM, et al. Video-assisted thoracoscopic surgery using single-lumen endotracheal tube anesthesia. Chest 2004;126:281-5. [DOI] [PubMed] [Google Scholar]
- 16.Alam N, Park BJ, Wilton A, et al. Incidence and risk factors for lung injury after lung cancer resection. Ann Thorac Surg 2007;84:1085-91; discussion 1091. [DOI] [PubMed] [Google Scholar]
- 17.Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003;97:1558-65. [DOI] [PubMed] [Google Scholar]
- 18.Arslantas MK, Kara HV, Tuncer BB, et al. Effect of the amount of intraoperative fluid administration on postoperative pulmonary complications following anatomic lung resections. J Thorac Cardiovasc Surg 2015;149:314-20, 321.e1. [DOI] [PubMed]
- 19.Mizuno Y, Iwata H, Shirahashi K, et al. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2012;41:e161-5. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139:530-7. [DOI] [PubMed] [Google Scholar]
- 21.García-de-la-Asunción J, García-del-Olmo E, Perez-Griera J, et al. Oxidative lung injury correlates with one-lung ventilation time during pulmonary lobectomy: a study of exhaled breath condensate and blood. Eur J Cardiothorac Surg 2015;48:e37-44. [DOI] [PubMed] [Google Scholar]
- 22.Takeda S, Funakoshi Y, Kadota Y, et al. Fall in diffusing capacity associated with induction therapy for lung cancer: a predictor of postoperative complication? Ann Thorac Surg 2006;82:232-6. [DOI] [PubMed] [Google Scholar]
- 23.Bonomi P, Faber LP, Warren W, et al. Postoperative bronchopulmonary complications in stage III lung cancer patients treated with preoperative paclitaxel-containing chemotherapy and concurrent radiation. Semin Oncol 1997;24:S12-123-S12-129. [PubMed]
- 24.Cerfolio RJ, Pickens A, Bass C, et al. Fast-tracking pulmonary resections. J Thorac Cardiovasc Surg 2001;122:318-24. [DOI] [PubMed] [Google Scholar]
- 25.Wright CD, Wain JC, Grillo HC, et al. Pulmonary lobectomy patient care pathway: a model to control cost and maintain quality. Ann Thorac Surg 1997;64:299-302. [DOI] [PubMed] [Google Scholar]
- 26.Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [DOI] [PubMed] [Google Scholar]
- 27.Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [DOI] [PubMed] [Google Scholar]
- 28.Cerfolio RJ, Bryant AS, Bass CS, et al. Fast tracking after Ivor Lewis esophagogastrectomy. Chest 2004;126:1187-94. [DOI] [PubMed] [Google Scholar]
- 29.Bryant AS, Cerfolio RJ. The analysis of a prospective surgical database improves postoperative fast-tracking algorithms after pulmonary resection. J Thorac Cardiovasc Surg 2009;137:1173-9. [DOI] [PubMed] [Google Scholar]
- 30.Nomori H, Horio H, Suemasu K. Early removal of chest drainage tubes and oxygen support after a lobectomy for lung cancer facilitates earlier recovery of the 6-minute walking distance. Surg Today 2001;31:395-9. [DOI] [PubMed] [Google Scholar]
- 31.Bjerregaard LS, Jensen K, Petersen RH, et al. Early chest tube removal after video-assisted thoracic surgery lobectomy with serous fluid production up to 500 ml/day. Eur J Cardiothorac Surg 2014;45:241-6. [DOI] [PubMed] [Google Scholar]
- 32.Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg 2008;135:269-73. [DOI] [PubMed] [Google Scholar]
- 33.Cerfolio RJ, Bass C, Katholi CR. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg 2001;71:1613-7. [DOI] [PubMed] [Google Scholar]
- 34.Marshall MB, Deeb ME, Bleier JI, et al. Suction vs water seal after pulmonary resection: a randomized prospective study. Chest 2002;121:831-5. [DOI] [PubMed] [Google Scholar]
- 35.Cerfolio RJ, Tummala RP, Holman WL, et al. A prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg 1998;66:1726-31. [DOI] [PubMed] [Google Scholar]
- 36.Cerfolio RJ, Minnich DJ, Bryant AS. The removal of chest tubes despite an air leak or a pneumothorax. Ann Thorac Surg 2009;87:1690-4; discussion 1694-6. [DOI] [PubMed]
- 37.Cerfolio RJ, Bryant AS, Skylizard L, et al. Optimal technique for the removal of chest tubes after pulmonary resection. J Thorac Cardiovasc Surg 2013;145:1535-9. [DOI] [PubMed] [Google Scholar]
- 38.Cerfolio RJ, Bryant AS. The benefits of continuous and digital air leak assessment after elective pulmonary resection: a prospective study. Ann Thorac Surg 2008;86:396-401. [DOI] [PubMed] [Google Scholar]
- 39.Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. [DOI] [PubMed] [Google Scholar]
- 40.Shackcloth MJ, McCarron E, Kendall J, et al. Randomized clinical trial to determine the effect of nasogastric drainage on tracheal acid aspiration following oesophagectomy. Br J Surg 2006;93:547-52. [DOI] [PubMed] [Google Scholar]
- 41.Daryaei P, Vaghef Davari F, Mir M, et al. Omission of nasogastric tube application in postoperative care of esophagectomy. World J Surg 2009;33:773-7. [DOI] [PubMed] [Google Scholar]