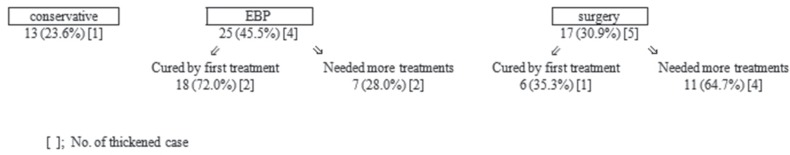Abstract
Spontaneous intracranial hypotension (SIH) has increasingly been recognized, and it is well known that SIH is sometimes complicated by chronic subdural hematoma (SDH). In this study, 55 cases of SIH with SDH were retrospectively analyzed, focusing on therapeutic strategies and outcomes. Of 169 SIH cases (75 males, 84 females), 55 (36 males, 19 females) were complicated by SDH. SIH was diagnosed based on clinical symptoms, neuroimaging, and/or low cerebrospinal fluid pressure. Presence of orthostatic headache and diffuse meningeal enhancement on magnetic resonance imaging were regarded as the most important criteria. Among 55 SIH with SDH cases, 13 improved with conservative treatment, 25 initially received an epidural blood patch (EBP), and 17 initially underwent irrigation of the hematomas. Of the 25 initially treated with EBP, 7 (28.0%) needed SDH surgery and 18 (72.0%) recovered fully without surgery. Of 17 SDH cases initially treated with surgery, 6 (35.7%) required no EBP therapy and the other 11 (64.3%) needed EBP and/or additional SDH operations. In the latter group, 2 cases had transient severe complications during and after the procedures. One of these 2 cases developed a hoarse voice complication. Despite this single, non-severe complication, all enrolled in this study achieved good outcomes. The present study suggests that patients initially receiving SDH surgery may need additional treatments and may occasionally have complications. If conservative treatment is insufficient, EBP should be performed prior to hematoma irrigation.
Keywords: spontaneous intracranial hypotension, chronic subdural hematoma, epidural blood patch
Introduction
The syndrome of intracranial hypotension as a result of cerebrospinal fluid (CSF) leakage from a spinal dural breach into surrounding tissue was reported by Leriche in 1920.1) In the 1990s, spontaneous intracranial hypotension (SIH) gradually gained recognition, particularly after Mokri reported on pachymeningeal gadolinium (Gd) enhancement accompanying low intracranial pressure headaches.2)
It is well known that SIH sometimes is associated with chronic subdural hematoma (SDH); the relationship between SIH and SDH started to be recognized in the 1950s.3,4) Many reports of SDH associated with SIH describe the condition as usually benign and having a good outcome.5,6) In contrast, there are several reports of transitional complications,7–16) morbid outcomes,7 ,8,17,18 ) and even death.7,19,20) Therefore, several reports warned that SIH with SDH is a potentially life-threating condition.7, 8,13 ,14,16 ,18,19 ,21)
Here we report a retrospective analysis of 55 cases of SIH with SDH, focusing on therapeutic strategies and outcomes compared to 114 cases of SIH without SDH. Of the cases enrolled, 2 experienced transient severe complications during and after treatment.
Materials and Methods
From 2004 to 2012, a total of 169 cases (75 males, 84 females) were diagnosed as SIH. SIH was diagnosed when all the following conditions described below were present, based on ICHD-322) and the diagnostic criteria reported by Schievink et al.23)
Evidence of CSF leakage on imaging as cranial MRI changes of intracranial hypotension (e.g., pachymeningeal enhancement) and/or low CSF pressure (≤ 60 mmH2O)
No recent history of dural puncture
Not attributable to another disorder
A total of 55 enrolled cases were complicated by SDH. The location and size of the SDH were determined by computed tomography (CT) scan and/or magnetic resonance imaging (MRI) as an iso- or high-intensity area in the subdural space on T1-weighted and fluid-attenuated inversion recovery (FLAIR) images.7,11,18) The volume of the hematoma was defined as “thick” when the maximum hematoma thickness was more than 15 mm.17)
Gd enhancement studies were also performed to check for diffuse meningeal enhancement.6,24–28) Thickened dura on T2-weighted image and/or FLAIR was another important finding for SIH diagnosis.5,29)
Radioisotope (RI) cisternography was performed for 24 cases of SIH with SDH by intrathecal lumbar injection of 1 ml (37 MBq at calibration time) of 111In (Nihon Medi-Physics Co. Ltd., Tokyo) under local anesthesia with 5–10 ml of 1% lidocaine using a disposable spinal needle (25G pencil point) (Unisis Corporation, Koshigaya, Saitama) or a spinal needle (23G or 25G) (Terumo Corporation, Tokyo) with the patient in the lateral decubitus position. Posteroanterior and anteroposterior whole-body planar scintigraphy was performed with a single-headed gamma camera (RC 2,500 IV; Hitachi Medical Corporation, Tokyo) at 1 h, 3 h, 5 h, and 24 h after injection.
The following findings of RI cisternography were regarded as significant: obvious CSF leaks (CLs) and early bladder filling (BF).5,6,21,24–26 ,30–34 )
Initial treatment consisted of bed rest and hydration. Some patients were administered pain killers, tranquilizers, and other compounds to enhance comfort.
When these conservative treatments were ineffective, an epidural blood patch (EBP) or surgery (irrigation of the hematoma) was performed.
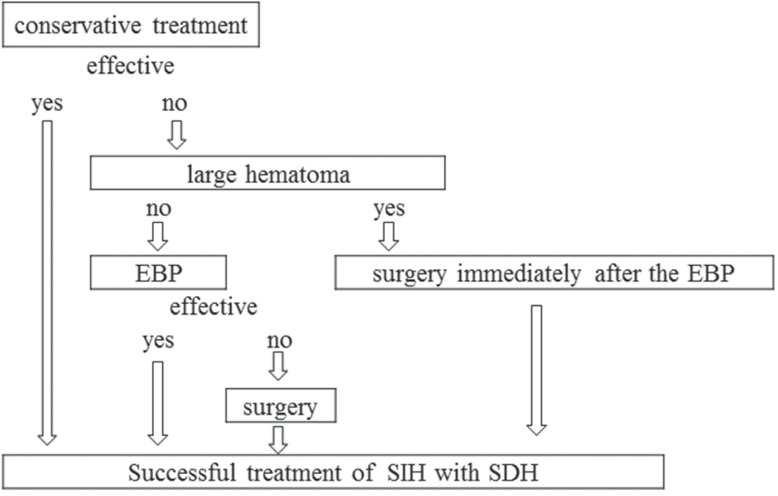
In total, 15 cases had already received and 40 cases did not receive SDH surgery, and none had undergone EBP before admission either here or at other hospitals. For 9 cases, we could have chosen EBP or SDH surgery for the first intervention. However, we did not have a definite treatment strategy until we experienced 2 severely troubling cases during and after SDH surgery in 2010 (see the section on Case Reports). Accordingly, we initiated the following therapeutic strategy: initial EBP and, if SDH is thick, SDH surgery is done immediately after EBP. As a result, we followed the treatment flowchart shown in Fig. 1 for our subsequent 23 cases.
Fig. 1.
Flowchart for management of SIH with SDH. EBP: epidural blood patch, SDH: subdural hematoma, SIH: spontaneous intracranial hypotension.
The EBP treatment consisted of autologous blood injected into the epidural space around the CL site, according to the RI cisternography results. If the CL was not clear, blood (about 30 ml for males and about 20 ml for females) was injected at the lumbar level in the prone position, usually under local anesthesia with 5–10 ml of 1% lidocaine. For EBP at the upper thoracic level, the volume of injected blood was ≤ 15 ml. This therapeutic strategy of performing serial EBP in patients was approved by the local ethics committee.31)
Outcomes were defined as follows: good recovery, for complete or almost complete recovery from symptoms, returning back to school or work (relief ≥ 75%); moderate recovery, for partial symptomatic recovery (relief ≥ 50%); or no change, for no apparent improvement (relief < 50%).31,35) Before conducting RI cisternography, EBP, or surgery, written informed consent was obtained from all patients and their parents, when appropriate.
Results
I. Age of the cases
Among 169 SIH cases seen at Sanno Hospital, 55 (32.5%: 36 males, 19 females) were diagnosed as SIH with SDH. Mean age at occurrence of SIH with SDH was 48.3 ± 11.2 years (range, 27–74 years).
SIH without SDH was diagnosed in 114 cases (67.5%: 39 males, 75 females); mean age at SIH occurrence was 38.0 ± 8.7 years (range, 22–57 years; Table 1).
Table 1.
Patient demographics and RI cisternography results
| SIH with SDH (n = 55) | SIH without SDH (n = 114) | |
|---|---|---|
| Male/female | 36/19 (65.5%) | 39/75 (34.2%) |
| Mean age (range) | 48.3 (24–74) | 38.0 (22–57) |
| CSF pressure | 6.6 (0–20.8), n = 23 | 5.4 (0–20.3), n = 30 |
| RI cisternography | n = 24 | n = 33 |
| CL | 22 (91.7%) | 27 (81.8%) |
| multiple | 14 | 16 |
| single | 8 | 11 |
| Cervical | 8 (22.9%) | 7 (15.6%) |
| Thoracic | 11 (31.4%) | 18 (40.0%) |
| Lumbar | 16 (45.7%) | 20 (40.4%) |
CL: CSF leak, CSF: cerebrospinal fluid, SDH: subdural hematoma, SIH: spontaneous intracranial hypotension.
II. Causes of SIH with SDH
A history of evident trauma was noted for 6 of the 55 SIH with SDH cases: 3 cases (1 of whom was also receiving warfarin treatment) had head injury a few months before onset; 2 cases had neck injury a few days before onset; and 1 case had received acupuncture therapy a day before onset. No connective tissue disorder was seen in the SIH with SDH cases, but 1 of the SIH without SDH cases was diagnosed with systemic lupus erythematosus (SLE).
III. Clinical symptoms
In total, 52 (94.5%) of SIH with SDH cases complained of typical orthostatic headache initially, and 2 cases suffered from non-postural headache as the initial symptom. Vertigo and/or hearing disturbance was present in 4 cases, and in 1 of these 4 cases, the initial symptom was hearing disturbance without an orthostatic headache. Only 1 case presented with dementia following orthostatic headache.
In SIH without SDH, 109 cases (95.6%) complained of typical orthostatic headache initially and 4 cases suffered from non-postural headache; 1 case complained of hearing disturbance without an orthostatic headache.
IV. MRI
MRI was performed in all 55 SIH with SDH cases. SDH was noted as a high-intensity area on T1-weighted image (T1WI) and FLAIR and slight low intensity on T2WI. A Gd-enhanced study was performed for 54 cases and all had diffuse meningeal enhancement. In the case for whom a Gd-enhanced study was not performed, FLAIR imaging revealed thickened dura. SDH was seen bilaterally in 48 cases (87.3%) and unilaterally in 7 (3 right, 4 left; 12.7%). A total of 10 cases (18.2%) were found to have a “thick” hematoma.
V. RI cisternography
RI cisternography was performed in 24 of the 55 cases of SIH with SDH. CL occurred in 22 cases (91.7%), and 14 of these 22 (63.6%) had multiple sites of CL. The most frequent site of CL was the lumbar level (16 cases; 45.7%) and the second most frequent site was the thoracic level (11 cases; 31.4%); 2 cases (8.3%) were categorized as early BF. Among the 114 cases of SIH without SDH, RI cisternography was performed in 33 cases. CL occurred in 27 cases (81.8%). In this group, 16 of 27 cases (59.3%) had multiple sites of CL. The most frequent site of CL was the lumbar level in 20 cases (44.4%), and the second most frequent site was the thoracic level in 18 cases (40.0%); 6 cases (18.2%) were categorized as early BF without CL findings (Table 1).
VI. Measurement of lumbar CSF pressure
Lumbar CSF pressure was measured in 22 of the 55 SIH with SDH cases in the lateral decubitus position. The mean lumbar CSF pressure was 6.6 ± 5.7 cmH2O (range, 0–20.3 cmH2O); 11 cases (47.8%) were at less than 6 cmH2O; and 1 case (4.3%) had high pressure of more than 20 cmH2O. In 30 of 114 SIH without SDH cases, the mean lumbar CSF pressure was 5.4 ± 5.8 cmH2O (range, 0–20.8 cmH2O); 17 cases (56.7%) were at less than 6 cmH2O; and 1 case (3.3%) had high lumbar CSF pressure (Table 1).
VII. Treatment and outcomes
Conservative therapy alone was effective for 13 cases (23.6%). Notably, 1 case had full recovery of symptoms although the hematoma volume was thick.
EBP was performed as the initial procedure in 25 cases, 18 (72.0%) were fully cured by the first EBP only, and 2 retained a thick hematoma. Consequently, 7 cases (28.0%) needed SDH surgeries (1 case needed two procedures), of whom 2 had a thick hematoma volume. The reasons for the necessity for SDH surgery were that the symptoms, such as headache, were severe and/or the SDH volume had increased.
Initial SDH surgery was performed in 17 cases. Among these, 2 were performed at our hospital while 15 cases were performed at other hospitals before being transferred to our hospital. Among 17 cases, SIH was diagnosed before and after surgery in 8 and 9 cases, respectively. In total, 6 cases (35.7%), including 1 case of thickened hematoma, were fully cured by this first surgery, whereas 11 cases (64.3%) needed additional treatment with EBP, including 7 cases who needed multiple hematoma irrigations and 1 case who needed two EBPs (Fig. 2).
Fig. 2.
Therapeutic strategies and outcomes. The group receiving surgery first tended to require more treatments. EBP: epidural blood patch.
According to our observation during SDH surgeries, the pressure associated with the SDH was not high when the CSF leakage was not treated well. In some cases, the intracranial pressure was very low, such that the SDH did not bulge out and the brain surface did not elevate, and so hematoma drainage was not adequate. On the other hand, when the CSF leak was adequately treated, intracranial pressure was higher, the hematoma bulged out, and hematoma drainage functioned well.
All 55 cases of SIH with SDH achieved good recovery. There were no serious complications and no recurrence of either SIH or SDH during the follow-up period (mean: 3.6 years, range: 1–8 years). However, one patient, described below (Case 1) had a persistent neurological disturbance (hoarse voice), and another case (Case 2) transiently experienced life-threatening conditions during SDH surgery.
Case Reports
I. Case 1
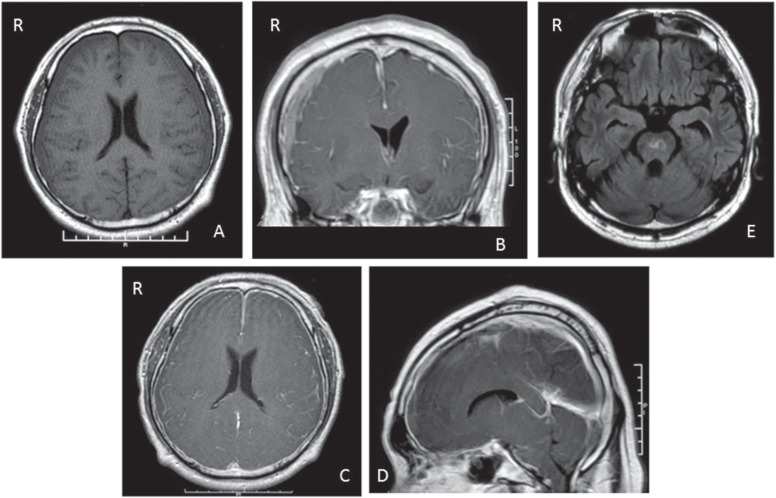
A 49-year-old male suffered from headaches in the summer. The headaches increased gradually. This was accompanied by a floating sensation, diplopia, and general fatigue. On October 12, he was admitted from another hospital. The admission neurological examination revealed slight consciousness disturbance (somnolence) but no other neurological deficits were seen. The MRI showed iso-intensity on T1WI in the subdural space bilaterally (Fig. 3A). Conservative therapy consisting of rest and intravenous hydration was begun; however, the consciousness disturbance progressed. On October 16 (2 months after the onset), irrigation of the hematoma bilaterally was performed. The intracranial pressure was low and hematomas did not bulge out. After the operation, the consciousness disturbance was improved. However, consciousness deteriorated 7 days after the first surgery (on October 23). On October 25, drainage of the SDH bilaterally was performed. After the second operation, the consciousness disturbance improved again. Operative findings were almost same as those of the first operation. On October 28, consciousness deteriorated again. The liquefied hematoma did not drain at all, although the drainage tubes on both sides of the subdural space were not occluded. On November 2, consciousness deteriorated into coma and respiration became ataxic, requiring intubation. Diffuse meningeal enhancement, descent of the cerebellar tonsil, and narrowing of the basal cistern were recognized on MRI (Fig. 3B–D). A whole spinal MRI was performed, but no apparent CSF leak was evident. EBP was performed with 20 ml of autogenous blood at the lumbar level (L2–3). After the EBP, the consciousness level rapidly improved to almost clear, and the descent of the brain had recovered on the MR images. Two weeks after the EBP, consciousness deteriorated and descent of the brain appeared again. Therefore, a second EBP was performed with 35 ml of blood at L 1–2. After the second EBP, consciousness improved; however, a high intensity lesion in the brain stem appeared on FLAIR imaging, probably due to downward displacement of the brain (Fig. 3E). Three months after the first EBP, the patient could go back to work and a hoarse voice remained as the only complication.
Fig. 3.
Case 1. Axial T1-weighted magnetic resonance (MR) image showing iso-intensity areas in bilateral subdural space (A). Coronal (B), axial (C), and sagittal (D) Gd-enhanced T1-weighted MR images showing bilateral subdural hematomas, diffuse meningeal enhancement, enlarged cerebral venous sinuses, and descent of cerebellar tonsil. Axial fluid attenuated inversion recovery (FLAIR) MR image showing high intensity lesion in brain stem (E).
II. Case 2
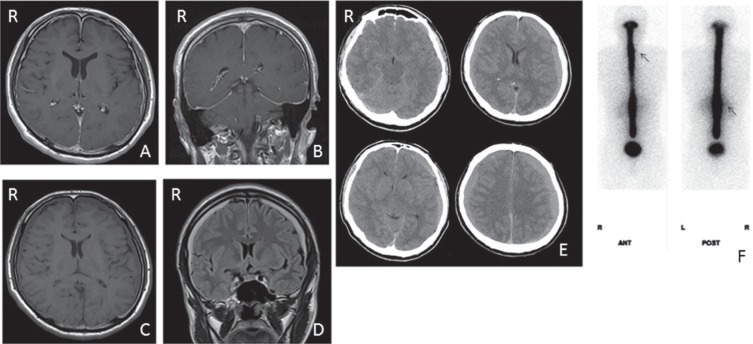
A 40-year-old male suffered from orthostatic headache and the MRI revealed diffuse meningeal enhancement (Fig. 4A, B). The diagnosis of SIH was made at another hospital. He was treated conservatively with bed rest and intravenous hydration; however, the headache increased and SDH was aggravated (Fig. 4C, D). Two months after the onset, he was hospitalized at Sanno Hospital with severe headaches even in the supine position. CT scan showed increasing hematoma volume (Fig. 4E). RI cisternography showed CL at the cervical level and apparent appearance of early RI filling in the bladder (Fig. 4F). The CSF pressure was 5.1 cmH2O. We planned initial irrigation of the hematoma, and that EBP would be performed immediately after the operation. Under local anesthesia, bilateral burr halls were opened and the hematoma was evacuated. Then, he started to complain of a severe headache and rapidly became drowsy with ataxic respiration. The blood pressure dropped to 50 mmHg, and heart rate decreased to 30 bpm. In operative findings, the hematoma did not bulge out and the brain was not elevated. Immediately, the burr holes were closed without irrigation of the hematomas. Subdural drains were inserted into both sides; however, the drainage was not functional. After closing the skin incision, his vital signs recovered to normal and stabilized; however, his headache and consciousness disturbance remained. We immediately performed EBP (15 ml at C7/Th1 and 20 ml at L2–3). Both headache and consciousness disturbance rapidly improved and subdural drainage started to function with liquefied blood draining out. One month later, his headaches increased and SDH recurred bilaterally. Irrigation of the hematomas was performed and the operating finding showed increasing ICP and a bulging hematoma. After the second hematoma irrigation, the patient recovered fully without any neurological deficits.
Fig. 4.
Case 2. Axial (A) and coronal (B) Gd-enhanced T1-weighted MR images showing diffuse meningeal enhancement. Axial T1-weighted MR image (C) and coronal FLAIR MR image (D) showing bilateral chronic SDHs. Axial CT scans (E) showing increased SDH, loss of subarachnoid cistern, and decrease in size of the ventricles. RI cisternography at 1 h after RI injection showed CSF leaks at cervical and lumbar lesions (arrows) (F). CSF: cerebrospinal fluid, CT: computed tomography, FLAIR: fluid attenuated inversion recovery, RI: radioisotope, SDH: subdural hematoma.
Discussion
The reported incidence of SDH among SIH patients ranges from 16% to 57%,21,28,36,37 ) predominantly in males,18,28,29) whereas SIH without SDH is predominant in females.17,21,24,25 ) Our results were similar to the previous reports.
Chung et al. reported that the onset age of SIH with SDH was 44.9 years old on average, which is significantly older than SIH without SDH (average: 36.6 years old).29) Our results were 48.6 years old for SIH with SDH, 38.0 years old for SIH without SDH. We may conclude that SIH with SDH occurs at an older age compared to SIH without SDH. We also have to note the fact that the onset age of SDH without SIH is definitely much older, occurring in the 70s or 80s.
It is reported that many cases have bilateral SDHs.5 ,18,21 ,25,35, 36) Our results also showed about 90% of the cases had bilateral SDH. Gd enhancement was useful to distinguish SIH with SDH from SDH unrelated to SIH.7,8, 15,21,36,37)
Several reports mentioned that RI cisternography is useful for detecting the CL site.5,6,21,24–26,30–34 ) In this study, most of the cases had obvious CL (SIH with SDH, 91.7%; SIH without SDH, 81.8%). Therefore, RI cisternography is also useful for assessing SIH with or without SDH.
To our knowledge, no cases of SIH with SDH have been reported to have elevated CSF pressure7–9 ,16,17, 19,21 ) and our results also indicated that CSF pressure tended to be low even though a large SDH was a complication. Previously reported operative findings included observations that intracranial pressure was not raised when CSF leakage was not yet sealed,8,19) similar to our findings. Those operative findings suggested that SIH with SDH occurs under the condition of low CSF pressure.
These facts may confirm that, even in SIH cases with large SDHs, EBP can be performed safely prior to drainage of the SDH.7)
However, whether to perform EBP or SDH surgery as the initial procedure remains controversial.7,8,21,25,29, 36,37)
De Noronha et al. reported 4 cases of SIH with large SDHs requiring urgent neurosurgical drainage, and suggested that in case of large subdural hemorrhage, surgical drainage is required initially, although treatment of the SIH also may be required.8)
On the other hand, some papers reported that surgery prior to EBP is more harmful. Patients undergoing SDH evacuation often had recurrences,25,27) persistent symptoms, and sometimes worsened clinical conditions. Improvement would be achieved by the adequate identification and sealing of the CL when SDH is also present.9,11–13 ,15,19, 38)
Chung et al. reported that SIH with SDH should be treated with EBP before surgery.29) Schievink et al. also suggested that the great majority of these SDHs associated with a significant mass effect can be managed by direct treatment of the spinal CSF leakage without hematoma evacuation.25,36,37) If a patient improves with EBP treatment, even a thick SDH can be left and will resolve spontaneously sooner or later.25)
Loya et al. reviewed 29 cases of SIH associated with coma. EBPs were successful in ameliorating the comatose state in 85%.7) Evacuation of SDHs may be performed for large SDH with mass effect,7,14,21) or for when dilated or asymmetric pupil is present.7) However, Loya et al. concluded that in most cases evacuation of the hematoma is not necessary and may give rise to worsened outcomes.
Schievink reported two fatal cases after craniotomy and mentioned that the strokes were due to downward displacement of the brain and can be precipitated by craniotomy for evacuation of associated SDH.19) Kelley et al. hypothesized that the deterioration is the result of acute brain stem displacement provoked by opening the intrathecal space to atmospheric pressure and that surgery increases the risk of brain herniation.10)
It is true that treatment with EBP only can resolve SDH without irrigation, but it has been reported that delayed SDHs can occur after EBP, and then additional surgery for SDH is needed.28,38–40) Persistent non-postural headache after EBP might be a predictive sign of progressive SDH.39) Those persistent headaches after EBP would be an important indication for SDH surgery.
In this study, 26.4% of the cases of SIH with SDH recovered with conservative therapy. Therefore, conservative treatment, such as hydration and rest, should be considered first. The group receiving surgery first tended to require more treatments and 2 cases had serious conditions during and after the operative periods (Cases 1 and 2 described above).
According to our results and literature review, we set up a flowchart of our strategy for treating SIH with SDH (Fig. 1). We treated 23 cases: 5 cases were cured by conservative therapy and 18 cases were treated by initial EBP, and we did not have any troubling cases. In accordance with previous reports, our results indicate that EBP should be performed prior to irrigation of the hematoma. In some cases in whom SDH becomes symptomatic or hematoma volume increases after EBP, irrigation of the hematoma should be considered. For cases of SIH with a large hematoma, irrigation of the hematoma would be performed immediately after the EBP.
In the past, SDH often might have been evacuated surgically prior to the treatment of the CSF leakage, probably because SIH was not well recognized as an underlying cause of SDH.7,16,21,25,29,36) Therefore, if a patient with bilateral SDH is middle-aged, and the case is non-traumatic, an MRI with Gd enhancement study should be considered in order to make a diagnosis of SIH. If the patient’s condition becomes critical during SDH surgery, the skin incision at the site of the burr hole opening should be quickly closed in order to prevent exposure of the intrathecal space to atmospheric pressure, and thereafter EBP should be performed immediately.
Conclusion
SIH with SDH can be managed with proper treatment. If non-traumatic and bilateral SDH at middle age is encountered, association with SIH should be considered as one of the causes and an MRI with Gd enhancement study should be performed. If conservative treatment is not sufficient, EBP should be considered prior to irrigation of the hematoma.
A summary of this article was presented at the 71st (Osaka in October, 2012), 72nd (Kanagawa in October, 2013), and 73rd (Tokyo in October, 2014) Annual Meetings of the Japan Neurosurgical Society.
References
- 1). Leriche R: De I’hypotension du liquid cephalorachidien dans certaines fractures de la base du crane et de son traitment par I’injection de serum sous la peau. Lyon Chir 17: 638, 1920. [Google Scholar]
- 2). Mokri B, Krueger BR, Miller GM, Piepgras DG: Meningeal gadolinium enhancement in low-pressure headaches. J Neuroimaging 3: 11– 15, 1993. [Google Scholar]
- 3). Holmes JM: Intracranial hypotension associated with subdural haematoma. Br Med J 1: 1363– 1366, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Page F: Intracranial hypotension. Lancet 1: 1– 5, 1953. [DOI] [PubMed] [Google Scholar]
- 5). Chung SJ, Kim JS, Lee MC: Syndrome of cerebral spinal fluid hypovolemia: clinical and imaging features and outcome. Neurology 55: 1321– 1327, 2000. [DOI] [PubMed] [Google Scholar]
- 6). Mokri B: Spontaneous low pressure, low CSF volume headaches: spontaneous CSF leaks. Headache 53: 1034– 1053, 2013. [DOI] [PubMed] [Google Scholar]
- 7). Loya JJ, Mindea SA, Yu H, Venkatasubramanian C, Chang SD, Burns TC: Intracranial hypotension producing reversible coma: a systematic review, including three new cases. J Neurosurg 117: 615– 628, 2012. [DOI] [PubMed] [Google Scholar]
- 8). de Noronha RJ, Sharrack B, Hadjivassiliou M, Romanowski CA: Subdural haematoma: a potentially serious consequence of spontaneous intracranial hypotension. J Neurol Neurosurg Psychiatr 74: 752– 755, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Whiteley W, Al-Shahi R, Myles L, Lueck CJ: Spontaneous intracranial hypotension causing confusion and coma: a headache for the neurologist and the neurosurgeon. Br J Neurosurg 17: 456– 458, 2003. [DOI] [PubMed] [Google Scholar]
- 10). Kelley GR, Johnson PL: Sinking brain syndrome: craniotomy can precipitate brainstem herniation in CSF hypovolemia. Neurology 62: 157, 2004. [DOI] [PubMed] [Google Scholar]
- 11). Sayer FT, Bodelsson M, Larsson EM, Romner B: Spontaneous intracranial hypotension resulting in coma: case report. Neurosurgery 59: E204; discussion E204 2006. [DOI] [PubMed] [Google Scholar]
- 12). Ferrante E, Arpino I, Citterio A, Savino A: Coma resulting from spontaneous intracranial hypotension treated with the epidural blood patch in the Trendelenburg position pre-medicated with acetazolamide. Clin Neurol Neurosurg 111: 699– 702, 2009. [DOI] [PubMed] [Google Scholar]
- 13). Souirti Z, Benzagmout M, Belahsen F, Chaoui ME: Spontaneous bilateral subacute subdural hematoma revealing intracranial hypotension. Neurosciences (Riyadh) 14: 384– 385, 2009. [PubMed] [Google Scholar]
- 14). Nardone R, Caleri F, Golaszewski S, Ladurner G, Tezzon F, Bailey A, Trinka E, Zuccoli G: Subdural hematoma in a patient with spontaneous intracranial hypotension and cerebral venous thrombosis. Neurol Sci 31: 669– 672, 2010. [DOI] [PubMed] [Google Scholar]
- 15). Dhillon AK, Rabinstein AA, Wijdicks EF: Coma from worsening spontaneous intracranial hypotension after subdural hematoma evacuation. Neurocrit Care 12: 390– 394, 2010. [DOI] [PubMed] [Google Scholar]
- 16). Ade S, Moonis M: Intracranial hypotension with multiple complications: an unusual case report. Case Rep Neurol Med 2013: 913465, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Chi NF, Wang SJ, Lirng JF, Fuh JL: Transtentorial herniation with cerebral infarction and duret haemorrhage in a patient with spontaneous intracranial hypotension. Cephalalgia 27: 279– 282, 2007. [DOI] [PubMed] [Google Scholar]
- 18). Lai TH, Fuh JL, Lirng JF, Tsai PH, Wang SJ: Subdural haematoma in patients with spontaneous intracranial hypotension. Cephalalgia 27: 133– 138, 2007. [DOI] [PubMed] [Google Scholar]
- 19). Schievink WI: Stroke and death due to spontaneous intracranial hypotension. Neurocrit Care 18: 248– 251, 2013. [DOI] [PubMed] [Google Scholar]
- 20). Yokosuka K, Matsubara S, Yamaguchi T, Toi H, Kuwayama K, Hirano K, Uno M: A fatal case of spontaneous intracranial hypotension with bilateral chronic subdural hematoma. Jpn J Neurosurg 21: 796– 800, 2012. [Google Scholar]
- 21). Yoon SH, Chung YS, Yoon BW, Kim JE, Paek SH, Kim DG: Clinical experiences with spontaneous intracranial hypotension: a proposal of a diagnostic approach and treatment. Clin Neurol Neurosurg 113: 373– 379, 2011. [DOI] [PubMed] [Google Scholar]
- 22). Headache Classification Committee of the International Headache Society : The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33: 629– 808, 2013. [DOI] [PubMed] [Google Scholar]
- 23). Schievink WI, Dodick DW, Mokri B, Silberstein S, Bousser MG, Goadsby PJ: Diagnostic criteria for headache due to spontaneous intracranial hypotension: a perspective. Headache 51: 1442– 1444, 2011. [DOI] [PubMed] [Google Scholar]
- 24). Mokri B: Low cerebrospinal fluid pressure syndromes. Neurol Clin 22: 55– 74, vi, 2004. [DOI] [PubMed] [Google Scholar]
- 25). Schievink WI: Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA 295: 2286– 2296, 2006. [DOI] [PubMed] [Google Scholar]
- 26). Shinonaga M: Chronic headache attributed to CSF hypovolemia (broadening of CSF hypotension). Cephalalgia 25: 1003, 2005. [Google Scholar]
- 27). García-Morales I, Porta-Etessam J, Galán L, Lagares A, Molina JA: Recurrent subdural haematomas in a patient with spontaneous intracranial hypotension. Cephalalgia 21: 703– 705, 2001. [DOI] [PubMed] [Google Scholar]
- 28). Hashizume K, Watanabe K, Kawaguchi M, Fujiwara A, Furuya H: Evaluation on a clinical course of subdural hematoma in patients undergoing epidural blood patch for spontaneous cerebrospinal fluid leak. Clin Neurol Neurosurg 115: 1403– 1406, 2013. [DOI] [PubMed] [Google Scholar]
- 29). Chung SJ, Lee JH, Kim SJ, Kwun BD, Lee MC: Subdural hematoma in spontaneous CSF hypovolemia. Neurology 67: 1088– 1089, 2006. [DOI] [PubMed] [Google Scholar]
- 30). Takahashi K, Mima T: Cerebrospinal fluid leakage after radioisotope cisternography is not influenced by needle size at lumbar puncture in patients with intracranial hypotension. Cerebrospinal Fluid Res 6: 5, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Takahashi K, Mima T: Intracranial hypotension in childhood and adolescence: clinical features and outcomes. Nervous System in Children 36: 552– 559, 2011. [Google Scholar]
- 32). Takahashi K, Mima T: Cerebrospinal fluid hypovolemia onset in childhood; A report of 9 cases and clinical features. Nervous System in Children 33: 426– 467, 2008. [Google Scholar]
- 33). Horikoshi T, Ikegawa H, Uchida M, Takahashi T, Watanabe A, Umeda T: Tracer clearance in radionuclide cisternography in patients with spontaneous intracranial hypotension. Cephalalgia 26: 1010– 1015, 2006. [DOI] [PubMed] [Google Scholar]
- 34). Moriyama E, Ogawa T, Nishida A, Ishikawa S, Beck H: Quantitative analysis of radioisotope cisternography in the diagnosis of intracranial hypotension. J Neurosurg 101: 421– 426, 2004. [DOI] [PubMed] [Google Scholar]
- 35). Kong DS, Park K, Nam DH, Lee JI, Kim JS, Eoh W, Kim JH: Clinical features and long-term results of spontaneous intracranial hypotension. Neurosurgery 57: 91– 96; discussion 91–96, 2005. [DOI] [PubMed] [Google Scholar]
- 36). Schievink WI, Maya MM, Moser FG, Tourje J: Spectrum of subdural fluid collections in spontaneous intracranial hypotension. J Neurosurg 103: 608– 613, 2005. [DOI] [PubMed] [Google Scholar]
- 37). Schievink WI, Maya MM, Pikul BK, Louy C: Spontaneous spinal cerebrospinal fluid leaks as the cause of subdural hematomas in elderly patients on anticoagulation. J Neurosurg 112: 295– 299, 2010. [DOI] [PubMed] [Google Scholar]
- 38). Mao YT, Dong Q, Fu JH: Delayed subdural hematoma and cerebral venous thrombosis in a patient with spontaneous intracranial hypotension. Neurol Sci 32: 981– 983, 2011. [DOI] [PubMed] [Google Scholar]
- 39). Takeuchi S, Takasato Y, Masaoka H, Hayakawa T, Otani N, Yoshino Y, Yatsushige H, Sugawara T: Progressive subdural hematomas after epidural blood patch for spontaneous intracranial hypotension. J Anesth 24: 315– 316, 2010. [DOI] [PubMed] [Google Scholar]
- 40). Wang HK, Liliang PC, Liang CL, Lu K, Hung KC, Chen HJ: Delayed subdural hematoma after epidural blood patching in a patient with spontaneous intracranial hypotension—case report Neurol Med Chir (Tokyo) 50: 479– 481, 2010. [DOI] [PubMed] [Google Scholar]