Abstract
The exposure-response for hexavalent chromium (Cr(VI))-induced lung cancer among workers of the Painesville Ohio chromate production facility has been used internationally for quantitative risk assessment of environmental and occupational exposures to airborne Cr(VI). We updated the mortality of 714 Painesville workers (including 198 short-term workers) through December 2011, reconstructed exposures, and conducted exposure-response modeling using Poisson and Cox regressions to provide quantitative lung cancer risk estimates. The average length of follow-up was 34.4 years with 24,535 person-years at risk. Lung cancer was significantly increased for the cohort (standardized mortality ratio (SMR)=186; 95% confidence interval (CI) 145–228), for those hired before 1959, those with >30-year tenure, and those with cumulative exposure >1.41 mg/m3-years or highest monthly exposures >0.26 mg/m3. Of the models assessed, the linear Cox model with unlagged cumulative exposure provided the best fit and was preferred. Smoking and age at hire were also significant predictors of lung cancer mortality. Adjusting for these variables, the occupational unit risk was 0.00166 (95% CI 0.000713–0.00349), and the environmental unit risk was 0.00832 (95% CI 0.00359–0.0174), which are 20% and 15% lower, respectively, than values developed in a previous study of this cohort.
Keywords: exposure-response modeling, hexavalent chromium, inhalation, lung cancer, risk assessment
INTRODUCTION
Hexavalent chromium (Cr(VI)) is a known human carcinogen associated with increased lung cancer risk among workers in certain industries.1 In the United States, >558,000 workers are exposed to airborne Cr(VI).2 In the EU, the estimated number of Cr(VI)-exposed workers is ~786,000 and that in Canada is 83,000, with the largest numbers exposed by welding.3 Environmental exposures occur in proximity to anthropogenic sources, including emissions from certain industries and combustion of petroleum products (e.g, automobile exhaust).4 In California, ambient monitoring for Cr(VI) from 1989 to 2013 has shown decreasing levels through time, and current levels generally below 0.1 ng/m3.3, 5 Similarly, in Texas ambient Cr(VI) is currently reported to range from 0.0059 to 0.17 ng/m3.3, 6
Data from two occupational cohorts—the Painesville, Ohio and Baltimore, Maryland chromate production workers—have been used in several quantitative risk assessments and are also the basis of the Occupational Safety and Health Administration (OSHA) Cr(VI) Rule.7, 8, 9, 10, 11 Painesville workers employed from 1931 to 1937 are the basis for the current US Environmental Protection Agency (US EPA) Cr(VI) cancer-risk assessment.12, 13 However, the exposure characterization for these workers was highly limited.12, 14, 15 In a subsequent study of Painesville workers by Luippold et al. (2003), those employed between 1940 and 1972 with at least 1 year of work tenure (n=482) were followed through 1997.16 As compared with the Mancuso (1975)12 study, the exposure reconstruction was greatly improved with the use of a job exposure matrix and quantitative measures of airborne Cr(VI).7, 16, 17, 18 These data were modeled in Crump et al. (2003), and a significant increase in lung cancer risk was observed at lifetime occupational exposures ⩾1.0 mg/m3 -years.7 A linear exposure-response was observed with cumulative exposure lagged 5 years. From the relative risk model of Poisson regression, the estimated lifetime additional risk of lung cancer mortality associated with 45 years of occupational exposure to 1 μg/m3 (occupational unit risk) was 0.00205 (90% CI 0.00134–0.00291).7 Extrapolating these findings to a continuous environmental exposure resulted in an environmental unit risk of 0.00978 (90% CI 0.00640–0.0138).7 However, short-term workers (<1 year of employment) were excluded from the analyses, limiting information in the low exposure range.
In this study, we expanded this cohort to include 198 short-term workers who worked <1 year, updated the mortality assessment through 2011, and conducted exposure-response modeling to quantify lung cancer risk from lifetime occupational and continuous environmental exposures to airborne Cr(VI).
METHODS
Ascertainment of Vital Status of the Painesville Cohort and Mortality Analysis
Similar to the earlier follow-up,16 only Painesville chromate production workers employed after 31 December 1939 and having a valid social security number and date of birth were included. Unlike the previous study,16 workers who worked for <12 months in the plant were also included. Workers employed before 1940 were excluded because work history and exposure data were too limited. In this study, 714 workers were identified as meeting the inclusion criteria. Supplementary Figure 1 shows the data sources used to identify the underlying causes of death for all deceased cohort members (n=658). We used Ancestry.com, which allowed searches of family genealogy to track vital status information and locate death certificates for workers who could not be identified through the National Death Index (NDI)19 or other sources. Twenty-four workers (3.4%) were considered loss to follow-up (LTF) because they could not be matched in NDI or tracked. Twenty-nine workers (4.1%) were confirmed to be deceased, but their death certificates could not be located. For all deaths identified in NDI (n=417), the underlying cause of death was coded using the International Classification of Disease (ICD) versions 8(a), 9, and 10 according to the instructions from the National Center for Health Statistics (NCHS).20 For all other deaths obtained from death certificates (n=212), an ICD-10 Conversion Analyst defined the ICD code associated with the underlying cause of death. Supplementary Table 1 provides the ICD-8(a), ICD-9, and ICD-10 code ranges that were used to identify specific causes of death evaluated in this study. It should be noted that mesothelioma was listed as the underlying cause of death for six workers. Three were coded as C45.9 (ICD-10) and fell under non-respiratory cancer classification. For three mesothelioma deaths, the dates of death corresponded to earlier ICD codes and were thus classified as lung cancer consistent with ICD-8(a) and 9.
Workers were followed from 1 January 1940 through 31 December 2011. Person-years at risk for each cohort member began the first day of hire and continued until the date of death, the last date of follow-up, or the last known date alive (that is, last day of employment) if considered LTF at the end of the employee's work tenure. Standardized mortality ratios (SMRs) and 95% confidence intervals (CI) for selected causes of death were calculated based on reference US and Ohio white male populations. For the 29 workers known to be deceased without death certificates (Supplementary Figure 1), their data were used to calculate the SMR for all-cause mortality only. The reference rates for white males were used because there were few females in the cohort and most workers were white, which was also the case for the previous study.16 Age- and cause-specific mortality rates for both US and Ohio reference populations for 1968–2010 by calendar period, were calculated from the NCHS Compressed Mortality File and the associated Population Files as well as from the Surveillance, Epidemiology and End Result Stat Database.21, 22, 23, 24
Using the Ohio mortality rates, lung cancer SMRs were further stratified by year of hire, duration of exposure, time since first exposure, and Cr(VI) exposures. For SMR analysis stratified by duration of exposure and time since hire, person-years were calculated in a time-dependent method, implying that employees could contribute person-years to all strata, if applicable. A Poisson trend statistic was calculated to test for monotonic exposure-response relationship of lung cancer mortality for the stratified variables. All SMR analyses and 95% CIs were calculated using SAS (Version 9.3; Cary, NC, USA). Institutional Review Board (IRB) approval (IRB #201207805) for the study was obtained from Schulman Associates IRB (Blue Ash, OH, USA).
Exposure-Response Modeling of Lung Cancer Mortality and Risk Estimation
We evaluated exposure-response using two exposure metrics, cumulative (mg/m3-years) and highest monthly average (mg/m3) exposures. Cumulative exposure of each worker was the sum of monthly average exposures across duration of employment. Highest monthly exposure was the highest of the monthly 8-h time-weighted average exposures.
The same modeling equations noted in Crump et al. (2003)7 were used in this study. Poisson regression was used to implement relative risk and additive risk models, and Cox regression was used to implement relative risk models. For Poisson regression, mortality analysis data were categorized into cells by age (10 categories: <45, 45–50, …, 80–85, >85) and by (possibly lagged) cumulative exposure (10 categories providing approximately equal numbers of expected lung cancer deaths from mortality rates of Ohio white males). The observed number of lung cancer deaths in a cell was assumed to have Poisson distribution with a mean of αE(1+βx+γx2) (relative risk model) or αE+p(βx+γx2) (additive risk model) where x was cumulative exposure, p was the number of person-years of observation in the cell, E was the expected number of lung cancer deaths based on Ohio death rates, and α, β, and γ were estimated parameters. When α≠1, the background lung cancer mortality risk in the cohort was different from that of the reference population. When γ≠0, exposure-response was non-linear; if γ=0, β was the measure of carcinogenic potency.
The relative risk model by Cox regression was assumed to have the form exp(βx+∑iβicovariatei) (exponential model) or (1+βx)exp(∑iβicovariatei) (linear model). Covariates explored included smoking, age at hire, and duration of exposure as a continuous variable categorized in two different ways (1–4, 4.1–7.9, ⩾8 years or <3.9, 4.0–20.7, ⩾20.8 years). Unless otherwise stated, smoking information was quantified using three categories: known smoker (n=157), known non-smoker (n=43), and no smoking information available (n=514).
One advantage of Cox regression in comparison to Poisson regression is that in Cox regression the cases are not categorized; individual responses are compared at the same age so age is fully controlled.25 On the other hand, Poisson regression is more convenient for developing non-relative-risk models.
Both the Poisson models and the Cox models were applied with cumulative exposure lagged 0, 5, 10, or 15 years. Parameters were estimated by the method of maximum likelihood, and likelihood ratio tests were used to test hypotheses.26 CIs were calculated mainly by the profile likelihood method.27
We quantified lung cancer risk for a contemporary population with birth cohort, sex, age, and race/ethnicity that are different from the Painesville cohort. Thus, additional lifetime risks of lung cancer mortality associated with occupational (45 years) or environmental (70 years) exposure were estimated using a life-table analysis based on the regression results and the reference US mortality rates (from 1968 to 2011) by 10-year age intervals for both sexes and all races. As described in Supplementary Material, the unit risk for occupational exposure was estimated as the additional lifetime risk of lung cancer mortality from occupational exposure to 1 μg/m3 Cr(VI) between ages of 20 and 65 years. The unit risk for environmental exposure was estimated as the additional lifetime risk of lung cancer mortality from continuous exposure to 1 μg/m3 Cr(VI) throughout life (24 h/day, 365.25 days/year). Trend tests were conducted to determine the lowest exposure for which a statistically significant increase in relative risk of lung cancer is observed for cumulative exposure or highest monthly exposure.
Microsoft Excel (Office 2011), SAS, and Epicure (Version 2.0) were used for exposure-response modeling of lung cancer mortality. Prism for Mac (Version 6, San Diego, CA, USA) was used to graph the modeling results.
RESULTS
Characteristics of the Painesville Cohort (n=714) and Mortality
The average length of follow-up was 34.4 years (range: 0.1–69.9 years) with 24 535 total person-years at risk (Table 1). Approximately 61% of the cohort (n=432) were first exposed to Cr(VI) between 1940 and 1954. Eighty-two workers (12%) were identified as having work tenures of 20 or more years, with 25 (33%) of these dying from lung cancer. The Cr(VI) concentration range for the cumulative exposure metric spans about five-orders of magnitude (0.0002–22.1 mg/m3-years) with the inclusion of the short-term workers. Although limited data from employee records were available on smoking status, 157 (79%) of 200 workers with available smoking data indicated that they were current smokers (yes/no) at the time of data collection. This may suggest that a large proportion of the cohort consisted of current smokers at the time of employment.
Table 1. Characteristics of Painesville, Ohio chromate production workers (n=714) and subset dead from lung cancer (n=77).
| Characteristic | Study cohort | Workers dead from lung cancer | ||
|---|---|---|---|---|
| n | % | n | % | |
| Year of birth | ||||
| 1877–1899 | 37 | 5.2 | 1 | 1.3 |
| 1900–1909 | 86 | 12.0 | 10 | 13.0 |
| 1910–1919 | 223 | 31.2 | 27 | 35.1 |
| 1920–1929 | 250 | 35.0 | 26 | 33.8 |
| 1930–1939 | 87 | 12.2 | 11 | 14.3 |
| 1940–1959 | 31 | 4.3 | 2 | 1.8 |
| Year first exposed | ||||
| 1940–1944 | 122 | 17.1 | 21 | 27.3 |
| 1945–1949 | 186 | 26.1 | 19 | 24.7 |
| 1950–1954 | 124 | 17.4 | 16 | 20.8 |
| 1955–1959 | 91 | 12.8 | 9 | 11.7 |
| 1960–1964 | 88 | 12.3 | 6 | 7.8 |
| 1965–1972 | 103 | 14.4 | 6 | 7.8 |
| Length of employment (years) | ||||
| <1 | 198 | 27.7 | 14 | 18.2 |
| 1–4 | 245 | 34.3 | 17 | 22.1 |
| 5–9 | 113 | 15.8 | 11 | 14.3 |
| 10–19 | 76 | 10.6 | 10 | 13.0 |
| 20–32 | 82 | 11.5 | 25 | 32.5 |
| Mean (SD) | Range | Mean (SD) | Range | |
| Cumulative exposure (mg/m3-years) | 1.1 (2.1) | 0.0002–22.1 | 2.5 (3.9) | 0.004–22.1 |
| Highest monthly exposure (mg/m3) | 0.3 (0.4) | 0.003–4.1 | 0.5 (0.7) | 0.01–4.1 |
| Age at hire (year) | 33.6 (11.0) | 12.9–69.4 | 30.6 (9.2) | 18.0–60.1 |
| Length of follow-up (years) | 34.4 (16.1) | 0.1–69.9 | 35.2 (13.6) | 4.3–62.7 |
Cancer deaths comprised 25% (n=167) of all known causes of death; of all cancer deaths, 46% (n=77) were identified as lung cancer (Table 1; Supplementary Table 2). After adjusting for both age and calendar year and using Ohio reference rates, there was elevated mortality from all causes, all cancers, cancers of the respiratory system, and other circulatory system diseases (Supplementary Table 2). In addition, all non-respiratory cancers were marginally elevated. Gastrointestinal tract cancers, which have been evaluated in other epidemiologic studies of Cr(VI) exposed workers and environmentally exposed populations,28, 29, 30, 31 were few and not significantly elevated (using Ohio reference rates, oral cancer: n=2, SMR of 77, 95% CI 0–183; stomach cancer: n=5, SMR of 144, 95% CI 18–270; and no small intestinal cancers were observed). Among 198 short-term workers, 185 were deceased with seven LTF (Supplementary Table 3). As expected, the short-term workers had relatively low cumulative exposures (Range: 0.0002–0.69 mg/m3-years). Using Ohio reference rates, short-term workers had a somewhat higher all-cause SMR (152, 95% CI 130–174) as compared with that for the whole cohort (132, 95% CI 127–148) although both were significantly elevated. Consistent with the lower Cr(VI) exposures experienced by short-term workers, the lung cancer SMR was lower (134, 95% CI 64–204) as compared with that of the full cohort (186, 95% CI 145–228). These observations might indicate that the short-term workers had generally poorer health status.
Lung Cancer Mortality
Lung cancer SMRs of 186 (95% CI 145–228) and 205 (95% CI 159–250) were observed based on the Ohio and US reference rates, respectively (Supplementary Table 2). Workers who died from lung cancer were similar to the entire cohort in terms of year of birth with ~70% born between the years 1910 and 1929 (Table 1). However, those who died from lung cancer were generally exposed to Cr(VI) in earlier years. In addition, those who died from lung cancer tended to have longer work tenures at the plant; 33% worked for 20 years or more compared to 12% of the entire cohort.
Several trends were observed with the stratified analyses of lung cancer (Supplementary Table 4). The SMRs decreased as the year of hire increased, consistent with the observation that airborne concentrations of Cr(VI) decreased over time.18 A significant trend was also observed when stratified by duration of employment (P<0.01); for the workers employed 20–32 years, lung cancer risk was five times that expected (SMR=502, 95% CI 305–698). Lung cancer risk was increased in association with Cr(VI) exposure for both exposure metrics. Among the cohort, 43 of 77 (56%) lung cancer deaths occurred among workers in the top three cumulative exposure categories.
Exposure-Response Modeling
The Poisson relative risk and additive risk models were applied to test for a nonlinear exposure-response (γ≠0) with both α and β estimated using all four exposure lags. None of these analyses indicated that γ was significantly different from 0. Thus, there was little statistical evidence that the exposure-response was not linear. Next, the same models were applied, but with γ=0, to test for the background rate of lung cancer mortality being different from the Ohio rate (α≠1). In none of these analyses was the estimate of α significantly different from 1. Thus, there is also little evidence that the background lung cancer mortality rate in this cohort is different from that of Ohio. β values from the relative risk model ranged 0.700–0.725 (mg/m3-years)−1, and those from the additive risk model ranged 0.00118–0.00169 (mg/m3-years per person-year)−1 (Table 2). These values were similar to those obtained previously with γ=0 and α=1, (β=0.794, 90% CI 0.518–1.20 from the relative risk model and β=0.00161, 90% CI 0.00107–0.00225 from the additive risk model) (Crump et al., 2003, Table II).7
Table 2. Potency factors for Cr(VI) obtained using Poisson regression.
| Poisson model | Lag (years) | β (Potency factor)a | −2LL |
|---|---|---|---|
| Relative | 0 | 0.725 | 232.31 |
| Additive | 0.00118 | 230.04 | |
| Relative | 5 | 0.732 | 230.20 |
| Additive | 0.00127 | 228.17 | |
| Relative | 10 | 0.703 | 226.35 |
| Additive | 0.00135 | 224.90 | |
| Relative | 15 | 0.700 | 226.91 |
| Additive | 0.00169 | 221.68 |
Abbreviation: –2LL, negative two log-likelihood.
The units of β for the relative risk model are (mg/m3-years)−1, and for the additive model are (mg/m3-years per person-year)−1, β obtained with γ=0 and α=1.
Using Poisson regression, evidence for a non-linear exposure-response for Cr(VI) could not be established, and the remainder of the analysis focuses on results obtained using Cox regression. Eight Cox regression models were applied involving both the exponential model and linear model and four lags for cumulative exposure (0, 5, 10, and 15 year lags). With each model, tests were conducted for the effect of including: (1) cumulative exposure alone; (2) smoking alone; (3) cumulative exposure and smoking together, (4) age at hire with cumulative exposure and smoking in the model; and (5) exposure duration (modeled three ways: as a continuous variable and two different categorizations) with cumulative exposure, smoking, and age at hire in the model. These eight modeling efforts all gave qualitatively very similar results: cumulative exposure and smoking were all highly significant, either alone or in combination. Age at hire was also always highly significant while adjusting for cumulative exposure and smoking; lung cancer mortality risk decreased with increasing age at hire. In all cases, the model with unlagged cumulative exposure gave the best fit (smallest deviance) among comparable models, and the exponential Cox models gave better fits than the comparable linear Cox models.
Modeling results for unlagged exposure are summarized in Table 3. We note that β (0.65, 95% CI 0.20–1.37) obtained from the linear Cox model with no additional covariates is very similar to that (0.66, 95% CI 0.11–1.2) obtained from the earlier analysis that did not include the short-term workers.7 Besides cumulative exposure, smoking and age at hire were significant parameters for predicting lung cancer mortality. Controlling for these two variables in the linear Cox models attenuated β estimates. In the linear Cox model, the β estimate was 0.40 (95% CI 0.12–0.97) when cumulative exposure, smoking, and age at hire were included.
Table 3. Exponential and linear models in Cox regression with unlagged cumulative Cr(VI) exposure.
| Variables in model | Exponential model | Linear model | ||||||
|---|---|---|---|---|---|---|---|---|
| Deviance | β (mg/m3-years)−1 |
P-valuea | Deviance | β (mg/m3-years)−1 |
P-valuea | |||
| MLE | 95% CI | MLE | 95% CI | |||||
| Cr(VI) | 1248.10 | 0.22 | (0.16, 0.28) | <0.0001 | 1252.26 | 0.65 | (0.28, 1.37) | <0.0001 |
| Cr(VI), smoking | 1234.95 | 0.19 | (0.12, 0.25) | 0.001 | 1236.45 | 0.58 | (0.22, 1.32) | <0.0001 |
| Cr(VI), smoking, age at hire | 1223.14 | 0.17 | (0.10, 0.24) | 0.0006 | 1225.75 | 0.40 | (0.12, 0.97) | 0.001 |
| Cr(VI), smoking, age at hire, years of exposure (continuous variable) | 1222.48 | 0.15 | (0.064, 0.23) | 0.42 | 1225.34 | 0.29 | (0.042, 1.03) | 0.52 |
| Cr(VI), smoking, age at hire, years of exposure (1–4, 4.1–7.9, ⩾8) | 1222.83 | 0.16 | (0.085, 0.23) | 0.85 | 1225.60 | 0.37 | (0.093, 1.11) | 0.93 |
| Cr(VI), smoking, age at hire, years of exposure (<3.9, 4.0–20.7, ⩾20.8) | 1223.00 | 0.18 | (0.092, 0.25) | 0.93 | 1225.27 | 0.51 | (0.118, 1.63) | 0.79 |
Abbreviations: Cr(VI), cumulative exposure, mg/m3-years; MLE, maximum likelihood estimate.
P-values are for the bolded variables that are italicized.
In an analysis of the Baltimore cohort, Gibb et al. (2011) found that exposure duration was a significant explanatory variable; lung cancer mortality risk was greater for those with high cumulative exposure over a short period of time (0.339 mg/m3-years achieved at 30 days) compared with the same cumulative exposure spread over a much longer duration (0.339 mg/m3-years achieved at 5 or 10 years).32 In order to determine whether dose-effect was present in the Painesville cohort, analyses were conducted using three indicators of exposure duration: exposure duration as a continuous variable and categorized two ways (Table 3). None of these analyses found statistical evidence of an effect of exposure duration in the Painesville cohort.
These analyses also included tests of β being age-dependent by estimating separate β values for ages <60, 60–72, and >72 (cut-points chosen to give equal numbers of lung cancer deaths in each range) while controlling for smoking, and testing whether the fits of these models were significantly improved over models employing a single β for all ages. None of these models had significantly improved fit indicating that there was no evidence of age-dependence (results not shown).
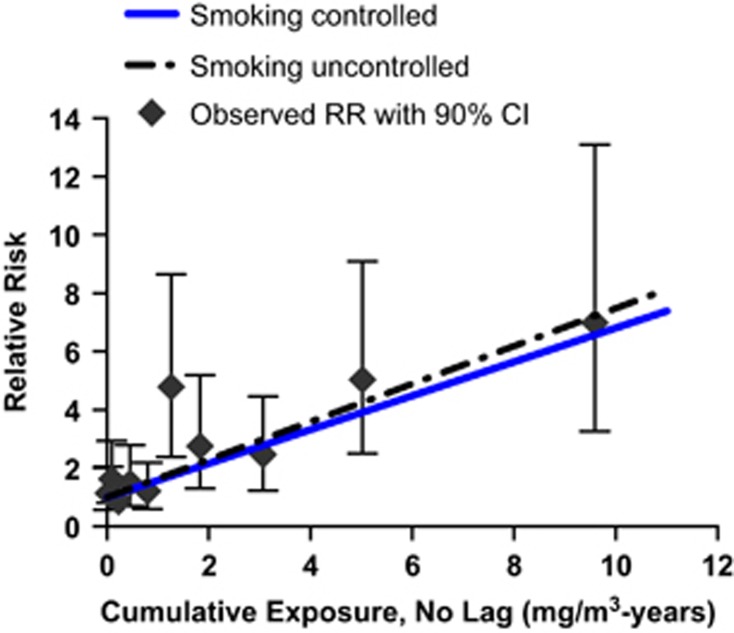
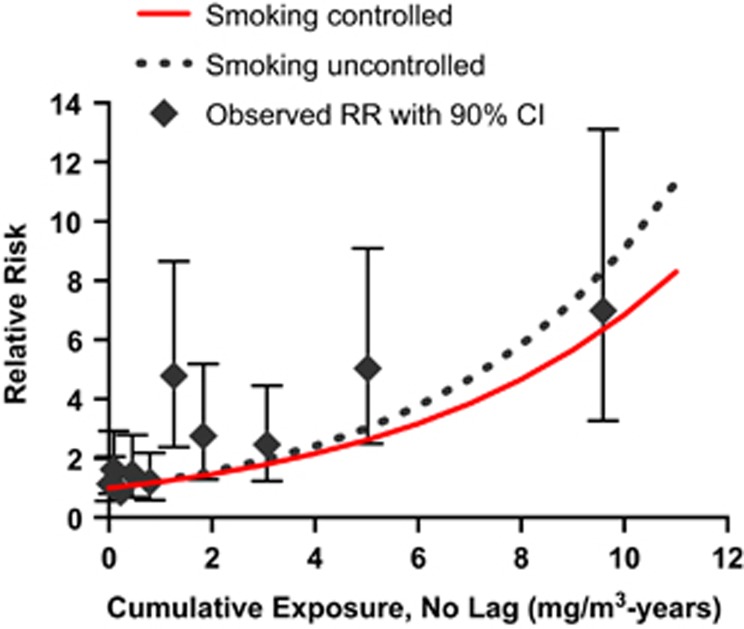
We were concerned that a few employees with very high cumulative exposures to Cr(VI) might be the reason why Cox exponential models fit slightly better than Cox linear models. Thus, to test the robustness of the model, all Cox analyses were repeated after removing the three subjects with the highest cumulative exposures, two of whom died of lung cancer. In these analyses, zero lag continued to provide better fit than lags of 5, 10, or 15 years, but with these three individuals removed, the linear Cox model gave better fits than the exponential Cox model for all four lag periods (results not shown). Figures 1 and 2 present graphs of the fit of the linear and exponential Cox models with zero lag using all cohort members to data categorized by unlagged cumulative exposure. Supplementary Figure 2 contains similar graphs showing the fit of the relative risk and additive risk Poisson models based on unlagged cumulative exposure. The linear Cox model appears to demonstrate the best fit, particularly in the intermediate exposure range (Figures 1 and 2).
Figure 1.
Linear Cox proportional hazard models of lung cancer mortality by unlagged cumulative exposure to Cr(VI) using all cohort members. Predicted relative risks are shown with smoking controlled or uncontrolled in the models. Observed relative risks with 90% confidence intervals are also shown. The Cox models were fit to individual data, and the highest cumulative exposure was 22.11 mg/m3-years, whereas the average exposure in the highest categorized groups, for this figure, was 9.59 mg/m3-years.
Figure 2.
Exponential Cox proportional hazard models of lung cancer mortality by unlagged cumulative exposure to Cr(VI) using all cohort members. Predicted relative risks are shown with smoking controlled or uncontrolled. Observed relative risks with 90% confidence intervals are presented. The Cox models were fit to individual data, and the highest cumulative exposure was 22.11 mg/m3-years, whereas the average exposure in the highest categorized groups, for this figure, was 9.59 mg/m3-years.
The lowest Cr(VI) exposures for which there was statistical evidence of an exposure-related increase in the risk of lung cancer mortality are shown in Table 4 (based on unlagged cumulative exposure) and Table 5 (based on highest average monthly exposure). In Table 4, there was scant statistical evidence of an effect of cumulative exposure until exposures at least exceeded 1.12 mg/m3-years, and statistical significance continued to increase as higher exposures were included in the analysis. These results, based on Cox modeling, are very similar to corresponding results obtained from Poisson modeling (Supplementary Table 5). The results for highest monthly exposures (Table 5) were less consistent. There was a significant trend when highest monthly exposures ⩽0.26 mg/m3 were included. However, as higher groupings of exposure were included, statistical evidence for an effect disappeared and did not reappear until highest monthly exposures ⩽0.57 mg/m3 were retained in the analysis. These analyses included smoking as a covariate, although similar results were obtained when smoking was not controlled (results not shown).
Table 4. Trend testa for significantly increased lung cancer risk with unlagged cumulative exposure.
| Cumulative exposures retained (mg/m3-years) | β (mg/m3-years)−1 | P-value | |
|---|---|---|---|
| MLE | 95% CI | ||
| ⩽0.14 | 4.9 | (−7.6, 16.5) | 0.42 |
| ⩽0.35 | –1.4 | (−6, 2.7) | 0.51 |
| ⩽0.47 | 0.43 | (−2.3, 3.1) | 0.75 |
| ⩽1.12 | 0.05 | (−1.2, 1.2) | 0.93 |
| ⩽1.41 | 0.89 | (0.06, 1.7) | 0.04 |
| ⩽2.14 | 0.48 | (−0.004, 0.93) | 0.05 |
| ⩽4.15 | 0.22 | (−0.02, 0.45) | 0.07 |
| ⩽6.27 | 0.29 | (0.13, 0.44) | 0.0004 |
| All | 0.19 | (0.12, 0.25) | <0.0001 |
Tests based on fit of the Cox exponential model adjusted for smoking.
Table 5. Trend testa for significantly increased lung cancer risk with highest monthly exposure.
| Highest monthly exposures retained (mg/m3) | β (mg/m3-years)−1 | P-valuea | |
|---|---|---|---|
| MLE | 95% CI | ||
| ⩽0.052 | 1.2 | (−7.9, 8.4) | 0.76 |
| ⩽0.104 | −1.3 | (−6.4, 1.5) | 0.43 |
| ⩽0.156 | 0.31 | (−1.7, 1.7) | 0.72 |
| ⩽0.208 | 0.31 | (−1.7, 1.7) | 0.72 |
| ⩽0.26 | 0.73 | (0.07, 1.2) | 0.03 |
| ⩽0.312 | 0.35 | (−0.092, 0.71) | 0.11 |
| ⩽0.416 | 0.01 | (−0.41, 0.34) | 0.97 |
| ⩽0.572 | 0.21 | (0.09, 0.31) | 0.0012 |
| All | 0.19 | (0.12, 0.25) | <0.0001 |
Tests based on fit of the Cox exponential model adjusted for smoking.
The effect of smoking was explored further in Cox models. The linear Cox model with unlagged cumulative exposure was restricted to 200 workers with known smoking history (157 smokers, 43 non-smokers) and smoking was controlled. The relative risk of lung cancer for smokers compared to non-smokers was 6.05 in the restricted model (Table 6). In the model including all workers regardless of known or unknown smoking status, relative risk of lung cancer for smokers compared with non-smokers was 5.01. For all linear Cox models, whether all workers were included or restricted to the workers with known smoking history, cumulative exposure to Cr(VI) added significantly to the lung cancer risk adjusted for smoking (P=0.000003 or 0.001, respectively). A χ2-test for non-homogeneity of smoking prevalence using 10 categories of cumulative Cr(VI) exposure was non-significant. Thus, the smoking data that were available showed no evidence of confounding. This is also suggested by the similarity of β estimates whether or not smoking is controlled (Table 3).
Table 6. The effect of smoking in linear Cox models with unlagged cumulative exposure to Cr(VI).
| Model 1: all workers (n=714) | β (Potency factor)a | ||
|---|---|---|---|
| Smoking | Deviance | MLE | 95% CI |
| Not controlled | 1252.26 | 0.649 | (0.279, 1.367) |
| Controlled | 1236.45 | 0.581 | (0.222, 1.322) |
|
Comparison groups |
RR | ||
| Non-smokers vs workers with unknown smoking status | 0.39 | ||
| Smokers vs workers with unknown smoking status | 1.94 | ||
| Smokers vs non-smokers | 5.01 | ||
|
Model 2: workers with known smoking history
(n=200) |
β (Potency factor)a |
||
| Smoking | Deviance | MLE | 95% CI |
| Not controlled | 504.28 | 0.434 | (0.0913, 1.541) |
| Controlled | 488.05 | 0.535 | (0.125, 1.922) |
|
Comparison groups |
RR | ||
| Smokers vs non-smokers | 6.05 | ||
Abbreviations: MLE, maximum likelihood estimate; RR, relative risk for lung cancer.
The unit of β for the relative risk is (mg/m3-years)−1.
Lung Cancer Risk Estimation
From the exponential and linear Cox models adjusted for smoking and age at hire, lifetime risk of lung cancer from occupational and environmental exposures were estimated (Tables 7 and 8). The unit risks calculated from linear Cox models with unlagged cumulative exposure were approximately three-times higher than comparable unit risk estimates calculated with the exponential Cox models, which is in keeping with the predictions from these models, showing lower risk for the exponential model (Figure 2) than the linear model (Figure 1). The unit risks derived using the linear Cox model were similar, but 15–20% lower, than those calculated in the previous study (Crump et al. 2003, Tables V and VI).7
Table 7. Unit risks of lung cancer mortality and effective concentrations associated with lifetime occupational exposurea to Cr(VI) from Cox models, controlled for smoking.
| Regression | Exposure lag (years) | EC10 (μg/m3)b | LEC10 (μg/m3)c | Unit riskd | 95% CI for unit risk |
|---|---|---|---|---|---|
| Exponential Cox | 0 | 123.2 | 18.4 | 0.000494 | (0.000314, 0.00338) |
| 5 | 131.7 | 98.2 | 0.000460 | (0.000281, 0.000618) | |
| 10 | 152.5 | 109.1 | 0.000395 | (0.000213, 0.000553) | |
| 15 | 191.7 | 127.6 | 0.000311 | (0.000128, 0.000468) | |
| Linear Cox | 0 | 64.4 | 30.6 | 0.00166 | (0.000713, 0.00349) |
| 5 | 70.6 | 33.4 | 0.00151 | (0.000639, 0.00320) | |
| 10 | 90.1 | 42.7 | 0.00119 | (0.000474, 0.00250) | |
| 15 | 123.2 | 56.4 | 0.000869 | (0.000300, 0.00190) |
Continuous occupational exposure (8 h/day, 240 days per year) from age 20 to 65.
EC10 is the estimated occupational exposure level associated with an additional lifetime lung cancer mortality risk of 0.1.
LEC10 is a lower 95% confidence limit for EC10.
Unit risk is the estimated additional lifetime risk from occupational exposure to 1 μg/m3. Both regressions included the three employees with the highest exposures.
Table 8. Unit risks of lung cancer mortality and effective concentrations associated with lifetime environmental exposurea to Cr(VI) from Cox models, controlled for smoking.
| Regression | Exposure lag (years) | EC10 (μg/m3)b | LEC10 (μg/m3)c | Unit riskd | 95% CI for unit risk |
|---|---|---|---|---|---|
| Exponential Cox | 0 | 24.3 | 20.4 | 0.00253 | (0.00160, 0.0191) |
| 5 | 27.0 | 20.2 | 0.00226 | (0.00137, 0.00305) | |
| 10 | 32.2 | 23.0 | 0.00189 | (0.00102, 0.00266) | |
| 15 | 14.6 | 6.7 | 0.00148 | (0.000609, 0.00225) | |
| Linear Cox | 0 | 12.8 | 6.1 | 0.00832 | (0.00359, 0.0174) |
| 5 | 14.6 | 6.9 | 0.00730 | (0.00309, 0.0154) | |
| 10 | 19.1 | 9.0 | 0.00560 | (0.00224, 0.0118) | |
| 15 | 26.1 | 11.9 | 0.00410 | (0.00142, 0.00892) |
Continuous environmental exposure (24 h/day, 365 days year−1) throughout life.
EC10 is the estimated environmental corresponding to an additional lifetime lung cancer mortality risk of 0.1.
LEC10 is a lower 95% confidence limit for EC10.
Unit risk is the estimated additional lifetime risk from environmental exposure to 1μg/m3. Both regressions included the three employees with the highest exposures.
DISCUSSION
The current study substantially increased the cohort size and person-years at risk from the previous follow-up, and captured the mortality status of short-term workers and those who started later in time and experienced lower exposure levels. As a result, statistical power in the lower exposure range was increased. The total number of lung cancer deaths in this study increased from the previous follow-up; however, the SMRs for the full cohort decreased, supporting that overall the updated cohort had lower exposures and decreased risk. Vital status was confirmed for 97% of workers with only 24 workers considered as LTF. As such, this study more completely describes the mortality experience of the Painesville cohort.
One unexpected observation from this study is that the exponential Cox model with unlagged exposure achieved the best model fit. In the previous assessment7 and in the Poisson regressions of the current data set, the linear model achieved optimal fit as expected. Because the exponential Cox model was particularly sensitive to the three workers with the highest cumulative exposure, we give preference to the linear model using Cox regression, with the three censored data points, because it is not reasonable that the outcome for the three most highly exposed workers informs the exposure-response in the low exposure range. However, the difference in β estimates between the linear and exponential model are noteworthy. Specifically, the β estimates are 2.3–3.4 times greater using the linear Cox model as compared with the exponential model.
Unit risks are used to assess the estimated increased cancer risk posed by occupational and environmental exposures to chemicals assuming low-dose linearity. Using the occupational unit risk factor derived herein, the cancer risk posed by continuous occupational exposure (every working day for 45 years) to the Cr(VI) OSHA permissible exposure limit (PEL) of 5 μg/m3 is 8.3 per 1000 (5 μg/m3 × 0.00166 (μg/m3)−1). However, the PEL is an 8-h average exposure that is not to be exceeded on any day; thus, long-term average exposures compliant with the PEL would certainly be lower than 5 μg/m3, as well as the calculated excess risk. Similarly, the theoretical risk associated with the current National Institute for Occupational Safety and Health recommended exposure limit of 0.2 μg/m3 is 3.3 × 10−4, and achieves the objective of obtaining increased cancer risk <1 per 1000.2 Using the environmental unit risk factor, the theoretical increased risk associated with environmental exposures can be calculated. Although environmental Cr(VI) data are relatively limited, the robust data sets from California and Texas suggests that current average ambient exposures are <0.0001 μg/m3.5, 6 Continuous exposure at this level is associated with an increased risk of 8.3 × 10−7, using the environmental unit risk factor of 0.0083 calculated herein (Table 8) and assuming low-dose linearity.
Although this study had increased power in the low exposure range by including short-term workers, consistent with the previous assessment7 lung cancer risk was not observed to be increased at cumulative exposures <1.4 mg/m3-years or highest monthly exposures <0.26 mg/m3. Conclusions of other studies based on the mode of action (MOA) and toxicokinetic data (specifically, detoxification by reduction prior to absorption) imply that the exposure-response may have a threshold in the low exposure range.33, 34, 35 Our analyses, based on low-dose linear non-threshold models, are not capable of detecting a threshold. As noted in Crump (2011),36 “any data set-no matter how extensive will be consistent with both threshold and low-dose linear responses (and also with low-dose sublinear responses).” Although not arguing against the existence of thresholds, the study author indicated there were serious problems with uses of thresholds in risk assessment and recommended a broader role for MOA.36
A recent review of the mechanistic, animal, human, and toxicokinetic data relevant to inhalation toxicity of Cr(VI) indicates that the evidence does not support a mutagenic MOA for Cr(VI)-induced lung cancer.35 Rather, review of the data support key events of the MOA including exceedance of pulmonary clearance mechanisms, tissue irritation, inflammation, and cytotoxicity, which ultimately result in chromosomal instability and tumor formation.35 Consistently, the recently updated mortality assessment for the Baltimore chromate production plant workers reported a significant associations between the occurrence of skin and nasal irritation and lung cancer mortality, concluding that irritation may have a role the MOA for lung cancer.37 Based on this MOA information, it is therefore reasonable to consider whether the exposure-response may be nonlinear in the low exposure range.
Recently, Haney et al. (2012) developed a cancer-based chronic inhalation reference value (ReV) for Cr(VI) based on supporting MOA and Cr(VI) toxicokinetic information.34 Candidate cumulative exposure points of departure (PODs) were identified from modeling results of several studies including the previous study of the Painesville workers.7, 38, 39 The daily and 3-month doses (total Cr(VI) mass) associated with the candidate PODs were then compared with the mean lung Cr(VI) reductive capacity estimates from the data of DeFlora et al. (1997).34, 40 It was shown that 3-month doses associated with the higher cumulative exposure candidate PODs were significantly greater than the extracellular reductive capacity (Table 9, Haney et al. 2012).34 The lower candidate POD (0.195 mg/m3-years which was developed from Birk et al. (2006)38) did not exceed estimates for extracellular reductive capacity. This exposure is also not associated with any observed increased risk in the Painesville cohort. Haney et al. concluded that doses exceeding the 3-month estimate for extracellular reductive capacity are of concern, and thus, the lower candidate POD was considered appropriate. With dosimetric adjustments and application of uncertainty factors (UFtotal=30), a general population POD of 7.1 μg/m3 and a chronic inhalation reference value for environmental exposure of 0.24 μg/m3 Cr(VI) were calculated.34
As with all mortality assessments, exposure reconstructions and risk assessments using epidemiologic data, there are a number of noteworthy limitations and uncertainties. Many of the uncertainties that applied to the previous analyses7, 16, 17, 18 also apply in this study, in particular the potential for misclassification of exposure due to limited monitoring data. Smoking information was also limited. However, several limitations faced in previous assessments were addressed in the current analysis. Statistical power, particularly in the low exposure range, was increased to the extent practical in the current study by extending follow-up and including short-term workers. Furthermore, through the use of ancestry.com, we were able to locate death certificates for workers who had previously been lost to follow-up. Although further efforts to retrieve death certificates may have further reduced the number lost to follow-up, it is not likely to have affected the results of the assessment.
Three deaths were identified as lung cancer were due to mesothelioma and coded as lung cancer under ICD-8(a) and 9, whereas three others were coded as mesotheliomas under ICD-10. In all cases of mesothelioma, latency from first exposure in the Painesville plant was long (25–55 years), and exposure to asbestos in the plant cannot be ruled out. This limitation is not exclusive to the Painesville cohort and likely affects lung cancer risk estimates from other studies where the cause of death is coded by earlier ICD codes.
With this investigation, we were also able to control for significant lung cancer risk factors including smoking and age at hire in the derivation of β values and unit risk estimates. The Painesville cohort was exposed to Cr(VI) at levels that are far in excess of the current occupational and environmental exposure levels. Hence, there is some uncertainty in extrapolating these results to present day exposures, and although the exposure-response was linear among this cohort, linearity may not hold at current environmental and occupational exposure levels. Nonetheless, owing to the use of updated mortality data and refined dose-response modeling approaches, this study provides improved information for assessing the potential cancer risk associated with exposure to Cr(VI).
Acknowledgments
This work was funded by the Electric Power Research Institute (EPRI), an independent nonprofit 501(c)3 organization that funds external research at a number of universities and institutes worldwide. Kevin Timp, who is a medical coder at Duke University Medical System, helped identify ICD codes associated with underlying cause of death. Finally, the authors acknowledge the thoughtful comments of the journal peer-reviewers.
Footnotes
Supplementary Information accompanies the paper on the Journal of Exposure Science and Environmental Epidemiology website (http://www.nature.com/jes)
This work was funded by the Electric Power Research Institute (EPRI), an independent non-profit 501(c)3 organization that funds external research at a number of universities and institutes worldwide. EPRI researcher and co-author, Dr. Annette Rohr, was given the opportunity to review the final draft manuscript. The purpose of the review was to allow input on the clarity of the science presented but not in interpretation of the research findings. DP, MS, and LM are employees of ToxStrategies. ToxStrategies is a private consulting firm providing services to private and public organizations on toxicology and risk assessment issues. DP and MS have presented study findings in meetings with regulators including public meetings on behalf of EPRI. DP has also been an expert in litigation involving Cr(VI). SH, RVS, CB, CVL, and KC are independent contractors to ToxStrategies and have no conflict of interest.
Supplementary Material
References
- 1IARC Chromium, nickel, and welding. IARC Monograph on the Evaluation of Carcinogenic Risks to Humans. Vol 49. World Health Organization: Lyon, France. 1990. [PMC free article] [PubMed] [Google Scholar]
- 2NIOSH Criteria for a Recommended Standard. Occcupational Exposure to Hexavalent Chromium. DHHS (NIOSH) Publication No. 2013–128. 2013.
- 3IARC IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Chromium (VI) Compounds. Volume 100C Lyon, France: International Agency for Research on Cancer, 2012. Available at: http://monographs.iarc.fr/ENG/Monographs/vol100C/mono100C-9.pdf.
- 4ATSDR Toxicological Profile for Chromium. Atlanta, GA: US Department of Health and Human Services, Public Health Service, 2012.
- 5CARB California Air Resources Board. ADAM: Annual Toxics Summary. North Long Beach, Hexavalent Chromium, 2015. Available at: http://www.arb.ca.gov/adam/toxics/sitepages/cr6nlbc.html.
- 6TCEQ Texas Commission on Environmental Quality. Development Support Document, Final. Hexavalent Chromium (Particulate Compounds), 2014. Available at: https://www.tceq.texas.gov/assets/public/implementation/tox/dsd/final/august2014/hexavalent_chromium.pdf.
- 7Crump C, Crump K, Hack E, Luippold R, Mundt K, Liebig E et al. Dose-response and risk assessment of airborne hexavalent chromium and lung cancer mortality. Risk Anal 2003; 23: 1147–1163. [DOI] [PubMed] [Google Scholar]
- 8Haney JT, Jr, Erraguntla N, Sielken RL, Jr, Valdez-Flores C. Development of an inhalation unit risk factor for hexavalent chromium. Regul Toxicol Pharmacol 2014; 68: 201–211. [DOI] [PubMed] [Google Scholar]
- 9Park RM, Bena JF, Stayner LT, Smith RJ, Gibb HJ, Lees PS. Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. Risk Anal 2004; 24: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 10OSHA Occupational Exposure to Hexavalent Chromium Final Rule. 29 CFR Parts 1910, 1915, 1917, 1918, and 1926, 2006. [PubMed]
- 11Seidler A, Jahnichen S, Hegewald J, Fishta A, Krug O, Ruter L et al. Systematic review and quantification of respiratory cancer risk for occupational exposure to hexavalent chromium. Int Arch Occup Environ Health 2012; 86: 943–955. [DOI] [PubMed] [Google Scholar]
- 12Mancuso TF. Consideration of chromium as an industrial carcinogen. International Conference on Heavy Metals in the Environment: Toronto, Ontario, Canada, 27–31 October, 1975, pp 343–356. [Google Scholar]
- 13U.S. EPA Toxicological review of hexavalent chromium in support of summary information on the integrated risk information system (IRIS). US Environmental Protection Agency: Washinton, DC, USA. 1998. [Google Scholar]
- 14Mancuso RF. Occupational cancer and other health hazards in a chromate plant: a medical appraisal. II. Clinical and toxicologic aspects. Ind Med Surg 1951; 20: 393–407. [PubMed] [Google Scholar]
- 15Mancuso TF. Chromium as an industrial carcinogen: part 1. Am J Ind Med 1997; 31: 129–139. [DOI] [PubMed] [Google Scholar]
- 16Luippold RS, Mundt KA, Austin RP, Liebig E, Panko J, Crump C et al. Lung cancer mortality among chromate production workers. Occup Environ Med 2003; 60: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17Proctor DM, Panko JP, Liebig EW, Paustenbach DJ. Estimating historical occupational exposure to airborne hexavalent chromium in a chromate production plant: 1940—1972. J Occup Environ Hyg 2004; 1: 752–767. [DOI] [PubMed] [Google Scholar]
- 18Proctor DM, Panko JP, Liebig EW, Scott PK, Mundt KA, Buczynski MA et al. Workplace airborne hexavalent chromium concentrations for the Painesville, Ohio, chromate production plant (1943–1971). Appl Occup Environ Hyg 2003; 18: 430–449. [DOI] [PubMed] [Google Scholar]
- 19NCHS National Center for Health StatisticsNational Death Index Users Guide. US Department of Health and Human Services: Hyattsville, MD, USA. 2013. [Google Scholar]
- 20NCHS National Center for Health Statistics. Instruction manual, part 2a: instructions for classifying the underlying cause of death. US Department of Health and Human Services: Hyattsville, MD, USA. 2008. [Google Scholar]
- 21DHHS Department of Health and Human Services. Centers for Disease Control and Prevention. National Center for Health Statistics. Compressed Mortality File 1989–1998, 2007..
- 22DHHS Centers for Disease Control and Prevention. National Center for Health Statistics. Compressed Mortality File 1999–2010, 2013.
- 23DHHS Department of Health and Human Services. Centers for Disease Control and Prevention. National Center for Health Statistics. Compressed Mortality File 1968–1988, 2013. Availabe at: http://www.cdc.gov/nchs/data_access/cmf.htm-data_release.
- 24SEER Surveillance, Epidemiology, and End Results (SEER) Program (http://www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969–2011) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released July 2014. Underlying mortality data provided by NCHS (http://www.cdc.gov/nchs), 2014.
- 25Crump KS, Allen B. Toward making epidemiologic data more useful for quantitative risk assessment. Open Epidemiol J 2011; 4: 30–44. [Google Scholar]
- 26Cox DR, Oakes D. Analysis of Survival Data. Chapman and Hall: London, UK. 1984. [Google Scholar]
- 27Venson D, Moolgavkar S. A method for computing profile-likelihood-based confidence intervals. Appl Stat 1984; 37: 87–94. [Google Scholar]
- 28Gatto NM, Kelsh MA, Mai DH, Suh M, Proctor DM. Occupational exposure to hexavalent chromium and cancers of the gastrointestinal tract: a meta-analysis. Cancer Epidemiol 2010; 34: 388–399. [DOI] [PubMed] [Google Scholar]
- 29Welling R, Beaumont JJ, Petersen SJ, Alexeeff GV, Steinmaus C. Chromium VI and stomach cancer: a meta-analysis of the current epidemiological evidence. Occup Environ Med 2015; 72: 151–159. [DOI] [PubMed] [Google Scholar]
- 30Linos A, Petralias A, Christophi CA, Christoforidou E, Kouroutou P, Stoltidis M et al. Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece—an ecological study. Environ Health 2011; 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31Beaumont JJ, Sedman RM, Reynolds SD, Sherman CD, Li LH, Howd RA et al. Cancer mortality in a Chinese population exposed to hexavalent chromium in drinking water. Epidemiology 2008; 19: 12–23. [DOI] [PubMed] [Google Scholar]
- 32Gibb HJ, Hoffman HJ, Haver C. Biologic implications from an epidemiologic study of chromate production workers. Open Epidemiol J 2011; 4: 54–59. [Google Scholar]
- 33De Flora S. Threshold mechanisms and site specificity in chromium(VI) carcinogenesis. Carcinogenesis 2000; 21: 533–541. [DOI] [PubMed] [Google Scholar]
- 34Haney JT, Erraguntla N, Sielken RL, Valdez-Flores C. Development of a cancer-based chronic inhalation reference value for hexavalent chromium based on a nonlinear-threshold carcinogenic assessment. Regul Toxicol Pharmacol 2012; 64: 466–480. [DOI] [PubMed] [Google Scholar]
- 35Proctor DM, Suh M, Campleman SL, Thompson CM. Assessment of the Mode of Aaction for hexavalent chromium-induced lung cancer following inhalation exposures. Toxicology 2014; 325: 160–179. [DOI] [PubMed] [Google Scholar]
- 36Crump KS. Use of threshold and mode of action in risk assessment. Crit Rev Toxicol 2011; 41: 637–650. [DOI] [PubMed] [Google Scholar]
- 37Gibb HJ, Lees PS, Wang J, Grace O'Leary K. Extended followup of a cohort of chromium production workers. Am J Ind Med 2015; 58: 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Birk T, Mundt KA, Dell LD, Luippold RS, Miksche L, Steinmann-Steiner-Haldenstaett W et al. Lung cancer mortality in the German chromate industry, 1958 to 1998. J Occup Environ Med 2006; 48: 426–433. [DOI] [PubMed] [Google Scholar]
- 39Park RM, Stayner LT. A search for thresholds and other nonlinearities in the relationship between hexavalent chromium and lung cancer. Risk Anal 2006; 26: 79–88. [DOI] [PubMed] [Google Scholar]
- 40De Flora S, Camoirano A, Bagnasco M, Bennicelli C, Corbett GE, Kerger BD. Estimates of the chromium(VI) reducing capacity in human body compartments as a mechanism for attenuating its potential toxicity and carcinogenicity. Carcinogenesis 1997; 18: 531–537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.