Abstract
The NIMH RDoC initiative calls for the incorporation of neurobiological approaches and findings into conceptions of mental health problems through a focus on biobehavioral constructs investigated across multiple domains of measurement (units of analysis). Though the constructs in the RDoC system are characterized in ‘process terms’ (i.e., as functional concepts with brain and behavioral referents), these constructs can also be framed as dispositions (i.e., as dimensions of variation in biobehavioral functioning across individuals). Focusing on one key RDoC construct, acute threat or ‘fear’, the current paper illustrates a construct-oriented psychoneurometric strategy to operationalizing this construct in individual-difference terms—as threat sensitivity (THT+). Utilizing data from 454 adult participants, we demonstrate empirically that: 1) a scale measure of THT+ design to tap general fear/fearlessness predicts effectively to relevant clinical problems (i.e., fear disorder symptoms), 2) this scale measure shows reliable associations with physiological indices of acute reactivity to aversive visual stimuli, and 3) a cross-domain factor reflecting the intersection of scale and physiological indicators of THT+ predicts effectively to both clinical and neurophysiological criterion measures. Results illustrate how the psychoneurometric approach can be used to create a dimensional index of a biobehavioral trait construct, in this case THT+, which can serve as a bridge between phenomena in domains of psychopathology and neurobiology. Implications and future directions are discussed with reference to the RDoC initiative and existing report-based conceptions of psycholological traits.
The National Institute of Mental Health’s Research Domain Criteria (RDoC; Kozak & Cuthbert, this issue) initiative calls for a shift away from traditional categorical conceptions of mental disorders—as embodied in current versions of the Diagnostic and Statistical Manual of Mental Disorders [DSM-5; American Psychiatric Association 2013] and International Classification of Disease [ICD-10, World Health Organization, 2004)—toward a continuous-dimensional approach directed at relating clinical symptomatology to process-oriented constructs deduced from biobehavioral research with animals and humans. More specifically, RDoC encourages research on mental health and illness using biobehavioral constructs such as acute threat [‘fear’], reward valuation, and response inhibition, grouped within broad ‘systems’ domains (negative affect, positive affect, cognition, social processes, arousal/regulation), that can be studied across multiple levels of analysis—from genes to brain circuits and physiology to observable behavior and self- or other-report. While ambitious in its scope and promising in its potential to reshape practices in psychopathology research, the RDoC initiative faces considerable (though conceivably addressable) challenges. As discussed in detail by Lilienfeld (2014), these include the strong emphasis of RDoC on biological measures (at the risk of ignoring other domains of measurement) and psychometric considerations such as measurement error and construct validity. The current paper describes an RDoC-compatible research strategy, the “psychoneurometric” paradigm, for addressing these crucial challenges. This strategy focuses on operationalizing trait-dispositional conceptions of RDoC constructs using indicators from differing domains of measurement, including biological and behavioral indicators together with report-based variables.
Building on themes featured in other recent writings (Nelson et al., 2015; Patrick et al., 2012, 2013; Venables et al, 2015.; Yancey, Vaidyanathan, & Patrick, 2014), the current paper focuses on psychoneurometric operationalization of the RDoC construct of acute threat, in the process highlighting three key points: (1) research on psychopathology, whether experimental or correlational in nature, is inherently individual differences research; (2) process constructs from the RDoC framework (or matrix; Kozak & Cuthbert, this issue) such as acute threat, response inhibition, and reward valuation can be framed and explicitly studied as individual difference (i.e., dispositional) constructs; and (3) the RDoC research initiative, by encouraging investigation of target constructs conceptualized in these alternative ways (i.e., both as dispositions and as processes), has the potential to reshape existing report-based conceptions of psychological traits hand-in-hand with reshaping ideas about clinical liabilities and pathophysiologies.
RDoC constructs as dispositional variables
Over a half-century ago, Cronbach (1957; see also Cronbach, 1975) drew attention to a persisting division between what he termed the “two disciplines of scientific psychology,” referring to smaller-N experimental research examining effects of manipulated variables and larger-N correlational studies evaluating relations among ‘variables-in-nature.’ His view was that these two disciplines, while proceeding largely along separate tracks to that point, possess complementary strengths and should be integrated to combine these strengths. A fundamental difference between the two disciplines noted by Cronbach was their focus on general (nomothetic) effects versus cross-subject (idiographic) variation: “Individual differences have been an annoyance rather than a challenge to the experimenter. His goal is to control behavior [to demonstrate a treatment effect]…Individual variation is cast into that outer darkness known as ‘error variance’…to be reduced by any possible device.” (p. 674). By contrast, “The correlational psychologist is in love with just those variables the experimenter left home to forget. He regards individual and group variations as important effects of biological and social causes.” (p. 674).
This distinction highlighted by Cronbach is important to consider in relation to the RDoC framework. On one hand, the experimentalist tradition is strongly evident in RDoC: The framework has a prominent biological-systems orientation and is designed to accommodate animal as well as human research. Target constructs specified in the RDoC matrix are framed in basic biobehavioral ‘process’ terms—e.g., as core functional concepts with referents in brain systems and behavior (e.g., acute threat, response inhibition)—rather than in individual difference terms. On the other hand, however, the phenomena that the RDoC initiative seeks to understand—clinical conditions—are inherently individual difference conceptions: Whether defined in terms of groupings based on traditional diagnostic criteria or scores on dimensions of impairment as advocated by RDoC (Kozak & Cuthbert, this issue), clinical problems are person factors reflecting naturally-occurring variability across individuals. From this standpoint, there are advantages to conceiving of RDoC constructs in alternative, dispositional terms. Framed this way, RDoC constructs can be investigated as biologically-based variations across people in tendencies that are related to clinical problems. For example, acute threat and response inhibition can be framed and studied, respectively, as proneness to react more less strongly to acute aversive stimuli (threat sensitivity) and capacity to suppress prepotent responses more or less effectively (inhibitory control).
Framing RDoC constructs in this way establishes concrete referents, in the form of dispositional concepts, for connecting process constructs to dimensions of clinical impairment. Dispositional threat sensitivity, for example, connects readily to phobic fear and avoidance, and weak inhibitory control (or disinhibition; Patrick et al., 2013) connects readily to impulsive-aggressive behavior. Framing RDoC constructs in this way also provides objective criteria for evaluating the relevance of dependent measures from experimental tasks to the aims of the initiative: Task measures should capture reliable person-variance that intersects with (i.e., predicts to) clinical problem dimensions. Viewed this way, dispositional dimensions corresponding to RDoC constructs can serve as valuable intermediaries for linking biobehavioral measures from lab tasks to real-life clinical phenomena.
Importantly, from the perspective of RDoC, dispositional counterparts to process constructs should incorporate data from domains other than self- or other-report (e.g., biological, behavioral) in order to contribute to a multi-domain understanding of clinical problems. And notably, per classic writings on the topic of construct validation (discussed further below), the use of indicators from other domains to operationalize individual difference concepts can lead to changes in the concepts themselves—through a process that Cronbach and Meehl (1955) termed bootstrapping. Viewed in this way, an RDoC approach to the investigation of clinically-relevant trait dispositions can contribute to the formulation of new, biobehaviorally-oriented individual difference concepts, as a complement to existing models of personality dispositions based mainly on self-report data.
Multi-domain operationalization of trait dispositions: The psychoneurometric approach
A core challenge that needs to be addressed in efforts to quantify clinically-relevant dispositions using biological or behavioral indicators is the issue of method variance. It is well known that measures of a common construct from differing domains of measurement (e.g., report, behavior) correlate only moderately, and that indicators of only somewhat related constructs from differing domains exhibit only modest associations (Campbell & Fiske, 1959; Mischel, 1968). This constraint accounts for the modest success to date of research aimed at identifying reliable physiological biomarkers of clinical problems or affiliated person-characteristics (Kalia & Costa e Silva, 2015; see also Miller & Rockstroh, 2013). A systematic strategy for addressing the issue of method variance in cross-domain assessment of clinically-relevant dispositions is the psychoneurometric approach (Patrick & Bernat, 2010; Patrick et al., 2012, 2013). This approach is grounded in classic perspectives on psychological assessment, which conceive of psychological attributes as constructs that transcend particular domains of measurement (Cronbach & Meehl, 1955; Loevinger, 1957). Viewed this way, ideas regarding the nature of a trait construct and how to measure it are considered provisional and subject to modification based on data.
Biobehavioral dispositions corresponding to RDoC constructs, such as threat sensitivity or inhibitory control, can serve as effective targets for this analytic approach. The starting point entails identifying reliable physiological indicators of the target trait construct operationalized psychometrically—i.e., through scores on an effective report-based scale that shows validity in predicting to relevant clinical problems. Work along this line consists of simple bivariate mapping of candidate physiological indicators to trait-scale scores. Once multiple physiological indicators of the scale-defined trait have been identified, these differing indicators can be combined with one another to form a composite neurometric index of the trait (cf. Nelson, Patrick, & Bernat, 2011), or with each other and one or more scale measures to form a composite psychoneurometric index of the trait (Patrick et al., 2013). Knowledge gained about the convergence of alternative physiological indicators from differing experimental tasks, and about underlying processes contributing to this convergence, in turn feeds back into conceptualization of the target construct and further ideas about how to operationalize it. In this recursive (“bootstrapping”) manner, the original self-report based conception of the trait shifts to accommodate findings for the physiological indicators.
Patrick et al. (2013) used this approach to develop a composite trait-scale/electrocortical-response index of weak inhibitory control (disinhibition) that predicted effectively to a criterion measure of brain response (target P3 amplitude) as well as to differing impulse control problems (i.e., child and adult antisocial behaviors, alcohol and drug problems, borderline personality tendencies). The current work was undertaken to develop and validate a counterpart psychoneurometric index of dispositional threat sensitivity.
Threat sensitivity: psychometric assessment and neurophysiological correlates
The psychological label attached to the biobehavioral construct of “acute threat” in the RDoC framework is “fear.” Assorted scale measures exist for assessing individual differences in fear/fearlessness as related to specific situations and stimuli (i.e., animals/objects, social contexts, circumstances of danger/uncertainty, and other stressors). Kramer et al. (2012) undertook a quantitative-structural analysis of scale measures of this type in an adult twin sample, and found evidence for a general factor on which all scales loaded; scores on this factor, interpretable as a dimension of dispositional threat sensitivity (THT+), were appreciably heritable (~.7) and accounted for relations of individual scales with a physiological indicator of threat reactivity—i.e., aversive startle potentiation (see also Vaidyanathan, Patrick, & Bernat, 2009). As regards clinical problems, other recent work by Nelson et al (2015) has demonstrated that scores on a scale measure of this general fear/fearlessness (THT+) factor effectively predict symptoms of differing ‘fear disorder’ conditions (i.e., specific phobia, social phobia, panic disorder, agoraphobia; cf. Krueger, 1999; Slade & Watson, 2006).
As regards physiological indices of THT+, basic emotion research has yielded evidence for reactivity of differing response systems to negatively-valent stimuli such as aversive pictures or imagery scripts (Vrana, Cuthbert, & Lang, 1986; Vrana, Spence, & Lang, 1988). Reliable indicators of negative activation include the startle blink reflex, corrugator muscle tension, and heart rate (HR) response (Bradley et al, 2001a; Cook, Davis, Hawk, Spence, & Gautier, 1992; Lang, 1995). The startle reflex is presumed to index the core action-priming component of emotional valence—increasing with activation of the brain’s acute threat (fear) system (Davis, 1989; Lang, Bradley, & Cuthbert, 1990)—and corrugator EMG reactivity and HR acceleration operate as facial-somatic and cardiac-visceral indices of unpleasant stimulus processing (Fridlund, Schwartz, & Fowler, 1984; Larson, Norris, & Cacioppo, 2003) and mobilization for defensive coping (Graham, 1979), respectively.
Other work showing enhanced physiological reactivity to aversive pictures or imagined scenarios in individuals with fear-related clinical conditions supports the idea that variation in indicators of these types reflects clinically-relevant person variance. For example, increased potentiation of the startle reflex during aversive cuing has been reported in patients with specific phobias (Hamm et al, 1997; Cuthbert et al, 2003; Lang & McTeague, 2009), social phobia (McTeague et al, 2009), and panic disorder (Melzig et al, 2007). As a counterpoint to this, decreased aversive startle potentiation has been reported for individuals high in affective-interpersonal features of psychopathy, presumed to reflect fearlessness (Benning, Patrick, & Iacono, 2005; Patrick, 1994). Building on these findings, Yancey et al. (2014) showed that the relationship between fear pathology and enhanced startle during aversive picture viewing was mediated by dispositional threat sensitivity (THT+) as indexed by a scale measure of Kramer et al.’s (2012) fear/fearlessness factor. Other work has reported enhanced corrugator EMG activation in patients with specific phobias and posttraumatic stress disorder (PTSD) during processing of fear-related imagery (Cuthbert et al., 2003), and among individuals high in state anxiety when viewing aversive pictures (Smith, Bradley, & Lang, 2005). Increased acceleratory HR response during aversive cuing has likewise been reported in individuals with phobia diagnoses (Cook, Melamad, Cuthbert, McNeil, & Lang, 1988; Hamm et al., 1997; Ruiz-Padial, Mata, Rodríguez, Fernández, & Vila, 2005) and participants high in dispositional fear (Cook et al., 1992). Other physiological variables linked to fear pathology or dispositional fear/fearlessness include baseline corrugator muscle tension (McTeague et al., 2009), differential late positive potential (LPP) brain response to aversive versus neutral picture stimuli (Venables, Hall, Yancey, & Patrick, 2015), and differential P3 brain response to noise probes occurring during aversive versus neutral picture stimuli (Drislane et al., 2013; Patrick, Durbin, & Moser, 2012).
In sum, evidence from differing sources suggests that: 1) a common dispositional factor underlies various scale measures of fear/fearlessness and scores on this factor are predictive of fear-related clinical problems, 2) activation in physiological systems including reflexive (startle), facial (corrugator EMG), and autonomic (HR) occurs to varying degrees during aversive cuing and this variation can be viewed as person-driven, and 3) variations in physiological responsiveness to aversive stimuli may reflect fear-related dispositional tendencies. Integrating these lines of evidence, we posited the existence of a common trait-dispositional factor contributing to variations in both self-reported fearfulness and physiological reactivity to phasic aversive stimuli.
Current Study Aims
Operating from the premise that biobehavioral threat sensitivity (THT+) is manifested in differing observable domains, the current work sought to: (a) operationalize THT+ through use of both psychometric scale and physiological response indicators—i.e., as a psychoneurometric composite, and (b) demonstrate the validity of THT+ indexed in this manner for predicting clinical and physiological criterion measures. Operationalized this way, THT+ and other dispositional constructs can serve as referents for linking RDoC process measures to clinical problem variables, as practical tools for cross-domain prediction, and as anchor points for an alternative biobehavioral model of psychological traits.
Method
Participants
The base sample for the study consisted of 508 adult twins (257 female) recruited from the greater Twin Cities metro area who participated for a payment of $100. Most participants were tested concurrently with their same gender co-twin on the same day, but by different experimenters in separate laboratory testing rooms. Participants were selected for participation in lab testing based on levels of THT+ as indexed by scores on the Trait Fear inventory described below, and as being free from visual or hearing impairments as assessed by a screening questionnaire. From among the full base sample, 22 participants were excluded from analyses due to missing individual difference data; 32 others were excluded due to missing or artifact-ridden data for two or all three of the main physiological indicators of THT+.1 These exclusions resulted in a final N of 454 for data analysis. The mean age of study participants, 51.3% of whom were female, was 29.5 (SD = 4.84). All participants provided informed written consent and study procedures were approved by the University of Minnesota’s Institutional Review Board.
Dispositional and Diagnostic Measures
Psychometric index of threat sensitivity: Trait Fear Inventory
Participants were assessed for THT+ in the self-report domain using a psychometric scale developed to index a broad dimension of fear/fearlessness identified through structural modeling analyses (Kramer et al., 2012; see also Vaidyanathan et al., 2009; Vizueta, Patrick, Jiang, Thomas, & He, 2012)—the 55-item Trait Fear inventory (TF-55). The items of the TF-55 are drawn from various established self-report inventories of fear and fearlessness, including the Fear Survey Schedule-III (Arrindell et al., 1984), the Fearfulness subscale of the EASI Temperament Survey (Buss and Plomin, 1984), the Harm Avoidance subscale of the Temperament and Personality Questionnaire (Cloninger, 1987), subscales comprising Factor 1 of the Psychopathic Personality Inventory (Lilienfeld and Andrews, 1996), and the Thrill/Adventure Seeking subscale of the Sensation Seeking Scale (Zuckerman, 1979). Scores on this scale measure correlate very highly (r > .9) with scores on the general fear/fearlessness factor from the structural model of these differing inventories (Kramer et al., 2012; see also: Patrick, Durbin, & Moser, 2012; Vaidyanathan et al., 2009). The items included in the TF-55 are inherently dispositional in nature and designed to index stable trait-like tendencies as opposed to transitional states. A total score was computed for each participant as the average score across individual items, each coded 0 to 3; descriptive statistics for this TF-55 score variable in the current sample were: M = 1.13, SD = .47, range = .04 to 2.51. Internal consistency reliability (Cronbach’s α) in the analysis sample was .96.
Participants for lab testing were selected from a larger base sample administered the TF-55 (N = 2,511; Kramer, et al. 2012). Half of the test sample (one member of each twin pair) was pre-selected based on TF-55 scores to ensure effective representation of individuals at high and low levels of THT+. Specifically, about one-third were chosen to be high in THT+ (i.e., highest 18% of screening sample), one third low (lowest 18%), and the remaining third intermediate (19th to 82nd percentile of scorers). The co-twins of these pre-selected individuals comprised the other half of the sample. The TF-55 was re-administered to all participants at the time of testing and scores from this administration were utilized in all analyses.
Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I)
All participants were assessed for the full range of lifetime Axis I psychiatric disorders, including anxiety and mood disorders, using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I; First et al., 2002). Each participant was interviewed by a PhD-level clinical psychologist or advanced clinical psychology graduate student trained in administration and scoring of the SCID-I diagnostic interview. Interviewers had no knowledge of other assessment data collected from interviewees. Symptom ratings were assigned through a consensus process involving meetings of the study interviewers (cf. Iacono, Carlson, Taylor, Elkins, & McGue, 1999), attended by the project PI (Christopher Patrick) and a licensed clinical psychologist who provided expert consultation on ratings and diagnostic decisions. Symptom count variables for the following ‘fear’ disorders (cf. Krueger et al., 1999; Nelson et al., 2015; Vaidyanathan, Patrick, & Iacono, 2011) were used in the current analyses: specific phobia, social anxiety disorder, panic disorder, and agoraphobia; 58.4% of the sample exhibited one or more symptoms of these fear disorders, with approximately 19% meeting full diagnostic criteria for one or more disorder. For each of these disorders, a proportion score was computed consisting of the number of symptoms endorsed for a participant divided by the maximum number possible, and these proportion scores were averaged across disorders to form a composite fear disorder score for each participant. For purposes of evaluating discriminant validity of variables expected to predict fear symptomatology (see below), we also computed scores for a substance disorder composite consisting of the average of symptom-proportion scores for alcohol and drug diagnoses (i.e., abuse and dependence).
Procedure and Experimental Paradigms
The data for the current analyses were collected as part of a larger physiological assessment protocol that included an affective picture-viewing task and a visual oddball task. While seated in a padded recliner, participants completed a series of questionnaires including the TF-55. During questionnaire administration, an electroencephalographic (EEG) cap and peripheral electrodes were attached to record EEG and peripheral physiological response (facial EMG and HR) data. During testing, participants viewed the task stimuli on a 21″ computer monitor, situated 1 m away at eye level. Stimuli were presented using a PC computer running E-Prime software (Psychology Software Tools), and physiological data were collected using a second PC computer running Scan 4 software (Neuroscan, Inc.).
The picture-viewing task included 90 pictures consisting of 30 pleasant, 30 neutral, and 30 aversive scenes selected from the International Affective Picture System (IAPS; Center for the Study of Emotion and Attention, 1999). Each picture stimulus was presented for 6 s, followed by an intertrial interval of 12 s preceding the next picture presentation, during which a fixation cross was displayed. Pleasant pictures included erotic, nurturant (babies and small animals), and adventure scenes (10 each). Neutral pictures included household objects, buildings, and neutral faces (also 10 each). Aversive scenes included 20 threat pictures (aimed guns and attacking animals) and 10 mutilation pictures (injured bodies, limbs, faces). During 81 of the 90 picture stimuli, noise probes (50 ms, 105 dB, 10 μs rise time) were presented at 3, 4, or 5 s into the 6 s presentation interval to elicit startle blink responses. Within and between orders, picture stimuli and noise probes were counterbalanced such that all picture valence categories (pleasant, neutral, and aversive) were represented equally across orders at each serial position, with the following constraints: no more than two pictures of the same valence occurred consecutively within any stimulus order; pictures of the same content category never appeared consecutively or across orders; and pictures were rotated so as to serve in both probed and unprobed conditions.
Data Acquisition
EEG and EMG activity were recorded from 54 scalp sites using Neuroscan Synamps 2 amplifiers and sintered Ag-AgCl EEG electrodes, positioned within a head-cap in accordance with the 10–20 system (Jasper, 1958). Separate electrodes were placed above and below the left eye to monitor vertical electrooculogram (VEOG) activity, and adjacent to the outer canthi of the left and right eyes to monitor horizontal electrooculogram (HEOG) activity. Facial EMG activity was measured using sintered Ag-AgCl electrodes filled with electrolyte paste and positioned above and below the left eye—over the corrugator supercilii muscle and the orbicularis oculi muscle, respectively. HR was recorded from Ag-AgCl electrodes placed on the forearms. All electrode impedances were kept below 10 KOhms. EEG/EMG signals were digitized on-line at 1000 Hz during data collection with an analog band pass filter of .05–200 Hz.
Data Processing and Reduction
Physiological Indicators of THT+: Startle blink, facial EMG, and heart rate measures
Corrugator and orbicularis EMG response during the affective picture-viewing task were each computed as average change in activity over the initial 3 s following picture onset, relative to a 1s pre-picture baseline—and mean response scores across trials were computed for each picture category (pleasant, neutral, aversive). Average corrugator EMG activity across all trials for the 1-s period preceding picture onset was computed as an index of general muscle tension. Startle blink reactivity was quantified as peak magnitude of orbicularis EMG occurring 30 – 120 ms after noise-probe presentation. Blink magnitude values were then standardized across picture trials within subject, with the mean across all trials for each participant scaled to equal 50 (cf. Patrick et al., 1993; for a more detailed discussion, see Yancey et al., 2014). HR data were processed using an automated Matlab protocol (Mathworks, Inc.) in which cardiac R-spikes were detected and interbeat intervals were used to compute HR in beats per minute during each picture trial. Based on prior work, (Bradley et al, 2001a), HR-change values were computed for 500-ms bins spanning the 6-s picture viewing interval, with change for each bin expressed relative to a 1-s pre-picture baseline. Consistent with Bradley et al. (2001a), inspection of the aggregate HR waveform for pictures of each type revealed an initial deceleratory component followed by a subsequent acceleratory component. For purposes of analysis, the acceleratory component was computed as the peak HR change from baseline across a window of 3 – 6 s after picture onset, and an average score across trials was computed for each picture valence condition.
Physiological criterion measures
In addition to quantifying aversive startle potentiation, corrugator reactivity, and heart rate acceleration as physiological indicators of THT+ to be combined with TF-55 scores into a psychoneurometric composite, we also quantified four other electrocortical and peripheral response variables as physiological criterion measures. These included general muscle tension (i.e., mean pre-picture corrugator EMG activity) and aversive-minus-neutral orbicularis EMG difference as described just above, and two brain event-related potential (ERP) measures—namely, the late positive potential (LPP) response elicited by picture presentations, and the P3 response elicited by intermittent noise probe stimuli.
As regards these latter ERP variables, procedures for recording and processing of EEG signal data from the picture-startle task followed those used in prior work by our group (Venables et al, 2014; Yancey et al, 2013). Raw EEG signals were referenced to electrode site Cz during on-line data collection, epoched offline from 1,000 ms before to 2,000 ms after stimulus onset, and then re-referenced to average mastoid activity. Trial-level EEG data were corrected for ocular activity and movement artifacts using the algorithm developed by Semlitsch, Anderer, Schuster, and Presslich (1986), as implemented in the Neuroscan Edit software routine, Version 4.5. Data were then imported into Matlab (Mathworks) for further processing, including resampling down to 128 Hz with application of an antialiasing filter prior to downsampling. For each picture valence category (pleasant, neutral, aversive), the LPP was computed from the aggregate cross-trial waveform as the average amplitude from 600 – 1000 ms after picture onset referenced to a 200 ms pre-picture baseline (cf. Weinberg et al., 2015). Probe P3 was quantified, again by valence category, as the peak amplitude of the aggregate waveform occurring during an interval of 250 – 351.56 ms following the onset of noise probes relative to a 300 ms pre-probe baseline (Patrick et al, 2013). For these two ERP variables, peak values at electrode site Pz for each picture valence category were used in analyses.
In addition, P300 response from the visual oddball task mentioned above was quantified as the maximal positive-going deflection within 297–602 ms (Yancey et al, 2013) following target infrequent stimuli within the task. This peak score served as a separate, discriminant validity criterion. Previous literature has shown P300 to be a well-established neurophysiological indicator of disinhibitory problems and proclivities (Iacono et al., 2003; Yancey et al., 2013). Since THT+ operationalized as dispositional fear/fearlessness is largely independent of disinhibitory tendencies (Patrick, Durbin, & Moser, 2012; Nelson et al., 2015), P300 amplitude was expected to be unrelated to THT+.
Data Analysis
Initial ANOVAs
Two-way mixed-model ANOVAs were first conducted to establish physiological variables from the picture-viewing task as correlates of THT+. The analysis for each physiological variable included scale-assessed THT+ (TF-55 score) as a continuous between-subjects factor and picture valence (neutral, aversive) as a discrete within-subjects factor.2,3 For all picture-task variables described in the preceding section except HR acceleration and general corrugator muscle tension, significant THT+ x Valence interaction effects were evident (all ps<.05)—and for these variables (i.e., blink startle, corrugator EMG reactivity, orbicularis EMG, LPP, and noise-probe P3), an aversive-minus-neutral difference score was computed for use in the main correlational analyses described below and reported on in the Results section.4 For HR acceleration, the THT+ x Valence interaction effect only approached significance (p<.10), but the THT+ main effect was clearly significant (p=.01)—with follow-up analyses showing this main effect to be driven more by HR response to aversive pictures (p<.005; see Table 1) than to neutral pictures (p=.29). Given these results, a HR variable consisting of mean acceleratory response to aversive pictures was used in the main correlational analyses described below. For general corrugator muscle tension, no interaction was expected given that pre-stimulus activation was the quantified variable, and the mixed-model ANOVA yielded only a highly robust main effect for THT+ (p<.0001). Thus, pre-stimulus EMG values were aggregated across picture trials of all types to create an optimally stable variable for use in the main correlational analyses.
Table 1.
Correlations among Psychometric and Physiological Indicators of Threat Sensitivity
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1. 55-Item Trait Fear Inventory (TF-55) | - | |||
| 2. Corrugator EMG (Aversive-Neutral) | .20** | - | ||
| 3. Heart Rate Acceleration (Aversive) | .14** | .09* | - | |
| 4. Aversive Startle Potentiation (Aversive-Neutral) | .10* | .08 | .14** | - |
Note: N = 454.
p< .05
p < .005
Correlational analyses
The Results section focuses on findings from correlational analyses examining (1) relations of a priori physiological indicator variables with scale-assessed THT+ (i.e., TF-55) and with one another, and (2) associations of scale, physiological, and joint scale/physiological (psychoneurometric) operationalizations of THT+ with diagnostic and physiological criterion measures. Zero-order correlations (Pearson’s r) are first reported to provide a picture of relationships among TF-55 scores and differing physiological indicators at a basic bivariate level. Results from a principal axis factor analysis incorporating TF-55 scale scores along with a priori physiological indicators of THT+ are then reported, to establish the presence of a single latent dimension accounting for observed covariance among indicators from these two measurement domains.
The final portion of the Results section focuses on convergent and discriminant validity of scores on the joint psychometric/neurophysiological (psychoneurometric) THT+ dimension from the factor analysis, in terms of relations with criterion measures from domains of clinical symptoms and physiological response. Convergent validity analyses focused on composite symptom and physiological criterion measures computed as averages, respectively, of standardized scores for fear disorder symptoms (specific phobia, social phobia, panic, agoraphobia) and scores for other response measures identified as indicators of THT+ (general corrugator muscle tension and aversive/neutral differentiation for orbicularis EMG, LPP, and probe P3 measures). Discriminant validity analyses focused as noted above on diagnostic and physiological measures known to be associated with the separate biobehavioral trait of disinihibition (i.e., substance problem composite, oddball P300 response). Associations for the psychoneurometric THT+ variable with these differing criterion measures were compared quantitatively to relationships for scores on the TF-55 and for a composite of the a priori physiological indicators of THT+. These comparisons were undertaken using software developed by Lee and Preacher (2013) for testing the difference between dependent correlations (rs); this software converts rs to z values using Fisher’s transformation, and then applies established equations (Steiger, 1980) to compute the asymptotic covariance of the estimates and then perform an asymptotic z test.
Results
Bivariate associations among psychometric and physiological indicators of threat sensitivity
Table 1 presents correlations among scores on the TF-55 scale measure of THT+ and a priori physiological indicators of THT+. As shown in the table, TF-55 scores showed significant positive associations with aversive/neutral corrugator differentiation and aversive/neutral startle potentiation, and also with HR acceleration to aversive pictures. Correlations between TF-55 scale scores and physiological indicators ranged from .10 to .20. Corresponding rs for the differing physiological indicators with one another ranged from .08 to .14 (median r = .12), two-tailed ps = .07 to .002, indicating a modest degree of covariation among these indicators.
Delineation of psychoneurometric THT+ dimension
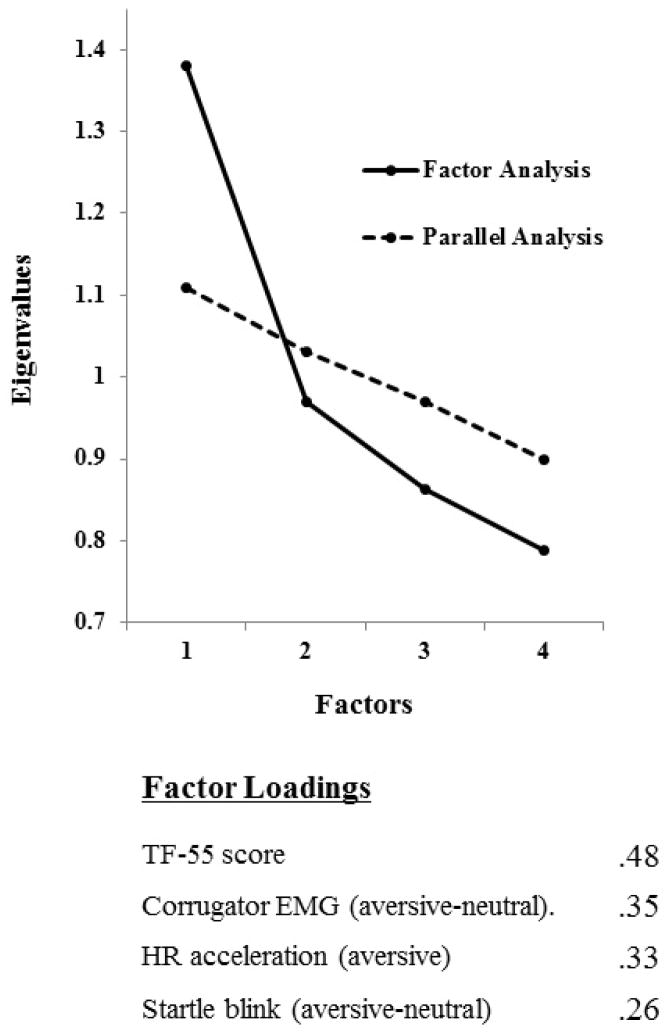
Exploratory principal axis factor analysis was used to formally evaluate whether, as suggested by the correlations shown in Table 1, the TF-55 scale measure and the three physiological indicators of THT+ index a common individual difference dimension. An initial factor analysis of scores for the physiological indicators only (aversive/neutral corrugator differentiation, aversive/neutral startle potentiation, aversive HR acceleration) revealed one dominant factor accounting for 40.12% of the variance in these indicators (loadings = .21, .36, and .39, respectively). A second analysis that included scores on the TF-55 scale measure along with scores for the three physiological indicators likewise revealed the presence of a single common factor (see Figure 1), in this case accounting for 34.4% of variance across the four indicators. As shown in Figure 1, loadings of the indicator variables on this common factor ranged from .26 for the startle potentiation variable6 to .48 for the TF-55 scale measure (median loading = .34).6
Figure 1.
Scree plot and variable loadings for factor analysis (N = 454) of TF-55 scores, corrugator EMG differentiation (aversive-neutral), heart rate acceleration (aversive), and startle blink potentiation (aversive-neutral). A one-factor solution is evident both by visual inspection of the scree plot and by parallel analysis, a technique for determining the number of factors to retain by comparing the eigenvalues of the sample data with those of randomly generated data (Horn, 1965). Actual eigenvalues are denoted in the plot by a solid line; eigenvalues estimated from a parallel analysis based on 1000 random samples are denoted by a dashed line.
To evaluate the stability of this four-variable factor solution, the sample was divided in half such that twin-pair members were assigned either to one half or the other, and the factor analysis was conducted separately for each half-sample. The two analyses each yielded a 1-factor solution, accounting for 34.27% in one case and 34.69% of the variance in the other. Factor loadings for THT+, HR response, corrugator differentiation, and startle modulation were .43, .36, .34, and .27, respectively, in the first subsample, and .51, .35, .32, and .26 in the second subsample.
Validity of the psychoneurometric index of threat sensitivity
As a point of reference for evaluating validity coefficients for the joint psychometric/neurophysiological (psychoneurometric) index of THT+, Table 2 shows correlations for the scale index alone (i.e., TF-55 scores) with clinical symptom and physiological criterion measures. The upper part of the table shows correlations with symptom scores for individual fear disorders and with the composite reflecting overall level of fear disorder symptomatology. The lower part of the table shows correlations with individual physiological criterion measures and with the composite reflecting overall degree of physiological activation/reactivity.7 As expected based on prior work (Nelson et al., 2011; Patrick et al., 2013), correlations with scale-assessed THT+ were generally higher for the clinical criterion measures (median r for individual symptom variables = .24; r for symptom composite = .43) than the physiological criterion measures (median r for individual variables = .12; r for physiological composite = .23).
Table 2.
Correlations between individual criteria measures and TF-55 scores
| r with TF-55 | |
|---|---|
|
Diagnostic Criterion Measures
| |
| Specific phobia | .34** |
| Social phobia | .45** |
| Panic disorder | .14** |
| Agoraphobia | .12** |
|
| |
| Fear Disorder Composite | .43** |
|
| |
|
Physiological Criterion Measures
| |
| General corrugator muscle tension | .22** |
| Orbicularis EMG to picture (aversive-neutral difference) | .09* |
| LPP (aversive-neutral difference) | .11* |
| Probe P3 (neutral-aversive difference) | .12** |
|
| |
| THT+ Physiological Composite | .26** |
Note: TF-55 = 55-item Trait Fear inventory. THT+ = threat sensitivity. N = 454 for all individual diagnostic criteria, and for composite criterion variables (Fear Disorder, THT+ Physiological). Ns for the four physiological criterion measures are, as follows: 450, 442, 414, 435.
p< .05,
p < .01
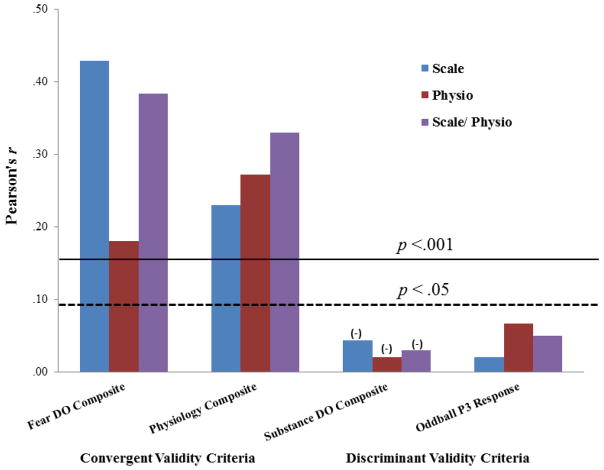
Convergent validity analyses focused on comparative associations of the psychoneurometric THT+ index (quantified as regression-estimated scores on the common factor from the analysis of scale and physiological indicators) with composite clinical and physiological criterion scores, which were more stable than individual symptom or reactivity measures. The left side of Figure 2 shows correlations for psychoneurometric THT+ scores with these composite criterion measures (purple bars), along with correlations for the scale (TF-55) index of THT+ (blue bars). Also shown, for purposes of additional comparison, are correlations for a physiology-only index of THT+ (red bars) consisting of regression-estimated scores on the common factor emerging from the analysis of scores for the three a priori physiological indicators of THT+ (corrugator differentiation, startle potentiation, HR acceleration).
Figure 2.
Depiction of convergent and discriminant correlations (N = 454) for three predictor variables, where bar amplitudes reflect r values: Blue bars = threat sensitivity (THT+) as indexed by TF-55 scale scores; red bars = THT+ as indexed by physiology-only factor scores (indicators = corrugator differentiation, aversive HR acceleration, and startle potentiation); purple bars = THT+ as indexed by psychoneurometric factor scores (indicators = TF-55, corrugator, HR, startle). Left and middle-left sets of bars reflect convergent rs with conceptually-relevant criterion measures, consisting of scores on (a) a composite of fear disorder symptoms (social phobia, specific phobia, panic disorder, agoraphobia), and (c) a composite of other physiological THT+ indicators (aversive-neutral difference scores for LPP, Probe P3, and orbicularis EMG; general corrugator muscle tension). Middle-right and right sets of bars reflect discriminant rs with conceptually-irrelevant criterion measures, consisting of scores on (a) a composite of substance use disorder symptoms (alcohol abuse and dependence; drug abuse and dependence), and (b) amplitude of P3 brain response to target stimuli within a visual oddball task.
As indicated by the horizontal threshold lines in Figure 2, scale-only, physiology-only, and scale/physiology (psychoneurometric) operationalizations of THT+ each predicted scores on clinical and physiological criterion measures to a robust degree (ps<.001). For the clinical criterion measure (Figure 2, far-left), tests of the comparative magnitude of validity coefficients (Preacher & Lee, 2013) revealed that the scale/physiology index of THT+ predicted more strongly to fear disorder symptomatology than the physiology-only index, z = 7.31, p < .001, and at a level only slightly (and nonsignificantly; z = −1.58, ns) below that for the scale-only (TF-55) index. For the physiological criterion measure (Figure 2, middle-left), comparisons of the magnitude of validity coefficients revealed that the scale/physiology index of THT+ predicted more strongly to measures of activity/reactivity (i.e., general corrugator tension and orbicularis EMG, LPP, and probe P3 differentiation) than the scale-only index, z = 3.25, p < .01, at a level also exceeding the physiology-only index, z = 2.12, p < .05. Of further note, additional comparisons revealed that whereas the scale-only index of THT+ showed a marked decrease in r when moving from prediction of fear symptomatology to prediction of physiological activity/reactivity (far-left and middle-left blue bars, respectively), z = −3.73, p < .001, the scale/physiology index did not show this same decrease (see far-left and middle-left purple bars), z = 1.0, ns.
Discriminant validity analyses focused on associations of the differing indices of THT+ (scale-only, physiology-only, and scale/physiology) with (a) a clinical criterion consisting of a composite of symptoms of substance use (alcohol, other drug) disorders (Figure 2, middle-right bars), and (b) a physiological criterion consisting of P3 brain response to oddball-task target stimuli (Figure 2, far-right bars). Consistent with expectation, the three indices of THT+ were unrelated to either of these externalizing-relevant (Patrick et al., 2013) criterion measures.
Discussion
The specific empirical aim of the current work was to establish an initial psychoneurometric operationalization of threat sensitivity (THT+) as a referent for further research. Extending prior published research (Patrick et al., 2013), we demonstrated that multiple physiological indicators of negative emotional reactivity assessed within an affective picture-viewing paradigm (i.e., startle potentiation, corrugator EMG reactivity, HR acceleration) can be combined with scores on a reported-based measure of dispositional fear/fearlessness (cf. Kramer et al., 2012) to delineate a composite individual difference dimension (factor), interpretable as a cross-domain index of THT+. We demonstrated that scores on this psychoneurometric THT+ factor exhibited robust associations both with other physiological measures of situational activation/reactivity (general corrugator muscle tension, and aversive/neutral differentiation for orbicularis EMG, LPP, and probe P3), and with symptoms of various DSM-IV-defined fear pathologies (specific and social phobias, panic disorder, agoraphobia).
Importantly, THT+ psychoneurometric factor scores also showed clear discriminant validity in terms of nonsignificant relations with symptoms of substance-related disorders and P3 brain response, a known physiological indicator of such disorders (and of proneness to externalizing problems more broadly; Iacono et al, 2003; Patrick et al., 2006, 2013; Yancey et al., 2013). This evidence for discriminant validity (see also Patrick et al., 2013) points to clear separation between THT+ and weak inhibitory control (INH-; Nelson et al., 2015; Patrick et al., 2013; Venables et al., 2015), both in terms of neural systems/correlates and prediction to clinical problems, and suggests differentiation of these constructs from broad general psychopathology factors (Caspi et al., 2014; Tellegen et al., 2003).
Key implications of the current work are that: (1) RDoC constructs can be profitably conceptualized and studied as dispositional dimensions (e.g., Nelson et al., 2015; Patrick et al., 2013; Venables et al., 2015), (2) RDoC constructs framed in this manner can be operationalized using indicators from differing domains of measurement, and (3) operationalizations of this type can serve as bridges between clinical problems and neurobiological processes, and also as referents for new cross-domain conceptions of trait constructs. Each of these points is considered in turn, followed by a discussion of current study limitations and directions for future research.
Dispositional counterparts to RDoC process constructs
Reflecting the RDoC initiative’s biological-systems focus, constructs specified in the RDoC matrix are characterized in biobehavioral ‘process’ terms—e.g., as functional concepts with clear referents in brain systems and behavior (e.g., acute threat, response inhibition). However, RDoC constructs can be framed alternatively as biobehavioral dispositions—e.g., as threat sensitivity or inhibitory control capacity. This approach is compatible both with the RDoC initiative’s goal of relating proclivities for clinical problems to variations in functioning of basic biobehavioral systems, and with historic efforts to characterize individual difference constructs in biological-systems terms (e.g., Collins & Depue, 1992; Depue & Iacono, 1989; see also Allport, 1937; Eysenck, 1967; Gray, 1987; Tellegen, 1985).
Conceiving of RDoC constructs in both psychological-process terms and trait-dispositional terms is valuable because it establishes a common framework for characterizing biobehavioral systems and variations in functioning of these systems across people. Individual differences of relevance to clinical problems can be studied in terms of systematic (i.e., reliable, trans-task/trans-measure) variation across people in core biobehavioral processes (and relevant neural systems) as specified in the RDoC framework. This combined trait/process approach also leads naturally to application of basic psychological measurement (‘psychometric’) principles and procedures to the task of reformulating mental disorder conceptions to interface more clearly with neurobiology. As described next, a construct-network perspective is helpful for addressing conceptual challenges confronting this task (e.g., issue of biological reductionism), and a measurement-oriented strategy is valuable for addressing core practical challenges (e.g., problem of method variance).
Operationalizing RDoC dispositions across measurement domains
Conceiving of RDoC process constructs in trait-dispositional terms provides a basis for developing cross-domain operationalizations of individual difference dimensions that predict effectively to neurophysiological variables of interest as well as to clinical problems. The current work illustrates a psychoneurometric approach to operationalizing clinically-relevant biobehavioral traits, entailing (a) bivariate mapping of physiological variables to a provisional psychometric (scale) operationalization of the target trait, (b) delineating a joint psychometric-neurophysiological (psychoneurometric) factor reflecting the covariance among indicators from these differing domains, and (c) deriving scores on this joint factor to serve as predictors of either clinical or neurophysiological target variables. Extending prior work on weak response inhibition (INH-) as relevant to externalizing psychopathology (Patrick et al., 2013), current analyses specifically demonstrate that a cross-domain operationalization of threat sensitivity (THT+) predicts at comparable robust levels to clinical and neurophysiological criterion variables.
The psychoneurometric approach is consistent with classic nomological network perspectives on construct validation and criterion prediction, and addresses a central concern regarding the focus of RDoC on neurobiologically-based conceptions of psychopathology—namely, the issue of biological reductionism (cf. Lilienfeld, 2014; Miller, 1996, 2010). Rather than seeking to ‘reduce’ phenomena in one domain (e.g., clinical or trait-dispositional) to phenomena in another (e.g., neural systems/processes), the psychoneurometric approach conceives of trait dispositions as biobehavioral tendencies expressed both in clinical problems and in task physiology. As such, dispositional dimensions can be operationalized using indicators from differing domains of measurement, including scale-assessed experience (e.g., “I get frightened easily”) and task-assessed physiological reactivity (e.g., aversive startle potentiation)—and other domains as well, such as interviewer/informant ratings and measured behaviors—without giving preference to one domain over the other. Dispositional tendencies quantified in this cross-domain manner are likely to be useful, for example, for pre-selecting research participants with differing degrees of biobehavioral risk for psychopathology, and in studies directed at evaluating relationships of biological variables of particular types with clinical problems.
Moving toward new biobehavioral conceptions of basic traits
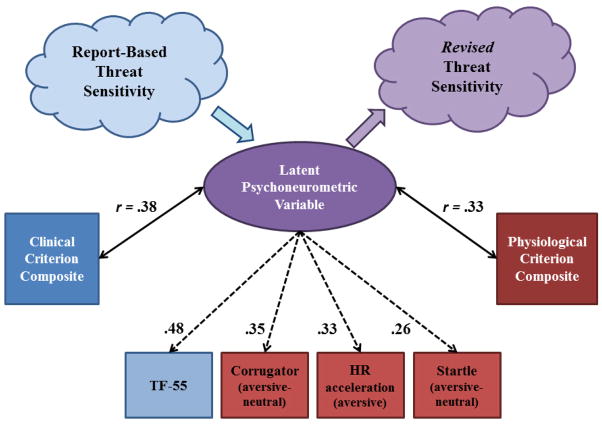
A further point is that the process of operationalizing traits using indicators from differing domains leads naturally to a shift in conceptions of the traits themselves. For example, as illustrated in Figure 3, the psychoneurometric operationalization of THT+ developed through the current work can be viewed as a step toward defining individual differences in reactivity to cue-specific aversive stimuli at the intersection of physiological reactivity and reported psychological experience. While serving as an initial referent for identifying physiological indicators of THT+, because of its known relations with fear symptomatology (Nelson et al., 2015) and acute-threat response (Vaidyanathan et al., 2009), the scale (TF-55) measure comprises only one indicator of the resultant psychoneurometric trait dimension—with the other three indicators coming from the domain of physiology. As a function of this, the nature of the new trait dimension has shifted from the report-based factor the scale measure was designed to index (cf. Kramer et al., 2012), now residing “in between” the domain of reported fear experience and that of affective-task physiology. Viewed this way, the latent psychoneurometric dimension depicted in Figure 3 becomes a referent for a revised psychobiological conception of THT+—one that reflects the interface between psychological conceptions of clinically-relevant traits (as operationalized by self-report) and conceptions of neural systems/processes reflective of defensive mobilization (operationalized as physiological reactivity to aversive stimuli).
Figure 3.
Conceptual-empirical depiction of results from analyses aimed at operationalizing a psychoneurometric index of threat sensitivity. The lower part of the figure depicts relations among observables across domains of self-report (scale, clinical symptom) and physiological response. Factor loadings of scale (dark blue square) and physiological (dark red squares) observables on the latent psychoneurometric factor are denoted by dashed arrows. Light blue and red squares represent composite scores for clinical (fear disorder) symptom criteria and physiological response criteria, respectively. Correlation coefficients between criterion composites and scores on the latent psychoneurometric factor (purple oval) are depicted by bidirectional arrows. The upper part of the figure depicts how the psychoneurometric approach can systematically shift the conceptualization of threat sensitivity from a construct rooted in self-report (light blue cloud) toward one that reflects the nexus of psychological and biological processes (light purple cloud). This process of bootstrapping (Cronbach & Meehl, 1955) is depicted by the light blue and light purple arrows.
In this way, by conceiving of target constructs both as dispositions and processes, relatable to one another and to clinical problems through systematic delineation of a conceptual-empirical network, the RDoC initiative has the potential to reshape existing conceptions of individual difference constructs hand-in-hand with reshaping conceptions of clinical problems and their causes. In contrast with existing conceptions of trait dispositions that are based predominantly on data from the domain of self-report, the RDoC framework encourages operationalization of dispositional constructs across multiple domains of measurement. Aligned with this emphasis, the psychoneurometric approach provides a concrete “bootstrapping” (Cronbach & Meehl, 1955) mechanism for establishing new multi-domain trait conceptions. In turn, a focus on multi-domain trait conceptions and counterpart process constructs can move the field toward a multi-level, process-oriented understanding of clinical problems.
Limitations and Future Directions
Some key limitations of the current work must be acknowledged, which highlight important directions for future research. One is that the current work did not attempt to account for other sources of variance in physiological indicators aside from that in common with the scale (TF-55) measure. For example, as noted in Footnote 5, the effectiveness of aversive startle potentiation as an indicator of THT+ in the current analysis was reduced by a known moderating effect of depression history (see Yancey et al., 2014). This highlights the need for further research on potential moderators of psychophysiological variables as effective indicators of RDoC constructs and corresponding dispositional traits.
Another point is that differing physiological variables may index differing aspects of affective reactivity. For example, facial indices of affective response such as corrugator EMG may be less indicative of core defensive-system activation than indices of reflex-priming such as aversive startle potentiation or visceral activation. More broadly, there are potential limitations to deriving physiological indices of threat sensitivity from passive cuing paradigms such as the affective picture-viewing task used here. We encourage future work utilizing measures from other paradigms such as shock-threat or aversive conditioning tasks to expand the range of known indicators of THT+ and clarify what contexts of affective-physiological assessment yield the most robust indicators.
A further point is that while scree plot inspection and parallel analysis (see Figure 1) clearly indicated a one factor solution, the factor loadings for individual indicator variables ranged from somewhat low to moderate in magnitude (i.e., from .26 to .48)—suggesting less than optimal specification of a common latent dimension. One likely source of this weakness is the low reliability of some physiological indicators used in the analysis (see Footnote 6). Further research is needed to establish optimal methods for quantifying variation in condition effects (e.g., affect-modulation scores), and in physiological response measures more broadly (e.g., Olvet & Hajcak, 2009), for use in individual difference analyses. As a point of comparison, work in classical psychological assessment has focused on developing highly reliable indices of constructs in domains of personality and intellect/ability, with data from large normative samples allowing for interpretation of scores at the individual level. Achieving similar precision with trait measures consisting partly or entirely of physiological indicators will require systematic efforts focusing on the measurement properties of such indicators in large participant samples. Relatedly, it is important to contextualize the findings from the current study as an initial step toward developing a multi-method (/’unit’) conception of threat sensitivity and a psychoneurometric operationalization of this key RDoC dispositional construct. Clearly, the indicators employed in the current work showed lower covariation than would be expected of well-developed scale-report measures designed to index a common construct (e.g., Kramer et al, 2012). Their level of covariation (i.e., .1 – .2 range) appears more consistent with that of individual items indexing somewhat related aspects of a broad construct (cf. Clark & Watson, 1995). Given this, the work reported here should be viewed as an illustration of a conceptual-methodological approach rather than as a formal, finished model.
Findings from the current study highlight the crucial need for research on biological processes in psychopathology to systematically evaluate the psychometric properties of physiological indicators in the same way as is routinely done for measures in the self-report domain. Improved reliability can help to increase the signal-to-noise ratio in indicators of these types, and in turn augment the modest associations of physiological measures with one another and with self-report variables, as observed in the current study and in other large-sample studies (Hicks et al, 2007; Weinberg et al, 2015; Vaidyanathan et al, 2009). With this point in mind, it is unsurprising that THT+ operationalized psychoneurometrically showed enhanced prediction to the physiological composite relative to the physiological variables alone. Requiring the biological measures to cohere with a highly reliable self-report measure harnesses the trait-relevant variance in those biological indices to create a more reliable index of a coherent psychobiological process than the physiological variables alone.
A further limitation of the current study is the use of simple exploratory techniques to delineate an initial psychoneurometric model of THT+. Future work should move toward utilization of more advanced quantitative techniques (e.g., structural equation modeling, item-response analysis) in order to clarify relations among indicators from physiological and non-physiological domains, and refine psychoneurometric quantification of trait constructs. For example, two of the criterion measures utilized in the current study consisted of brain ERP variables, whereas physiological indicators of THT+ consisted of somatic (startle, corrugator EMG) and visceral (HR) measures. It will be important in future research to utilize techniques beyond EFA to delineate trait-relevant variance versus measure-specific variance in physiological indicators of differing types in order to optimize psychoneurometric assessments of trait constructs. A further advantage of using advanced quantitative techniques is that they allow for the formal testing of model invariance as function of important demographic variables such as gender. Tests of this type cannot be formally conducted in an EFA context. Studies using quantitative modeling methods will require larger samples with multiple psychophysiological indicators of established reliability.
Another limitation of the current study vis-à-vis RDoC’s stated objective of moving away from existing conceptions of mental disorders is its focus on clinical criteria consisting of DSM disorder symptoms. Up to now, RDoC writings have referred mainly to examples of symptom variables that might serve as viable targets for research (e.g., anhedonia, sleep disturbance, rumination)—and so the question of how best to operationalize clinical outcomes more broadly in RDoC-oriented research has not been entirely clear. However, views expressed in the current issue paper by Kozak and Cuthbert point to an emerging perspective on clinical problems consistent with a classical ‘network’ view of psychological constructs—i.e., as conceptions of non-normative, maladjusted tendencies operationalizable in differing ways (e.g., through self-report, interviewer or informant ratings, observable behaviors, etc.) and relatable to process constructs of various types, also quantifiable in differing ways. In line with this, further research on psychoneurometric operationalizations of THT+ and other RDoC dispositional constructs should seek to include alternative measures of clinical problems—in particular, continuous-score variables assessed through means other than questionnaire or interview. Inclusion of more objective measures of impairment such as physical health, mortality, and work productivity will be useful for further evaluating the clinical utility of THT+.
One potential criticism of the psychoneurometric approach pertains to clinical utility. While THT+ was reliably associated with symptoms of fear disorders in the current study sample, the magnitude of the association was no larger (and perhaps smaller, though not significantly so) than the association between scale THT+ and fear disorders. It may be a concern to some that incorporating physiological measurement has not contributed incrementally to prediction of clinical problems. However, the goal of this research endeavor is not to enhance prediction to established diagnostic variables. Doing so would serve in large part to reify the existing diagnostic framework. The aim of the psychoneurometric approach, consistent with the mission of RDoC, is to progress toward new conceptions of problems and problem-related processes that connect more clearly to neurobiological systems. In this light, reliable prediction to fear symptomatology observed in the current study is desirable, as clinical problems are essential to consider in a predictive framework (or ‘nomological net’; Cronbach & Meehl, 1955) for psychopathology—but increased predictive utility is not necessarily desirable, as report-based symptoms serve mainly as criteria for validation of report-based THT+. The more important aim of the psychoneurometric approach is to model a latent variable that can effectively predict across differing units of analysis (e.g., reported problems, task performance, brain or bodily reactivity, etc.) without giving preference to one domain over others.
Yet another limitation of the current study is its cross-sectional design. As such, the current study was not equipped to address whether high dispositional fear (i.e., THT+, as indexed by report and reactivity) represents a liability toward the development of fear-related conditions, or rather an emergent (pathophysiological) aspect of such conditions. Systematic longitudinal work will be needed to determine this. However, the view of RDoC constructs as dispositions leads naturally to questions about liability versus pathophysiology that can serve to guide hypotheses, participant selection, and choice of predictor and criterion variables in longitudinal-developmental studies.
Notwithstanding these limitations, the current study serves to illustrate how a dispositional dimension corresponding to the RDoC construct of acute threat can be operationalized in joint psychometric/neurophysiological (psychoneurometric) terms. This initial work, and the psychoneurometric approach more broadly, has important implications for understanding affective individual differences contributing to clinical problem dimensions. While current analyses focused on relations with symptoms of fear disorders as defined in DSM-IV, we expect that a psychoneurometric index of THT+ will also show predictive relations with distress-related clinical conditions (Nelson et al., 2015), ‘fearful/anxious’ personality pathology (Patrick et al., 2012), suicide risk (Venables et al., 2015), and affective-interpersonal features of psychopathy (Vaidyanathan et al., 2009). Supportive findings would help to establish THT+ as a key transdiagnostic trait construct. More broadly, we anticipate that psychoneurometric operationalizations of a select subset of RDoC dispositional constructs—including reward- (e.g., Proudfit, 2014) and affiliation-related constructs (Patrick, Drislane, & Strickland, 2012) from the RDoC domains of Positive Valence and Social Processes, respectively, along with threat sensitivity (highlighted here) and response inhibition (Patrick et al., 2013)—can serve as anchor dimensions for new, neurobiologically-oriented structural models of psychopathology and of normative personality.
Acknowledgments
This work was supported by grant W911NF-14-1-0027 from the US Army and grants MH072850 and MH089727 from the National Institute of Mental Health.
Footnotes
For participants with one missing physiological indicator, maximum likelihood estimation (as implemented in MPLUS 6) were used to generate imputed values on these scores.
These analyses focused on the aversive versus neutral comparison because THT+ effects were expected to emerge for aversive stimuli specifically (relative to neutral as a control). However, for purposes of completeness, we ran counterpart analyses incorporating pleasant versus neutral pictures as the within-subjects factor; none of these analyses yielded either a significant THT+ x Valence interaction or a significant THT+ main effect.
Supplemental analyses including gender as a second between-subjects factor (along with scale-assessed THT+) were also conducted for each physiological variable. No moderating impact of gender (i.e., no significant Gender x THT+ effect) was evident for the THT+ indicators (HR, startle modulation, corrugator differentiation) or the brain-response criterion variables (LPP differentiation, probe-P3 modulation). Moderating effects of gender (p<.05) were evident, however, for the two EMG criterion variables (general corrugator tension, orbicularis differentiation), with the THT+/physiology association stronger for women in each case. While in need of replication, these findings suggest the possibility of gender differences in the functioning of certain physiological variables (e.g., facial activation measures; cf. Bradley et al., 2001b) as indicators of THT+.
Yancey, Vaidyanathan, and Patrick (2014) provide a detailed report of results from the mixed-model ANOVA for the blink startle variable. More complete descriptions including statistical details for these other variables, commensurate with the description provided by Yancey et al. for startle blink, are available from the authors upon request.
While aversive startle potentiation emerged as the weakest indicator of the common factor in the analysis incorporating all participants (N = 454), prior work with this sample (Yancey et al., 2014) revealed a significant moderating effect of depression history on the relationship between startle potentiation and TF-55 scores, F(1, 417) = 6.05, p<.05 (i.e., participants with no history of major depression showed a positive startle-potentiation/TF-55 relationship, r = .15, p < .01, whereas those with a prior depression history did not, r = −.15, ns). We therefore conducted a supplemental factor analysis including only participants without a history of depression (n = 370). This analysis also yielded a single common factor, on which startle potentiation loaded .32. These findings highlight the possibility of moderating influences on factor loadings for particular indicator variables. In the case of aversive startle potentiation, the inclusion of participants with prior depression in the analysis resulted in a lower loading for this variable, because startle potentiation operates as an effective indicator of THT+ only in participants without a history of major depression (see also Taylor-Clift, Morris, Rottenberg, & Kovacs, 2011) or pervasive distress disorders more broadly (Lang, McTeague, & Cuthbert, 2007).
To examine score reliability as a possible contributor to variation in factor loadings, split-half correlations were computed for each of the indicator variables—i.e., between scores based on odd versus even trials in the case of the physiological variables, and between scores based on odd versus even items in the case of the scale variable (TF-55). Split-half correlations were significant (p<.001) for all physiological variables, but varied in magnitude from modest to moderately high: r’s = .27, .39, and .58 for startle potentiation, HR acceleration, and corrugator reactivity, respectively. The split-half coefficient for the TF-55 scale measure was = .94. Notably, the split-half coefficient for a composite of the three physiological indicators (i.e., r between the unit-weighted average of the three for odd trials and the corresponding average for even trials) was .50, and the split-half coefficient for a composite incorporating the scale measure as well (i.e., scores for odd and even items) was even higher, r = .66. These findings indicate that (a) variations in score reliability likely contributed to factor loading magnitudes (i.e., loadings were stronger for indicators exhibiting higher reliabilities), and (b) aggregating across indicators increased score reliability.
The median r among individual physiological criterion measures listed in the lower part of Table 2 was .12; the median r between these individual measures in Table 2 and individual physiological indicators of threat sensitivity (THT+) listed in Table 1 was .13.
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Government, Department of Defense, Department of the Army, Department of Veterans Affairs, or U.S. Recruiting Command.
Financial Disclosures: We have no conflicts of interest or financial disclosures to report.
References
- Allport GW. Personality: A psychological interpretation. New York, NY: Holt, Rinehart, & Winston; 1937. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: Author; 2013. [DOI] [Google Scholar]
- Arrindell W, Emmelkamp P, Van der Ende J. Phobic dimensions: I. Reliability and generalizability across samples, gender and nations. Advances in Behaviour Research and Therapy. 1984;6:207–254. doi: 10.1016/0146-6402(84)90001-8. [DOI] [Google Scholar]
- Benning S, Patrick CJ, Iacono WG. Psychopathy, startle blink modulation, and electrodermal reactivity in twin men. Psychophysiology. 2005;42:753–762. doi: 10.1111/j.1469-8986.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001a;1:276–298. doi: 10.1037/1528-3542.1.3.276. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001b;1:300–319. doi: 10.1037/1528-3542.1.3.300. [DOI] [PubMed] [Google Scholar]
- Buss AH, Plomin R. Temperament: early developing personality traits. Hillsdale: Lawrence Erlbaum Associates; 1984. [Google Scholar]
- Campbell DT, Fiske DW. Convergent and discriminant validation by the multitrait-multimethod matrix. Psychological Bulletin. 1959;56:81–105. doi: 10.1037/h0046016. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky WM, Goldman-Mellor SJ, Harrington H, Israel S, Meier MH, Ramrakha S, Shalev I, Poulton R, Moffitt TE. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2:119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention. The international affective picture system: Digitized photographs. Gainsville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- Clark LA, Watson D. Constructing validity: Basic issues in objective scale development. Psychological Assessment. 1995;7(3):309–319. doi: 10.1037/1040-3590.7.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Archives of General Psychiatry. 1987;44:573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- Collins PF, Depue RA. A neurobehavioral systems approach to developmental psychopathology: Implications for disorders of affect. In: Toth DCSL, editor. Developmental perspectives on depression. Rochester symposium on developmental psychopathology. Rochester NY: University of Rochester Press; 1992. pp. 29–101. [Google Scholar]
- Cook EW, III, Davis TL, Hawk LW, Spence EL, Gautier CH. Fearfulness and startle potentiation during aversive visual stimuli. Journal of Psychophysiology. 1992;29:633–645. doi: 10.1111/j.1469-8986.1992.tb02038.x. [DOI] [PubMed] [Google Scholar]
- Cook EW, III, Melamed BG, Cuthbert BN, McNeil DW, Lang PJ. Emotional imagery and differential diagnosis of anxiety. Journal of Counsulting and Clinical Psychology. 1988;56:734–740. doi: 10.1037/0022-006X.56.5.734. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ. The two disciplines of scientific psychology. American Psychologist. 1957;12:671–684. doi: 10.1037/h0043943. [DOI] [Google Scholar]
- Cronbach LJ. Beyond the two disciplines of scientific psychology. American Psychologist. 1975;30:116–127. doi: 10.1037/h0076829. [DOI] [Google Scholar]
- Cronbach LJ, Meehl PE. Construct validity in psychological tests. Psychological Bulletin. 1955;52:281–301. doi: 10.1037/h0040957. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: fear memory imagery. Psychophysiology. 2003;40:407–422. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- Davis M. Neural systems involved in fear-potentiated startle. In: Davis M, Jacobs BL, Schoenfeld RI, editors. Annals of the New York Academy of Sciences, vol. 563: Modulation of defined neural vertebrate circuits. New York: Author; 1989. pp. 165–183. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Review of Psychology. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Drislane LD, Vaidyanathan U, Patrick CJ. Reduced cortical call to arms differentiates psychopathy from antisocial personality disorder. Psychological Medicine. 2013;43:825–835. doi: 10.1017/S0033291712001547. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. The biological basis of personality. Springfield, IL: Charles C. Thomas; 1967. [Google Scholar]
- First MB, Spitzter RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR axis I disorders, non-patient edition. (SCID-I/NP) New York, NY: Biometrics; 2002. [Google Scholar]
- Fridlund AJ, Schwartz GE, Fowler SC. Pattern recognition of self-reported emotional state from multiple-site facial EMG activity during affective imagery. Psychophysiology. 1984;21:622–637. doi: 10.1111/j.1469-8986.1984.tb00249.x. [DOI] [PubMed] [Google Scholar]
- Graham FK. Distinguishing among orienting, defense, and startle reflexes. In: Kimmel HD, Van Olst EH, Orelbeke JF, editors. The orienting reflex in humans. Hillsdale, NJ: Erlbaum; 1979. pp. 137–168. [Google Scholar]
- Gray JA. The psychology of fear and stress. 2. Cambridge, UK: Cambridge University Press; 1987. [Google Scholar]
- Hamm AO, Cuthbert BN, Globisch J, Vaitl D. Fear and startle reflex: Blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology. 1997;34:97–107. doi: 10.1111/j.1469-8986.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30:179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11:869–900. doi: 10.1017/S0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/S0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalographic Clinical Neurophysiology. 1958;10:370–375. [PubMed] [Google Scholar]
- Kalia M, Costa e Silva J. Biomarkers of psychiatric diseases: Current status and future prospects. Metabolism: Clinical and Experimental. 2015;64:S11–S15. doi: 10.1016/j.metabol.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Kramer MD, Patrick CJ, Krueger RF, Gasperi M. Delineating physiological defensive reactivity in the domain of self-report: Phenotypic and etiologic structure of dispositional fear. Psychological Medicine. 2012;42:1305–1320. doi: 10.1017/S0033291711002194. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–285. doi: 10.1037/0003-066X.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychology Review. 1990;97:377–398. doi: 10.1037/0033-295X.97.3.377. [DOI] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM. The anxiety disorder spectrum: Fear imagery, physiological reactivity, and differential diagnosis. Anxiety, Stress & Coping. 2009;22:5–25. doi: 10.1080/10615800802478247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM, Cuthbert BN. Fear, anxiety, depression, and the anxiety disorder specturm: A psychophysiological analysis. In: Treat RBT, Baker T, editors. Psychological clinical science: Papers in honor of Richard M. McFall. New York, NY: Psychology Press; 2007. pp. 167–195. [Google Scholar]
- Larson JT, Norris CJ, Cacioppo JT. Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology. 2003;40:776–785. doi: 10.1111/1469-8986.00078. [DOI] [PubMed] [Google Scholar]
- Lee IA, Preacher KJ. Calculation for the test of the difference between two dependent correlations with one variable in common [Computer software] 2013 Sep; Available from http://quantpsy.org.
- Lilienfeld SO, Andrews B. Development and Preliminary Validation of a Self-Report Measure of Psychopathic Personality Traits in Noncriminal Population. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO. The Research Domain Criteria (RDoC): An analysis of methodological and conceptual challenges. Behaviour Research and Therapy. 2014;62:129–139. doi: 10.1016/j.brat.2014.07.019. [DOI] [PubMed] [Google Scholar]
- Loevinger J. Objective tests as instruments of psychological theory. Psychological Reports. 1957;3:635–694. doi: 10.2466/pr0.1957.3.3.635. [DOI] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful Imagery in social phobia: Generalization, comorbidity, and physiological reactivity. Biological Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzig CA, Weike AI, Zimmermann J, Hamm AO. Startle reflex modulation and autonomic responding during anxious apprehension in panic disorder patients. Psychophysiology. 2007;44:846–854. doi: 10.1111/j.1469-8986.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- Miller GA. How we think about cognition, emotion, and biology in psychopathy. Toronto: 1996. [DOI] [PubMed] [Google Scholar]
- Miller GA. Mistreating psychology in the decades of the brain. Perspectives on Psychological Science. 2010;5:716–743. doi: 10.1177/1745691610388774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Rockstroh B. Endophenotypes in psychopathology research: Where do we stand? Annual Review of Clinical Psychology. 2013;9:177–213. doi: 10.1146/annurev-clinpsy-050212-185540. [DOI] [PubMed] [Google Scholar]
- Mischel W. Personality and Assessment. New York: Wiley; 1968. [Google Scholar]
- Nelson LD, Strickland C, Krueger RF, Arbisi PA, Patrick CJ. Neurobehavioral traits as transdiagnostic predictors of clinical problems. Assessment. 2015 doi: 10.1177/1073191115570110. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Bernat EM. Operationalizing proneness to externalizing psychopathology as a multivariate psychophysiological phenotype. Psychophysiology. 2011;48:64–72. doi: 10.1111/j.1469-8986.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. Reliability of error-related brain activity. Brain Research. 2009;1284:89–99. doi: 10.1016/j.brainres.2009.05.079. [DOI] [PubMed] [Google Scholar]
- Patrick CJ. Emotion and psychopathy: Startling new insights. Psychophysiology. 1994;31:319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Bernat E, Millon T, Krueger RF, Simonsen E, editors. Contemporary directions in psychopathology: Toward the DSM-V. New York: Guilford Press; 2010. Neuroscientific foundations of psychopathology. [Google Scholar]
- Patrick CJ, Bernat EM, Malone S, Iacono W, Krueger R, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Drislane LE, Strickland C. Conceptualizing psychopathy in triarchic terms: Implications for treatment. International Journal of Forensic Mental Health. 2012;11:253–266. doi: 10.1080/14999013.2012.746761. [DOI] [Google Scholar]
- Patrick CJ, Durbin E, Moser JS. Reconceptualizing antisocial deviance in neurobehavioral terms. Development and Psychopathology. 2012;24:1047–1071. doi: 10.1017/S0954579412000533. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: application to assessment of externalizing psychopathology. Journal of Abnormal Psychology. 2013;122:902–916. doi: 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit GH. The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology. 2015;52:449–459. doi: 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- Ruiz-Padial E, Mata JL, Rodríguez S, Fernández MD, Vila J. Non-conscious modulation of cardiac defense by masked phobic pictures. International Journal of Psychophysiology. 2005;56:271–281. doi: 10.1016/j.ijpsycho.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Smith JC, Bradley MM, Lang PJ. State anxiety and affective physiology: effects of sustained exposure to affective pictures. Biological Psychology. 2005;69:247–260. doi: 10.1016/j.biopsycho.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. doi: 10.1037/0033-2909.87.2.245. [DOI] [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Tuma AH, Maser JD, editors. Anxiety and the anxiety disorders. Hillsdale, N. J: Lawerence Erlbaum Assoc; 1985. pp. 681–706. [Google Scholar]
- Tellegen A, Ben-Porath YS, McNulty JL, Arbisi PA, Graham JR, Kaemmer B. MMPI-2 Restructured Clinical (RC) Scales: Development, validation, and interpretation. Minneapolis, MN: University of Minnesota Press; 2003. [Google Scholar]
- Vaidyanathan U, Patrick CJ, Bernat E. Startle reflex potentiation during aversive picture viewing as an index of trait fear. Psychophysiology. 2009;46:75–85. doi: 10.1111/j.1469-8986.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan U, Patrick CJ, Iacono WG. Patterns of comordibity among common mental disorders: A person-centered approach. Comprehensive Psychiatry. 2011;52:527–535. doi: 10.1016/j.comppsych.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Hall JR, Yancey JR, Patrick CJ. Factors of psychopathy and electrocortical response to emotional pictures: Further evidence for a two-process theory. Journal of Abnormal Psychology. 2015;124:319–328. doi: 10.1037/abn0000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ. Reconciling discrepant findings for P3 brain response in criminal psychopathy through reference to the concept of externalizing proneness. Psychophysiology. 2014;51:427–436. doi: 10.1111/psyp.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Sellbom M, Sourander A, Kendler KS, Joiner TE, Drislane LE, Sillanmaki L, Parkkola K, Multimaki P, Patrick CJ. Separate and interactive contributions of weak inhibitory control and threat sensitivity to prediction of suicide risk. Psychiatry Research. 2015 doi: 10.1016/j.psychres.2015.01.018. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizueta N, Patrick CJ, Jiang Y, Thomas KM, He S. Trait fear and negative affectivity as predictors of neuroimaging response to invisible fear faces. NeuroImage. 2012;59:761–771. doi: 10.1016/j.neuroimage.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Cuthbert BN, Lang PJ. Fear imagery and text processing. Psychophysiology. 1986;23:247–253. doi: 10.1111/j.1469-8986.1986.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: A new measure of emotion? Journal of Abnormal Psychology. 1988;97:487–491. doi: 10.1037/0021-843X.97.4.487. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Venables NC, Proudfitt GH, Patrick CJ. Heritability of the neural response to emotional pictures: Evidence from ERPs in an adult twin sample. Social Cognitive and Affective Neuroscience. 2015;10:424–434. doi: 10.1093/scan/nsu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. International statistical classification of diseases and health related problems. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]
- Yancey JR, Vaidyanathan U, Patrick CJ. Aversive startle potentiation and fear pathology: Mediating role of threat sensitivity and moderating impact of depression. International Journal of Psychophysiology. 2014 doi: 10.1016/j.ijpsycho.2014.10.014. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey JR, Venables NC, Hicks BM, Patrick CJ. Evidence for a heritable brain basis to deviance-promoting deficits in self-control. Journal of Criminal Justice. 2013;41:309–317. doi: 10.1016/j.jcrimjus.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking: Beyond the optimal level of arousal. Hillsdale, NJ: Lawrence Erlbaum Association; 1979. [Google Scholar]