Abstract
The ability to assess the perfusion territories of major cerebral arteries can be a valuable asset to the diagnosis of a number of cerebrovascular diseases. Recently, several arterial spin labeling (ASL) techniques have been proposed to obtain the cerebral perfusion territories of individual arteries according to three different approaches: (1) using a dedicated labeling RF coil; (2) applying selective inversion of spatially confined areas; or (3) employing multi-dimensional RF pulses. Methods that use a separate labeling RF coil have high SNR, low RF power deposition and unrestricted 3-dimensional coverage, but are mostly limited to separation of the left and right circulation, and do require extra hardware, which may limit their implementation in clinical systems. Alternatively, methods that utilize selective inversion have higher flexibility of implementation and higher arterial selectivity, but suffer from imaging artifacts resulting from interference between the labeling slab and the volume of interest. The goal of the present review is to provide the reader with a critical survey of the different ASL approaches proposed to date to obtain cerebral perfusion territories, by discussing the relative advantages and disadvantages of each technique, so as to serve as a guiding resource towards future refinements of this promising methodology.
Keywords: brain, cerebral blood flow, cerebrovascular diseases, magnetic resonance imaging, vascular territory
Introduction
Neuroimaging techniques capable of measuring cerebral hemodynamics are finding increasing applications in the clinical setting (1,2), particularly for the diagnosis and therapeutic management of brain tumors (3) and that of a variety of cerebrovascular diseases, including cerebral vasospasm (4), obstructive arterial disease (5) and stroke (6). Compared to other imaging modalities, MRI of cerebral perfusion has the advantages of not exposing patients to ionizing radiation and for providing quantitative values for regional cerebral blood flow (rCBF), volume (rCBV) and mean transit time (rMTT) at relatively high spatial resolution and within a few minutes (1,2). When combined to other MRI techniques such as angiography (MRA), diffusion-weighted imaging (DWI) and T2-weighted MRI, perfusion MRI techniques can provide a comprehensive evaluation of the cerebral circulation in normal and pathological conditions, by allowing a direct comparison of regions of altered perfusion with the ischemic territory and the vascular source of compromise (7). Such strategy has been widely used for the early evaluation of stroke patients (8), allowing the assessment of infarcted versus salvageable tissue and guiding the treatment strategy (9).
Several MRA and MRI techniques and methods have been proposed for non-invasive imaging of individual perfusion territories (5,10–31). The ability to assess the perfusion territories of major cerebral arteries can be a valuable asset to the diagnosis of a number of cerebrovascular diseases, in which maps of vascular territories provide complimentary information to angiography on the status of blood flow. Mapping of perfusion territories can be quite informative both in prognostic as well as in diagnostic applications. On a prognostic basis, mapping of perfusion territories allow the identification of flow abnormalities and aid in the prediction of the extent of regions at increased risk of ischemic damage. For example, a large inter-individual variability in perfusion territories of 115 normal volunteers has been recently reported in vivo, with anatomical variations of the circle of Willis (COW) being responsible for most of the variation in flow territories of the anterior and posterior circulation (5). In addition, prognostic mapping of the vascular territories allows assessment of collateral circulation, a parameter of critical importance in surgical planning for patients in need of vascular interventions such as bypass of the feeding cerebral arteries (22). On a diagnostic basis, the use of vascular territories in patients with cerebrovascular diseases, such as occlusion of the internal carotid artery (ICA), allows verification of the extent of the ischemic region and the adaptation of the vasculature as a result of flow redistribution resulting from compensatory collateral flow, of re-canalization of the occluded artery, or of bypass surgery to correct the occlusion (24).
MRI of cerebral vascular territories require selective labeling of blood in the artery (ies) of interest, and thus arterial spin labeling (ASL) techniques have been used with exclusivity to map the vascular territories of major cerebral arteries (5,14–31). Ideally, techniques dedicated to map cerebral perfusion territories should allow a flexible selection of which arterial territory to demarcate, and to do so within the short time constraints available for clinical exams, providing images of the whole brain with high SNR and devoid of artifacts resulting from labeling the arteries of interest. However, even though a number of different ASL approaches to measure vascular territories have been proposed, the relative advantages and disadvantages of each method have not been extensively investigated. The goal of the present review is to provide the reader with a critical survey of the different ASL techniques proposed to date to obtain cerebral perfusion territories, and to serve as a guiding resource towards future refinements of this promising methodology.
General Principles of Mapping Vascular Territories with ASL
ASL is a completely non-invasive methodology that uses magnetically labeled arterial blood water as an endogenous diffusible tracer. In ASL, two images are acquired with (labeled image) and without (control image) a suitable preparation of the magnetization of arterial blood proximal to the area of interest. Generally speaking, two different ASL strategies have been developed: pulsed ASL (PASL), in which a thick, but well defined, slab of blood is inverted prior to entering the volume of interest in the brain; or continuous ASL (CASL), in which arterial blood flowing proximal to the COW is continuously inverted prior to entering the volume of interest in the brain. Comprehensive reviews have been written on the technical details of implementation and applications of ASL (32–35), to which the reader is referred for further reference.
The mapping of vascular territories with ASL is based in labeling only blood flowing through the artery or arteries of interest, while leaving the other arteries unlabeled. Thus, the difference between the labeled and the control images will return the perfusion territory supplied only by those labeled arteries. To date, this strategy has been implemented according to three different approaches: (1) using a dedicated labeling RF coil positioned over the arteries of interest (14–16,19,30,31); (2) employing selective inversion of spatially confined areas where the arteries of interest are located (5,17,20–29); and (3) using multi-dimensional RF pulses to directly label the artery of interest (18). Each approach will be described and critically discussed in the following sections.
Use of a Separate Labeling RF Coil
The first perfusion imaging studies were performed using a single RF coil to perform both the ASL as well as to excite the imaging volume of interest in the brain (36,37). When a single labeling/imaging RF coil is used, the off-resonance RF pulse required for ASL saturates macromolecules in the brain, resulting in a large spatially dependent decrease of the signal intensity due to magnetization transfer (MT) effects (38). The original way to account for MT effects was to acquire a control image with RF power applied on a plane symmetrically opposed to the labeling plane (37,38), which limited data acquisition to a single slice centered between the labeling and control planes. While some methods were proposed to extend the coverage of both PASL and CASL to multiple slices (39–41), MT effects still imposed a few hard to overcome constraints, such as imaging slice orientation restricted to the labeling direction (39,40) and reduction of the effective degree of spin labeling (41). Complete elimination of MT effects could only be achieved with the use of a separate, dedicated labeling RF coil (15,30,31,42–44). The use of a small labeling RF coil located outside the imaging region eliminated MT effects, turning the multi-slice implementation trivial and allowing the imaging planes to be arbitrarily positioned in space. In addition, the need to apply RF power during acquisition of the control images was eliminated, resulting in a significant drop in the total RF power deposited on the subject (15). Furthermore, the use of a separate RF coil allowed for selective labeling of specific arteries.
Both in small animals as well as in humans, the labeling RF coil is positioned over the neck (15,30,31,42–44), producing ASL of the common carotid arteries (CCA) and the vertebral arteries (VA), which supply the anterior and posterior brain circulation, respectively. High degrees of labeling efficiency, in the range of 0.75 – 0.92, have been obtained with a separate labeling RF coil and the average RF power deposited on the neck was smaller than 2 W both in animals (42, 44) as well as in humans (43), corresponding to less than 3.8W/kg specific absorption rate (SAR). However, because of the close proximity of the labeling RF coil to the skin in the neck and the individual variability in the location of the CCAs and VAs, a careful calibration of the mean RF power required to produce efficient labeling is advised to minimize quantification errors of CBF. In addition, monitoring of the skin temperature during the experiment (44) is advised to avoid local RF burns.
The first use of ASL to map arterial territories was made by Detre et al. (14). They explored the limited spatial coverage of a separate butterfly-shaped labeling RF coil placed on the neck, combined with the use of an oblique labeling gradient, as shown in Fig. 1a–b, to obtain territory maps of the left and right cerebral hemispheres of the rat. The oblique labeling gradient ensures that ASL occurs only over the artery of choice, as the endogenous water spins flowing in the contralateral arteries are out of resonance within the longitudinal field of view of the labeling RF coil (FOVz, as shown in Fig. 1b). By varying the angle θ between the labeling plane and the plane orthogonal to the arteries of interest, ASL of the right only, the left only, or both CCAs can be achieved, providing perfusion territories of the right, left or both cerebral hemispheres, respectively. This approach has been recently extended by Paiva et al to obtain maps of cerebral perfusion territories maps both in animal as well as in human subjects (30,31). Fig. 1c shows CBF images of a normal human subject, obtained by setting θ = 0° (top row), θ = +60° (middle row) and θ = −60° (bottom), corresponding to the CBF images of the whole brain, the right and the left circulation, respectively. Usually each VA is labeled with its collateral CCA, such that the unilateral vascular territories obtained with this method include contributions from both the anterior and posterior circulation. However, if a double oblique labeling plane is used, the territory of the VAs can be separated from their corresponding collateral CCAs, provided they are situated sufficiently apart from each other. In fact, the minimum arterial separation one can differentiate with the method is determined by the longitudinal field of view FOVz of the labeling RF coil and the angle θ between the labeling plane and the plane orthogonal to the arteries of interest, as shown in Fig. 1b. This minimum separation is given by:
| [1] |
Figure 1.
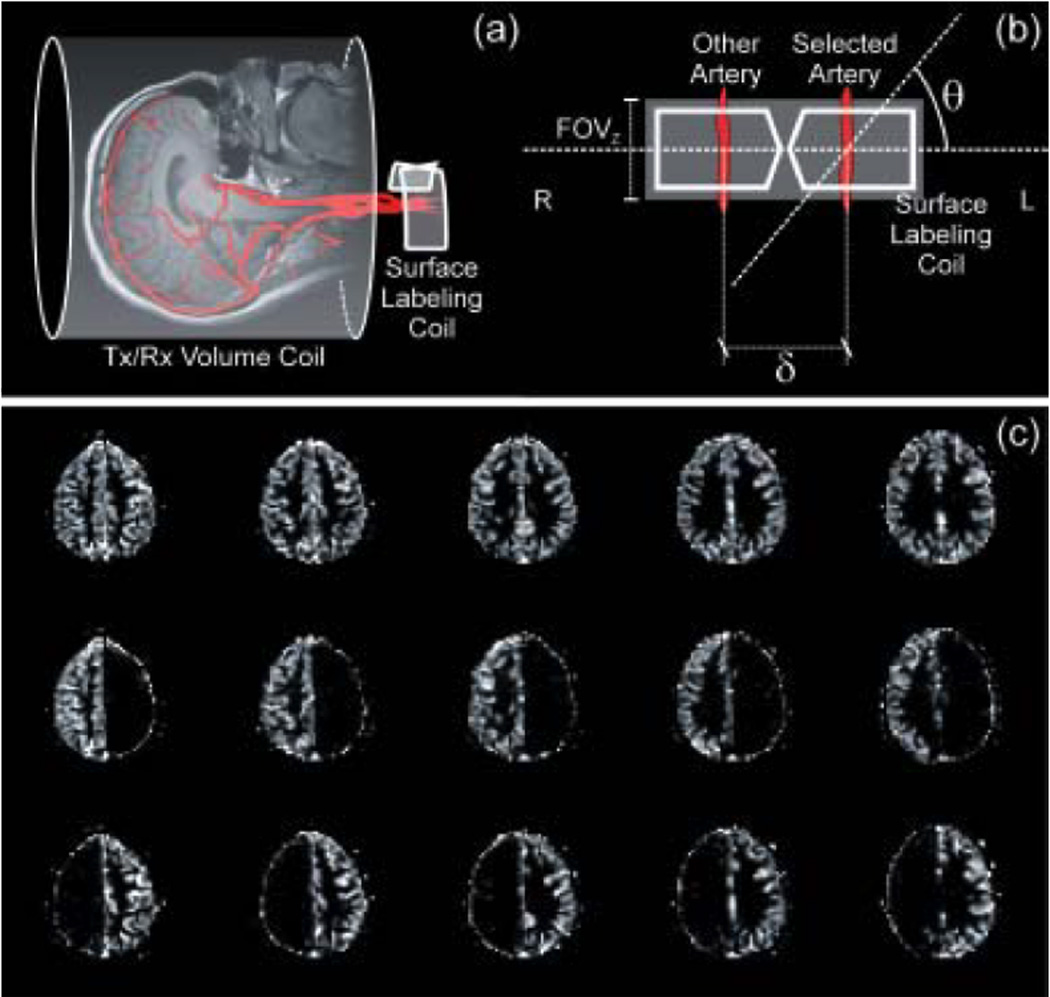
(a) Schematic representation of the separate labeling/imaging coil approach. The labeling RF coil is placed right over the neck to access both CCAs and VAs. (b) An oblique labeling plane, employed at an angle θ with respect to a plane perpendicular to blood flow, allows for selective labeling of the desired arteries. Positive values of θ target the left circulation, while negative values target the right circulation. (c) CBF images of a healthy human subject, obtained by setting θ = 0° (top row), θ = −60° (middle row) and θ = +60° (bottom), corresponding to the CBF images of the whole brain, the right and the left circulation, respectively.
Therefore, higher lateral separability is obtained using narrow RF coils (an essential feature to avoid MT effects (42)) and high angles.
One drawback of using a separate labeling RF coil placed on the neck is that mapping of vascular territories is restricted to the neck arteries. This precludes the use of the method to independently obtain territories of the branches of the COW, although in a few cases it is possible to obtain information about perfusion territories of vessels that are not uniquely accessible by properly combining acquisitions of different territories that share the vessel that is not uniquely accessible in order to infer its own territory.
The same principles as described above were applied by Zaharchuk et al (15) and by the group of David Norris (16,19) to measure the vascular territories of the CCAs with the use of a separate labeling RF coil placed directly over a single individual artery, as illustrated in Fig. 2a. In this case, the need for an oblique labeling plane to select the artery of interest was eliminated, at a cost of not being able to access internal arteries without affecting the more superficial ones. Furthermore, differently from when a full neck coil is employed, this approach does not allow the acquisition of both left and right perfusion and arterial territory maps without repositioning of the subject or of the coil, and it does not provide a perfusion map of the whole brain. Figure 2b shows a set of multi-slice perfusion territory images acquired in a 3T human system using a small coil right over the left carotid, showing perfusion contrast in the ipsilateral hemisphere of a healthy subject (15).
Figure 2.
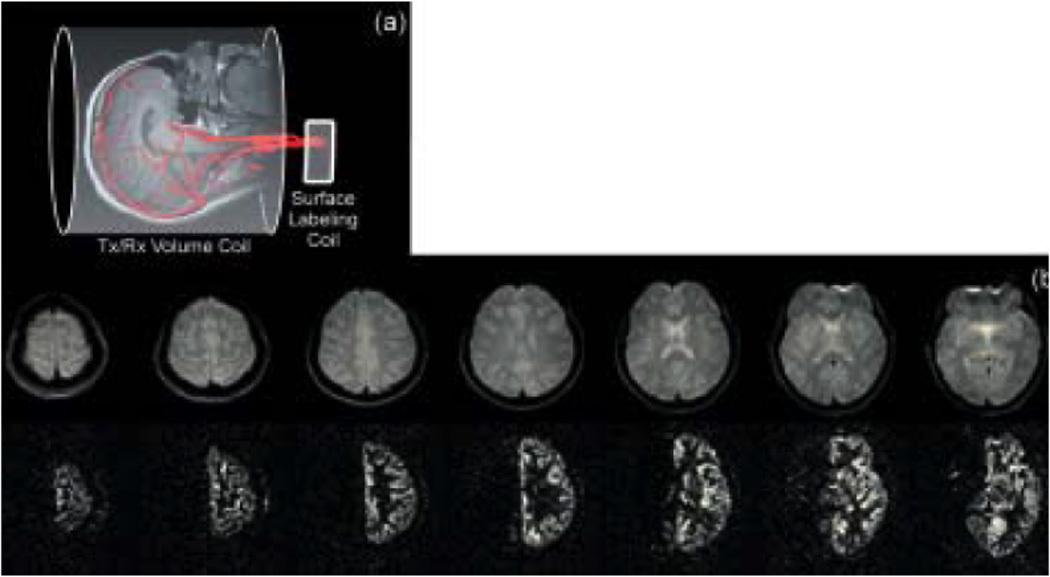
(a) An alternative option that excludes the need of the oblique labeling plane is to place the labeling RF coil right over one of the carotids. (b) Multi-slice axial control (top) and subtraction (bottom) images created using a unilateral carotid labeling RF coil and spin echo EPI (TR/TE/labeling period/post-labeling delay = 4s/22ms/3s/0.5s). As expected in the normal brain, perfusion from one carotid artery supplies only the ipsilateral hemisphere. The excellent subtraction of the contralateral hemisphere serves as a demonstration of the elimination of magnetization transfer effects. This method requires repositioning the coil to selectively label the contra lateral carotid. (Reproduced by permission of Zaharchuk et al (15)).
In short, specific advantages of the use of a separate labeling RF coil to obtain perfusion territories of the major cerebral arteries are high SNR, lower RF power deposition and 3-dimensional coverage due to the absence of MT effects that make it unnecessary to apply RF power during the control phase of the experiment. Full separation of the left and right circulation is easily achieved, while separation of the anterior and posterior circulation is possible with the use of additional scans. The main disadvantage is that, due to the limited spatial coverage of the labeling RF coil, distal branches of the COW are not accessible. Furthermore, the separate labeling RF coil requires the MRI scanner to be equipped with extra hardware, which can impose a significant limitation for the implementation of the method in clinical systems. Careful calibration of the RF power levels and the degree of labeling efficiency are also required to prevent excessive local RF power densities and to minimize CBF quantification errors.
Selective Inversion of Spatially Confined Areas
The most explored ASL method for measurement of vascular territories uses selective labeling of the brain region containing the artery of interest (5,17,20–29). These techniques employ a single RF coil to perform the labeling of the arterial spins, as well as the imaging of the volume of interest. The use of a single transmit RF coil in ASL carries an intrinsic disadvantage related to RF power deposition. In contrast to a separate labeling RF coil, which has a confined excitation profile, the volume RF coil encompasses the entire subject, causing a global RF deposition. Furthermore, to account and control for MT effects, it is necessary to apply RF also while acquiring the control images, doubling the required RF duty cycle when compared to separate labeling RF coil methods (15). Because the SAR is proportional to the magnetic field, application of these methods at higher magnetic fields can become restricted. On the other hand, a single labeling/imaging RF coil approach has the advantage of not requiring additional hardware, making it easier to implement in the clinical setting.
Using an approach similar to the one described for a separate labeling RF coil, Werner et al. (20) implemented a method in which they explored the limited coverage of a head transmit/receive RF coil over the human neck to selectively label either the left of the right CCAs and VAs. Based on the fallout of the RF volume coil excitation profile over the neck (Fig. 3a) and aided by the use of an oblique labeling plane, ASL occurs just over the left- or right-sided CCA and VA (Fig. 3b). Therefore, this method constitutes the single-coil implementation of the previously described use of a separate labeling RF coil. However, because of the head coil’s homogeneous excitation profile on the head, branches of the COW can not be separated and thus the method is limited to distinguishing left versus right circulation only. Fig. 3c shows perfusion-weighted images from a healthy volunteer with left and right-sided circulation labeled, respectively. Good separation between left and right hemispheres is achieved, but the method exposes its limitations regarding the isolation of the contribution from the posterior circulation.
Figure 3.
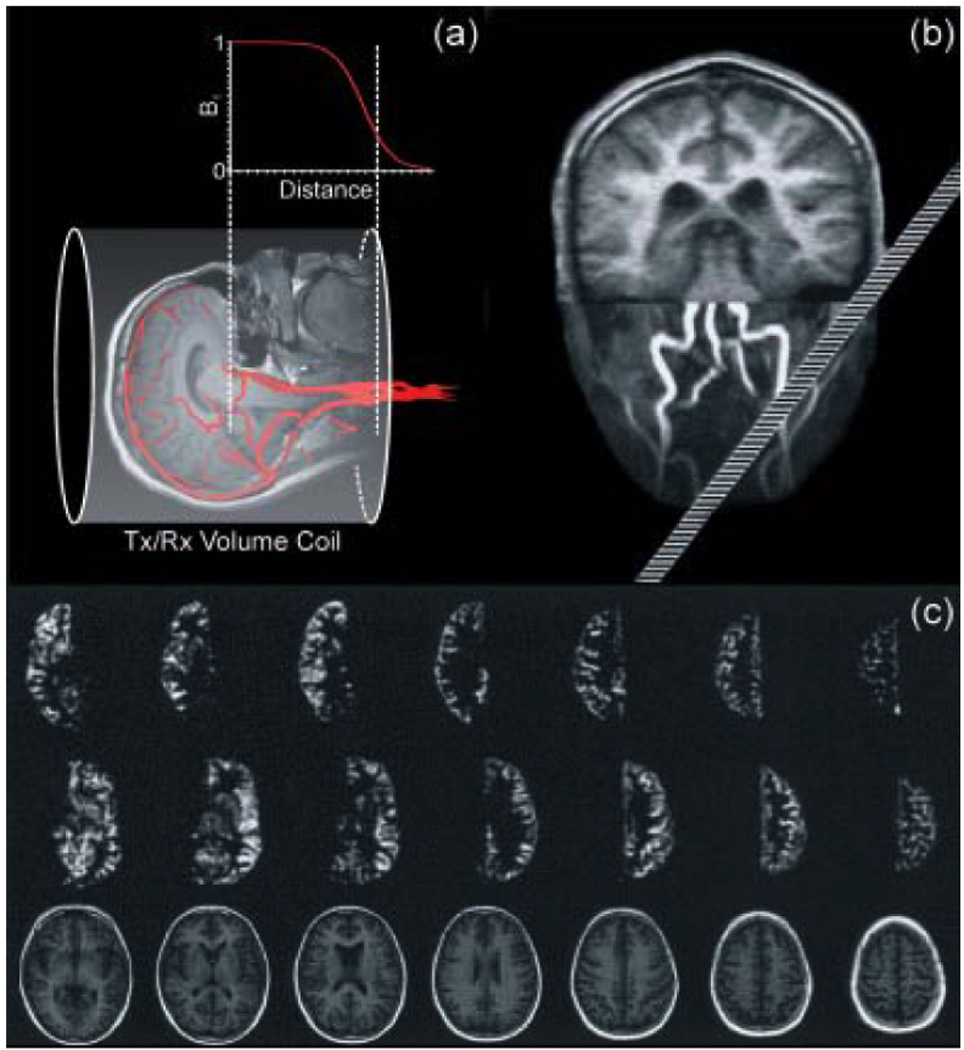
(a) Schematic illustration of the amplitude profile along the longitudinal axis of a standard transmitter/receive volume coil. The selective label of the desired arteries is achieved exploring the limited longitudinal field of the volume coil in the neck’s region combined with an oblique labeling plane. (b) Positioning of the labeling plane during the MR experiment based on maximum intensity projection of angiography data. The image of the brain was added for illustration purposes. The oblique plane intersects the selected arteries 80 mm, the contralateral arteries 160 mm below the coil center. Adapted from Werner et al (20). Perfusion-weighted images as acquired in a volunteer. First/second row: perfusion images with left/right-sided arteries labeled; bottom row: anatomical images. The oblique-plane selection mechanism yields clearly delineated perfusion territory images of the anterior circulation. Gray and white matter can easily be distinguished even in the smaller structures. (Reproduced by permission of Werner et al (20)).
In contrast to the CASL methods described above, PASL sequences were introduced to selectively label the anterior circulation (left and right ICAs), as well as the posterior circulation, formed by the VAs and the basilar artery (BA) (5,21,22,24–28). Figure 4a shows the basics of the labeling scheme. First, an angiogram of the COW is acquired and used to prescribe the labeling slabs over the left ICA (green), the right ICA (red), and the posterior circulation (blue). Each labeling slab reaches the whole path of the cerebral arteries, so that there is no significant decrease in SNR compared to ASL of the entire brain. However, both in these methods, as well as in that one proposed by Taoka et al. (21), saturation modules and strong dephasing gradients must be employed to minimize artifacts introduced by overlapping of the labeling plane with the imaging slices.
Figure 4.
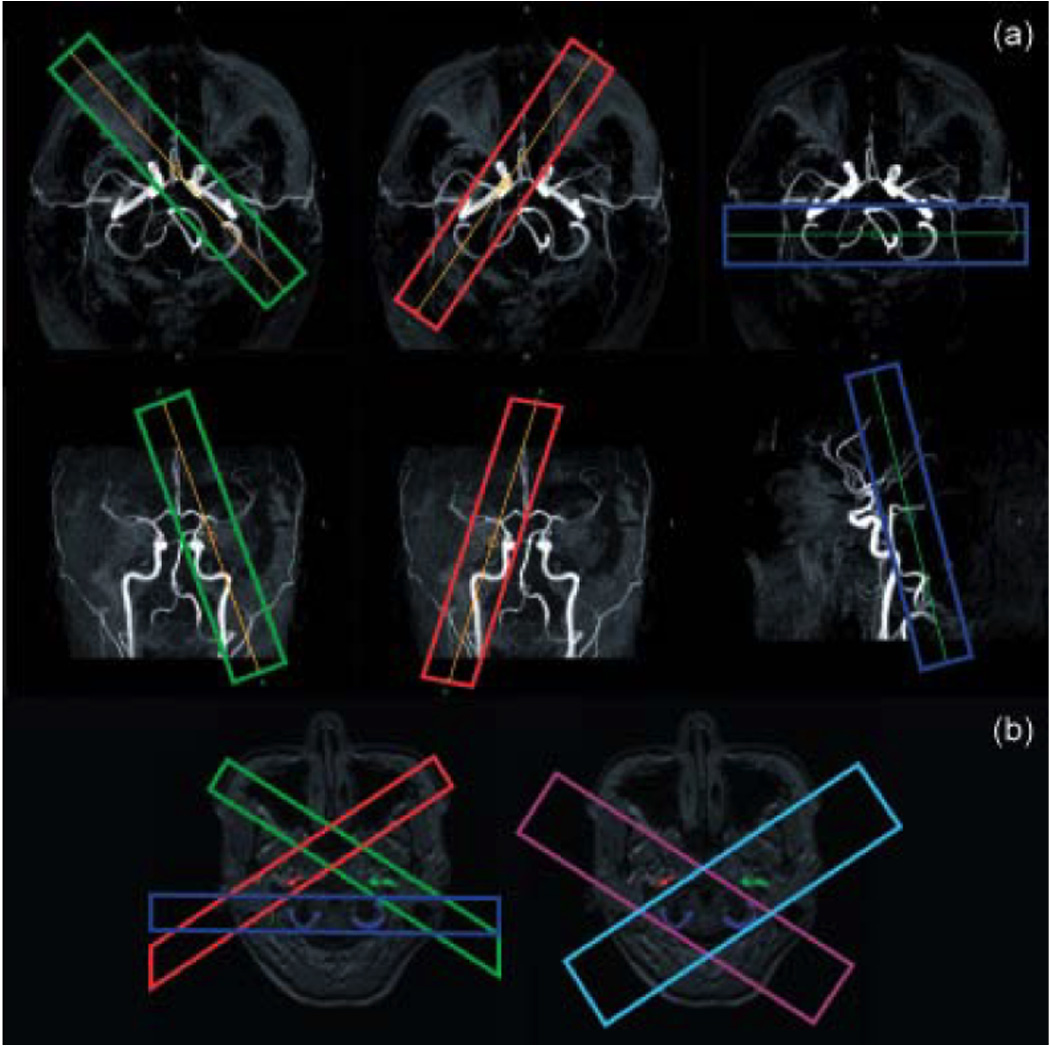
(a) Planning of the respective labeling of the left ICA (green), right ICA (red), and posterior circulation (blue) on the MIPs of the circle of Willis of a healthy volunteer. (Reproduced by permission of Golay et al (25)) (b) Planning of the labeling scheme used in the dual vessel approach, in which two acquisitions (light blue and purple), both including the posterior circulation, replace the three acquisitions (red, green and blue) from the previous method. (Courtesy of Dr. Xavier Golay).
Initially, such methods were developed with the intent of directly labeling only the desired arteries (Fig. 3a), while trying to avoid contamination from different arteries (5,21,22,24,25). More recently, however, Zimine et al. (26), Günther (27) and Wong (28) introduced variations in this approach tagging combinations of arteries and obtaining the desired territories using post-processing. Zimine and colleagues replaced the three acquisitions used in prior methods by two acquisitions, both of which included the posterior circulation (Fig. 4b). In doing so, total image acquisition was shortened (26). Color-coded maps were introduced to better visualization of the territories and are exemplified in Figure 5a. The scheme proposed by Günther introduced a gradient cycling where the sign of the longitudinal spin magnetization of the inflowing blood changes (27). Thus, the information from the different territories is achieved within a single experiment that is less time consuming. In addition, independent component analysis was utilized as a post-processing tool to increase the accuracy of delineation of the different vascular territories, diminishing the problems caused by imprecise slab labeling, typical of previous similar methods (Fig. 5b). These methods have shown their usefulness both at 1.5T and 3T human systems.
Figure 5.
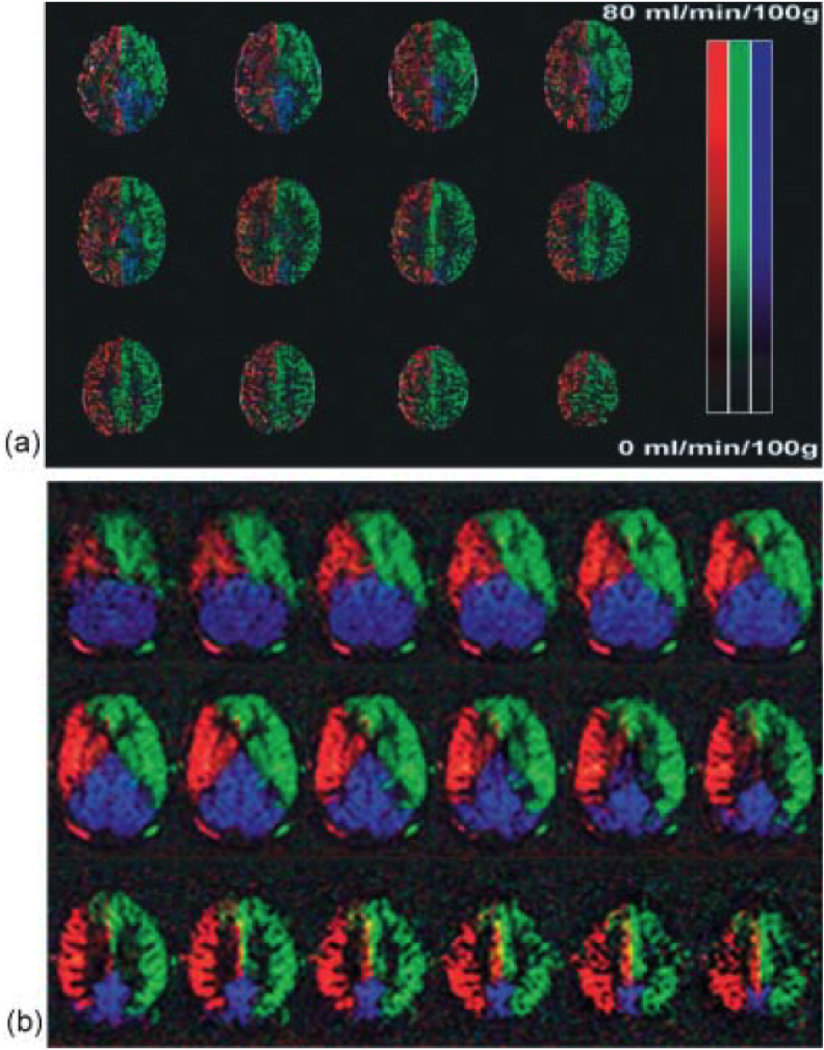
Color-coded based territories maps of the left ICA (green), right ICA (red), and posterior circulation (blue) from healthy volunteers. (a) Maps acquired based on three independent acquisitions. (Reproduced by permission of Golay et al (25)). (b) Maps acquired in a single cycled ASL experiment and processed using independent component analysis. (Reproduced by permission of Günther (27)).
While the use of MRA for prescribing the position of the labeling slabs is a fundamental step in the above-mentioned methods, there are several caveats that should be considered. First, there is the potential for movements of the patient between the acquisition of the MRA and the successive regional perfusion imaging, resulting in inaccurate mapping of the regional vascular territories or in decreased SNR due to incomplete inversion of the spins in the arteries of choice. This potential increases with the duration of the scan, so that prolonged experiments may benefit from frequent re-acquisitions of the MRA. Second, in some subjects it may be impossible to clearly separate the anterior and posterior perfusion territories due to the tortuosity of the vessels and the position of the subject’s head in the scanner. Finally, the accurate positioning of the labeling slabs is subject to user-dependent variations, leading to inconsistent results. The latter problem can be improved by the development of automatic positioning systems of the different labeling slabs (such as described in “I. Zimine, et al., Automatic Planning for Regional Perfusion Imaging, Proc. 14th ISMRM, 3426 (2006)”), or by the use of dedicated post-processing techniques such as the ICA routines proposed by Günther (27).
To achieve better definition of the vascular territory, Eastwood et al. (17) and Jones et al. (29) used a more elaborated PASL scheme, in which inversion slabs were combined with saturation of the imaging slices, as shown in Fig. 6. The former reinforced the importance of selective ASL using this approach to extend angiographic techniques instead of expressly generate perfusion data (17), while the latter effectively explored the specific advantages of these methods, which includes less vulnerability to uncertainties in labeling efficiency due to variations in arterial geometry and blood flow velocity, to separate right and left perfusion hemispheres (29). Nevertheless, both approaches are restricted to acquisition of a single imaging slice, and are highly sensitive to the slice profile and power calibration of the RF pulses used to obtain inversion of the imaging plane and saturation of the stationary spins.
Figure 6.
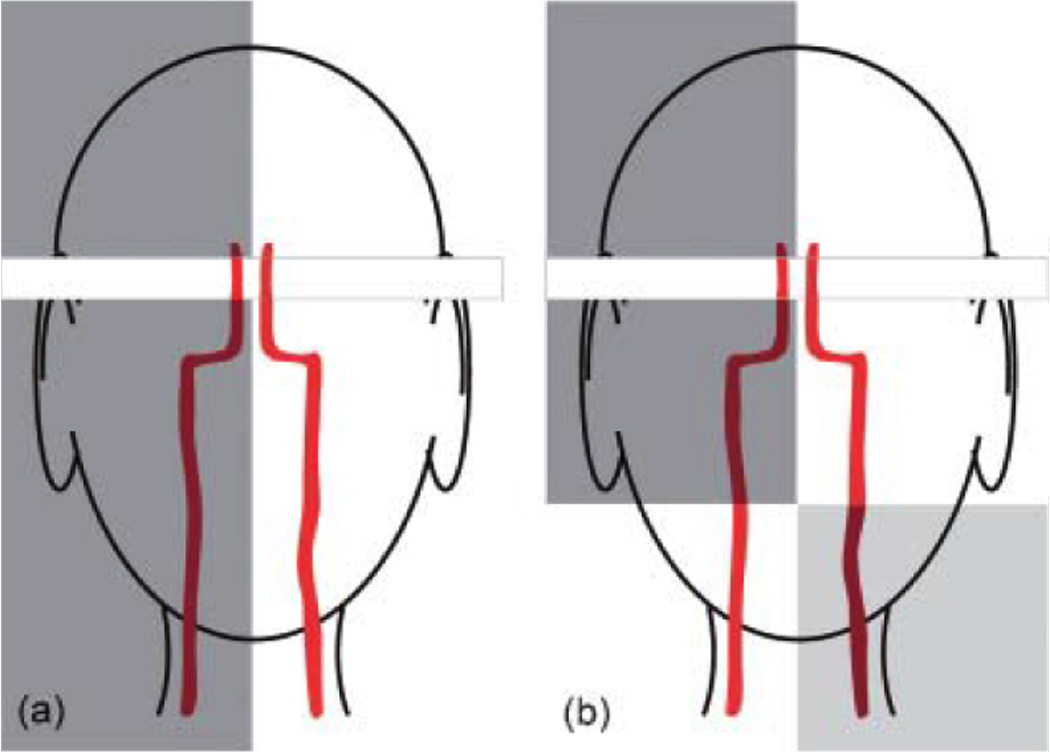
Schematic representation of selectively labeling schemes that combine RF pulses and saturation of the imaging slices to achieve better definition of the vascular territory. (a) A sagittal unilateral selective inversion pulse inverts the spins in the selected carotid. A saturation pulse followed by a spoiler gradient is applied to destroy the effects of the inversion pulse in the imaging slice. (b) A sagittal unilateral selective inversion pulse is followed by a transverse selective inversion pulse that swaps the labeling scheme in the region located below the acquisition slice. A saturation pulse followed by a spoiler gradient is applied to destroy the effects of the inversion pulse in the imaging slice.
A novel labeling mechanism, called continuous artery-selective spin labeling (CASSL), was recently proposed by Werner et al. (23), who introduced a rotating labeling plane centered over the artery of interest to spoil labeling of unwanted arteries (Fig. 7a). As shown in Fig. 7b, a good level of selectivity can be reached with the method, including labeling of the BA, the middle cerebral artery (MCA) and both anterior cerebral arteries (ACAs). However, even though individual territories appeared well delineated, they could be contaminated by interferences of the labeling scheme and the imaged regions, shown as dark bands in the perfusion-weighted images (Fig. 7b). Furthermore, CASSL requires a long time to scan each independent branch of the arteries distal to COW, which is not really possible within a standard clinical acquisition time.
Figure 7.
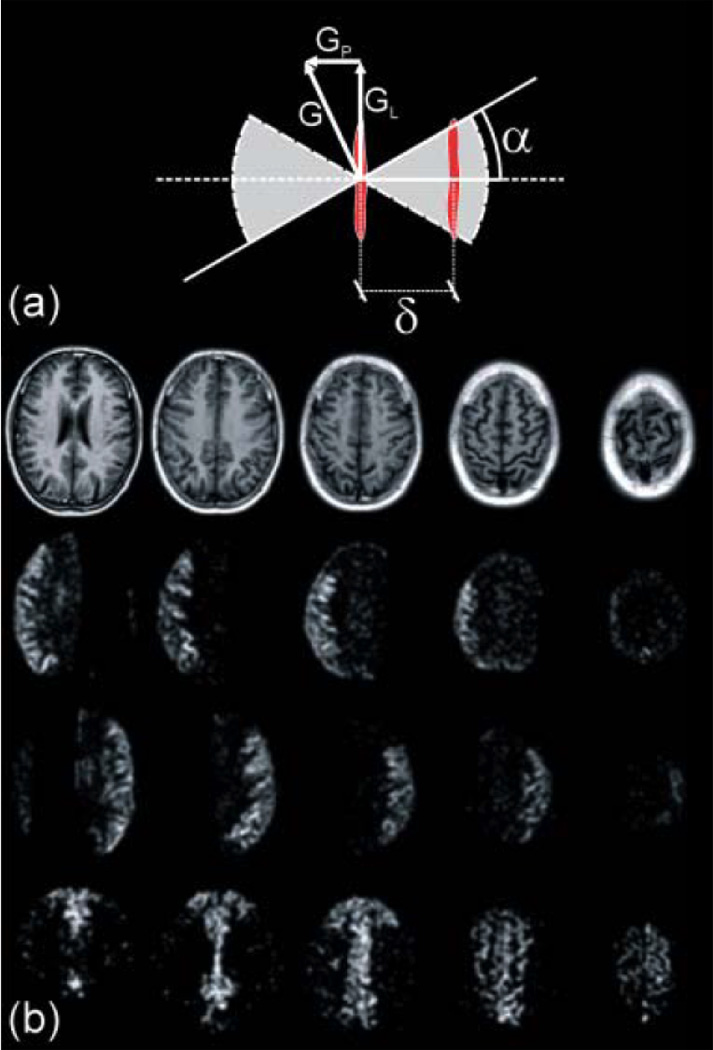
(a) Schematic illustration of the rotating labeling plane scheme used to selectively label the desired artery (23). A rotating labeling gradient G combined with a frequency-modulated labeling RF pulse sets the position of the labeling plane to the selected artery while the adjacent areas observes a sinusoidally-variable resonant behavior. The shaded area illustrates the moving labeling plane coverage. (b) Perfusion territory images acquired in a health volunteer, obtained with the Continuous Artery-Selective Spin Labeling (CASSL) scheme (23). Top row: T1-weighted anatomical reference images. Second row: right-sided MCA selected. Third row: left-sided MCA selected. Bottom row: left- and right-sided ACAs selected. The perfusion territories are clearly delineated. Regions in which the labeling mechanism intersects the imaging volume appear dark. (Reproduced by permission of Werner et al (23)).
Phantom experiments realized in a clinical 1.5T system demonstrated that the method can achieve a relatively high labeling efficiency (~80% of a regular continuous ASL experiment). However, the method proved to be high sensitive to errors in the labeling position, especially in vivo. Small deviations in the labeling plane position can produce strong degrading effects on the labeling efficiency, making absolute quantification quite challenging (23). Other sources of imprecision in the labeling position may be introduced by susceptibility effects, as well as by B0 field inhomogeneities. In both cases, the inhomogeneity gives rise to a field gradient, whose component pointing perpendicular the selected artery can act shifting the labeling point away from the desired location, reducing the labeling efficiency and even the selectivity of the artery of interest.
In summary, the single label/imaging approaches to obtain perfusion territories have higher flexibility of choice than the methods that use a separate labeling RF coil, allowing selectivity of cerebral arteries upstream of the neck, including the BA, the different branches of the COW and even upstream of the COW. Disadvantages include imprecision in delineation of the vascular territories stemming from incomplete or imprecise selectivity of the arteries of interest, as well as the presence of artifacts associated with overlap of the labeling and imaging regions, especially when targeting arteries distal of the COW. The use of a single volume RF coil to perform the ASL and imaging may suffer of excessive RF power deposition in CASL sequences, especially at high field strengths.
Two-Dimensional Labeling RF Pulses
Two-dimensional (2D) RF pulses, initially proposed by Bottomley and Hardy (45), arose as a feasible option to achieve the most flexible method for mapping cerebral vascular territories in terms of selectivity and specificity (18). Since their proposition, 2D RF pulses have been employed in different applications in MRI, including restricted field-of-view imaging (46), angiographic (47) and cardiac imaging (48). The main advantage in using two-dimensional RF pulses is that they can provide a simultaneous spatially selective label in two dimensions that, in principle, makes it possible to label any desired artery, including those ones located above the COW.
In spite of all flexibility and selectivity achieved by the employment of 2D RF pulses in ASL to obtain perfusion territories, there are some practical disadvantages that must be mentioned. Two-dimensional RF pulses have intrinsic side-lobes that must be placed outside the imaging field-of-view to avoid MT effects and to minimize contamination of the perfusion signal. However, the narrower the pulse bandwidth is (which improves the specificity), the smaller the radius of the side lobe. This relation plays a constraint in how selective the method can be without causing interfering effects in the perfusion signal. In addition, in vivo applications impose some extra inherent challenges related to the anatomy of the labeling region. Usually the algorithms used for design 2D RF pulses do not take in consideration the blood flow, which translates into bulk motion of the spins. This makes it difficult to achieve the desired inversion profile in regions where the blood flow is changing directions, causing distortions of the 2D RF pulse profile due to off-resonance effects and diminishing the accuracy of the labeling profile.
An example of the low accuracy of 2D RF pulses in delineating perfusion territories is illustrated in the Fig. 8. The difference images shown in the second and third rows resulted from a 2D labeling pulse applied to the right and left ICA, respectively. There is significant contamination and consequently poor definition of the vascular territories. This feature constitutes the biggest practical challenge related to the implementation of the method.
Figure 8.
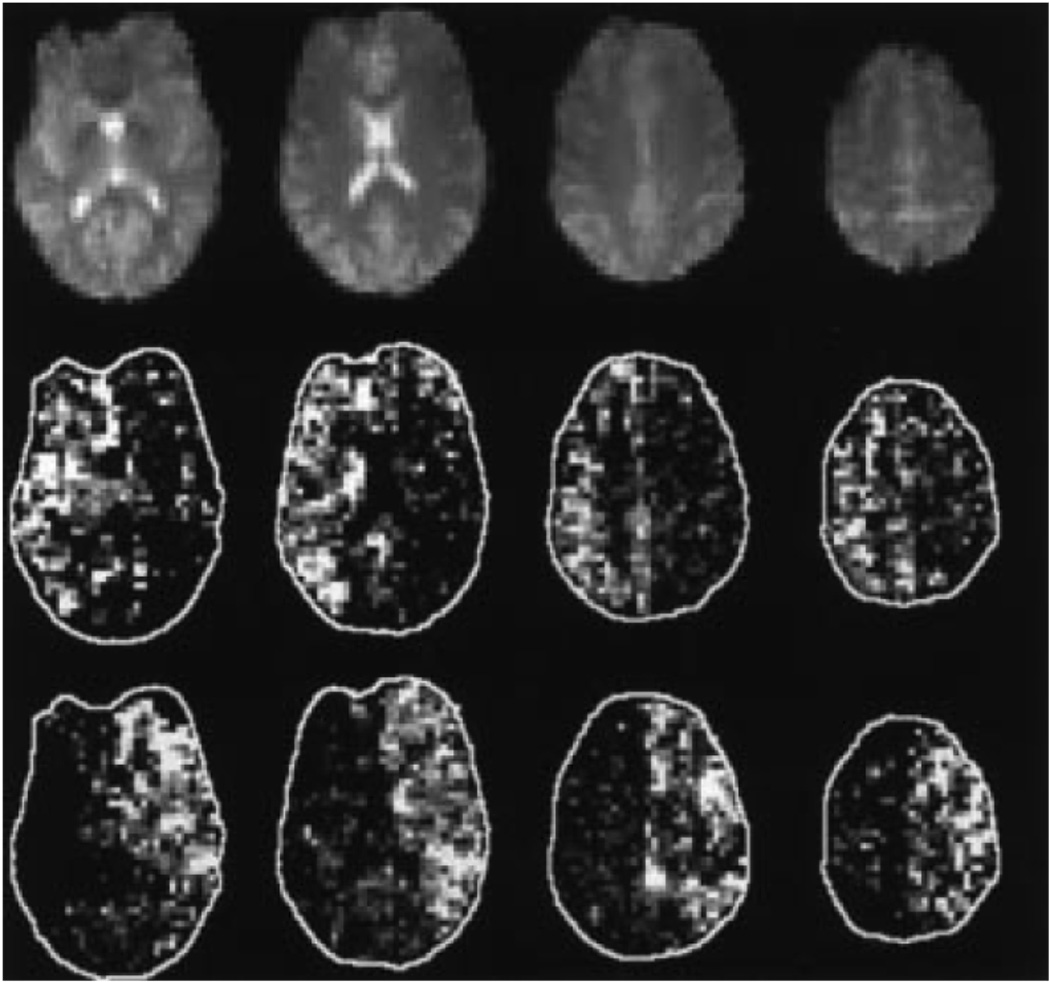
The four superior slices of baseline EPI readout (M0) from one subject (top panel). Also shown are mean pairwise difference images normalize to baseline EPI (M/M0) resulting from a 2D tag applied to the right ICA (middle panel) and the left ICA (bottom panel), selected with the use of a tailored 2-dimensional RF pulse. (Reproduced by permission of Davies and Jezzard (18)).
Conclusions
Several ASL methods have been proposed to map cerebral perfusion territories. Table 1 lists the major advantages and disadvantages of each method. In essence, methods that use a separate labeling RF coil have high SNR, low RF power deposition and 3-dimensional coverage due to the absence of MT effects, but are mostly limited to providing full separation of the left and right circulation, and do require extra hardware, which may significantly limit their implementation in clinical systems. Methods that utilize standard clinical hardware have higher flexibility of implementation and higher arterial selectivity, and may be the best choice for a more detailed picture of territories of the different branches of the COW. However, it is hard to achieve selective labeling inside the volume of interest without causing significant imaging artifacts that may interfere both with the mapping of the desired territory and with quantification of CBF.
Table 1.
Summary of the advantages, disadvantages and limitations of each method to obtain cerebral perfusion territories.
| Method | References | Territories | Advantages | Disadvantages | Limitations | |
|---|---|---|---|---|---|---|
|
Separate Labeling RF Coil (CASL) |
Full Neck (butterfly) | 14, 30, 31 | Left and right anterior and middle cerebral circulation (ICA) Posterior circulation (VAs & BA) with post-processing |
High SNR 1Lower RF power deposition Unrestricted 3-D coverage due to absence of MT effects |
Requires use of additional hardware, not easy to implement in clinical scanner | Can only label circulation of the neck, proximal to COW; distal branches are not accessible |
| Single Loop | 15, 16, 19 | Left and right anterior and middle cerebral circulation (ICA, requires repositioning of RF coil) | Posterior circulation not separable from anterior and middle circulation | |||
| Selective Inversion of Spatial Slabs | Single-coil CASL | 20 | Left and right anterior and middle cerebral circulation (ICA) Posterior circulation (VAs & BA) with post-processing |
Does not require additional hardware, easy to implement in clinical scanner | High RF power deposition | Can only label circulation of the neck, proximal to COW; distal branches are not accessible |
| PASL techniques | 5, 21, 22, 24–28 | Left and right anterior and middle cerebral circulation (ICA) Posterior circulation (VAs & BA) Arteries located proximal and distal to the COW |
High flexibility of selective labeling of proximal and distal branches of COW | Imprecise delineation of the vascular territories due to imprecise selectivity Artifacts due to overlap of labeling and imaging regions |
Tortuosity of vessels may impede clear identification of territories Spatial coverage significantly limited by interference of labeling region with measurement slices |
|
| 2D Labeling RF pulses | 18 | In principle, any artery of choice | Highest theoretical flexibility of selective labeling of any artery of choice | Hard to obtain selective 2D pulse without artifacts from side lobes | Moving arterial spins due to blood flow distort the profile of the 2D RF pulse and diminish selectivity | |
When compared to single-coil CASL methods;
In the past few years, there has been an explosion of new ASL methods dedicated to mark the perfusion territories of major cerebral arteries. This is due, in part, to significant technological advances in the hardware and software capabilities of MRI scanners. However, to the greatest extent, due to increased demand by clinicians to use not only the arterial patency status provided by MRA, but also the information about the extent of tissue supplied by each artery, a parameter that can only be assessed with a selective CBF measurement. Thus, it is certain that further research will continue in two main directions, which are: (1) to eliminate most technical limitations associated with each technique, and to improve the sensitivity and the spatial coverage; and (2) to bring these techniques to the bedside, making them available in clinical scanners to be routinely used in patients.
Vascular territory mapping is a promising tool for the study of cerebrovascular diseases. The development and use of ASL techniques to obtain cerebral perfusion territories came as a natural solution to the need to selectively visualize the flow of blood through only a few arteries of interest, a feature that is very hard to accomplish with DSC-MRI. The major advantages of ASL-based mapping of vascular territories are that ASL does not require injections of contrast agents, allowing multiple, repetitive measurements to be carried out within a single clinical visit or across several visits. Furthermore, in addition to offering a map of the vascular territory, ASL also provides quantitative information on the perfusion status of the tissue. The combination of the dynamic measurements of vascular territories with the quantification of CBF may open up important interventional strategies to verify, in real time, the change in perfusion territory and perfusion status of the tissue in treatment of acute stroke, as well as to follow up the evolution of the ischemic area and changes in compensatory collateral flow. Application of these methods in clinical practice will aid furthering the understanding, diagnosis and treatment of cerebrovascular diseases.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NINDS (Alan P. Koretsky, Scientific Director).
Abbreviation List
- ACA
anterior cerebral artery
- ASL
arterial spin labeling
- BA
basilar artery
- CASL
continuous arterial spin labeling
- CASSL
continuous artery-selective spin labeling
- CCA
common carotid artery
- COW
circle of Willis
- DWI
diffusion-weighted imaging
- ICA
internal carotid artery
- MCA
middle cerebral artery
- MRA
magnetic resonance angiography
- MT
magnetization transfer
- PASL
pulsed arterial spin labeling
- rCBF
regional cerebral blood flow
- rCBV
regional cerebral blood volume
- rMTT
regional mean transit time
- SAR
specific absorption rate
- VA
vertebral arteries
References
- 1.Hoeffner EG. Cerebral perfusion imaging. J Neuroophthalmol. 2005;25(4):313–320. doi: 10.1097/01.wno.0000189832.00129.2e. [DOI] [PubMed] [Google Scholar]
- 2.Wintermark M, Sesay M, Barbier E, Borbely K, Dillon WP, Eastwood JD, Glenn TC, Grandin CB, Pedraza S, Soustiel JF, Nariai T, Zaharchuk G, Caille JM, Dousset V, Yonas H. Comparative overview of brain perfusion imaging techniques. Stroke. 2005;36(9):e83–e99. doi: 10.1161/01.STR.0000177884.72657.8b. [DOI] [PubMed] [Google Scholar]
- 3.Cha S. Perfusion MR imaging: basic principles and clinical applications. Magn Reson Imaging Clin N Am. 2003;11(3):403–413. doi: 10.1016/s1064-9689(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 4.Lad SP, Guzman R, Kelly ME, Li G, Lim M, Lovbald K, Steinberg GK. Cerebral perfusion imaging in vasospasm. Neurosurg Focus. 2006;21(3):E7. doi: 10.3171/foc.2006.21.3.7. [DOI] [PubMed] [Google Scholar]
- 5.van Laar PJ, Hendrikse J, Golay X, Lu H, van Osch MJ, van der Grond J. In vivo flow territory mapping of major brain feeding arteries. Neuroimage. 2006;29(1):136–144. doi: 10.1016/j.neuroimage.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Latchaw RE. Cerebral perfusion imaging in acute stroke. J Vasc Interv Radiol. 2004;15(1 Pt 2):S29–S46. doi: 10.1097/01.rvi.0000112976.88422.86. [DOI] [PubMed] [Google Scholar]
- 7.Rowley HA, Roberts TP. Clinical perspectives in perfusion: neuroradiologic applications. Top Magn Reson Imaging. 2004;15(1):28–40. doi: 10.1097/00002142-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen AG, Buonanno FS, Gonzalez RG, Schwamm LH, Lev MH, Huang-Hellinger FR, Reese TG, Weisskoff RM, Davis TL, Suwanwela N, Can U, Moreira JA, Copen WA, Look RB, Finklestein SP, Rosen BR, Koroshetz WJ. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology. 1996;199(2):391–401. doi: 10.1148/radiology.199.2.8668784. [DOI] [PubMed] [Google Scholar]
- 9.Warach S. Measurement of the ischemic penumbra with MRI: it's about time. Stroke. 2003;34(10):2533–2534. doi: 10.1161/01.STR.0000092395.19554.9A. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura DG, Macovski A, Pauly JM, Conolly SM. MR angiography by selective inversion recovery. Magn Reson Med. 1987;4(2):193–202. doi: 10.1002/mrm.1910040214. [DOI] [PubMed] [Google Scholar]
- 11.Edelman RR, Mattle HP, O'Reilly GV, Wentz KU, Liu C, Zhao B. Magnetic resonance imaging of flow dynamics in the circle of Willis. Stroke. 1990;21(1):56–65. doi: 10.1161/01.str.21.1.56. [DOI] [PubMed] [Google Scholar]
- 12.Sardashti M, Schwartzberg DG, Stomp GP, Dixon WT. Spin-labeling angiography of the carotids by presaturation and simplified adiabatic inversion. Magn Reson Med. 1990;15(2):192–200. doi: 10.1002/mrm.1910150203. [DOI] [PubMed] [Google Scholar]
- 13.Furst G, Steinmetz H, Fischer H, Skutta B, Sitzer M, Aulich A, Kahn T, Modder U. Selective MR angiography and intracranial collateral blood flow. J Comput Assist Tomogr. 1993;17(2):178–183. doi: 10.1097/00004728-199303000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Detre JA, Zhang W, Roberts DA, Silva AC, Williams DS, Grandis DJ, Koretsky AP, Leigh JS. Tissue specific perfusion imaging using arterial spin labeling. NMR Biomed. 1994;7(1–2):75–82. doi: 10.1002/nbm.1940070112. [DOI] [PubMed] [Google Scholar]
- 15.Zaharchuk G, Ledden PJ, Kwong KK, Reese TG, Rosen BR, Wald LL. Multislice perfusion and perfusion territory imaging in humans with separate label and image coils. Magn Reson Med. 1999;41(6):1093–1098. doi: 10.1002/(sici)1522-2594(199906)41:6<1093::aid-mrm4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Trampel R, Mildner T, Goerke U, Schaefer A, Driesel W, Norris DG. Continuous arterial spin labeling using a local magnetic field gradient coil. Magn Reson Med. 2002;48(3):543–546. doi: 10.1002/mrm.10228. [DOI] [PubMed] [Google Scholar]
- 17.Eastwood JD, Holder CA, Hudgins PA, Song AW. Magnetic resonance imaging with lateralized arterial spin labeling. Magn Reson Imaging. 2002;20(8):583–586. doi: 10.1016/s0730-725x(02)00536-2. [DOI] [PubMed] [Google Scholar]
- 18.Davies NP, Jezzard P. Selective arterial spin labeling (SASL): perfusion territory mapping of selected feeding arteries tagged using two-dimensional radiofrequency pulses. Magn Reson Med. 2003;49(6):1133–1142. doi: 10.1002/mrm.10475. [DOI] [PubMed] [Google Scholar]
- 19.Mildner T, Trampel R, Moller HE, Schafer A, Wiggins CJ, Norris DG. Functional perfusion imaging using continuous arterial spin labeling with separate labeling and imaging coils at 3 T. Magn Reson Med. 2003;49(5):791–795. doi: 10.1002/mrm.10438. [DOI] [PubMed] [Google Scholar]
- 20.Werner R, Alfke K, Schaeffter T, Nabavi A, Mehdorn HM, Jansen O. Brain perfusion territory imaging applying oblique-plane arterial spin labeling with a standard send/receive head coil. Magn Reson Med. 2004;52(6):1443–1447. doi: 10.1002/mrm.20253. [DOI] [PubMed] [Google Scholar]
- 21.Taoka T, Iwasaki S, Nakagawa H, Fukusumi A, Hirohashi S, Sakamoto M, Kichikawa K, Murata K. Distinguishing between anterior cerebral artery and middle cerebral artery perfusion by color-coded perfusion direction mapping with arterial spin labeling. AJNR Am J Neuroradiol. 2004;25(2):248–251. [PMC free article] [PubMed] [Google Scholar]
- 22.Hendrikse J, van der Grond J, Lu H, van Zijl PC, Golay X. Flow territory mapping of the cerebral arteries with regional perfusion MRI. Stroke. 2004;35(4):882–887. doi: 10.1161/01.STR.0000120312.26163.EC. [DOI] [PubMed] [Google Scholar]
- 23.Werner R, Norris DG, Alfke K, Mehdorn HM, Jansen O. Continuous artery-selective spin labeling (CASSL) Magn Reson Med. 2005;53(5):1006–1012. doi: 10.1002/mrm.20475. [DOI] [PubMed] [Google Scholar]
- 24.Hendrikse J, van der Zwan A, Ramos LM, van Osch MJ, Golay X, Tulleken CA, van der Grond J. Altered flow territories after extracranial-intracranial bypass surgery. Neurosurgery. 2005;57(3):486–494;. doi: 10.1227/01.neu.0000170563.70822.10. [DOI] [PubMed] [Google Scholar]
- 25.Golay X, Petersen ET, Hui F. Pulsed star labeling of arterial regions (PULSAR): a robust regional perfusion technique for high field imaging. Magn Reson Med. 2005;53(1):15–21. doi: 10.1002/mrm.20338. [DOI] [PubMed] [Google Scholar]
- 26.Zimine I, Petersen ET, Golay X. Dual vessel arterial spin labeling scheme for regional perfusion imaging. Magn Reson Med. 2006;56(5):1140–1144. doi: 10.1002/mrm.21049. [DOI] [PubMed] [Google Scholar]
- 27.Gunther M. Efficient visualization of vascular territories in the human brain by cycled arterial spin labeling MRI. Magn Reson Med. 2006;56(3):671–675. doi: 10.1002/mrm.20998. [DOI] [PubMed] [Google Scholar]
- 28.Wong EC. Vessel encoded arterial spin labeling using pseudo-continuous tagging. ISMRM 14th Scientific Meeting & Exhibition; 2006; Seattle, WA. p. 668. [Google Scholar]
- 29.Jones CE, Wolf RL, Detre JA, Das B, Saha PK, Wang J, Zhang Y, Song HK, Wright AC, Mohler EM, 3rd, Fairman RM, Zager EL, Velazquez OC, Golden MA, Carpenter JP, Wehrli FW. Structural MRI of carotid artery atherosclerotic lesion burden and characterization of hemispheric cerebral blood flow before and after carotid endarterectomy. NMR Biomed. 2006;19(2):198–208. doi: 10.1002/nbm.1017. [DOI] [PubMed] [Google Scholar]
- 30.Paiva FF, Nascimento GC, Tannús A, Silva AC. Arterial spin labeling measurement of arterial perfusion territories using a localized labeling RF coil. ISMRM 14th Scientific Meeting & Exhibition; 2006; Seatlle, WA. p. 1004. [Google Scholar]
- 31.Paiva FF, Nascimento GC, Tannús A, Talagala SL, Silva AC. Arterial perfusion territory mapping using a localized labeling RF coil. Neuroimage; 12th Annual Meeting of the Organization for Human Brain Mapping; 2006; Florence, ITA. p. S173. [Google Scholar]
- 32.Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab. 1999;19(7):701–735. doi: 10.1097/00004647-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Barbier EL, Lamalle L, Decorps M. Methodology of brain perfusion imaging. J Magn Reson Imaging. 2001;13(4):496–520. doi: 10.1002/jmri.1073. [DOI] [PubMed] [Google Scholar]
- 34.Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Top Magn Reson Imaging. 2004;15(1):10–27. doi: 10.1097/00002142-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Williams DS. Quantitative perfusion imaging using arterial spin labeling. Methods Mol Med. 2006;124:151–173. doi: 10.1385/1-59745-010-3:151. [DOI] [PubMed] [Google Scholar]
- 36.Detre JA, Leigh JS, Williams DS, Koretsky AP. Perfusion imaging. Magn Reson Med. 1992;23(1):37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- 37.Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci U S A. 1992;89(1):212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Williams DS, Detre JA, Koretsky AP. Measurement of brain perfusion by volume-localized NMR spectroscopy using inversion of arterial water spins: accounting for transit time and cross-relaxation. Magn Reson Med. 1992;25(2):362–371. doi: 10.1002/mrm.1910250216. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Frank JA, Hou L, Ye FQ, McLaughlin AC, Duyn JH. Multislice imaging of quantitative cerebral perfusion with pulsed arterial spin labeling. Magn Reson Med. 1998;39(5):825–832. doi: 10.1002/mrm.1910390520. [DOI] [PubMed] [Google Scholar]
- 40.Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39(5):702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- 41.Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208(2):410–416. doi: 10.1148/radiology.208.2.9680569. [DOI] [PubMed] [Google Scholar]
- 42.Silva AC, Zhang W, Williams DS, Koretsky AP. Multi-slice MRI of rat brain perfusion during amphetamine stimulation using arterial spin labeling. Magn Reson Med. 1995;33(2):209–214. doi: 10.1002/mrm.1910330210. [DOI] [PubMed] [Google Scholar]
- 43.Talagala SL, Ye FQ, Ledden PJ, Chesnick S. Whole-brain 3D perfusion MRI at 3.0 T using CASL with a separate labeling RF coil. Magn Reson Med. 2004;52(1):131–140. doi: 10.1002/mrm.20124. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X, Nagaoka T, Auerbach EJ, Champion R, Zhou L, Hu X, Duong TQ. Quantitative basal CBF and CBF fMRI of rhesus monkeys using three-coil continuous arterial spin labeling. Neuroimage. 2007;34(3):1074–1083. doi: 10.1016/j.neuroimage.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bottomley PA, Hardy CJ. Two-dimensional spatially selective spin inversion and spin-echo refocusing with a single nuclear magnetic resonance pulse. J Appl Phys. 1987;62(10):4284–4290. [Google Scholar]
- 46.Rieseberg S, Frahm J, Finsterbusch J. Two-dimensional spatially-selective RF excitation pulses in echo-planar imaging. Magn Reson Med. 2002;47(6):1186–1193. doi: 10.1002/mrm.10157. [DOI] [PubMed] [Google Scholar]
- 47.Alley MT, Pauly JM, Sommer FG, Pelc NJ. Angiographic imaging with 2D RF pulses. Magn Reson Med. 1997;37(2):260–267. doi: 10.1002/mrm.1910370220. [DOI] [PubMed] [Google Scholar]
- 48.Cline HE, Hardy CJ, Pearlman JD. Fast MR cardiac profiling with two-dimensional selective pulses. Magn Reson Med. 1991;17(2):390–401. doi: 10.1002/mrm.1910170211. [DOI] [PubMed] [Google Scholar]


