Abstract
Evidence is presented supporting a dimension of defensive reactivity that varies across the anxiety disorder spectrum and is defined by physiological responses during threat-imagery challenges that covary with objective measures of psychopathology. Previous imagery studies of anxiety disorders are reviewed, highlighting that, regardless of contemporary diagnostic convention, reliable psychophysiological patterns emerge for patients diagnosed with circumscribed fear compared to those diagnosed with pervasive anxious-misery disorders. Based on the heuristic outlined by the Research Domain Criteria (RDoC) initiative, an exploratory transdiagnostic analysis is presented, based on a sample of 425 treatment-seeking patients from across the spectrum of DSM-IV anxiety diagnoses. Using a composite index of startle reflex and heart rate reactivity during idiographic-fear imagery for each patient, a defensive dimension was defined by ranking patients from most defensively reactive to least reactive and then creating five groups of equivalent size (quintile; N = 85). Subsequent analyses showed significant, parallel trends of diminishing reactivity in both electrodermal and facial EMG reactions across this defensive dimension. Negative affectivity, defined by questionnaire, and extent of functional interference, however, showed consistent, inverse trends with defensive reactivity -- as reports of distress increased, defensive reactivity was increasingly attenuated. Notably, representatives of each principal diagnosis appeared in each quintile, underscoring the reality of pronounced within-diagnosis heterogeneity in defensive reactivity. In concluding, we describe our new RDoC research project, focusing on the assessment of brain circuit function as it determines hypo/hyper reactivity to challenge—somatic and autonomic—and may relate to patients’ stress history and genetic inheritance.
Keywords: RDoC, imagery, anxiety, phobia, fear, comorbidity, depression, anhedonia, anxiety sensitivity, emotional reactivity, narrative imagery, mental imagery, psychophysiology, startle, heart rate, facial expressivity, skin conductance, corrugator, EMG, SCL
Measuring Anxiety: DSM & RDoC
How are the anxiety disorders defined? According to the Diagnostic and Statistical Manual of Mental Disorders (5th ed.; DSM-5; American Psychiatric Association [APA], 2013), inclusion criteria for anxiety disorder diagnoses are based on shared “features of excessive fear and anxiety and related behavioral disturbances” (p.189, APA, 2013). The presence of these features is determined by the patient’s report of symptoms at interview and the diagnostician’s evaluation of their significance. It is further suggested (p. 189) that fear and anxiety disorders are characterized by changes in the patient’s physiology, that fear responses to threat cues prompt “surges of autonomic arousal,” and that anxiety is “associated with muscle tension and vigilance in preparation for future danger…” Clinical assessment does not, however, routinely include measurement of these physiological variables. Indeed, the diagnosis of significant mental distress in general, from schizophrenia and depression to conduct disorders, and despite similar conjectures about physiological factors in diathesis and symptom presentation, assessment is not in general practice abetted by biological measurement.
The National Institute of Mental Health (NIMH) now directly addresses this diagnostic lacuna, beginning a new program of research support (see this issue, Kozak & Cuthbert, 2015; Insel & Cuthbert, 2009) called the Research Domain Criteria (RDoC) initiative. Goal 1.4 of NIMH’s Strategic Plan questions the heuristic value for researchers of organizing their data exclusively around “clinical syndromes based on subjective symptoms,” suggesting that investigators “develop, for research purposes, new ways of classifying mental disorders based on dimensions of observable behavior and neurobiological measures.” Our contribution to Psychophysiology’s special RDoC issue is an assessment of research defining a physiological dimension across anxiety disorders consistent with this RDoC aim. We first briefly consider genetic and factor analytic studies that suggest such a dimension exists, and then present a series of studies examining physiological reactivity to “fear” challenge as responding varies over DSM anxiety diagnoses. We conclude with an exploratory, dimensional analysis of affective physiological reactivity, assessing the dimension’s relation to questionnaire findings and symptom patterns in a large sample of patients reporting principal disordered anxiety and mood.
An anxiety spectrum dimension
For the anxiety disorders, it is increasingly apparent that the DSM-5 (APA, 2013) and the International Classification of Diseases (ICD-10, revised; World Health Organization [WHO], 2015) diagnoses are not restrictive, unitary categories, and that significant comorbidity—with dysthymia/depression, as well as with other clinically significant anxiety diagnoses—is the norm. Unfortunately, DSM’s categorical structure has encouraged research programs that are organized around a single diagnosis, comparing how patients diagnosed with a specific disorder differ from healthy control participants, rather than evaluating differences among disorders. As such, much of our collective understanding is that of disordered processes in relation to rigorously screened healthy participants, who are often negative for even mild symptom elevations. While differences between patients of a given disorder and a comparison group of those with “ideal” mental health are often pronounced in symptom and biomarker indices, questions remain as to the specificity of abnormalities in a given disorder. Furthermore, too often differences have been interpreted as indexing “pure” manifestations of a single principal disorder, ignoring the comorbidities that characterize pathology in most treatment-seeking anxiety patients.
Factor analytic studies have suggested that there may be a latent dimension across the anxiety spectrum, overlapping with mood disorders, which might better capture the anxiety diathesis. For example, in a study of the National Comorbidity Survey, Krueger (1999; see also, Clark & Watson, 2006) reported dramatically high disorder covariation among “internalizing (anxiety/depression) disorders within two discriminable factor subsets, one characterized by intense “fear” (phobic disorders) and a grouping factor that included generalized anxiety disorder (GAD), dysthymia, and major depression, labeled “anxious misery.” Krueger also noted the positive association between comorbidity and severity of psychopathological dysfunction, and proposed that the factor analytic model that grouped disorders with shared variance might better guide the search for a “genetic etiology.”
Subsequent genetic epidemiological research has since significantly advanced this approach (e.g., Hettema, Prescott, Myers, Neale, & Kendler, 2005; Kendler, Prescott, Myers, & Neale, 2003; Tambs et al., 2009). In a study of more than 5,000 twin pairs, Hettema et al. (2005) determined that the genetic influences on anxiety were best explained by two additive genetic factors common across disorders. The first (A1) loaded most strongly in generalized anxiety disorder, panic disorder, and agoraphobia, whereas the second (A2) loaded primarily in the two specific phobias. It has been further suggested that comorbidity patterns among the internalizing disorders might reflect underlying personality traits that extend from healthy/adaptive levels in the general population to pathological levels in the anxious and mood disorders (e.g., Bienvenu et al., 2001).
Taken together, these findings suggest that there may be a spectrum dimension of pathology extending from diagnoses primarily associated with specific fears to more severe, generalized, highly comorbid diagnoses that can be characterized as chronic “anxious misery.” The hypothesis explored here is that psychophysiological reactions to a fear-challenge can serve as a defining marker for a related dimension.
Comparative Studies of Anxiety Spectrum Disorders
Measuring emotional imagery
The psychophysiological research program presented here assessed fear memory imagery in anxiety/mood-disordered patients. Several factors contributed to the selection of this paradigm. First, imagery is clinically relevant: It is a significant part of many re-learning based therapeutic interventions. That is, instructed imagery is central to treatment through exposure, and the spontaneous evocation and reprocessing of remembered distress is an inevitable component of cognitive treatments. Second, it affords the use of idiographic material in the experimental task that is central to the patient’s reported symptoms and experience. Third, translation to clinical phenomena was a natural transition from our experimental program aimed at developing an effective protocol for the psychophysiological measurement of emotional memories (e.g., Lang, 1977; Lang, Kozak, Miller, Levin, & McLean, 1980; Vrana, Cuthbert, & Lang, 1986; 1989; Vrana & Lang, 1990). Importantly, imagery research with healthy volunteers supported the hypothesis that psychophysiological reactions to threat during imagery, though diminished in amplitude, parallel the physiological pattern observed during actual threat exposure (e.g., Lang, Levin, Miller, & Kozak, 1983).
As a cognitive event, an emotional memory may be conceived as an associative network (Lang, 1979) that codes sensory information (what, where, who), semantic information (interpretive elaborations), and importantly, response information (physiological arousal and action). Thus, when emotional memories are activated, response information (cf., “procedural knowledge” stored in memory) is expressed as measurable, sub-overt somatic and autonomic changes, paralleling the physiology of the remembered, actual, life events. Neuroscience research supports the view that these diagnostically-relevant, physiological changes (cardiovascular, glandular, and neuromuscular) that accompany the processing of fearful/aversive memories are mediated by the brain’s cortico-limbic defense circuitry. Extensively explored in animal subjects (e.g., Amaral, Price, Pitkanen, & Carmichael, 1992; Davis, 2000; Fanselow, 1994; Kapp, Wilson, Pascoe, Supple, & Whalen, 1990; LeDoux, Iwata, Cicchetti, & Reis, 1988; Namburi, et al., 2015) the circuit’s bi-lateral amygdala is seen as a central structure mediating survival-motivated behavior. That is, the basolateral amygdala receives sensory and memorial input from the cortex, thalamus, and hippocampus. When threat signals occur, the central nucleus of the amygdala projects to and activates a series of neural target sites, e.g., the lateral hypothalamus and insula, connecting to the autonomic nervous system (modulating heart rate, blood pressure, endocrine and other glandular activity); the sensory cortices (visual, auditory, etc), increasing attentive and perceptual processing; the central grey and striatum that variously initiate “freezing” or active escape; and projections to a pontine center prompting an enhanced startle reflex—an escape response in many species (e.g., Hoy, Nolen, & Brodfuehrer, 1989). Importantly, many features of this survival circuitry have been confirmed in research with human participants, using functional magnetic resonance imagery (fMRI) to study defensive reactivity to threat-cues (e.g., Büchel & Dolan, 2000; Phelps et al., 2001; Sabatinelli, Bradley, Fitzsimmons, & Lang, 2005), and pertinent here, in fMRI studies of mental imagery with both anxiety patients and healthy participants (e.g., Costa, Lang, Sabatinelli, Versace, & Bradley, 2010; Phan, Wager, Taylor, & Liberzon, 2002).
The patho-physiology of fear and anxiety—DSM-III & DSM-III-R
Research with anxiety patients has been a focus of our laboratory for over four decades. In this work, it was apparent at the outset that we faced a fundamental measurement problem. The early, clinical model of anxiety presumed its basic pathology was a mind state of experienced distress, in which different disorders reflected different internal diatheses—subjective phenomena to which there was no direct access. To approach the problem from the perspective of natural science we needed to reformulate the concept of anxiety in terms of objective measurement. From this perspective, the basic data of anxiety were considered threefold (Lang, 1968; 1977; 1978; 1985, 1988): (1) Verbal report: Interview reports of subjective experience, questionnaires, psychophysical ratings, etc.. Importantly, in this analysis “the patient is not treated here as an observer (of an internal state)…rather the reports themselves are considered to be a component of the primary response of anxiety” (Lang, 1985, p. 134); (2) “Fear/threat” related behavioral actions—e.g., avoidance, escape hypervigilance, dysfunctional immobility, performance deficits; (3) Patterns of visceral and somatic activation, e.g., heart rate, skin conductance, electromyographic responses.
It was soon apparent in this early research that although anxiety disorders in general were presumed to show strong autonomic nervous system (ANS) arousal to “fear” challenges, the data suggested otherwise. For example, heart rate increase during fear imagery was significantly reduced in DSM-III (APA, 1980) defined agoraphobia patients, compared to patients with other phobic diagnoses (Lang, 1985). The research also suggested that patients who were more physiologically reactive were also more likely to have a successful therapeutic experience (Lang, Melamed, & Hart, 1970; Levin, Cook, & Lang, 1982). In reviewing these early psychophysiological findings, we hazarded the speculation, “that the anxiety disorders may be distributed along a continuum,” from hyper-reactivity in “focal phobias” to markedly diminished responding in “panic and generalized anxiety states” (Lang, 1985, p.165–66).
Subsequent studies were broadly consistent with the early reports: Cook, Melamed, Cuthbert, McNeil, & Lang (1988) studied three DSM-III (APA, 1980) patient groups: simple (specific) phobia, social phobia, and agoraphobia, recording physiological reactivity during imagery, along with affective ratings and a battery of symptom questionnaires. Again, responding to personal fear narrative cues, specific phobia patients were the most physiologically reactive. Heart rate increase was least in agoraphobia patients, with social phobia patients falling between. Dimensional questionnaire scores, including the indices of broad fearfulness (Fear Survey Schedule; FSS; Wolpe & Lang, 1964) and cognitive and somatic symptoms of depression (Beck Depression Inventory; BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) also differentiated among the diagnostic groups, but inversely—with significantly lower scores for the ANS-responsive phobia patients and the highest scores for the agoraphobia patients. In a subsequent follow-up study, McNeil, Vrana, Melamed, Cuthbert, & Lang (1993) took a purposefully transdiagnostic approach dividing a group of 87 participants based on dimensional questionnaire scores into a “fearful” group—characterized by a history of active avoidance of a specific feared object or group of objects—and an “anxious” group—with higher scores on measures of broad distress (e.g. passive avoidance, restlessness, negative self-talk). The physiological response to imagery challenges by the “anxious” group was significantly lower than that of the “fear” group, as well as discordant with verbal reports (i.e., the groups had similarly high fear intensity ratings).
Encouraged by these results, we undertook a yet more extensive anxiety spectrum study (Cuthbert et al., 2003) with over 100 treatment-seeking patients, organized by DSM-III-R (APA, 1987) principal diagnoses into similar-sized sub-samples: Specific phobia, Social phobia, posttraumatic stress disorder (PTSD), and panic disorder with agoraphobia (PDA), plus a healthy control group. Again the focus was on the imagery challenge paradigm, including standard narrative cues (fearful and neutral), and interview-determined idiographic narratives, defined as the participants’ most fearful memories. Physiological measures included heart rate, skin conductance, and a more recently developed measure of emotion, the probe startle reflex response (e.g., Lang, 1995; Davis & Lang, 2003).
As previously observed, heart rate change during the fear imagery task differed significantly over diagnoses. Specific and social phobia patients responded consistently with the greatest increases to the fear imagery challenge; PDA patients and unexpectedly, PTSD patients, were the least responsive. A similar pattern was found for probe startle reflexes. For probes presented during personal fear imagery, startle responses differed markedly across diagnostic groups: Significant startle potentiation was observed for both specific and social phobia, but not for PDA or PTSD patients.
The diagnostic groups also differed significantly in anxiety disorder comorbidity—the lowest comorbidity percentage was found for specific phobia (39%), and the highest for PTSD and PDA (82% and 85%, respectively). The incidence of depressive disorder comorbidity was 11% for the specific phobia group, 42% for PDA patients and 55% for PTSD. Social phobia patients fell between these extremes for both comorbidities. The distribution of scores on several anxiety and depression questionnaires also discriminated among diagnoses. The highest scores were for PTSD and PDA and lowest for specific phobia—again, with social phobia in between.
“Fear” imagery between and within diagnoses- DSM-IV
In addition to re-examining between-group differences in a broader spectrum of anxiety diagnoses, an important aim of our most recent research project was to examine variation in physiological reactivity, as symptom patterns varied within principal diagnoses (i.e., diagnostic sub-type, severity, or comorbidity) as defined by DSM-IV (APA, 1994). Aspects of these research findings are reported in separate analyses of patient groups with a common principal diagnosis (McTeague et al., 2009 [social phobia]; 2010 [PTSD]; McTeague, Lang, Laplante, & Bradley, 2011 [panic disorder]; McTeague, Lang, Wangelin, Laplante, & Bradley, 2012 [specific phobia]), and in overviews comparing differences among diagnoses (Lang, McTeague, & Bradley, 2014; McTeague & Lang, 2012). In addition to these principal diagnoses, the overall patient cohort included patient samples diagnosed with GAD and obsessive-compulsive disorder (OCD). Notably, the psychophysiological imagery assessment occurred on the same day as the structured diagnostic intake procedures completed as part of treatment planning and intake. Thus, the results are reflective of the objective and subjective profiles of patients whose functional interference is pronounced enough to motivate treatment.
Regarding the specifics of the psychophysiological assessment, participants listened to brief narrative scripts (6-seconds duration) describing events that varied selectively in affective valence and arousal. Participants were instructed to imagine being actively engaged in the narrative, as a participant rather than observer, for a subsequent twelve-second interval that was terminated by a tone cue. A group of standard scripts were presented to all participants—some survival-related (e.g., being attacked by an animal or menaced by a street gang); other scripts were affectively neutral, everyday events (e.g., watching a documentary on TV). Importantly, two idiographic narratives were also included. These personal scenarios were developed in a structured interview, and targeted to represent each patient’s clinically relevant “worst fear” experience (Table 1).
Table 1.
Sample Personal Threat Scene Exemplars by Principal Disorder
| Principal Disorder | Personal Threat Exemplar |
|---|---|
| Specific Phobia | As I move closer to the cage, I see a large hairy spider. My heart is pounding and my body is shaking. |
| Social Phobia | I don’t know anyone at this party. I feel sweaty and clammy as I realize that everyone is staring at me. |
| Panic Disorder without Agoraphobia | I wake up suddenly—frozen in fear. My heart pounds, I’m dizzy, nauseous, short of breath, choking. |
| Panic Disorder with Agoraphobia | I am sweaty and I feel like I am about to faint, standing in the middle of a crowded mall. I need to get out. |
| OCD | My heart pounds as liquid from the garbage drips on my hands. The germs are spreading. I need to get clean |
| GAD | As I watch the ambulance drive away my heart pounds and I begin to panic. What if my children are hurt? |
| PTSD | My leg is trapped between the seats. This is it, the van is full of smoke and I am going to die in this fire. |
| Major Depression | I am sweaty and dizzy while reading my speech to the class. I can’t stop panicking. I have lost control. |
A complete research protocol dictated collection of three data sets: Interview measures: The Anxiety Disorders Interview Schedule (ADIS-IV; Brown, DiNardo, & Barlow, 1994) was administered, establishing principal diagnosis, assessing comorbidities, and providing ratings of diagnosis-specific severity; Questionnaire measures and patient ratings: These included dimensional symptom measures as well as valence and arousal ratings of the imagery scripts; Reflex physiology: Responses in heart rate, skin conductance level, and facial electromyograpy were recorded during the imagery challenge session. Furthermore, brief acoustic startle probes were administered during imagery, and blink-response magnitude was measured.
In this new study, the modulation of startle reactivity across the different principal diagnoses was in many ways similar to that observed in the earlier, DSM-III (Cook et al., 1988; McNeil et al., 1993) and DSM-III-R (Cuthbert et al., 2003) diagnosed samples. Thus, patients with principal specific phobia showed strong potentiation during the fear imagery challenge (McTeague & Lang, 2012), accompanied by marked ANS reactivity (heart rate and skin conductance increases), and again, PDA patients overall were physiologically less responsive. With this larger PDA sample, however, the patients could be divided into three distinct subgroups—those diagnosed as panic disorder without agoraphobia, and two agoraphobia groups, moderate and severe (McTeague et al., 2011). The severe agoraphobic group was defined by significantly higher interview-based severity ratings than the moderate group in agoraphobic apprehension and avoidance. Severe agoraphobic patients also showed greater comorbidity (anxiety and recurrent depression) than the other groups, greater pathology based on questionnaire scores, and the poorest prognosis ratings. Startle reactivity during fear imagery (compared to neutral content), however, was inversely related to this symptom pattern: Fear potentiation was greatest for panic-only patients (closer in reactivity to specific phobia) and smallest for those diagnosed with severe agoraphobia—with moderate agoraphobia falling between.
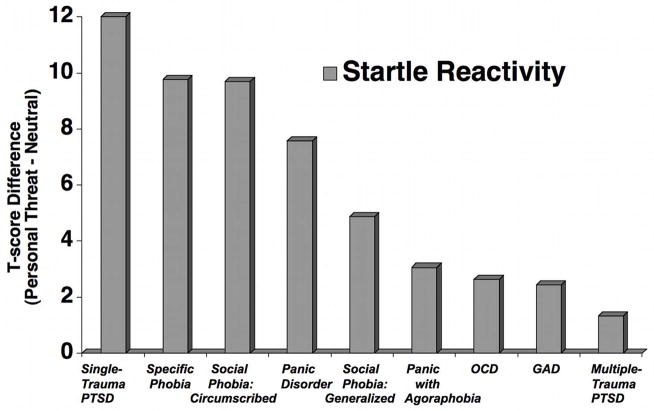
Figure 1 illustrates mean startle potentiation when imagining personal fear scenes across the full sample of DSM-IV principal diagnoses. In our previous research (Cuthbert et al., 2003), social anxiety patients showed significant fear potentiation during personal fear imagery, similar to that found for specific phobia patients. When sub-groups of social anxiety are considered, however, marked potentiation is found primarily for patients with circumscribed (performance) pathology (McTeague et al., 2009). Generalized social phobia patients, on the other hand, show a blunted modulation that was even further reduced among socially anxious patients with high comorbidity (depression and other anxiety diagnoses). Surprisingly, the most dramatic within-diagnosis differences were found for patients diagnosed with PTSD (McTeague et al., 2010): Those whose disorder was initiated by a single trauma prompted the greatest startle potentiation aross all diagnoses, whereas patients with cumulative trauma were the least reactive. Again, multiple trauma patients, relative to single trauma PTSD, were characterized by higher severity of disorder, poorer prognosis, and greater chronicity and Axis I comorbidity.
Figure 1.
Mean fear potentiation of startle reflexes (startle response magnitude during personal fear and survival fear minus neutral imagery) for patients by principal disorder (OCD=obsessive compulsive disorder; GAD=generalized anxiety disorder; PTSD=posttraumatic stress disorder) as determined with the Anxiety Disorders Interview Schedule for DSM-IV (Brown et al., 1994), arranged in order of decreasing response magnitude.
To summarize these findings and their implications, (1) A biological measure (startle potentiation) varies across principal DSM diagnoses that broadly parallels the variations in a dimension of fear to anxious-misery that has been suggested by factor analytic and genetic findings (e.g., Krueger, 1999; Hettema, et al., 2005; Hettema, Neale, Myers, Prescott, & Kendler, 2006). (2) These within-diagnosis data, however, also show that principal diagnoses are far from firm linchpins on this continuum, that indeed, coherent symptom sub-samples within a common principal diagnosis can be widely dispersed—even as for PTSD—to the dimension’s opposite extremes. (3) Considered from an RDoC perspective, the extent of systematic within-diagnosis variance raises a question: To what extent is the dimension being explored related to DSM diagnostic categories? The differences in pathology within diagnoses that relate to decreased physiological reactivity are broadly similar—increased comorbidity, higher pathology questionnaire scores, greater symptom severity. The data invite reanalysis. Is there a dimension defined by physiological response—independent of DSM categories—that might better track objective measures of pathology across the anxiety spectrum?
An RDoC Dimensional Analysis
Considered from the RDoC perspective, this reanalysis was organized according to Matrix, v. 5.1 under Negative valence systems, the construct addressed is Acute Threat (“fear”); the experimental Paradigm is emotional imagery; the Units of Analysis are Physiology & behavior: Fear potentiated startle, heart rate, skin conductance, corrugator and orbicularis muscle action [facial expressive behavior]. Self Reports: Dimensional symptom questionnaire measures and structured diagnostic and assessment scales (ADIS-IV, Brown et al., 1994). The question addressed is: Does a dimension of increasing reflex reactivity systematically relate to other measures of symptomatic distress?
The current consideration of these data includes 425 treatment-seeking patients diagnosed with principal disorders that included: specific phobia N = 66; circumscribed social phobia N = 27; generalized social phobia N = 47; panic disorder without agoraphobia (PD) N = 37; panic disorder with agoraphobia (PDA) N = 64; obsessive compulsive disorder (OCD) N = 43; generalized anxiety disorder (GAD) N = 64; single-trauma posttraumatic stress disorder (PTSD) N = 20; multiple-trauma PTSD N = 25; major depressive disorder (MDD) N = 32.
Imagery response concordance
In our previous research, startle modulation and heart rate change were the psychophysiological measures most reliably differentiated among symptom patterns, both within and across diagnostic categories (e.g., Cook et al., 1988; Cuthbert et al., 2003; McTeague & Lang, 2012). Thus, these reflex data were taken as the starting point for our exploratory dimensional analysis. Startle blink magnitude for each patient was defined using the within-subject standardized (relative to the distributions of blinks acquired between trials) eye-blink magnitude recorded for probes presented during imagery; heart rate modulation was defined as the residual change on each trial, after accounting for individual variance in baseline heart rate. Then, startle potentiation and heart rate change during personal fear imagery were each deviated from reactions measured during standard neutral imagery and each of these difference scores standardized across all patients.
A composite reactivity measure, using both startle and heart rate reactivity, was then defined as the sum of the individual indices, and the distribution (i.e. all patients) rank ordered by the composite scores. A defensive dimension was created by dividing patients into five groups (quintiles) of equivalent size (N = 85), with those showing hyper-reactivity during fear imagery in the initial group (quintile 1) and those showing hypo-reactivity in the final group (quintile 5), with intermediate responders in between (quintiles 2–4). Thus, the composite defined a robust dimension of multimodal physiologically-defined defensive reactivity, with a substantial number of patients at each of five levels of defensive response1. Analyzing the relationship of other units of analysis (i.e., physiology, symptoms, diagnoses) to the defensive reactivity dimension was the object of the next set of tests.
Importantly, participants in each quintile did not differ in terms of age, (F(4,420) = 1.37, ns; M = 33.1; SD = 12.5), gender (Χ2(4) = 3.5, ns; female = 63.1%), attainment of college degree (Χ2(4) = 4.95, ns; 42.6%), marital/cohabitating status (Χ2(4) = 4.98, ns; 63.1%), or race (Χ2(4) = 3.71, ns; Caucasian = 81.6%). In terms of psychotropic medication usage, selective serotonin reuptake inhibitors (SSRIs; 32.5%) and benzodiazepines (32%) were the most frequently endorsed and in a manner similar across quintiles (SSRIs, X2 (4) = 1.01, ns; benzodiazepines X2 (4) = 2.02, ns).
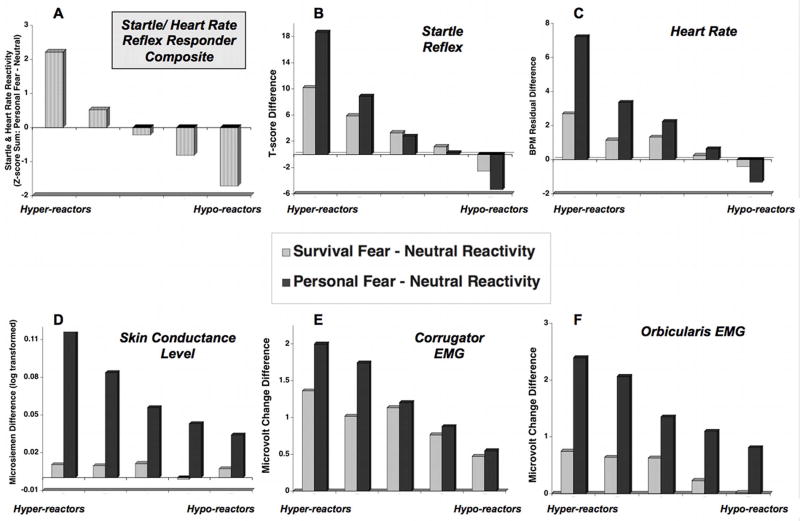
Figure 2a shows the mean composite score at each of the five points across the defensive dimension, as well as the separate distributions for startle potentiation (Figure 2b) and heart rate change (Figure 2c) at each point. An analysis using dimensional group (i.e., quintile) as a between-subject factor and imagery content (i.e., survival fear, personal fear) as a within-subject factor explored how this defensive dimension, based on personal fear imagery reactivity, relates to reactions when imagining situations involving more general survival fear. Consistent with the dimension’s construction, the individual measures of startle reactivity (Figure 2b) and heart rate change (Figure 2c) decreased significantly across the defensive dimension (quintile, startle: F(4,420) = 47.39, p < .001; quintile, heart rate: F(4,410) = 94.12, p < .001).
Figure 2.
Panel A: Mean scores by responder quintile groupings on composite measure of startle and heart rate reactivity (i.e., startle and heart rate reactivity during personal fear minus neutral differences standardized across sample (N = 425) and summed); Mean difference score by quintile group for personal fear and survival fear minus neutral imagery in startle reflex (Panel B), heart rate change (Panel C), SCL change, (Panel D), corrugator EMG change (Panel E), and orbicularis EMG change (Panel F).
Of special interest are the findings for defensive reactions during general scenes of survival fear, as these data did not contribute to defining defensive reactivity. As illustrated in Figures 2b and 2c, both startle reflex potentiation and heart rate change decreased systematically and significantly across the defensive dimension, with significant inverse linear trends found during personal fear imagery for both (startle: F(1,420) = 217.47, p < .001; heart rate F(1,414) = 526.65, p < .001). However, a similar inverse linear pattern was evident for survival fear imagery (startle: F(1,420) = 63.95, p < .001; heart rate F(1,414) = 84.75, p < .001). Moreover, the relationship between defensive reactions during imagery of personal fear and standard survival scenes differed for patients at either end of the defensive dimension (category x quintile, F(4,420) = 11.76, p < .001), with hyper-responsive patients showing heightened defensive reactions during personal fear, compared to survival imagery. Hypo-responsive patients showed the counter-intuitive pattern of blunted defensive reactivity when imagining personal fear, compared to standard survival scenes (category, F(1,420) = 6.25, p < .05; category x quintile, F(4,420) = 11.76, p < .001). Impressively, heart rate change showed the same pattern of modulation as did startle potentiation (category x quintile, F(4,410) = 63.22, p < .001), with hyper-responders again showing more defensive reactivity when imagining personally-relevant, compared to survival, fear scenes, whereas hypo-responders showed a significant effect in the other direction.
Most importantly, this defensive dimension, defined jointly by startle and heart rate reactions during personally relevant fear imagery, showed consistent relationships to additional objective psychophysiological measures of defensive reactivity, including skin conductance change and facial expressivity. As Figure 2c illustrates, the magnitude of electrodermal reactions elicited during personally relevant fear imagery showed a significant decrease across the defensive dimension (quintile F(4,410) = 5.61, p < .001), with hyper-responders showing greater differentiation than hypo-responders which became progressively more pronounced across the dimension (category x quintile, F(4,410) = 8.10, p < .001). On the other hand, for this sympathetically-mediated measure of emotional arousal, differences were more pronounced when imagining personal fear scenes (category, F(1,410) = 143.58, p < .001; linear F(1,410) = 26.15, p < .001) compared to general survival fear scenes, (F(1,410) = 1.68, ns).
Facial expressivity also varied significantly across the defensive dimension. As illustrated in Figure 2e, differential corrugator EMG activity during personal fear imagery decreased significantly across the defensive dimension, with the largest reactions for hyper-reactive patients and the least in the hypo-reactive group (Figure 2e, quintile F(4,414) = 5.14, p < .05). Unlike skin conductance, however, a linear relationship between corrugator EMG activity (e.g. “frowning”) and defensive reactivity was found both during personal fear imagery (F(1,410) = 11.99, p < .01), and survival imagery, (F(1,414) = 8.40, p < .01), for all groups (category x quintile, F(4,414) = 1.79, ns). Consistent with Figure 2e, however, a post-hoc analysis indicated that heightened facial EMG activity for personal, compared to standard, fear scenes was significant for the more hyper-reactive patients (i.e., quintiles 1 and 2; category F(1,165) = 11.49, p < .001).
A second facial muscle measured (which indexes the reflexive startle blink) was the orbicularis oculi muscle, situated just beneath the eye, which is also a component of a facial grimace found when people view frightening or disgusting scenes (e.g. Bradley, Codispoti, Cuthbert, & Lang, 2001). As illustrated in Figure 2f, differences in orbicularis oculi reactivity during imagery showed a significant decrease across the defensive dimension (quintile F(4,415) = 2.57, p < .05) and results in a significant linear trend both for personal fear imagery (F(1,415) = 15.35, p < .0012) and survival fear imagery (F(1,415) = 13.84, p < .001). Overall, orbicularis oculi activity was heightened when imagining personal compared to survival fear scenes (category, F(1,415) = 87.31, p < .001), which, once again, was most pronounced for hyper-responders (category x quintile, F(4,415) = 2.57, p < .05).
Unlike physiological measures of defensive activation, patients’ self-reports of emotional arousal (Self-Assessment Manikin [SAM], ratings 1–9, Bradley & Lang, 1994) did not differ across the defensive dimension, with all patients reporting higher arousal (category, F(4,409) = 226.48, p < .001) when imagining personally relevant fear scenes (M = 8.13; SD = 1.28) or scenes of survival fear (M = 7.03; SD = 1.38), compared to imagining neutral, everyday scenes (M = 2.80; SD = 1.60). Thus, there was no evidence of reports of emotional intensity decreasing across the defensive dimension that ranged from hyper-responders to hypo-responders (quintile F < 1). Relatedly, pleasure ratings reflected intense subjective aversion for all patients both when imagining personal fear scenes (M = 2.09; SD = 1.35) and scenes of survival fear (M = 2.84; SD = 1.19), compared to neutral imagery (M = 6.69; SD = 1.42), with no significant diffenence across the defensive dimension (quintile F(4,407) = 1.50, ns). Modest evidence suggested slightly higher aversiveness ratings for more reactive patients (compared to all other groups) that was specific to personal fear imagery (category, F(4,412) = 5.48, p < .001; posthoc comparisons to other quintiles ps < .05).
Questionnaire units of analysis
In our prior work (Cuthbert et al., 2003; McTeague & Lang, 2012) we have noted that rather than a single symptom dimension or comorbid disorder, it is the confluence of dysphoria dimensions that maps onto defense system hypo-responsivity. As such, we have termed this non-specific self-reported symptom array negative affectivity to highlight the influence of multiple pathologies in modulating defensive reflex physiology. We also found that cumulative life stressors and trauma exposure is related to blunted reactions. Guided by these findings, and endeavoring to meaningfully reduce the array of questionnaires to underlying dimensions, we conducted a principal components analysis using a variety of questionnaire measures previously found to vary with physiological reactivity in our separate within-diagnosis examinations (McTeague et al., 2009; 2010; 2011; 2012), which included the Anxiety Sensitivity Index (ASI; Reiss, Peterson, Gursky, & McNally, 1986), the Beck Depression Inventory (BDI-II; Beck, Steer, & Brown, 1996), the Mood and Anxiety Symptom Questionnaire subscales (i.e., mixed symptoms of anxiety and depression, anxious mood, depressed mood, anhedonia, anxious arousal; Watson et al., 1995), the trait form of the State-Trait Anxiety Inventory (STAI-trait; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), the trait form of the State-Trait Anger Inventory (STAI-trait; Spielberger, 1988), and the Fear Survey Schedule (FSS; Wolpe & Lang, 1964). In addition, the Illness Intrusiveness Rating Scale (IIRS; Devins, 2010), a measure of transdiagnostic functional impairment, and several indices of cumulative life stress were included in the analysis. The latter specifically included subscales of stressors in the last six months and lifetime weighted for stressor impact on wellbeing (Social Readjustment Rating Scale; SRRS; Holmes & Rahe, 1967) and a 17-item checklist of early life stressor occurrence (e.g., separation from caregivers, sexual/physical abuse, neglect).
Following varimax rotation (based on three unrotated factors with eigenvalues greater than 1), the analysis resulted in three factors of: 1) general distress/negative affectivity (λ = 6.99), which accounted for the most variance (49.94%), 2) anxious/hyperarousal (λ = 1.46; 10.4% of variance), and 3) life stress (λ = 1.09, 7.75% of variance). Table 2 lists the factor loadings for individual questionnaires, which were consistently high (0.58–0.87) and showed clear single-component loading with the exception of the nearly equivalent cross-loadings for the Fear Survey Schedule (i.e., 0.38–0.44). Interestingly, the questionnaires also loaded onto specific factors in a manner largely consistent with discriminable face validity.
Table 2.
Varimax-Rotated Factor Loadings of Questionnaires Across all Patients (N=425)
| Scale/Subscale Total | Component 1: Negative Affectivity | Component 2: Anxious Arousal | Component 3: Cumulative Life Stress |
|---|---|---|---|
| STAI: Trait Anxiety | .85 | .30 | .10 |
| MASQ: Anhedonia | .85 | .22 | .04 |
| MASQ: Depressed Mood | .81 | .36 | .10 |
| Beck Depression Inventory-II | .78 | .33 | .30 |
| MASQ: Mixed Symptoms | .71 | .55 | .13 |
| Illness Intrusiveness Rating Scale | .67 | .36 | .21 |
| STAXI: Trait Anger | .58 | −.01 | .12 |
| MASQ: Anxious Arousal | .25 | .87 | .09 |
| MASQ: Anxious Mood | .45 | .77 | .08 |
| Anxiety Sensitivity Index | .24 | .73 | .28 |
| SRRS: Stressors Lifetime | .05 | .12 | .79 |
| Count Early Life Stressors | .25 | .39 | .59 |
| SRRS: Stressors Last 6 Months | .03 | .39 | .59 |
| Fear Survey Schedule Total | .41 | .39 | .44 |
Note. MASQ = Mood & Anxiety Symptom Questionnaire; SRRS = Social Readjustment Rating Scale; STAI = State-Trait Anxiety Inventory.
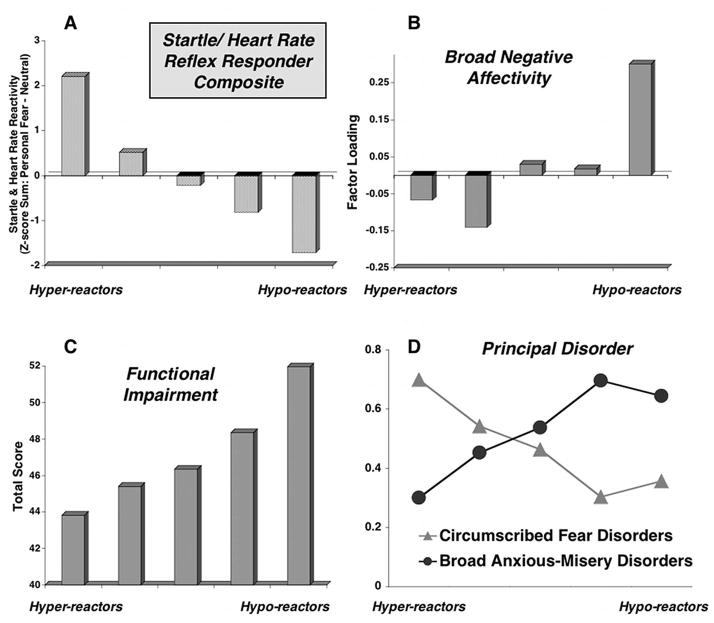
Linear trend tests were conducted for each of the mean factor scores across the defensive dimension (e.g. quintiles) which resulted in a significant relationship only for the factor accounting for the most variability among the questionnaire measures -- the negative affect factor. Figure 3b illustrates the mean negative affect factor scores for each group of patients arrayed along the defensive reactivity dimension. Only the broad distress/negative affectivity factor showed a reliable change that systematically increased from hyper-responders to hypo-responders (linear F(1,420) = 4.71, p < .05). Interestingly, the broad measure of functional impairment (i.e., the Illness Intrusiveness Rating Scale; IIRS; Devins, 2010) loaded on this factor, together with measures of diffuse affective, cognitive, and somatic symptoms. Given the uniquely transdiagnostic aspect of the IIRS, further analyses were conducted to explore potential domain-specific impairments.
Figure 3.
Mean scores by responder quintile groupings on composite measure of startle and heart rate reactivity (i.e., startle and heart rate reactivity during personal fear minus neutral differences standardized across sample [N = 425] and summed) (Panel A); Responder quintile group by mean regression-based factor scores for broad distress/negative affectivity factor (Panel B), by Illness Intrusiveness Rating Scale scores (Panel C; Devins, 2010), and proportion of fear (specific phobia, circumscribed social phobia, single-trauma PTSD) versus anxious-misery (GAD, major depression, multiple-trauma PTSD) principal disorders.
The IIRS scale was originally developed to assess the impact of physical health conditions on an individual’s life, and queries the extent of impairment in multiple domains (e.g., health, diet, work/school, relationship with partner, sex life, family relations, etc). To adapt it for anxiety patients, the term “illness” is replaced by “mental health problems (e.g., anxiety, depression)” 3. Using a sample of anxiety disorder patients, Bieling, Rowa, Antony, Summerfeldt, & Swinson (2001) described a three-factor solution that targets domains of lifestyle (e.g., health, diet, recreation), activity involvement (e.g., community/civic involvement), and intimacy (e.g., social relations). As Figure 3c illustrates, in our sample, the IIRS total score showed a reliable increase in impairment ratings across the defensive dimension (linear F(1,419) = 7.71, p < .01), and subscale scores indicated this relationship was found for the domains of lifestyle, F(1,419) = 6.83, p < .01 and activity involvement, F(1,419) = 5.73, p < .05, but not for reports of interference in intimate or social relations, F(1,419) = 1.25, ns.
Interview units of analysis
Thus far, the data suggest that a factor of broad distress/negative affectivity—including disorder-nonspecific and transdiagnostic functional impairment—is inversely related to defensive physiological reactivity during an emotional imagery challenge. Thus, reports of greater broad distress are related to lower levels of defensive activation, whereas more pronounced defensive mobilization is related to reports of less broad distress and impairment. Based on our prior findings (McTeague & Lang, 2012), we expected that patients with a principal fear disorder (i.e., specific phobia, circumscribed social phobia, single-trauma PTSD) and those with a principal anxious-misery disorder (i.e., major depression, GAD, multiple-trauma PTSD) would fall at opposite ends of the defensive reactivity spectrum. Supporting this, the proportion of patients diagnosed with either principal fear or anxious misery-disorders (N = 238) differs in each of the five groups across the defensive dimension, Χ2(4) = 20.83, p < .001. As illustrated in Figure 3d, the proportion of patients diagnosed with principal anxious-misery disorders progressively increases when moving from the hyper- to the hypo-responsive end of the continuum, whereas the opposite is the case for patients with circumscribed fear disorders. Notably, while these data illustrate how patients diagnosed with circumscribed fear and anxious-misery disorders tend to show different defensive reactions, it is also critical to underscore the tremendous heterogeneity in the proportion of principal disorders in each quintile.
In general, measures derived from the patient interview have not, so far, proved to vary significantly with the defensive reactivity dimension as it is defined psychophysiologically. The measures tested included the percentage of patients with/without (as well as the number of) comorbid anxiety or depressive disorders, clinician ratings of disorder severity and prognosis, and patient reported disorder chronicity. None of these measures varied significantly over the quintiles. These data contrast with our prior DSM analysis, which found that physiological reactivity varied meaningfully with these measures within diagnoses. Further analysis suggests that the failure to find these effects reflects, in part, the fact that a clinician makes judgments about a given patient relative to other patients with the same principal diagnosis, i.e., they are not transdiagnostic. Thus, for example, the 91 patients in the current sample with the highest clinician-rated severity, transdiagnostic questionnaire measures of symptom intensity varied dramatically. Specific phobia patients in this subset had mean BDI scores of 12.7, trait anxiety scores of 41.6, and IIRS scores of 31.8. The multiple trauma PTSD patients with equivalent severity ratings had far higher scores, with mean BDI scores of 36.6, trait anxiety of 74.4, and IIRS of 62.8. Relatedly, when 59 (of the 425) patients whose prognosis was rated as “excellent,” the subset of patients with specific phobia scored a mean of 6.3 on the BDI, 35.5 on trait anxiety and 22.6 on the IIRS, whereas multiple trauma PTSD patients with the same excellent prognosis ratings had much higher scores on all measures of distress and interference (BDI = 28, trait anxiety = 57.2, IIRS = 44.1).
Discussion: Interpretations and Future Directions
Interpretations: DSM and RDoC
The analyses presented here represent a first “RDoC-ian” look at data that were previously analyzed and organized using DSM-IV categories. The open-ended nature of RDoC is an analytic challenge, as it will be for all investigators, and we do not propose this exploratory foray as a model methodology. Considering the large, multi-measure, multi-method nature of the data that investigators are likely to accumulate, many analytic strategies will need to be essayed. As our own anxiety research program developed, we became increasingly aware of the high variability within and between diagnoses and measures, and the need for ever larger samples. Furthermore, considering the RDoC emphasis on integrative science—a matrix that includes seven units of analysis—vast numbers of very different, independently measured, discrete and continuous, linear and non-linear data will inevitably be obtained. With measurables ranging from genes and molecules, cells and circuits, to physiology, behavior and self-reports, it is not expected that investigators will limit their proposals to the stated minimum of two units of analysis (RFA-MH-12-100). Very likely, we will soon be in the domain of “big data”, and variations of more complex methodologies (e.g., cluster computing [Freeman et al., 2014; Wang & Krystal, 2014], support vector machine classifiers [Fung & Mangasarian, 2005]) will be required.
Despite its limitations, however, significant new findings have emerged from this RDoC analysis. Heart rate change during imagery was not a measure that varied consistently across diagnoses in the previous DSM-driven analyses. Rather, only focal fear disorders tended to show a strong correspondence between heart rate and startle reactivity, whereas most of the other DSM classifed disorders showed varying degrees of discordance. Furthermore, corrugator EMG (“frown”) responses also did not reliably differ across or within the diagnostic groups in previous analyses. For example, both single and multiple trauma PTSD patients showed pronounced corrugator increases during personal fear imagery, despite different patterns in startle and autonomic reactivity. In general, in the previous DSM analyses, facial action measures co-varied with subjective ratings of distress during imagery (McTeague & Lang, 2012) and both were consistently high, but invariant, over diagnoses—a finding that suggested a linked verbal/facial, social communication system perhaps independent of bodily arousal.
The new analyses, however, point to systematic variations in the concordance between physiological measures, which now includes both skin conductance and facial action. Furthermore, concordance is highest in the most reactive patients and palpably diminishes as negative affect increases across the defensive reactivity dimension. Notably, these different patterns of concordance do not emerge clearly when diagnostic category is used to define anxiety groups. It is still possible that certain phenotypes are marked by discordance in specific defensive measures during personal fear imagery (e.g., exaggerated corrugator EMG and diminished startle), which would not be apparent in the current analyses, as they are defined by concordant reactivity in startle modulation and heart rate change. It will be useful in the future to consider within-participant data coherence as another dimension in exploring its relationship to other units of analysis.
Imagery of standard survival fear scenes prompted palpable defense reactions that similarly decreased across the physiologically defined defensive dimension, indicating some generality in defensive reactivity across different imagery scenarios. On the other hand, imagining general scenes of survival fear often prompted lower defensive reactions than imagining idiographic scenes, and not all physiological measures (e.g., skin conductance) varied systematicaly across the defensive dimension when imagery did not target the personally relevant fear. Interestingly, and consistent with previous DSM-driven analyses, patients’ ratings of emotional arousal evoked by imagery were uniformly high across the physiology dimension, with no difference between hyper- and hypo-responders for either scenes of personal fear or survival scenarios.
The questionnaire data are also newly informative, particularly the finding of increasing functional impairment across the defensive dimension (from hyper- to hypo-reactive) as measured by the IIRS. As noted, unlike many of the questionnaires in our battery, the IIRS is not a report of emotional distress but has been used most frequently to evaluate impairment secondary to physical health disorders (e.g., rheumatoid arthritis, sequelae to heart transplantation; Devins et al., 2001), rather than mental disorders. Our results suggest that anxiety patients who are physiologically least-reactive to the imagery challenge suffer the greatest difficulty in navigating their daily lives, reporting broad functional interference in health, diet, work, recreation, financial situation, and in religious expression and community involvement. Interestingly, this lifestyle dysfunction measure, rather than loading independently or with the life stress or anxious hyperarousal factors, loaded on the broad distress/negative affectivity factor in the PCA analysis.
In our research protocol, all patients first participated in an extended (2–4 hours), structured clinical interview (ADIS) in which distress and interference was assessed for every anxiety and mood disorder as well as substance abuse/dependence, psychotic, and somatoform disorders before assignment of a principal diagnosis. Not surprisingly, a significantly greater proportion of the patient sample with principal focal fears (specific and circumscribed social phobia, single trauma PTSD) were defensive hyper-responders, while the largest proportion of the anxious-misery sample (depression, GAD, multiple-trauma PTSD) were hypo-responders. Nevertheless, specific diagnoses were widely distributed, with a substantial number of patients with each principal diagnosis appearing in each quintile. Moreover, the absolute number of patients diagnosed with focal fear disorders that fell into into the most defensively reactive group (e.g. quintile 1; n = 23) was greatly exceeded by the number of patients in this quintile diagnosed with other principal diagnoses (n = 62), including those diagnosed with principal GAD (n = 10) or depression (n = 3).
In general, clinician ratings were not systematically related to defensive reactivity during the fear imagery challenge. Considering that these measures are designed to establish a principal diagnosis, the absence of cross-spectrum relevance might be expected. That is, the rating of the severity of a panic patient’s symptoms or prognosis, for example, is evaluated with respect to the range expected for this disorder. Similarly, a patient diagnosed with specific phobia is considered as an exemplar of the class of specific phobia patients. As current clinical assessments are typically conducted, few interview measures of pathology can be considered transdiagnostic—as are, for instance, the IIRS and other questionnaires of functional interference. The RDoC initiative may encourage a broader perspective in interview practice, inviting a new consideration of symptom reports, viewed both in the context of an assigned principal diagnosis and importantly, also transdiagnostically, as they may relate to the range of pathology across the anxiety/mood spectrum.
Future Directions
In our view, the symptomatic, affective physiology of anxiety/mood disorders reflects a modulation of motivational circuits that evolved in mammalian brains to ensure the survival of individuals and their progeny—circuits that, when activated, are associated with reports of expressed emotion in humans. Much of our understanding of circuit function is based on extensive research with animal models, in studies investigating brain activation patterns and associated physiological reactions in animals under physical threat (e.g., Amaral, et al., 1992; Davis, 2000; Fanselow, 1994; Kapp, et al., 1990; LeDoux et al., 1988). Using modern imaging technologies in the study of a variety of fear/threat challenges in humans, research has generally confirmed activation of similar neural circuits in the brains of human participants (e.g., Costa et al., 2010).
The hypothesis underlying our new studies of the RDoC spectrum is that the differing patterns of defensive reactivity during an imagery challenge indicates circuit hyper-function, or alternatively, a diminishing reactivity that signals an increasingly dysfunctional survival circuit. It is held, furthermore, that the mediated defensive response to palpable threat-cues, danger signs, fearful memories and imagery are a normal, adaptive response in humans. That is, circuit output prepares the organism for threat confrontation, focusing attentional resources and mobilizing autonomic and somatic systems for defensive action (Lang & Bradley, 2010; Lang, 2010). The anxiety/mood spectrum under study reflects a dimension that extends from patients characterized by dysfunctional, exaggerated fear-cue reactivity to dysfunctional, markedly diminished responses—both of which are viewed here, as the output of a compromised neural circuit.
Thus, the new research begins, as before, with clinical interview and a subsequent assessment of psychophysiological responses to imagery challenges. However, consistent with the above hypotheses, we have added significantly to the units of analysis that are collected: To directly assess brain circuit function, patients participate in an MRI session in which brain structural scans are gathered, functional scans are recorded during the imagery challenge, and diffusion tensor imaging (DTI) is used to assess white-matter connectivity. A complementary brain-based analysis acquires dense electrode electroencephalography (EEG) during emotional imagery in a laboratory psychophysiological (i.e., startle reflexes, heart rate, etc.) session which will allow assessement in differences in brain oscillatory activity during emotional imagery (e.g., Bartsch, Hamuni, Miskovic, Lang, & Keil, 2015). Furthermore, considering that blunted ANS responses (heart rate, skin conductance) might be a consequence of chronic dysfunction in the hypothalamic/pituitary/adrenal (HPA) system, hair samples are collected which allows us to assess sustained cortisol differences (see Kirschbaum, Tietze, Skoluda, & Dettenborn, 2009), and saliva samples provide a look at possible genetic contributions.
Factors that might determine the hypothesized circuit dysfunction and its coordinate differences in defensive reactivity are not yet understood. While interpretations based on this cross-sectional study must be contrained, findings tentatively suggest that premorbid genetic liabilities, accumulating life stress and enduring negative affectivity may, in isolation or conjunction produce patterns of hypo-/hyper-reactivity. Activation levels of key neural structures in the circuit may be reduced or exaggerated (amygdala)5, or the circuit may be altered in connectivity, or structural volumes may be reduced (e.g., hipppocampus) —all of these possibilities have been conjectured (e.g., Admon et al., 2009; Etkin & Wager, 2007). Long-term stress—particularly salient in multiple trauma PTSD—could well be an environmental contributor, changing the brain’s neuro-hormonal milieu. Twin studies (e.g., Hettema et al., 2005; 2006) suggest genetic factors that relate either to “fear” disorders or to disorders of “anxious misery.” Although studies of candidate genes have not yet proved as productive as originally hoped, collecting genetic material will be available for new guidance (e.g., epigenotyping: Puglia, Lillard, Morris, & Connelly, 2015) that comes with advances in genetic research. Again, however, large samples are needed to draw conclusions based on genetic data, and may depend on the coordination of results from many research groups (such archival collection of data from RDoC researchers is already being implemented at NIMH).
In summary, RDoC opens new avenues for anxiety researchers, and also introduces formidable new challenges. Unconstrained by presumptive interview-based categories, it invites a dimensional exploration of individual differences, stretching from adaptively-coping healthy participants, and patients with varying success in surmounting life’s stressors, to those suffering the most profound, incapacitating misery and distress. As Kozak & Cuthbert (2015) emphasize in this issue, the aims of RDoC are to begin “formulating and evaluating explanatory hypotheses for clinical phenomena that psychopathologists estimate are ripe for biopsychological explanation.” We have tried to show here that the anxiety and mood disorders are such clinical phenomena, and that their biopsychological explication is a highly promising research aim. Evidence has been presented that the phenomenology of anxiety can be conceived bio-dimensionally, and that the defined dimension of defensive reactivity is to a considerable extent psychophysiologically grounded. The continuing aim of our current research is to advance a biological understanding of the anxiety/mood disorders, with a focus on how observed measurable patterns of defensive reactivity in anxiety disorders relate to individual differences in the functional activation/connectivity of the brain’s motivational circuits, to effects of stress on neuro-hormonal systems, and to genetic inheritance, which collectively determine differences in mood and defensive behavior in humans.
Acknowledgments
This work was supported in part by a National Institute of Mental Health grants MH098078 and MH094386 to Peter J. Lang, and F31 MH069048 and K23 MH104849 to Lisa M. McTeague. Special thanks to Bruce N. Cuthbert for assistance with study design and interpretation of findings. Special thanks to the following individuals for their assistance in data collection: Marie-Claude Laplante, Cyd C. Strauss, Denise M. Sloan, Eleni Dimoulas, Jose M. Soler-Baillo, Reid Scott, Greg Perlman, Bethany Wangelin, Joshua R. Shumen, Esther Jean-Baptiste, and Hailey W. Bulls.
Footnotes
Due to the nature by which the startle-heart rate composite was constructed (i.e., personal fear – neutral difference scores), startle responses, F(4,420) = 2.78, p < .05, and heart rate change, F(4,413) = 46.72, p < .001, during neutral imagery reliably differed across quintiles such that responding decreased from the most reactive to least reactive extreme. Differences between quintiles during neutral processing were not evident on other physiological or subjective measures.
There were no pre-stimulus baseline (i.e., average of two half-seconds prior to script onset) differences across quintiles for any physiological measure, Fs = 0.31–8.87, ns. A difference in ITI startle magnitude was evident across quintiles, F(4,424) = 2.44, p < .05, attributable to a trend for the two most reactive quintiles to differ, p = .08. All other pairwise comparisons did not differ.
“The following items ask about how much your mental health problems (e.g., anxiety, depression) interfere with different aspects of your life.”
An effort to introduce a transdiagnostic dimensional model into DSM was made by the Personality Disorders Workgroup (Krueger, Hopwood, Wright, & Markon, 2014), which unfortunately was rejected by the American Psychiatric Asociation’s Board of Trustees. Nevertheless, Krueger et al. (2014) recommend “continued efforts to better incorporate dimensional elements” that are connected to “the empirical literature (p. 256)”, and his group’s proposal is described in DSM-V, Section III (2013), Emerging Methods and Models. In introducing a series of articles evaluating DSM-V, Lilienfeld (2014) noted further tlhat “DSM’s categorical measurement system… is not consistent with taxometric (Haslam, Holland, & Kuppens, 2012) or psychometric (Markon, Chmielewski, & Miller, 2011) data, suggesting that its structure might only reflect the fact that humans “more easily processed” categories than dimensions (p. 275), and significantly, ended his review advising the “next generation of researchers and practitioners” to insist “on the development of alternative systems that map more closely onto the state of nature (p. 276).”
We report brieflly (McTeague & Lang, 2012) a study from our laboratory comparing two groups of non-patient volunteers, one significantly higher than the other for both BDI-II and STAI scores, analyzing their reactivty during emotional imagery with fMRI. Defense circuit activation was observed overall. However, significant amygdala activion (fear minus neutral imagery) was not found for the group high in mood/anxiety symptoms.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Peter J. Lang, University of Florida
Lisa M. McTeague, Medical University of South Carolina
Margaret M. Bradley, University of Florida
References
- Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-Ami H, Hendler T. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proceedings of the National Academy of Sciences. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. New York, NY: Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Association; 1987. Revised. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013. [DOI] [Google Scholar]
- Bartsch F, Hamuni G, Miskovic V, Lang PJ, Keil A. Oscillatory brain activity in the alpha range is modulated by the content of word-prompted mental imagery. Psychophysiology. 2015 doi: 10.1111/psyp.12405. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1007/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bieling PJ, Rowa K, Antony MM, Summerfeldt LJ, Swinson RP. Factor structure of the Illness Intrusiveness Rating Scale in patients diagnosed with anxiety disorders. Journal of Psychopathology and Behavioral Assessment. 2001;23:223–230. [Google Scholar]
- Bienvenu OJ, Brown C, Samuels JF, Liang KY, Costa PT, Eaton WW, Nestadt G. Normal personality traits and comorbidity among phobic, panic and major depressive disorders. Psychiatry Research. 2001;102:73–85. doi: 10.1016/S0165-1781(01)00228-1. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The Self-Assessment Manikin and the semantic differential. Journal of Behavior Therapy & Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–98. doi: 10.1037//1528-3542.1.3.276. [DOI] [PubMed] [Google Scholar]
- Brown TA, DiNardo PA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV) San Antonio, TX: Psychological Corporation; 1994. [Google Scholar]
- Büchel C, Dolan RJ. Classical fear conditioning in functional neuroimaging. Current Opinion in Neurobiology. 2000;10:219–223. doi: 10.1016/S0959-4388(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Distress and fear disorders: An alternative empirically based taxonomy of the “mood” and “anxiety” disorders. The British Journal of Psychiatry. 2006;189:481–483. doi: 10.1192/bjp.bp.106.03825. [DOI] [PubMed] [Google Scholar]
- Cook EW, Melamed BG, Cuthbert BN, McNeil DW, Lang PJ. Emotional imagery and the differential diagnosis of anxiety. Journal of Consulting and Clinical Psychology. 1988;56:734–740. doi: 10.1037/0022-006X.56.5.734. [DOI] [PubMed] [Google Scholar]
- Costa VS, Lang PJ, Sabatinelli D, Versace F, Bradley MM. Emotional imagery: Assessing pleasure and arousal in the brain’s reward circuitry. Human Brain Mapping. 2010;31:1446–1457. doi: 10.1002/hbm.20948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: Fear memory imagery. Psychophysiology. 2003;40:407–422. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The amygdala: A functional analysis. Oxford, UK: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- Davis M, Lang PJ. Emotion. In: Gallagher M, Nelson RJ, editors. Handbook of psychology, Vol. 3. Biological Psychology. New York, NY: Wiley; 2003. pp. 405–439. [DOI] [Google Scholar]
- Devins GM. Using the Illness Intrusiveness Ratings Scale to understand health-related quality of life in chronic disease. Journal of Psychosomatic Research. 2010;68:591–602. doi: 10.1016/j.jpsychores.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Devins GM, Dion R, Pelletier LG, Shapiro CM, Abbey S, Raiz LR, Edworthy SM. Structure of lifestyle disruptions in chronic disease: A confirmatory factor analysis of the Illness Intrusiveness Ratings Scale. Medical Care. 2001;39:1097–1104. doi: 10.1097/00005650-200110000-00007. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–439. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Freeman J, Vladimirov N, Kawashima T, Mu Y, Sofroniew NJ, Bennett DV, Ahrens MB. Mapping brain activity at scale with cluster computing. Nature Methods. 2014;11:941–950. doi: 10.1038/nmeth.3041. [DOI] [PubMed] [Google Scholar]
- Fung GM, Mangasarian OL. Multicategory proximal support vector machine classifiers. Machine Learning. 2005;59:77–97. doi: 10.1007/s10994-005-0463-6. [DOI] [Google Scholar]
- Haslam N, Holland E, Kuppens P. Categories versus dimensions in personality and psychopathology: A quantitative review of taxometric research. Psychological Medicine. 2012;42:903–920. doi: 10.1017/S0033291711001966. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Archives of General Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS. A population-based twin study of the relationship between neuroticism and internalizing disorders. American Journal of Psychiatry. 2006;163:857–864. doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- Holmes TH, Rahe RH. The social readjustment rating scale. Journal of Psychosomatic Research. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Hoy R, Nolen T, Brodfuehrer P. The neuroethology of acoustic startle and escape in flying insects. Journal of Experimental Biology. 1989;146:287–306. doi: 10.1242/jeb.146.1.287. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN. Endophenotypes: Bridging genomic complexity and disorder heterogeneity. Biological Psychiatry. 2009;66:988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Kapp BS, Wilson A, Pascoe JP, Supple WF, Whalen PJ. A neuroanatomical systems analysis of conditioned bradycardia in the rabbit. In: Gabriel M, Moore J, editors. Neurocomputation and learning: Foundations of adaptive networks. New York, NY: Bradford Books; 1990. pp. 55–90. [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production – Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Kozak, Cuthbert The NIMH Research Domain Criteria Initiative: Background, issues, and pragmatics. Psychophysiology. 2015 doi: 10.1111/psyp.12518. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hopwood CJ, Wright AGC, Markon KE. DSM-5 and the path toward empirically based and clinically useful conceptualization of personality and psychopathology. Clinical Psychology: Science and Practice. 2014;21:245–261. doi: 10.1111/cpsp.12073. [DOI] [Google Scholar]
- Lang PJ. Fear reduction and fear behavior: Problems in treating a construct. In: Schlien JM, editor. Research in psychotherapy. Vol. 3. Washington, DC: American Psychological Association; 1968. pp. 90–103. [DOI] [Google Scholar]
- Lang PJ. Psychophysiological assessment of anxiety and fear. In: Cone JD, Hawkins RP, editors. Behavioral assessmen: New directions in clinical psychology. New York, NY: Brunner-Mazel; 1977. pp. 178–195. [Google Scholar]
- Lang PJ. Anxiety: Toward a psychophysiological definition. In: Akiskal HS, Webb WL, editors. Psychiatric diagnosis: Exploration of biological predictors. New York, NY: Spectrum; 1978. pp. 368–389. [Google Scholar]
- Lang PJ. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16:495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The cognitive psychophysiology of emotion: Fear and anxiety. In: Tuma AH, Maser JD, editors. Anxiety and the anxiety disorders. Hillsdale, NJ: Erlbaum; 1985. pp. 131–170. [Google Scholar]
- Lang PJ. What are the data of emotion? In: Hamilton V, Bower GH, Frijda N, editors. Cognitive perspectives on emotion and motivation. Boston, MA: Martinus Nijhoff; 1988. pp. 173–194. [DOI] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037/0003-066X.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Emotion and motivation: Toward consensus definitions and a common research purpose. Emotion Review. 2010;2:229–233. doi: 10.1177/1754073910361984. [DOI] [Google Scholar]
- Lang PJ, Melamed BG, Hart JD. A psychophysiological analysis of fear modification using an automated desensitization procedure. Journal of Abnormal Psychology. 1970;76:220–234. doi: 10.1037/h0029875. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: Conceptual structure and pattern of somato-visceral response. Psychophysiology. 1980;17:179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Levin DN, Miller GA, Kozak MJ. Fear behavior, fear imagery, and the psychophysiology of emotion: The problem of affect response integration. Journal of Abnormal Psychology. 1983;92:276–306. doi: 10.1037/0021-843X.92.3.276. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biological Psychology. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Wangelin BC, Bradley MM, Versace F, Davenport PW, Costa VD. Threat of suffocation and defensive reflex activation. Psychophysiology. 2011;48:393–396. doi: 10.1111/j.1469-8986.2010.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, McTeague LM, Bradley MM. Pathological anxiety and function/dysfunction in the brain’s fear/defense circuitry. Restorative Neurology and Neuroscience. 2014;32:63–77. doi: 10.3233/RNN-139012. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. Journal of Neuroscience. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DN, Cook EW, III, Lang PJ. Fear imagery and fear behavior: Psychophysiological analysis of clients receiving treatment for anxiety disorders. Psychophysiology. 1982;19:571–572. [Google Scholar]
- Lilienfeld SO. DSM-5: Centripetal scientific and centrifugal antiscientific forces. Clinical Psychology: Science and Practice. 2014;21:269–279. doi: 10.1111/cpsp.12075. [DOI] [Google Scholar]
- Markon KE, Chmielewski M, Miller CJ. The reliability and validity of discrete and continuous measures of psychopathology: A quantitative review. Psychological Bulletin. 2011;137:856–879. doi: 10.1037/a0023678. [DOI] [PubMed] [Google Scholar]
- McNeil DW, Vrana SR, Melamed BG, Cuthbert BN, Lang PJ. Emotional imagery in simple and social phobia: Fear versus anxiety. Journal of Abnormal Psychology. 1993;102:212–225. doi: 10.1037/0021-843X.102.2.212. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: Generalization, comorbidity, and physiological reactivity. Biological Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsch.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: Trauma recurrence, comorbidity, and physiological reactivity. Biological Psychiatry. 2010;67:346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Bradley MM. Aversive imagery in panic disorder: Agoraphobia severity, comorbidity, and defensive physiology. Biological Psychiatry. 2011;70:415–424. doi: 10.1016/j.biopsych.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ. The anxiety spectrum and the reflex physiology of defense. From circumscribed fear to broad distress. Depression and Anxiety. 2012;29:264–281. doi: 10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Wangelin BC, Laplante MC, Bradley MM. Defensive mobilization in specific phobia: Fear specificity, negative affectivity, and diagnostic prominence. Biological Psychiatry. 2012;72:8–18. doi: 10.1016/j.biopsych.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namburi P, Beyeler A, Yorozu S, Calhoon GG, Halbert SA, Wichmann R, Tye KM. A circuit mechanism for differentiating positive and negative associations. Nature. 2015;520:675–678. doi: 10.1038/nature14366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2001;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby C, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation fo fear. Nature Neuroscience. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Puglia MH, Lillard TS, Morris JP, Connelly JJ. Epigenetic modification of the oxytocin receptor gene influences the perception of anger and fear in the human brain. Proceedings of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1422096112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behaviour Research and Therapy. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. NeuroImage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Mannual for the State-Trait Anger Expression Inventory. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tambs K, Czajkowsky N, Røysamb E, Neale MC, Reichborn-Kjennerud T, Aggen SH, Kendler KS. Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. The BritishJournal of Psychiatry. 2009;195:301–307. doi: 10.1192/bjp.bp.108.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Cuthbert BN, Lang PJ. Fear imagery and text processing. Psychophysiology. 1986;23:247–253. doi: 10.1111/j.1469-8986.1986.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Cuthbert BN, Lang PJ. Processing fearful and neutral sentences: Memory and heart rate change. Cognition and Emotion. 1989;3:179–195. [Google Scholar]
- Vrana SR, Lang PJ. Fear imagery and the startle-probe reflex. Journal of Abnormal Psychology. 1990;99:189–197. doi: 10.1037/0021-843X.99.2.189. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Krystal JH. Computational psychiatry. Neuron. 2014;84:638–654. doi: 10.1016/j.neuron.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. http://psycnet.apa.org/doi/10.1037/0021-843X.104.1.3. [DOI] [PubMed] [Google Scholar]
- Wolpe J, Lang PJ. A fear survey schedule for use in behavior therapy. Behavior Research Therapy. 1964;2:27–30. doi: 10.1016/0005-7967(64)90051-8. [DOI] [PubMed] [Google Scholar]
- World Health Organization. International statistical classification of diseases and related health problems. (10) 2015 Revised. Retrieved from http://apps.who.int/classifications/icd10/browse/2015/en.