Abstract
Background
Systemic inflammation is involved in the development of acute kidney injury (AKI) after cardiac surgery with cardiopulmonary bypass (CPB). Ulinastatin, a urinary trypsin inhibitor (UTI), possesses a variety of anti-inflammatory effects. Therefore, we hypothesized that the administration of ulinastatin would reduce the occurrence of AKI in patients undergoing cardiac surgery with CPB.
Methods
A retrospective propensity score matched analysis was used to evaluate the effect of ulinastatin on the development of AKI in patients undergoing first documented cardiac surgery with CPB between January 2008 and December 2012 in our hospital. Multiple logistic regression models were also employed to identify the association between UTI administration and development of AKI.
Results
A total of 2072 patients who underwent cardiac surgery with CPB met the inclusion criteria. Before propensity score matching, variables such as age, baseline creatinine, CPB duration, red blood cells transfused, and hematocrit were statistically different between the ulinastatin (UTI) group and the control group. On the basis of propensity scores, 409 UTI patients were successfully matched to the 409 patients from among those 1663 patients without UTI administration. After propensity score matching, no statistically significant differences in the baseline characteristics were found between the UTI group and the control group. The propensity score matched cohort analysis revealed that AKI and the need for renal replacement therapy occurred more frequently in the control group than in the UTI group (40.83 % vs. 30.32 %, P = 0.002; 2.44 % vs. 0.49 %, P = 0.02, respectively). However, there were no significant differences in mortality, length of intensive care unit stay, and length of hospital stay between the UTI group and the control group. Using multivariate logistic regression analysis, we found ulinastatin played a protective role in the development of AKI after cardiac surgery (odds ratio 0.71, 95 % confidence interval 0.56–0.90, P = 0.005).
Conclusions
This study shows that ulinastatin was associated with a lower incidence of AKI after cardiac surgery, suggesting that the administration of ulinastatin may be favorable for those patients undergoing cardiac surgery with CPB.
Keywords: Acute kidney injury, Ulinastatin, Cardiac surgery, Cardiopulmonary bypass
Background
Acute kidney injury (AKI) commonly occurs in patients undergoing cardiac surgery, especially those treated with cardiopulmonary bypass (CPB) [1]. The incidence of cardiac surgery–associated (CSA) AKI ranges from 8.9 % [2] to 39 % [3], depending on the definition used. Renal replacement therapy (RRT) is needed in 1–5 % of patients [4], and the mortality rate is 1.4 % [5]. Previous studies demonstrated that even mild elevation in serum creatinine (SCr) levels after cardiac surgery is significantly associated with higher mortality [6–8]. However, the treatment for CSA-AKI still remains challenging [9]; thus, prevention is the mainstay of effective treatment for patients at high risk of developing AKI.
There are multiple factors involved in the development of CSA-AKI, including hemodynamic, inflammatory, metabolic, and nephrotoxic factors [10]. Emerging evidence shows that systemic inflammation plays a key role in the development of CSA-AKI and participates in renal injury, especially tubular lesions [11–15].
To date, pharmacological renal protection strategies remain limited [16]. Ulinastatin, a urinary trypsin inhibitor (UTI) that inhibits various inflammatory proteases, including chymotrypsin, trypsin, and neutrophil elastase, has been widely used in China, Japan, and Korea for the treatment of patients with inflammatory disorders, postoperative organs protection, shock, and pancreatitis [17–19]. A previous study has shown that ulinastatin can decrease cytokine concentrations in patients undergoing cardiac surgery [20].
However, studies investigating its renoprotective role in the development of AKI in patients undergoing cardiac surgery with CPB are limited [21]. Thus, the aim of the present study was to test the hypothesis that the administration of ulinastatin would reduce the incidence of CSA-AKI.
Methods
Patients
This retrospective study included patients aged 18 years or older who underwent cardiac procedures with CPB at the Nanjing First Hospital in Nanjing, China, between January 2008 and December 2012. A total of 2072 patients were selected for analysis. Patients with end-stage kidney disease needing RRT before surgery were excluded.
This study was performed in accordance with the Declaration of Helsinki and was approved by the Regional Human Research Ethics Committee of Nanjing First Hospital (reference 201409–1). Individual patient consent was waived on condition that all patient data were deidentified before analysis, because this study was a retrospective analysis. The most recent examined SCr value within 7 days before surgery was defined as the baseline creatinine level.
Anesthesia
Patients were premedicated with intramuscular morphine 0.2 mg/kg and scopolamine 0.3 mg. Anesthesia induction was performed with midazolam 0.2 mg/kg, fentanyl 10 μg/kg, and vecuronium 0.15 mg/kg, and then tracheal intubation was performed. Anesthesia was maintained with continuous propofol (4–8 mg/kg/h) and intermittent intravenous fentanyl, midazolam, vecuronium, and inhalation of 1–2 % isoflurane. Right internal jugular vein was cannulated for transfusion. Electrocardiography, central venous pressure, mean arterial pressure (MAP), arterial oxygen saturation, and nasopharynx and rectal temperature were monitored.
Ulinastatin administration
Patients who were prescribed with and without ulinastatin were identified and correspondingly divided into the UTI group and the control group. Patients in the UTI group received a bolus dose of ulinastatin 500,000 KIU intravenously in 50 ml of saline for 15 minutes after induction of anesthesia.
Anticoagulation
The dose of unfractionated heparin via central venous catheter used for anticoagulation during CPB was 300–400 U/kg plus additional doses to achieve and maintain an activated clotting time between 480 and 600 seconds. Protamine sulfate was used to reverse heparin-induced anticoagulation after separation from CPB.
CPB management and surgical procedures
The CPB circuit was primed with a solution containing 500 ml of crystalloid solution, 1000 ml of hydroxyethyl starch, and 200 ml of 20 % mannitol. Management of CPB included alpha-stat pH management, MAP in the range of 50–80 mmHg, hematocrit of 20–25 %, and a non-pulsatile flow rate of 2.0–2.4 L/min/m2. The surgical procedures included coronary artery bypass grafting, mitral valve replacement, mitral and aortic valve replacement, and aortic valve replacement.
Data collection
The demographic, preoperative, intraoperative, and postoperative data were collected from an electronic medical record database. SCr was recorded each day until the seventh day after surgery. Urine output data were not collected, owing to absence of these data in the records.
Outcome endpoint
The primary endpoints were set as CSA-AKI, which was defined using Kidney Disease: Improving Global Outcomes criteria as an increase in SCr ≥0.3 mg/dl (≥26.5 μmol/L) within 48 h, and an increase in SCr to ≥1.5 times baseline known or presumed to have occurred within the prior 7 days. Overall mortality, need for RRT, intensive care unit (ICU) length of stay, and hospital length of stay were also recorded.
Assessment of adverse events
Adverse events associated with ulinastatin, such as nausea, vomiting, leukocytopenia, hypersensitivity reactions, and elevation of transaminase, were recorded.
Statistical methods
The data were analyzed using SPSS version 18.0 software (SPSS, Chicago, IL, USA) and the MatchIt package in R (version 2.8.1; http://www.r-project.org/). Continuous variables following a normal distribution were expressed as mean ± standard deviation, and categorical variables were expressed as percentages. An unpaired t test was employed to compare means between two groups. The χ2 test or Fisher’s exact test was used to compare categorical variables between two groups of subjects. The Mann–Whitney U test was used to compare medians. Multiple regression binary logistic regression with the backward stepwise method was performed to evaluate the relationship between the administration of ulinastatin and the occurrence of AKI. The significant acceptance and removal levels for a covariate were set at 0.05 and 0.1, respectively. Data were listed as odds ratios (ORs) with 95 % confidence intervals (CIs). Adjusted variables were age, sex, body mass index (BMI), history of hypertension, history of diabetes, insulin-controlled diabetes, chronic obstructive pulmonary disease (COPD), chronic kidney disease, cerebrovascular disease, MAP, history of coronary angiography, ejection fraction, preoperative baseline creatinine level, hematocrit, red blood cells (RBCs) transfused, CPB duration, body temperature (>38 °C) after surgery within 3 days, and mechanical ventilation time.
Propensity score matching
Propensity scores for each subject were generated using a multivariable logistic regression analysis model to compute the probability of ulinastatin administration based on the following covariates: age, sex, BMI, diabetes, insulin-controlled diabetes, hypertension, COPD, chronic kidney disease, cerebrovascular disease, coronary angiography, preoperative baseline creatinine level, CPB duration, MAP, erythrocyte transfusion, ejection fraction, history of coronary angiography, hematocrit, and mechanical ventilation. Propensity scores were then employed to create 1:1 matched pairs (matching the UTI users to non-UTI users) using a nearest neighbor matching algorithm without a caliper method.
Results
Patient characteristics
A total of 2072 patients who underwent cardiac surgery with CPB met the inclusion criteria. Characteristics of the study subjects before and after propensity score matching are listed in (Table 1). Age, baseline creatinine, CPB duration, RBCs transfused, and hematocrit were statistically different between the UTI and control groups. On the basis of the propensity score, 409 patients who received UTI were successfully matched to 409 patients who did not have the UTI treatment (Fig. 1). After propensity score matching, no statistically significant baseline characteristics between the UTI group and the control group were found.
Table 1.
Baseline characteristics of subjects before and after propensity score matched analysis
| Variable | Before matching | Propensity score matched | ||||
|---|---|---|---|---|---|---|
| Control group (n = 1663) | UTI group (n = 409) | P value | Control group (n = 409) | UTI group (n = 409) | P value | |
| Age, yr | 56 ± 13 | 54 ± 14 | 0.008 | 54 ± 14 | 54 ± 14 | 0.880 |
| Male sex, n (%) | 883 (53) | 234 (57) | 0.135 | 235 (57.45) | 234 (57.21) | 0.944 |
| BMI, kg/m2 | 23.6 ± 3.6 | 23.4 ± 3.6 | 0.315 | 23.5 ± 3.7 | 23.4 ± 3.6 | 0.757 |
| History of hypertension, n (%) | 581 (36) | 125 (31) | 0.094 | 119 (29.1) | 125 (30.6) | 0.647 |
| History of diabetes mellitus, n (%) | 175 (10.5) | 40 (9.8) | 0.315 | 44 (10.8) | 40 (9.8) | 0.363 |
| Insulin-controlled diabetes, n (%) | 99 (6.0) | 26 (6.4) | 0.563 | 27 (6.6) | 26 (6.4) | 0.887 |
| COPD, n (%) | 27 (1.6) | 11 (2.7) | 0.150 | 9 (2.2) | 11 (2.7) | 0.490 |
| Chronic kidney disease, n (%) | 30 (1.8) | 5 (1.2) | 0.414 | 3 (0.73) | 5 (1.2) | 0.722 |
| Cerebrovascular disease, n (%) | 80 (4.8) | 15 (3.7) | 0.322 | 14 (3.4) | 15 (3.7) | 0.850 |
| Coronary angiography, n (%) | 590 (35.5) | 150 (36.7) | 0.651 | 165 (40.3) | 150 (36.7) | 0.281 |
| Ejection fraction, % | 59.2 ± 8.4 | 59.1 ± 9.4 | 0.953 | 58.9 ± 8.7 | 59.1 ± 9.4 | 0.761 |
| Creatinine, μmol/L | 75.0 ± 33.3 | 82.4 ± 26.9 | <0.001 | 82.9 ± 53.9 | 82.4 ± 26.9 | 0.889 |
| CPB duration, min | 112.9 ± 48.0 | 106.3 ± 42.0 | 0.006 | 103.2 ± 40.2 | 106.3 ± 42.0 | 0.285 |
| MAP, mmHg | 62.3 ± 7.3 | 62.1 ± 5.6 | 0.515 | 62.2 ± 7.8 | 62.1 ± 5.6 | 0.803 |
| RBCs transfused, U | 4.3 ± 4.2 | 5.8 ± 4.9 | <0.001 | 5.3 ± 5.3 | 5.8 ± 4.9 | 0.241 |
| Hematocrit, % | 23.4 ± 4.6 | 25.0 ± 5.6 | <0.001 | 25.1 ± 5.3 | 25.0 ± 5.6 | 0.736 |
| Mechanical ventilation, median (range) | 7.8 (5.5–10.6) | 7.8 (5.9–11.2) | 0.073 | 7.8 (5.2–11.7) | 7.8 (5.9–11.2) | 0.078 |
Abbreviations: BMI body mass index, CPB cardiopulmonary bypass, MAP mean arterial pressure, COPD chronic obstructive pulmonary disease, UTI urinary trypsin inhibitor, RBCs red blood cells
Fig. 1.
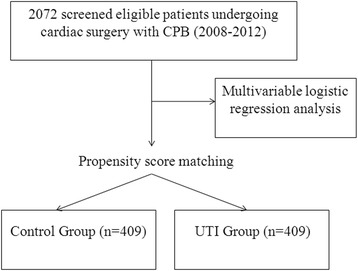
Flowchart showing patients included in the analysis. CPB cardiopulmonary bypass, UTI urinary trypsin inhibitor
Comparison of patient outcomes
Cohort analysis revealed that AKI and RRT occurred more frequently in the control group (40.83 % vs. 30.32 %, P = 0.002; 2.44 % vs. 0.49 %, P = 0.02, respectively) (Fig. 2). No statistically significant differences in ICU length of stay, in-hospital length of stay, and mortality were found between the control group and the UTI group (P > 0.05) (Table 2).
Fig. 2.
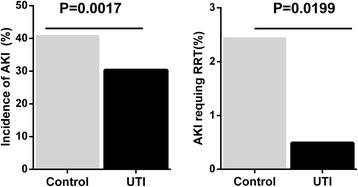
Incidence of acute kidney injury (AKI) and AKI requiring renal replacement therapy between the control group and urinary trypsin inhibitor (UTI) group
Table 2.
Comparison of outcomes between the control group and the UTI group (propensity score matching)
| Outcome | Control group (n = 409) | UTI group (n = 409) | P value |
|---|---|---|---|
| AKI, n (%) | 167 (40.83) | 124 (30.32) | 0.002 |
| RRT, n (%) | 10 (2.44) | 2 (0.49) | 0.020 |
| Death, n (%) | 11 (2.69) | 4 (0.98) | 0.068 |
| Length of ICU stay, d | 2.5 ± 7.1 | 2.0 ± 2.09 | 0.161 |
| Length of in-hospital stay d | 22.5 ± 10.3 | 21.6 ± 7.4 | 0.147 |
Abbreviations: AKI acute kidney injury, RRT renal replacement therapy, UTI urinary trypsin inhibitor, ICU intensive care unit
Ulinastatin administration is a protective factor in the development of AKI
Multivariate logistic regression analysis was used to determine the possible protective role of ulinastatin in the development of AKI. The results are listed in Table 3. Notably, the administration of ulinastatin was found to be beneficial for protecting against CSA-AKI development (OR 0.71, 95 % CI 0.56–0.90, P = 0.005). The independent risk factors for CSA-AKI were as follows: male sex (OR 1.36, 95 % CI 1.12–1.66, P = 0.002), BMI (OR 1.28, 95 % CI 1.10–1.48, P = 0.001), history of hypertension (OR 1.58, 95 % CI 1.23–1.96, P < 0.001), insulin-dependent diabetes mellitus (OR 1.59, 95 % CI 1.07–2.35, P = 0.021), CPB duration ≥110 minutes (OR 1.38, 95 % CI 1.13–1.68, P = 0.001), body temperature (>38 °C) after surgery within 3 days (OR 1.22, 95 % CI 1.016–1.49, P = 0.044), RBC units transfused (OR 1.09, 95 % CI 1.06–1.12, P < 0.001), and mechanical ventilation time ≥9 h (OR 1.01, 95 % CI 1.00–1.02, P = 0.007).
Table 3.
Multivariable analysis determining covariate factors associated with AKI development
| Variable | Adjusted OR | 95 % CI | P value |
|---|---|---|---|
| Male sex | 1.36 | 1.12–1.66 | 0.002 |
| BMI | 1.28 | 1.10–1.48 | 0.001 |
| History of hypertension | 1.58 | 1.23–1.96 | <0.001 |
| Insulin-dependent diabetes | 1.59 | 1.07–2.35 | 0.021 |
| RBCs transfused, U | 1.09 | 1.06–1.12 | <0.001 |
| CPB duration ≥110 min | 1.38 | 1.13–1.68 | 0.001 |
| Mechanical ventilation ≥9 h | 1.01 | 1.00–1.02 | 0.007 |
| Body temperature (>38 °C) after surgery within 3 days | 1.22 | 1.01–1.49 | 0.044 |
| Ulinastatin administration | 0.71 | 0.56–0.90 | 0.005 |
Abbreviations: AKI acute kidney injury, OR odds ratio, CI confidence interval, BMI body mass index, RBCs red blood cells, CPB cardiopulmonary bypass
Assessment of adverse events
No adverse events associated with UTI were found.
Discussion
In the present study, we investigated the role of ulinastatin in the occurrence of AKI in patients undergoing cardiac surgery with CPB. Using a propensity score matching method, we found that patients receiving UTI treatment had a significantly lower incidence of AKI than those in the control group (30.32 % vs. 40.83 %, P = 0.002). Multivariate logistic regression analysis also revealed that ulinastatin played a beneficial role in the development of AKI. The independent risk factors found in our study, such as history of hypertension, insulin dependent diabetes, mechanical ventilation, CPB duration, and erythrocyte transfusion, were in accord with a previous study [9], while early postoperative fever (>38 °C in the first 72 h), which is rarely caused by an infection [22], was found for the first time (to the best of our knowledge) to be an independent risk factor for CSA-AKI. The underlying mechanism was thought to be related to systemic inflammatory responses, which is one of the postoperative complications in patients undergoing cardiac surgery with CPB [23]. These findings may be of importance in light of the renal protection in this setting due to CSA-AKI being significantly associated with higher postoperative mortality [8].
Systemic inflammatory response syndrome caused by cardiac surgery was found to play a central role in the development of AKI [24, 25] by deteriorating ischemia-reperfusion injury, resulting in tubular epithelial and vascular endothelial injury [11]. A large randomized clinical trial has demonstrated that patients undergoing cardiac surgery with CPB are more likely to have CSA-AKI than those treated with surgery using a beating-heart (off-pump) technique [1]. The possible mechanism may be associated with systemic inflammatory response, which occurs more frequently in patients who undergo CPB than in those treated with off-pump surgery [26]. The pathogenesis of systemic inflammatory responses involves multiple factors, such as surgical trauma, blood loss, transfusion, hypothermia, activation of the immune system, ischemia-reperfusion injury and endotoxemia [27]. The activation of humoral and cellular cascades results in an increase of proinflammatory cytokines, and eventually leads to cellular damage and organ injury [28].
Currently, the prophylactic pharmacologic agents aimed at attenuating the inflammatory response mainly include propofol, statins, and glucocorticoids [29]. The benefits of using corticosteroid prophylaxis as an anti-inflammatory agent in patients undergoing cardiac surgery with CPB remain controversial [30]. Although evidence indicates that corticosteroids are effective in reducing the risk of atrial fibrillation and mechanical ventilation duration [31], no difference in major outcomes was found, such as myocardial infarction, renal failure, and postoperative 30-day mortality [32, 33].
In the clinical practical setting, steroids should be prescribed cautiously for those patients with contraindications to steroids, such as peptic ulcer, diabetes mellitus, infection, and fracture. In contrast to steroids, ulinastatin, a powerful protease inhibitor derived from human urine, has unique anti-inflammatory properties, which include inhibition of neutrophil elastase and other proteases and suppression of the production of cytokines, such as interleukin (IL)-6, IL-8, and tumor necrosis factor-α, as well as endothelial leukocyte adhesion molecule-1 [34–38]. The possible side effects of ulinastatin, such as digestive symptoms, leukocytopenia, and hypersensitivity reactions, have rarely been observed in clinical studies [39–41].
On the basis of these properties of ulinastatin, it has been used to prevent postoperative complications and post–pump organ injury in patients undergoing cardiac surgery with CPB [42]. Nakanishi and coworkers [43] demonstrated that prepump administration of ulinastatin can suppress the elevation of postoperative IL-6 and IL-8 in patients undergoing coronary artery bypass graft surgery with CPB in a prospective, randomized, double-blind, placebo-controlled study. A meta-analysis of randomized controlled trials also indicates that ulinastatin can significantly decrease cytokine concentrations in patients undergoing cardiac surgery compared with those who received placebo [44]. Of note, a study of 60 subjects in Korea showed that ulinastatin administration has no cardiac or renal protective effects in patients undergoing aortic valve replacement with CPB [45]. In the study, the sample size of 30 patients in the UTI group and 30 patients in the control group was relatively small. Furthermore, the observation time points were only postoperative day 1 and day 2. The mean levels of SCr, cystatin C, and neutrophil gelatinase-associated lipocalin were employed to determine renal injury was present, instead of using the generally accepted Acute Kidney Injury Network or RIFLE (risk, injury, failure, loss, and end-stage kidney disease) criteria, making the study not so convincing. In addition, a letter to the editor in the same journal also raised concerns that the result should be interpreted with caution due to the relatively small sample size and the multifactorial causes of AKI [46].
Our data show that the administration of ulinastatin during CPB played a protective role in reducing the risk of AKI after cardiac surgery. Although the ICU and hospital lengths of stay seemed longer and mortality in the control group was higher, no statistically significant differences were found between the two groups. One possible reason is that the postoperative patients admitted to the ICU were then transferred to common wards on the second day as routine practice, unless the patients were in critical condition. Therefore, despite the fact that some patients developed AKI, a minor impact on the length of ICU stay resulted. The length of in-hospital stay was also influenced by the policy in our hospital, which limited the average length of hospital stay. Another aspect is that multiple factors including all kinds of complications, such as low cardiac output syndrome, bleeding, infection, and heart failure, can affect mortality and length of hospital stay. Further prospective randomized controlled trials with large sample sizes and perioperative administration of ulinastatin are warranted to confirm the protective role of ulinastatin in the development of CSA-AKI.
Our study has notable limitations. First, our study is a retrospective, single-center study, making it prone to bias. Second, due to the lack of urine output values, only creatinine was used to define AKI criteria. In addition, determining AKI on the basis of urine output was less than practical due to the urinary catheters’ usually being removed about 2 days after surgery.
Conclusions
Our study shows that ulinastatin administration was associated with a lower incidence of CSA-AKI, suggesting that the administration of ulinastatin may be favorable for those patients undergoing cardiac surgery with CPB.
Key messages
Our results reveal ulinastatin administration was associated with a lower incidence of CSA-AKI using a propensity score methodology.
Ulinastatin administration may play a protective role against the development of CSA-AKI in patients undergoing cardiac surgery with CPB.
Acknowledgements
We thank the surgeons and nursing staff of the Department of Cardiothoracic Surgery for providing consultation and useful information. This study was supported by Jiangsu Provincial Special Program of Medical Science (grant BL2014015). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- AKI
acute kidney injury
- BMI
body mass index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CPB
cardiopulmonary bypass
- CSA-AKI
cardiac surgery–associated acute kidney injury
- ICU
intensive care unit
- IL
interleukin
- MAP
mean arterial pressure
- OR
odds ratio
- RBC
red blood cell
- RRT
renal replacement therapy
- SCr
serum creatinine
- UTI
urinary trypsin inhibitor
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CC designed the study, performed statistical analysis, interpreted the data, and drafted the manuscript. XW conceived the study, analyzed and interpreted the data, and drafted the manuscript. XX participated in the design of the study, performed the statistical analysis, and drafted the manuscript. YG participated in study design and helped to draft the manuscript. XC analyzed and interpreted the data and helped to draft the manuscript. XJ performed statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Contributor Information
Xin Wan, Email: xinwan73@yeah.net.
Xiangcheng Xie, Email: freemaple@126.com.
Yasser Gendoo, Email: yassergendoo@sohu.com.
Xin Chen, Email: doctchenxin@163.com.
Xiaobing Ji, Email: xiaobingji78@163.com.
Changchun Cao, Email: changchuncao@yeah.net.
References
- 1.Garg AX, Devereaux PJ, Yusuf S, Cuerden MS, Parikh CR, Coca SG, et al. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA. 2014;311(21):2191–8. doi: 10.1001/jama.2014.4952. [DOI] [PubMed] [Google Scholar]
- 2.Parolari A, Pesce LL, Pacini D, Mazzanti V, Salis S, Sciacovelli C, et al. Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. Ann Thorac Surg. 2012;93(2):584–91. doi: 10.1016/j.athoracsur.2011.09.073. [DOI] [PubMed] [Google Scholar]
- 3.Brown JR, Kramer RS, Coca SG, Parikh CR. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg. 2010;90(4):1142–8. doi: 10.1016/j.athoracsur.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, et al. Acute renal failure following cardiac surgery. Nephrol Dial Transplant. 1999;14(5):1158–62. doi: 10.1093/ndt/14.5.1158. [DOI] [PubMed] [Google Scholar]
- 5.Robert AM, Kramer RS, Dacey LJ, Charlesworth DC, Leavitt BJ, Helm RE, et al. Cardiac surgery-associated acute kidney injury: a comparison of two consensus criteria. Ann Thorac Surg. 2010;90(6):1939–43. doi: 10.1016/j.athoracsur.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Elmistekawy E, McDonald B, Hudson C, Ruel M, Mesana T, Chan V, et al. Clinical impact of mild acute kidney injury after cardiac surgery. Ann Thorac Surg. 2014;98(3):815–22. doi: 10.1016/j.athoracsur.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol. 2004;15(6):1597–605. doi: 10.1097/01.ASN.0000130340.93930.DD. [DOI] [PubMed] [Google Scholar]
- 8.Kolli H, Rajagopalam S, Patel N, Ranjan R, Venuto R, Lohr J, et al. Mild acute kidney injury is associated with increased mortality after cardiac surgery in patients with eGFR < 60 ml/min/1.73 m2. Ren Fail. 2010;32(9):1066–72. doi: 10.3109/0886022X.2010.510616. [DOI] [PubMed] [Google Scholar]
- 9.Mao H, Katz N, Ariyanon W, Blanca-Martos L, Adýbelli Z, Giuliani A, et al. Cardiac surgery-associated acute kidney injury. Blood Purif. 2014;37(Suppl 2):34–50. doi: 10.1159/000361062. [DOI] [PubMed] [Google Scholar]
- 10.Vives M, Wijeysundera D, Marczin N, Monedero P, Rao V. Cardiac surgery-associated acute kidney injury. Interact Cardiovasc Thorac Surg. 2014;18(5):637–45. doi: 10.1093/icvts/ivu014. [DOI] [PubMed] [Google Scholar]
- 11.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg JH, Whitlock R, Zhang WR, Thiessen-Philbrook HR, Zappitelli M, Devarajan P, et al. Interleukin-6 and interleukin-10 as acute kidney injury biomarkers in pediatric cardiac surgery. Pediatr Nephrol. 2015;30(9):1519–27. doi: 10.1007/s00467-015-3088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gueret G, Lion F, Guriec N, Arvieux J, Dovergne A, Guennegan C, et al. Acute renal dysfunction after cardiac surgery with cardiopulmonary bypass is associated with plasmatic IL6 increase. Cytokine. 2009;45(2):92–8. doi: 10.1016/j.cyto.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Scrascia G, Guida P, Rotunno C, de Luca Tupputi Schinosa L, Paparella D. Anti-inflammatory strategies to reduce acute kidney injury in cardiac surgery patients: a meta-analysis of randomized controlled trials. Artif Organs. 2014;38(2):101–12. doi: 10.1111/aor.12127. [DOI] [PubMed] [Google Scholar]
- 15.McBride WT, Prasad PS, Armstrong M, Patterson C, Gilliland H, Drain A, et al. Cytokine phenotype, genotype, and renal outcomes at cardiac surgery. Cytokine. 2013;61(1):275–84. doi: 10.1016/j.cyto.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol. 2015;10(3):500–14. doi: 10.2215/CJN.07830814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong CW, Lee CS, Lee SH, Jeung HJ, Kwak SH. Urinary trypsin inhibitor attenuates liver enzyme elevation after liver resection. Korean J Anesthesiol. 2012;63(2):120–3. doi: 10.4097/kjae.2012.63.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim NY, Shim JK, Bang SO, Sim JS, Song JW, Kwak YL. Effects of ulinastatin on coagulation in high-risk patients undergoing off-pump coronary artery bypass graft surgery. Korean J Anesthesiol. 2013;64(2):105–11. doi: 10.4097/kjae.2013.64.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZF, Yang N, Zhao G, Zhu L, Zhu Y, Wang LX. Preventive effect of ulinastatin and gabexate mesylate on post-endoscopic retrograde cholangiopancreatography pancreatitis. Chin Med J (Engl) 2010;123(18):2600–6. [PubMed] [Google Scholar]
- 20.Chen TT, Jiandong-Liu, Wang G, Jiang SL, Li LB, Gao CQ. Combined treatment of ulinastatin and tranexamic acid provides beneficial effects by inhibiting inflammatory and fibrinolytic response in patients undergoing heart valve replacement surgery. Heart Surg Forum. 2013;16(1):E38–47. doi: 10.1532/HSF98.20121072. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Zeng Z, Cao Y, Du X, Wan Z. Effect of urinary protease inhibitor (ulinastatin) on cardiopulmonary bypass: a meta-analysis for China and Japan. PLoS One. 2014;9(12):e113973. doi: 10.1371/journal.pone.0113973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesperance R, Lehman R, Lesperance K, Cronk D, Martin M. Early postoperative fever and the “routine” fever work-up: results of a prospective study. J Surg Res. 2011;171(1):245–50. doi: 10.1016/j.jss.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Corral-Velez V, Lopez-Delgado JC, Betancur-Zambrano NL, Lopez-Suñe N, Rojas-Lora M, Torrado H, et al. The inflammatory response in cardiac surgery: an overview of the pathophysiology and clinical implications. Inflamm Allergy Drug Targets. 2015;13(6):367–70. doi: 10.2174/1871528114666150529120801. [DOI] [PubMed] [Google Scholar]
- 24.Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med. 2007;357(8):797–805. doi: 10.1056/NEJMra064398. [DOI] [PubMed] [Google Scholar]
- 25.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62(5):1539–49. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 26.Jongman RM, Zijlstra JG, Kok WF, van Harten AE, Mariani MA, Moser J, et al. Off-pump CABG surgery reduces systemic inflammation compared with on-pump surgery but does not change systemic endothelial responses: a prospective randomized study. Shock. 2014;42(2):121–8. doi: 10.1097/SHK.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 27.Cremer J, Martin M, Redl H, Bahrami S, Abraham C, Graeter T, et al. Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg. 1996;61(6):1714–20. doi: 10.1016/0003-4975(96)00055-0. [DOI] [PubMed] [Google Scholar]
- 28.Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21(2):232–44. doi: 10.1016/S1010-7940(01)01099-5. [DOI] [PubMed] [Google Scholar]
- 29.Kraft F, Schmidt C, Van Aken H, Zarbock A. Inflammatory response and extracorporeal circulation. Best Pract Res Clin Anaesthesiol. 2015;29(2):113–23. doi: 10.1016/j.bpa.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Heyn J, Beiras-Fernandez A, Luchting B, Briegel J, Weis F. Inflammatory reactions and hydrocortisone in the setting of cardiac surgery: an overview. Cardiovasc Hematol Agents Med Chem. 2011;9(1):56–61. doi: 10.2174/187152511794182800. [DOI] [PubMed] [Google Scholar]
- 31.Ho KM, Tan JA. Benefits and risks of corticosteroid prophylaxis in adult cardiac surgery: a dose–response meta-analysis. Circulation. 2009;119(14):1853–66. doi: 10.1161/CIRCULATIONAHA.108.848218. [DOI] [PubMed] [Google Scholar]
- 32.Dieleman JM, Nierich AP, Rosseel PM, van der Maaten JM, Hofland J, Diephuis JC, et al. Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA. 2012;308(17):1761–7. doi: 10.1001/jama.2012.14144. [DOI] [PubMed] [Google Scholar]
- 33.Cappabianca G, Rotunno C, de Luca Tupputi Schinosa L, Ranieri VM, Paparella D. Protective effects of steroids in cardiac surgery: a meta-analysis of randomized double-blind trials. J Cardiothorac Vasc Anesth. 2011;25(1):156–65. doi: 10.1053/j.jvca.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Inoue K, Takano H, Shimada A, Yanagisawa R, Sakurai M, Yoshino S, et al. Urinary trypsin inhibitor protects against systemic inflammation induced by lipopolysaccharide. Mol Pharmacol. 2005;67(3):673–80. doi: 10.1124/mol.104.005967. [DOI] [PubMed] [Google Scholar]
- 35.Shu H, Liu K, He Q, Zhong F, Yang L, Li Q, et al. Ulinastatin, a protease inhibitor, may inhibit allogeneic blood transfusion-associated pro-inflammatory cytokines and systemic inflammatory response syndrome and improve postoperative recovery. Blood Transfus. 2014;12(Suppl 1):s109–18. doi: 10.2450/2013.0224-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aosasa S, Ono S, Mochizuki H, Tsujimoto H, Ueno C, Matsumoto A. Mechanism of the inhibitory effect of protease inhibitor on tumor necrosis factor alpha production of monocytes. Shock. 2001;15(2):101–5. doi: 10.1097/00024382-200115020-00004. [DOI] [PubMed] [Google Scholar]
- 37.Nakatani K, Takeshita S, Tsujimoto H, Kawamura Y, Sekine I. Inhibitory effect of serine protease inhibitors on neutrophil-mediated endothelial cell injury. J Leukoc Biol. 2001;69(2):241–7. [PubMed] [Google Scholar]
- 38.Gao C, Huan J, Li W, Tang J. Protective effects of ulinastatin on pancreatic and renal damage in rats following early scald injury. Burns. 2009;35(4):547–52. doi: 10.1016/j.burns.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Karnad DR, Bhadade R, Verma PK, Moulick ND, Daga MK, Chafekar ND, et al. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: a multicenter randomized controlled study. Intensive Care Med. 2014;40(6):830–8. doi: 10.1007/s00134-014-3278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park KH, Lee KH, Kim H, Hwang SO. The anti-inflammatory effects of ulinastatin in trauma patients with hemorrhagic shock. J Korean Med Sci. 2010;25(1):128–34. doi: 10.3346/jkms.2010.25.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanai T, Ishiwata T, Kobayashi T, Sato H, Takizawa M, Kawamura Y, et al. Ulinastatin, a urinary trypsin inhibitor, for the initial treatment of patients with Kawasaki disease: a retrospective study. Circulation. 2011;124(25):2822–8. doi: 10.1161/CIRCULATIONAHA.111.028423. [DOI] [PubMed] [Google Scholar]
- 42.Song J, Park J, Kim JY, Kim JD, Kang WS, Muhammad HB, et al. Effect of ulinastatin on perioperative organ function and systemic inflammatory reaction during cardiac surgery: a randomized double-blinded study. Korean J Anesthesiol. 2013;64(4):334–40. doi: 10.4097/kjae.2013.64.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakanishi K, Takeda S, Sakamoto A, Kitamura A. Effects of ulinastatin treatment on the cardiopulmonary bypass-induced hemodynamic instability and pulmonary dysfunction. Crit Care Med. 2006;34(5):1351–7. doi: 10.1097/01.CCM.0000215110.55899.AE. [DOI] [PubMed] [Google Scholar]
- 44.He S, Lin K, Ma R, Xu R, Xiao Y. Effect of the urinary tryptin inhibitor ulinastatin on cardiopulmonary bypass-related inflammatory response and clinical outcomes: a meta-analysis of randomized controlled trials. Clin Ther. 2015;37(3):643–53. doi: 10.1016/j.clinthera.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 45.Oh SY, Kim JC, Choi YS, Lee WK, Lee YK, Kwak YL. Effects of ulinastatin treatment on myocardial and renal injury in patients undergoing aortic valve replacement with cardiopulmonary bypass. Korean J Anesthesiol. 2012;62(2):148–53. doi: 10.4097/kjae.2012.62.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lema G. Ulinastatin treatment and renal injury in patients undergoing aortic valve replacement with cardiopulmonary bypass: a note of caution. Korean J Anesthesiol. 2013;64(1):91–2. doi: 10.4097/kjae.2013.64.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]


