Abstract
The aim of this study is to evaluate the effect of peptide cyclization on the BBB modulatory activity and plasma stability of HAV peptides, which are derived from the EC1 domain of human E-cadherin. The activities to modulate the intercellular junctions by linear HAV4 (Ac-SHAVAS-NH2), cyclic cHAVc1 (Cyclo(1,8)Ac-CSHAVASC-NH2), and cyclic cHAVc3 (Cyclo(1,6)Ac-CSHAVC-NH2) were compared in in vitro and in vivo BBB models. Linear HAV4 and cyclic cHAVc1 have the same junction modulatory activities as assessed by in vitro MDCK monolayer model and in-situ rat brain perfusion model. In contrast, cyclic cHAVc3 was more effective than linear HAV4 in modulating MDCK cell monolayers and in improving in vivo brain delivery of Gd-DTPA upon i.v. administration in Balb/c mice. Cyclic cHAVc3 (t1/2 = 12.95 h) has better plasma stability compared to linear HAV4 (t1/2 = 2.4 h). The duration of the BBB modulation was longer using cHAVc3 (2–4 h) compared to HAV4 (<1 h). Both HAV4 and cHAVc3 peptides also enhanced the in vivo brain delivery of IRdye800cw-PEG (25 kDa) as detected by near IR imaging. The result showed that cyclic cHAVc3 peptide had better activity and plasma stability than linear HAV4 peptide.
Keywords: blood-brain barrier, BBB, brain delivery, cadherin peptides, HAV peptide, paracellular pathway, tight junction, adherens junction, BBB modulation
INTRODUCTION
It is challenging to treat brain diseases such as Alzheimer's and Parkinson's and brain tumors because drugs used to treat these disorders have difficulty in crossing the blood-brain barrier (BBB).1-3 The microvascular endothelial cells of the brain have tight junctions that limit paracellular diffusion3 and there are various efflux transport proteins (P-glycoprotein, breast cancer resistance protein, multidrug resistance-associated proteins) and metabolic enzymes that reduce transcellular routes of entry to the brain.4-6 To cross the BBB, the drug molecule must have optimal physicochemical properties; for example, many large molecules such as peptides and proteins cannot readily cross the BBB due to their size and hydrophilicity.1,3,7. For example, nerve growth factor (NGF) and brain derived neurotrophic factor (BDNF) have been investigated to treat neurodegenerative diseases.8-13 Unfortunately, as with other proteins, the brain delivery of NGF and BDNF is challenging. Therefore, there is a need for safe and effective methods to improve the delivery of proteins and peptides to the brain.
Modulation of the BBB paracellular pathway is one way to improve brain delivery of proteins and peptides. blood-brain barrier modulation can be achieved in a variety of different ways; one example is the use of vasoactive agents (i.e., bradykinin, bradykinin analogs, histamine, lysophosphatidic acid) to disrupt the BBB.14 However, these agents produce their effects through activation of receptors on the brain endothelial cells and can potentially cause rapid desensitization, pathological changes in receptor density, and damage to the BBB.14 Infusion of hyperosmolar mannitol (25% solution) has also been used to temporarily open the BBB tight junctions to allow both lower (i.e., methotrexate) and higher molecular weight (i.e., Evan blue-albumin) molecules to enter the brain.15,16
A more selective method to modulate the paracellular pathway of the BBB uses peptides to inhibit protein-protein interactions in the intercellular junctions of the BBB. Examples include inhibitory peptides targeting claudin-1 (i.e., C1C2 peptide) and occludin (i.e., OCC1 and OCC2 peptides) that modulate tight junctions in various in vitro models of the BBB17,18 and increase the brain delivery of the opioid receptor agonist [D-Ala2, N-MePhe4, Gly5-ol]-enkephalin (DAMGO).19 In the case of C1C2 peptide, the mechanism of action involves relocalization of claudin-1 from the cell surface to cytosol, resulting in a long-lasting modulation of the BBB.20,21 Our work has focused on cadherin peptides that modulate cadherin interactions in the adherens junctions of both endothelial and epithelial cells. The His-Ala-Val (HAV) and Ala-Asp-Thr (ADT) peptides derived from the extracellular-1 (EC-1) domain of E-cadherin have been shown to enhance delivery of 14C-mannitol in vitro in Madin-Darby canine kidney (MDCK) cell monolayers.22,23 Recently, both HAV6 (Table 1) and ADTC5 peptides have been shown to enhance the brain delivery of both small and large molecules into the brain of rats and mice. 24-26
Table 1.
The Names, Sequences and Molecular Weights of Cadherin Peptides
| Peptide Name | Sequence | Molecular weight *(Da) |
|---|---|---|
| HAV6 | Ac-SHAVSS-NH2 | 627.2 |
| HAV4 | Ac-SHAVAS-NH2 | 611.3 |
| cHAVc1 | Ac-CSHAVASC-NH2 | 815.3 |
| cHAVc3 | Ac-CSHAVC-NH2 | 657.2 |
| ADTC5 | Ac-CDTPPVC-NH2 | 772.2 |
In this study, cyclic HAV peptides (cHAVc1 and cHAVc3) were designed to improve BBB modulatory activity compared to that of linear HAV4 peptide (Table 1). The hypothesis was that the increased backbone rigidity provided by cyclic HAV peptides would result in improved binding affinity for the extracellular domain of cadherin as well as improved plasma stability compared to that of the linear peptide. Therefore, cyclic HAV peptides (i.e., cHAVc1, cHAVc3) were synthesized by forming a disulfide bond between two cysteine residues added to the N- and C-termini of the original linear peptide (Table 1). The plasma stabilities of linear HAV4 and cyclic cHAVc3 peptides were determined in rat plasma, and the peptide degradation was detected and quantified by mass spectrometry. The adherens junction modulatory activity of cyclic cHAVc3 peptide was compared to that of linear HAV4 using in vitro MDCK cell monolayers, and the modulatory effects on BBB permeability were examined using the in-situ rat brain perfusion model as well as in the in vivo Balb/c mouse model. The results indicated that cyclic cHAVc3 peptide has better BBB modulatory activity and plasma stability than does the linear HAV4 peptide.
MATERIALS AND METHODS
Peptide Synthesis
Cyclic and linear HAV peptides were synthesized using solid-phase method with Fmoc chemistry in a Perceptive Pioneer Peptide synthesizer as previously described.22,23 HAV peptides were cleaved from resin and purified by reversed-phase HPLC using a C18 column. The disulfide bond in cyclic peptides was formed by bubbling air into a dilute solution of precursor linear peptides in ammonium bicarbonate buffer at pH 8.5. The identity of each peptide was determined by mass spectroscopy.
In Vitro Peptide Modulatory Activity in MDCK Cell Monolayers
Cell Culture
The in vitro modulatory activities of linear and cyclic HAV peptides were evaluated in MDCK cell monolayers and this model was selected to evaluate the effect of cadherin peptides in modulating the intercellular junctions of the cell monolayers. The MDCK-II cells (cat.# 00062107) were acquired from ECACC, Salisbury, UK and were seeded into Corning flasks until they reached 80% confluency as a monolayer. Then the cell monolayer was washed twice with PBS followed by treatment with trypsin-EDTA solution (0.25% trypsin, 1.0 mM EDTA in Hank's Balanced Salt Solution (HBSS)). The detached single cells were then resuspended, collected, and counted. They were then added into each well (75,000 cells/well) of a Transwell® plate (Permeable Supports, 0.4 μM polyester membrane, 12-well plates) and were incubated for 5–8 days. TEER values were measured before and at the day of the experiment to check the monolayer integrity.
Inhibition of Junction Resealing
The inhibition of junction-resealing in MDCK-II cell monolayers was used to compare the modulatory activity of linear and cyclic HAV peptides.23 In this study, the changes in TEER values were followed using an EVOM™ voltohmmeter (World Precision Instruments) in the presence and absence of HAV peptides. Each experiment was started at TEER values of 280–320 ohm.cm2 for the monolayers. After the cell monolayers were confluent, they were washed with HBSS solution containing 25 mM glucose, 2 mM CaCl2, 0.75 mM MgSO4, and 10 mM HEPES. The cell monolayers were incubated with HBSS for 1.5 h, and the TEER values were recorded. Then the cell monolayers were washed and incubated with Ca2+-free buffer to open the tight junctions. The intercellular junction opening caused a decrease in TEER values of 50–60%. The cells were then incubated in Ca2+-sufficient medium to reseal the intercellular junctions in the presence and absence of HAV peptides (1.0 mM) on the apical and basolateral sides. During the resealing of the intercellular junctions, the TEER values were recorded every hour for 6–8 h.
Direct Junction Modulation
The activities of HAV peptides were evaluated by directly modulating intact MDCK-II cell monolayers. In this assay, only Ca2+-sufficient buffer was used. The Transwells were washed with HBSS buffer containing 25 mM glucose, 2 mM CaCl2, 0.75 mM MgSO4 and 10 mM HEPES. The MDCK cell monolayers were incubated with and without HAV peptides (1.0 mM) on the apical and basolateral sides followed by measurement of TEER values every hour for 6–8 h.
Peptide Stability in Rat Plasma
The rat plasma (lot. no. 19595; NaHeparin) used for plasma stability studies was purchased from Innovative Research, Inc., Novi, Michigan. The plasma degradations of cyclic cHAVc3 and linear HAV4 were determined using LC-MS/MS, and the half-life of each peptide in plasma was calculated. Briefly, HAV4 or cHAVc3 peptide in 1.2 μL of DMSO was added into 200 μL of rat plasma to make the final concentration of DMSO 0.6%. Each peptide solution in rat plasma was incubated and agitated using an orbital shaker at 50 rpm at 37 °C for up to 2–3 half-lives, which were 72 h for cyclic cHAVc3 and 8 h for linear HAV4. The peptides were extracted from plasma using liquid-liquid extraction. In this case, 201.2 μL of plasma containing peptide was added into a 1.0 mL solvent mixture of ACN:H2O:EtOAc (6:1:1) to precipitate plasma proteins; this was followed by centrifugation at 17226 g (12000 PRM) using Centrifuge-5415D (Eppendorf AG-22331 Hamburg, Germany). The supernatant was collected and evaporated under nitrogen at moderate temperature (35 °C). The resulting residue containing HAV peptide was resuspended in 1 mL of a solution mixture of 14% ACN and 1% formic acid (FA) in water. Then, 2% heptafluorobutyric acid (HFBA) was added as an ion-pairing agent to enhance the partitioning of HAV peptides in a C-18 column (dimensions: 150 × 2 mm; pore size, 100 Å; particle size, 5.0 μm). The internal standard for cHAVc3 was HAV4 (~300 M) while the internal standard for HAV4 was 58 μM cHAVc3.
In-Situ Rat Brain Perfusion
The in-situ rat brain perfusion studies were done following the previous method developed by Takasato et al.27 and using three groups of Sprague-Dawley rats for vehicle, linear HAV4 and cyclic cHAVc1 peptide. The protocol to perform the animal studies was approved by the IACUC at The University of Kansas (AUS#75-05). Before starting the animal surgery, the rats were anesthetized by administration of 100 mg/kg ketamine and 5 mg/kg xylazine via the intraperitoneal route. A cannula was inserted into the left common carotid artery (LCCA) for perfusion of the brain microvessels with vehicle or peptide solution (1.0 mM) at a flow rate of 5.0 mL/min. For ligation of the left common carotid artery (LCCA), a surgical silk thread was used to encircle the artery while the pterygopalatine, occipital, and superior thyroid arteries were coagulated and cut. Then the LCCA was catheterized with a polyethylene catheter (PE-50) for retrograde perfusion with a heparinized saline (100 IU/mL). The perfusion was started immediately after a cardiac puncture under anesthesia. The perfusion protocol was carried out as follows: 20-s pre-perfusion with saline, 240-s perfusion with peptide or vehicle, 240-s perfusion of 14C-mannitol and 5-s post-perfusion of wash with saline. The perfusate was sterilized by filtration and placed in an incubator for oxygenation with 95% air and 5% CO2 at 37 °C. Throughout the experiment, the rectal temperature was kept at 36.5 ± 0.5 °C using a heat lamp with a monitoring device (YSI model 73 ATD indicating controller).24
In Vivo Studies
MRI Studies
The activities of cyclic cHAVc3 and linear HAV4 peptides in modulating the BBB were compared in adult Balb/c mice. The animal study protocol to detect brain delivery and deposition of Gd-DTPA with and without HAV peptides followed with detection using MRI was approved by the IACUC at the University of Manitoba (Number 11-069). A previous MRI procedure by On et al. to monitor the BBB permeability was used in this study.28 The enhancement of Gd-DTPA brain deposition caused by cHAVc3 or HAV4 peptides was determined using MRI pixel intensities of the brain images. Before administration of Gd-DTPA, T1- and T2-weighted brain images were obtained as background.25 Then Gd-DTPA (0.4 mmol/kg) along with cHAVc3 (0.001–0.10 mmol/kg), HAV4 (0.001–0.10 mmol/kg), or PBS was administered via tail vein. Every 3 min, a T1-weighted image was obtained up to 21-min imaging session followed by a second dose of Gd-DTPA at 21 min; then, T1-weighted images were collected every 3 min for another 21-min. Marevisi 3.5 software (Institute for Biodiagnostics, National Research Council, Ottawa, Ontario, Canada) was used to quantified the intensity of Gd-DTPA in the brain at the outlined regions of interest (ROI) within coronal brain slices. Paravision 3.0 software package was used to quantify the percent difference of Gd-DTPA in the images of brain slices using following equation:
The percent difference analysis was expressed as fold-enhancement of Gd-DTPA at different time intervals.
To determine the duration of BBB modulation achieved with the cadherin peptides, Gd-DTPA contrast MRI experiments were performed following various pretreatment periods. The dose of each peptide was 0.01 mmol/kg and the dose of Gd-DTPA was 0.4 mmol/kg. Three groups of mice were administered with vehicle, cHAVc3, or HAV4, respectively, via i.v. 1 h prior to administration of Gd-DTPA followed by an MRI imaging session. Another three groups of mice were injected i.v. with vehicle, cHAVc3, or HAV4, respectively, 2 h prior to i.v. administration of Gd-DTPA followed by MRI imaging of the brain. Finally, two groups of mice were administered vehicle and cHAVc3 via i.v. 4 h prior to the administration of Gd-DTPA followed by brain imaging with MRI.
NIR Imaging Studies
The BBB permeability increases for a large molecular weight compound and a P-glycoprotein (P-gp)-sensitive agent cause by HAV peptide were also determined using NIRF imaging agents.25,28,29 IRDye 800CW PEG (0.01 μmol/kg), a pegylated dye of approximately 25 kDa molecular weight, and R800 (0.032 μmol/kg) as P-gp substrate were delivered and detected in the brain using NIRF.6,30 These marker molecules were delivered via i.v, route into mice in three different treatment regimens. In treatment regimen A, the mice received only vehicle injection (PBS); in treatment regimen B, they received 0.010 mmol/kg of cHAVc3; in treatment regime C, they received 0.010 mmol/kg of HAV4. Twenty minutes after treatment, cardiac perfusion of 10% formaldehyde solution was delivered to sacrifice the mice. Then, the brain was removed for NIRF analysis and the deposition of NIRF dye in the brain was determined ex vivo using an Odyssey near-infrared imaging system (Licor, Lincoln, NE). For quantitative determination, the fluorescence intensity at ROI from the tissue was normalized to fluorescence from the blood sample at the same time point. The results were presented as relative fluorescence units per unit of tissue divided by relative fluorescence units per microliter of blood.
RESULTS
Modulatory Activity Comparison between Linear HAV4 and Cyclic cHAVc1 Peptides
The activity of linear and cyclic peptides in inhibiting junction resealing was evaluated using MDCK cell monolayers. In this experiment, the cell monolayers were treated with calcium-free medium, resulting in the opening of intercellular junctions and a decrease in TEER values for the cell monolayers. After one hour, calcium-free medium was replaced with calcium-containing medium to reseal the intercellular junctions. The junction resealing was reflected by the steady increase in the TEER values of the cell monolayers measured over time. To evaluate cadherin peptide activity, the peptide was added into the calcium-containing medium prior to addition into the disrupted cell monolayers. Both the linear HAV4 and cyclic cHAVc1 peptides at 1.0 mM significantly inhibited the resealing of the intercellular junction compared to HBSS control, and both peptides had the same activity (Figure 1A). At various concentrations (20 to 800 μM) and measuring %Δ TEER between the 2-h and 5-h time points, cHAVc1 inhibited the resealing in a concentration-dependent manner and significant inhibitory activity was observed as low as 40 μM (Figure 1B).
Figure 1.
Modulatory activity comparison of linear HAV4, cyclic cHAVc1, and HBSS in MDCK cell monolayers. (A) Comparison of peptide activities to inhibit junction resealing. At 5- and 6-h time points, both linear HAV4 and cyclic cHAVc1 peptide significantly inhibited the junction resealing compared to HBSS (*, p = 0.011 for HAV4 vs. HBSS; #, p = 0.035 cHAVc1 vs. HBSS). There was no significant difference in the activity of linear HAV4 and cyclic cHAVc1 peptides. (B) The concentration-dependent activity of cHAVc1 peptide at 20–800 μM determined as %Δ TEER value. The %Δ TEER value was the difference of TEER values between 2-h and 5-h time points for each concentration and %Δ TEER of HBSS was set at 100%. (*) considered significant change with p < 0.013. (C) The effect of cHAVc1 and HAV4 in increasing brain delivery of 14C-mannitol in the in-situ rat brain perfusion model. Both linear HAV4 and cyclic cHAVc1 peptides significantly enhanced 14C-mannitol transport to the brain compared to vehicle (*, p < 0.05). No difference was observed in the delivery enhancement of 14C-manitol by HAV4 and cHAVc1.
The in-situ rat brain perfusion studies were used to compare the efficacy of linear HAV4 and cyclic cHAVc1 in enhancing brain transport of 14C-mannitol compared to vehicle (Figure 1C). Both linear HAV4 and cyclic cHAVc1 peptides significantly enhanced the passage of 14C-mannitol into the rat brain compared to vehicle (Figure 1C). However, as observed in the in vitro junction annealing assay, there was no significant difference in the BBB permeability of 14C-mannitol caused by linear HAV4 and cyclic cHAVc1 peptides.
Modulatory Activity Comparison between Linear HAV4 and Cyclic cHAVc3 Peptides
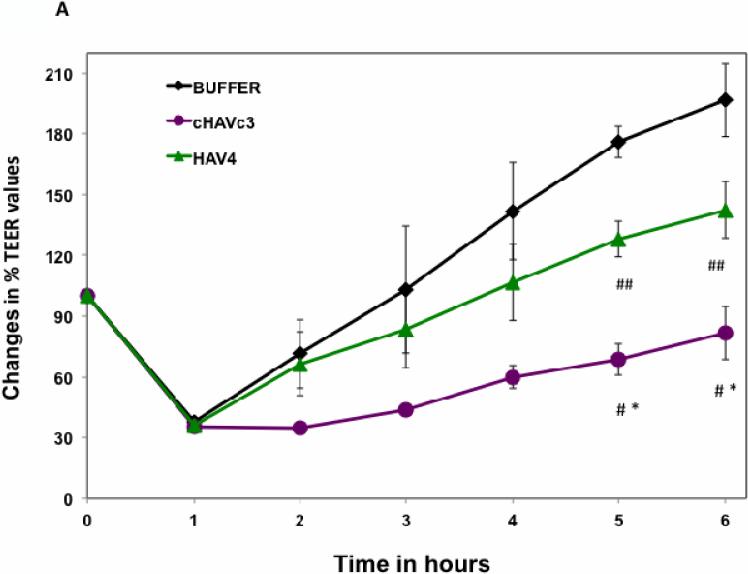
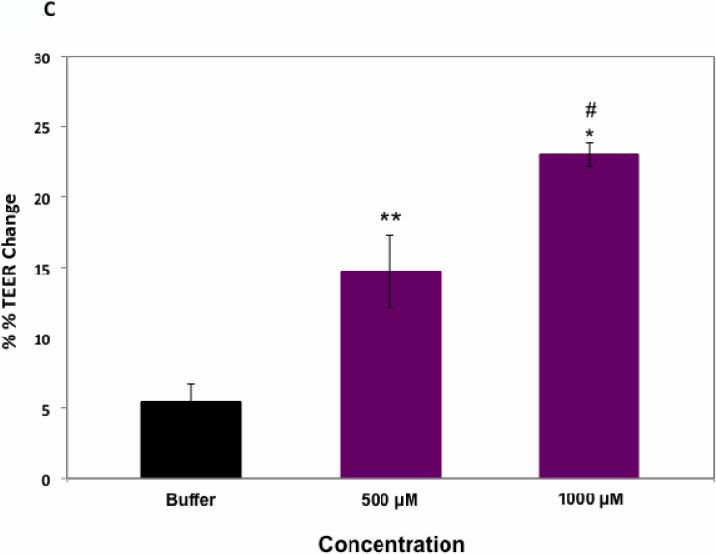
Both HAV4 and cyclic cHAVc3 peptides inhibited the junction resealing of MDCK cell monolayers compared to HBSS-treated controls (Figure 2A). However, cHAVc3 was a significantly better inhibitor of junction resealing than HAV4; at the 5- and 6-h time points, the cHAVc3-treated cells had lower TEER values than those of HAV4-treated cell monolayers. Next, the activities of these peptides were compared in the intact MDCK cell monolayers (Figure 2B). At the 7- and 8-h time points, both HAV4 and cHAVc3 peptides lowered the TEER values significantly compared to HBSS (Figure 2B). In addition, the cHAVc3 peptide had significantly better modulatory activity than HAV4 peptide at the 7- and 8-h time points. The cHAVc3 peptide was active in a concentration-dependent manner; the modulatory activity of 1000 μM peptide was significantly different from that of 500 μM peptide, and the activity of 500 μM peptide was significantly different than that of HBSS (Figure 2C).
Figure 2.
Modulatory activity comparison of linear HAV4, cyclic cHAVc3, and HBSS in MDCK cell monolayers. (A) Comparison of peptide activities to inhibit junction resealing. Cyclic cHAVc3 peptide has significantly higher activity than linear HAV4 peptide at the 6-h time point. #, p = 0.029, cHAVc3 vs. HAV4; *, p = 0.002, cHAVc3 vs. HBSS; and ##, p = 0.044, HAV4 vs. HBSS. (B) Junction modulatory activity of linear HAV4 and cyclic cHAVc3 peptide in normal MDCK cell monolayers. Both peptides significantly decreased the TEER values compared to HBSS at time points 7- and 8-h. (*) considered significantly different, HAV4 vs. HBSS (7-h, p = 0.006; 8-h, p = 0.008) and cHAVc3 vs. HBSS (7-h and 8-h, p = 0.001). (#) considered significantly different, cHAVc3 vs. HAV4 (#, 7- and 8-h, p = 0.009). (C) The effect of concentrations of cHAVc3 in lowering the TEER values (% TEER change) at 7-h time point (*, p = 0.001, 0 vs. 1000 μM; **, p = 0.003, 0 vs. 500 μM; #, p = 0.012, 500 vs. 1000 μM).
Plasma Stability Comparison of Linear HAV4 and Cyclic cHAVc3 Peptides
The in vitro plasma stabilities of HAV4 and cHAVc3 peptides were determined in rat plasma using LC/MS-MS. Linear HAV4 degraded faster than cyclic cHAVc3—2.4 h and 12.95 h, respectively (Figure 3). These results suggest that cyclization increases peptide backbone rigidity, which suppresses enzymatic degradation of HAV peptides in plasma.
Figure 3.
The stability of linear HAV4 (▲) and cyclic cHAVc3 (■) peptides in rat plasma. Peptides were detected using LC-MS/MS to quantify the half-lives. The results indicated that the half-life of linear HAV4 peptide (▲) is 2.4 h with kd = 0.2858 ± 0.0162, and the half-life for cyclic cHAVc3 peptide (■) is 12.9 h with kd = 0.053 ± 0.0016. The extraction efficiency for cHAVc3 is 92.94% and for HAV4 is 101.0%.
Comparison of HAV4 and cHAVc3 Peptides in Enhancing Brain Delivery of Gd-DTPA
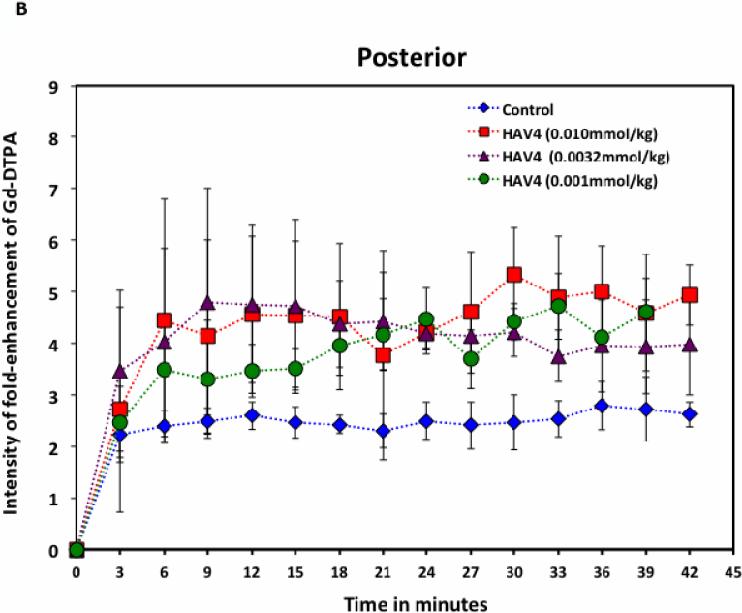
The in vivo effects of HAV4 and cHAVc3 peptides on the brain delivery of Gd-DTPA were determined using MRI throughout a 42-min brain imaging session (Figure 4). Dose-dependent effects of the peptides (0.001, 0.0032, 0.01 mmol/kg) on Gd-DTPA brain delivery were monitored in the posterior, midbrain, and anterior regions of the brain and were compared to those of mice receiving vehicle (PBS). In mice receiving vehicle, minimal amounts of Gd-DTPA were detected in the brain, resulting in mostly dark images except for the ventricle regions observed in mid-brain coronal slices (Figure 4A). The white/grey areas in the MRI images following peptide treatment represent areas of the brain having increased deposition of Gd-DTPA contrast agent (Figure 4A). Treatment with either the linear HAV4 or the cyclic cHAVc3 peptides rapidly enhanced the brain deposition of Gd-DTPA, with increases in brain deposition observed as early as 3 min following the Gd-DTPA+peptide administration (Figure 4B-G). The effect on BBB permeability was dose-dependent for both the linear and cyclic cadherin peptides. For both peptides, the highest brain deposition of Gd-DTPA was found in the posterior region followed by the midbrain and subsequently the anterior region (Figure 4B-G). At 0.010 mmol/kg in posterior region, the maximum enhancement of Gd-DTPA compared to the background at the 0 time point was up to fivefold for linear HAV4 peptide and about eightfold for cyclic cHAVc3 peptide while the maximum deposition when treated with vehicle was 2.5-fold.
Figure 4.
The effects of HAV4, cHAVc3, and vehicle on enhancing brain deposition of Gd-DTPA after i.v. administration in Balb/c mice. (A) Representatives of brain deposition of Gd-DTPA shown as T1-weighted MR images for the posterior region of the brain at 0- and 30-min time points after adminstration of Gd-DTPA (0.4 mmol/kg) with vehicle and 0.001 mmol/kg of peptides (HAV4 and cHAVc3). The red arrows indicate the brain depositions of the contrast agents. (B–G) The effects of (B–D) linear HAV4 peptide and (E–G) cyclic cHAVc3 peptide on the fold-enhancement of Gd-DTPA brain vs. time of imaging at (B, E) posterior, (C, F) midbrain, and (D, G) anterior regions.
The area under the curve (AUC) for Gd-DTPA contrast enhancement in the brain was used to compare the efficacy of HAV4 and cHAVc3 peptides compared to vehicle (Figure 5). For HAV4 treatment group at the posterior and anterior regions, the AUCs of Gd-DTPA deposition were significantly higher than those of the vehicle group at all peptide doses examined; however, for the midbrain, only treatment with 0.01 mmol/kg showed significant enhancement compared to vehicle (Figure 5A). In addition, the effects of different HAV4 peptide doses could be distinguished in the anterior part of the brain but not in the other regions (i.e., posterior and midbrain). For cHAVc3 peptide, all peptide doses were significantly better than control in the posterior and midbrain regions. In the posterior region, the effect of each dose was significantly different from the others (Figure 5B). In the anterior region, only the highest peptide dose showed significantly enhanced delivery of contrast agent compared to vehicle.
Figure 5.
The effect of peptide dose (0.001, 0.0032, 0.01 mmol/kg) on the area under the curve (AUC) for Gd-DTPA fold-enhancement at the posterior, midbrain, and anterior regions for the entire 42-min session as a result of BBB modulation with (A) linear HAV4 and (B) cyclic cHAVc3 peptides (* represents p < 0.05 between peptide and vehicle; ‡ represents p < 0.05 between two different concentrations). (C) Comparison of AUC of Gd-DTPA in the brain posterior after treatment with HAV4 (0.01 mmol/kg), cHAVc3 (0.01 mmol/kg), and vehicle (* represents p < 0.05 between peptide and vehicle; ‡ represents p < 0.05 between two peptides).
Effect of Linear HAV4 and Cyclic cHAVc3 Peptides on the Duration of BBB Disruption
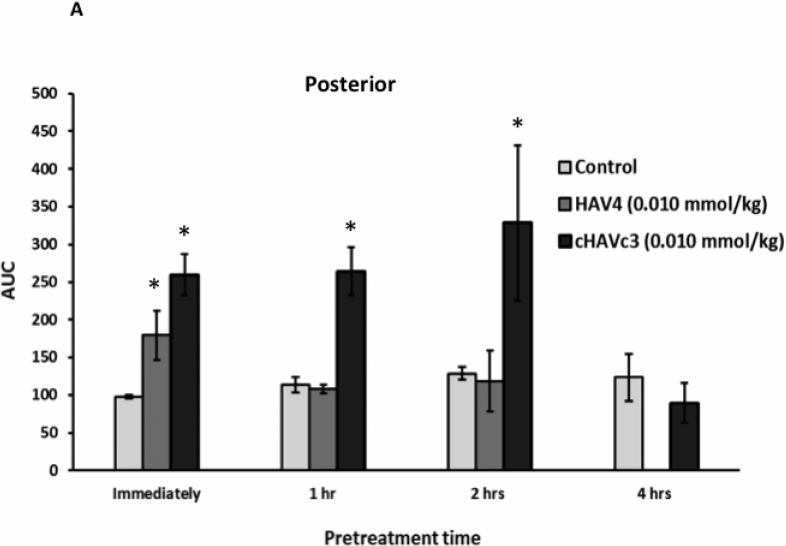
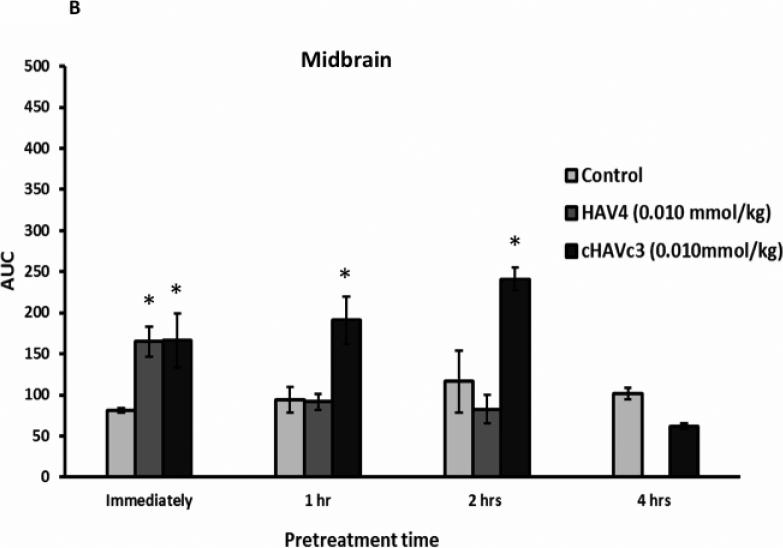
The duration of BBB disruption caused by each peptide was examined by administration of 0.01 mmol/kg of either HAV4 or cHAVc3 peptides at various pretreatment times (Figure 6). The AUCs of Gd-DTPA over a 42-min imaging period were plotted to compare the treatment effects following administration of vehicle, HAV4, or cHAVc3. When Gd-DTPA was delivered immediately after treatment with peptide or vehicle, there were clear increases in Gd-DTPA brain depositions in the posterior (Figure 6A), midbrain (Figure 6B), and anterior (Figure 6C) regions for both peptide treated groups (i.e., HAV4, cHAVc3) compared to the control group. However, administration of Gd-DTPA following a 1- or 2-h pretreatment with HAV4 did not produce any Gd-DTPA enhancement in any regions of the brain examined. In contrast, both the 1-h and 2-h cHAVc3 pretreatments caused significant enhancement of the Gd-DTPA contrast agent in all three brain regions. Only those mice receiving the 4-h pretreatment with cHAVc3 showed no significant increase in contrast enhancement compared to vehicle-treated control mice (Figure 6). These results demonstrate that the time frame for BBB modulation is longer for cHAVc3 than for HAV4 peptide.
Figure 6.
Comparison of length of BBB modulation by linear HAV4 and cyclic cHAVc2 peptides at 0.01 mmol/kg dose as measured by the AUC of Gd-DTPA brain deposition as a function of time in the (A) posterior, (B) midbrain, and (C) anterior regions. The brain depositions of Gd-DTPA were evaluated at different time points for HAV4 (immediately, and following 1-h, and 2-h pretreatment) and cHAVc3 (immediately, and following 1-h, 2-h, and 4-h pretreatment). * represents p < 0.05
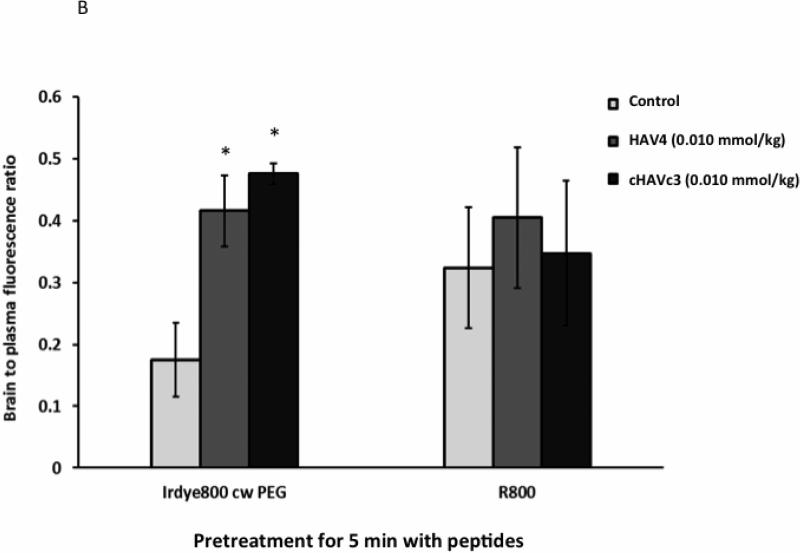
The extent of BBB modulation with large macromolecules and drug efflux transport substrates was also examined using NIRF imaging agents IRdye800cw-PEG and R800, respectively. In these studies, mice were pretreated with the peptide and, after 5 min or 1 h, R800 and IRdye800cw-PEG were delivered and the brain deposition of these molecules was quantitatively assessed using ex vivo brain images. Following 5-min pretreatment with both linear HAV4 and cyclic cHAVc3 peptides, the brain deposition of IRdye800cw-PEG was higher than in saline-treated controls (Figures 7A-B). However, the brain delivery of efflux pump substrate R800 was not enhanced by either peptide at the 5-min time point. After pretreatment with both peptides for 1 h, there were no significant differences in brain deposition of either IRdye800cw-PEG or R800 molecules (Figures 7C-D).
Figure 7.
The effects of time and characteristics of marker molecules such as IR-dye-800cw PEG and R800 on their brain deposition when the BBB was modulated with HAV4 and cHAVc3 peptides (0.01 mmol/kg). The brain images (A, C) and brain-to-plasma fluorescence ratio (B, D) of marker molecules when the marker molecules were delivered 5 min (A, B) or 1 h (C, D) after the delivery of each peptide. * represents p < 0.05
DISCUSSION
The identification and development of methods to improve the delivery of molecules into the brain for diagnosis and treatment of brain diseases is a major challenge. This study was focused in evaluating the effect of linear (i.e., HAV4) and cyclic HAV peptides (i.e., cHAVc1 and cHAVc3) in modulating the intercellular adherens junctions using both in vitro cell culture models and in vivo BBB models. The hypothesis for these studies is that HAV peptides bind to E-cadherin and disrupt E-cadherin-mediated cell adhesion in the intercellular junctions, resulting in increased paracellular porosity of the BBB. HAV4 is a derivative of HAV6 in which the Ser5 residue in HAV6 was mutated with the Ala5 residue (Table 1).22 The sequence of HAV6 peptide was derived from the original sequence (SHAVSS) of extracellular 1 (EC1) domain of human E-cadherin. This original sequence is conserved in the E-cadherin from different species such as canine, human, murine and chicken.31,32 Based on the previous studies in MDCK monolayers, HAV4 displayed better modulation of the intercellular junctions of MDCK cell monolayers.22 Similarly, HAV4 peptide enhanced the in vivo brain delivery of Gd-DTPA better than HAV6 in Balb/c mice as detected by MRI.26
The formation of cyclic peptides has been shown to increase the peptide receptor binding selectivity and biological activity. For example, the antimicrobial activity of cyclic peptide BPC194 is higher than that of linear peptide BPC193.33 Similarly, a cyclic RGD peptide (e.g., cyclo(1,5)KRGDf) was specific toward integrin receptors αvβ3 and αvβ5 while a linear RGD peptide was not.34 Furthermore, formation of cyclic peptide has been shown to improve chemical stability as well as plasma proteolytic stability of peptides. The chemical stability of cyclic RGD peptide was higher than linear RGD peptide in solution close to neutral pH = 7.0.35 In the present study, a cyclic peptide, derived from the linear HAV-based sequence, was formed to induce peptide backbone rigidity, increase binding selectivity to target the extracellular domain of the E-cadherin protein, and enhance BBB modulatory activity in vitro and in vivo.
Initially, the cyclic cHAVc1 peptide was designed and synthesized based on the linear HAV4 sequence with addition of cysteine residues at both N- and C-termini to make a cyclic octapeptide (Table 1). A disulfide bond was formed upon oxidation of thiol groups between the Cys1 and Cys8 residues. Examination of intracellular junction modulation in MDCK cell monolayers showed no significant difference in activity between cHAVc1 and HAV4 (Figure 1A). However, the cHAVc1 peptide did display concentration dependency with an IC50 of approximately 80 μM (Figure 1B). In this assay, the monolayers were first treated with Ca2+-deficient medium to open the intercellular junctions and then exposed to the peptides and Ca2+-containing medium to examine re-establishment of intracellular junctions and TEER values. Thus, although cHAVc1 could have better cadherin-binding affinity than HAV4, the larger size of cHAVc1 compared to HAV4 may have impeded its permeation through the tight junctions and influenced its ability to modulate the cadherin-cadherin interactions. However, as both the HAV4 and cHAVc1 peptides produced similar increases in the BBB permeability of 14C-mannitol in the in-situ rat brain perfusion experiments where the junctions are intact, differences in the abilities of the peptides to access the adherens junction appears unlikely. A more plausible explanation for the similar efficacies observed with the linear HAV4 and cyclic cHAVc1 peptides is that the large ring size of cyclic cHAVc1 did not impose sufficient backbone rigidity to enhance selectivity and affinity for targeting of E-cadherin protein.
In contrast to cHAVc1, cyclic cHAVc3 had better activity than linear HAV4 in inhibiting resealing of the intercellular junctions in MDCK cell monolayers (Figure 2A) and in modulating the intact MDCK cell monolayers (Figure 2B). Cyclic cHAVc3 was a derivative of cHAVc1 with deleted Ala6 and Ser7 residues; this deletion increased the backbone rigidity while reducing the size to a hexapeptide. It is interesting that cHAVc3 has previously been investigated and not found to inhibit the N-cadherin-mediated cell adhesion process.36 This suggests that cHAVc3 peptide is selective for E-cadherin over N-cadherin. Thus, the conformational rigidity of cyclic cHAVc3 peptide may contribute to its activity and selectivity for E-cadherin compared to HAV4 and cHAVc1.
The BBB modulatory activities of cHAVc3 and HAV4 peptides were further compared in enhancing brain delivery of Gd-DTPA in Balb/c mice. The brain deposition of Gd-DTPA in mice was enhanced by both peptides in a concentration-dependent manner (Figures 4 and 5). Cyclic cHAVc3 had significantly higher activity than HAV4 in enhancing the delivery of Gd-DTPA. In the posterior region, cHAVc3 showed an eightfold enhancement of Gd-DTPA (Figure 4E) while HAV4 had only a fivefold enhancement (Figures 4B). Increased activity of cHAVc3 was also supported by the AUC comparison of Gd-DTPA entry into the brain in the posterior region (Figure 5C). Taken together, both in vitro and in vivo studies indicated that cyclic cHAVc3 peptide had a greater ability to modulate the intercellular junctions than the linear HAV4 peptide.
The MRI studies measuring the brain deposition of Gd-DTPA showed regional differences in BBB permeability with the posterior having the greatest accumulation, followed by the mid-brain and anterior regions. Although not assessable with many of the methods used to measure BBB permeability, regional differences in both capillary density and permeability exist.37 Despite these regional differences in basal BBB permeability, the cadherin peptides were able to modulate BBB permeability in all regions examined. These results were consistent with previous studies with other cadherin peptides such as HAV625 and cyclic ADTC5.26
The dynamic range of BBB modulation with the cadherin peptides was evaluated with several different imaging agents. The effect of the cadherin peptides on BBB permeability was most evident for Gd-DTPA. As it is a hydrophilic compound of approximately 900 dalton size, there is limited BBB permeability of this agent under normal conditions. However, substantial increases (3-4-fold) in the brain delivery of Gd-DTPA were observed following both HAV4 and cHAVc3 treatments. While both cadherin peptides also increased the delivery of IRdye800cwPEG, an approximately 30,000 dalton near-infrared fluorescence contrast agent, maximal increases were only around twofold in magnitude. The BBB permeability of the near-infrared fluorescent P-gp contrast agent R800 was not affected by either cadherin peptide. As R800 BBB permeability is influenced by transcellular diffusion and active drug efflux transport processes, the impact of the cadherin peptides on brain delivery of R800 was expected to be minimal.
The plasma stability of cHAVc3 was also better than that of HAV4 with t1/2 = 12.95 h for cHAV3 and t1/2 = 2.4 h for HAV4. This result suggests that cHAVc3 is more stable in systemic circulation than HAV4 after i.v. administration. The structural rigidity of cyclic cHAVc3 suppressed its rate of proteolysis in the blood compared to linear HAV4 peptide.35,38 Previous studies have shown that peptide cyclization prevented proteolytic degradation in plasma and improved the half-life of the peptide in systemic circulation.39 The longer residence time of cyclic cHAVc3 peptide in the systemic circulation compared to the linear HAV4 peptide could influence the magnitude and duration of BBB modulation observed with the peptides.
The duration of BBB opening caused by both peptides was determined by measuring peptide response when given as a pretreatment. While both the linear and cyclic cadherin peptides were able to modulate BBB permeability, there were notable differences in the duration of BBB opening with the two peptides in terms of the size of delivered molecules. For Gd-DTPA, the small molecular weight paracellular marker, the duration of BBB modulation observed with HAV4 was less than 1 h (Figure 6). In contrast, based on the MRI studies examining effects of various pretreatment times, cHAVc3 had a window of BBB opening from 2–4 h (Figure 6). These findings are similar to those of our previous studies with the cyclic cadherin peptide, ADTC5, in which BBB modulation was observed for 2 h, but was completely reversed by 4 h following treatment.26 Such differences in duration of opening with the linear and cyclic cadherin peptides may be due to improved molecular interactions of the cyclic peptide with the extracellular domain of cadherin and/or improved plasma stability.
Interestingly, studies with IRdye800cw-PEG, the large molecular weight paracellular marker, indicated restoration of BBB permeability within 1 h for both peptides (Figure 7). For applications involving delivery of larger therapeutic molecules, infusion along with cadherin peptide may be required to insure an adequate duration of BBB modulation. However, for small molecule brain delivery applications, the relatively limited duration of BBB modulation to the larger molecular weight molecules could be an advantage as it would reduce plasma protein deposition into the brain, which is a major contributor to toxicity observed with other BBB disruption methods. Our hypothesis is that the cadherin peptide modulates the BBB to create large, medium, and small size pores in the intercellular junctions as soon as the peptide is administered. As time progress, the large pores immediately collapse to medium and small size pores and followed by the collapse of medium pores to small pores. Finally, it leads to the resealing of the intercellular junctions to a normal condition. In other words, the rate of large pores disappearance (collapse) is faster than the rates of the collapse of medium and small size pores in the intercellular junctions. This could be the possible reason of why a large molecule such as IRdye800cw-PEG could not be delivered after 1 h peptide pretreatment while a small molecule such as Gd-DTPA could be delivered after 1 h peptide pretreatment.
CONCLUSIONS
Cyclic cHAVc3 peptide has a better adherens junction interaction profile and longer activity in modulating the BBB in vitro and in vivo compared to the linear HAV4 peptide. The BBB modulatory activity of both peptides is reversible with restoration of BBB integrity within 1–4 hours following exposure. The size of the intercellular junction opening is dynamic, with larger pores being present at early time points in BBB modulation. As time passes, the larger pores created by the cadherin peptides collapse to smaller pores within the intercellular junctions. The better activity of cHAVc3 compared to HAV4 is proposed to be due to its rigidity and lower number conformers for selective binding to E-cadherin. In the future, cHAVc3 will be used to deliver functional peptides and proteins into the brain for diagnosis and treatment of animal models for brain disorders such as multiple sclerosis, Alzheimer's, Parkinson's, and brain tumors.
ACKNOWLEDGMENTS
This research was funded by an R01-NS075374 grant from National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH). We would like to thank Nancy Harmony for proofreading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12(1-2):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Di L, Kerns EH, Carter GT. Strategies to assess blood-brain barrier penetration. Expert Opin Drug Discov. 2008;3(6):677–687. doi: 10.1517/17460441.3.6.677. [DOI] [PubMed] [Google Scholar]
- 3.Laksitorini M, Prasasty VD, Kiptoo PK, Siahaan TJ. Pathways and progress in improving drug delivery through the intestinal mucosa and blood-brain barriers. Ther Deliv. 2014;5(10):1143–1163. doi: 10.4155/tde.14.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardridge WM. Brain metabolism: A perspective from the blood-brain barrier. Physiol Rev. 1983;63(4):1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- 5.McCaffrey G, Staatz WD, Sanchez-Covarrubias L, Finch JD, Demarco K, Laracuente ML, Ronaldson PT, Davis TP. P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J Neurochem. 2012;122(5):962–975. doi: 10.1111/j.1471-4159.2012.07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.On NH, Miller DW. Transporter-based delivery of anticancer drugs to the brain: Improving brain penetration by minimizing drug efflux at the blood-brain barrier. Curr Pharm Des. 2014;20(10):1499–1509. doi: 10.2174/13816128113199990458. [DOI] [PubMed] [Google Scholar]
- 7.Pardridge WM. Crossing the blood-brain barrier: Are we getting it right? Drug Discov Today. 2001;6(1):1–2. doi: 10.1016/s1359-6446(00)01583-x. [DOI] [PubMed] [Google Scholar]
- 8.Isaacson LG, Saffran BN, Crutcher KA. Intracerebral NGF infusion induces hyperinnervation of cerebral blood vessels. Neurobiol Aging. 1990;11(1):51–55. doi: 10.1016/0197-4580(90)90062-5. [DOI] [PubMed] [Google Scholar]
- 9.Friden PM, Walus LR, Watson P, Doctrow SR, Kozarich JW, Backman C, Bergman H, Hoffer B, Bloom F, Granholm AC. Blood-brain barrier penetration and in vivo activity of an NGF conjugate. Science. 1993;259(5093):373–377. doi: 10.1126/science.8420006. [DOI] [PubMed] [Google Scholar]
- 10.Hefti F, Weiner WJ. Nerve growth factor and Alzheimer's disease. Ann Neurol. 1986;20(3):275–281. doi: 10.1002/ana.410200302. [DOI] [PubMed] [Google Scholar]
- 11.Nosrat CA, Fried K, Ebendal T, Olson L. NGF, BDNF, NT3, NT4 and GDNF in tooth development. Eur J Oral Sci. 1998;106(Suppl 1):94–99. doi: 10.1111/j.1600-0722.1998.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 12.Pardridge WM, Kang YS, Buciak JL. Transport of human recombinant brain-derived neurotrophic factor (BDNF) through the rat blood-brain barrier in vivo using vector-mediated peptide drug delivery. Pharm Res. 1994;11(5):738–746. doi: 10.1023/a:1018940732550. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Pardridge WM. Blood-brain barrier targeting of BDNF improves motor function in rats with middle cerebral artery occlusion. Brain Res. 2006;1111(1):227–229. doi: 10.1016/j.brainres.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Cloughesy TF, Black KL. Pharmacological blood-brain barrier modification for selective drug delivery. J Neurooncol. 1995;26(2):125–132. doi: 10.1007/BF01060218. [DOI] [PubMed] [Google Scholar]
- 15.Neuwelt EA, Maravilla KR, Frenkel EP, Rapaport SI, Hill SA, Barnett PA. Osmotic blood-brain barrier disruption. Computerized tomographic monitoring of chemotherapeutic agent delivery. J Clin Invest. 1979;64(2):684–688. doi: 10.1172/JCI109509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuwelt EA, Barnett PA, Bigner DD, Frenkel EP. Effects of adrenal cortical steroids and osmotic blood-brain barrier opening on methotrexate delivery to gliomas in the rodent: the factor of the blood-brain barrier. Proc Natl Acad Sci U S A. 1982;79(14):4420–4423. doi: 10.1073/pnas.79.14.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong V, Gumbiner BM. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. The Journal of cell biology. 1997;136(2):399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tavelin S, Hashimoto K, Malkinson J, Lazorova L, Toth I, Artursson P. A new principle for tight junction modulation based on occludin peptides. Molecular pharmacology. 2003;64(6):1530–1540. doi: 10.1124/mol.64.6.1530. [DOI] [PubMed] [Google Scholar]
- 19.Zwanziger D, Hackel D, Staat C, Bocker A, Brack A, Beyermann M, Rittner H, Blasig IE. A peptidomimetic tight junction modulator to improve regional analgesia. Mol Pharm. 2012;9(6):1785–1794. doi: 10.1021/mp3000937. [DOI] [PubMed] [Google Scholar]
- 20.Zwanziger D, Staat C, Andjelkovic AV, Blasig IE. Claudin-derived peptides are internalized via specific endocytosis pathways. Ann N Y Acad Sci. 2012;1257:29–37. doi: 10.1111/j.1749-6632.2012.06567.x. [DOI] [PubMed] [Google Scholar]
- 21.Staata C, Coisneb C, Dabrowskia S, Stamatovicc SM, Andjelkovicc AV, Wolburgd H, Engelhardtb B, Blasiga IE. Mode of action of claudin peptidomimetics in the transient opening of cellular tight junction barriers. Biomaterials. 2014;50:9–20. doi: 10.1016/j.biomaterials.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Makagiansar IT, Avery M, Hu Y, Audus KL, Siahaan TJ. Improving the selectivity of HAV-peptides in modulating E-cadherin-E-cadherin interactions in the intercellular junction of MDCK cell monolayers. Pharm Res. 2001;18(4):446–453. doi: 10.1023/a:1011094025008. [DOI] [PubMed] [Google Scholar]
- 23.Sinaga E, Jois SD, Avery M, Makagiansar IT, Tambunan US, Audus KL, Siahaan TJ. Increasing paracellular porosity by E-cadherin peptides: discovery of bulge and groove regions in the EC1-domain of E-cadherin. Pharm Res. 2002;19(8):1170–1179. doi: 10.1023/a:1019850226631. [DOI] [PubMed] [Google Scholar]
- 24.Kiptoo P, Sinaga E, Calcagno AM, Zhao H, Kobayashi N, Tambunan US, Siahaan TJ. Enhancement of drug absorption through the blood-brain barrier and inhibition of intercellular tight junction resealing by E-cadherin peptides. Mol Pharm. 2011;8(1):239–249. doi: 10.1021/mp100293m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.On NH, Kiptoo P, Siahaan TJ, Miller DW. Modulation of blood-brain barrier permeability in mice using synthetic E-cadherin peptide. Mol Pharm. 2014;11(3):974–981. doi: 10.1021/mp400624v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laksitorini MD, Kiptoo PK, On NH, Thliveris JA, Miller DW, Siahaan TJ. Modulation of Intercellular Junctions by Cyclic-ADT Peptides as a Method to Reversibly Increase Blood-Brain Barrier Permeability. J Pharm Sci. 2015;104(3):1065–1075. doi: 10.1002/jps.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takasato Y, Rapoport SI, Smith QR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am J Physiol. 1984;247(3 Pt 2):H484–493. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- 28.On NH, Savant S, Toews M, Miller DW. Rapid and reversible enhancement of blood-brain barrier permeability using lysophosphatidic acid. J Cereb Blood Flow Metab. 2013;33(12):1944–1954. doi: 10.1038/jcbfm.2013.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.On NH, Mitchell R, Savant SD, Bachmeier CJ, Hatch GM, Miller DW. Examination of blood-brain barrier (BBB) integrity in a mouse brain tumor model. J Neurooncol. 2013;111(2):133–143. doi: 10.1007/s11060-012-1006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.On NH, Chen F, Hinton M, Miller DW. Assessment of P-glycoprotein activity in the Blood-Brain Barrier (BBB) using Near Infrared Fluorescence (NIRF) imaging techniques. Pharm Res. 2011;28(10):2505–2515. doi: 10.1007/s11095-011-0478-6. [DOI] [PubMed] [Google Scholar]
- 31.Noe V, Willems J, Vandekerckhove J, Roy FV, Bruyneel E, Mareel M. Inhibition of adhesion and induction of epithelial cell invasion by HAV-containing E-cadherin-specific peptides. J Cell Sci. 1999;112(Pt 1):127–135. doi: 10.1242/jcs.112.1.127. [DOI] [PubMed] [Google Scholar]
- 32.Kister AE, Roytberg MA, Chothia C, Vasiliev JM, Gelfand IM. The sequence determinants of cadherin molecules. Protein Sci. 2001;10(9):1801–1810. doi: 10.1110/ps.37001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mika JT, Moiset G, Cirac AD, Feliu L, Bardaji E, Planas M, Sengupta D, Marrink SJ, Poolman B. Structural basis for the enhanced activity of cyclic antimicrobial peptides: the case of BPC194. Biochim Biophys Acta. 2011;1808(9):2197–2205. doi: 10.1016/j.bbamem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Cheng KT, Razkin J, Josserand V, Jin Z, Foillard S, Boturyn D, Favrot PMC, Dumy P, Coll JL. Molecular Imaging and Contrast Agent Database (MICAD) Bethesda (MD): 2004. Self-quenched-regioselectively addressable functionalized template-[cyclo-(RGD-d-Phe-Lys)]4 peptide-Cy5-fluorescence quencher QSY21. [PubMed] [Google Scholar]
- 35.Bogdanowich-Knipp SJ, Chakrabarti S, Williams TD, Dillman RK, Siahaan TJ. Solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53(5):530–541. doi: 10.1034/j.1399-3011.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 36.Williams E, Williams G, Gour BJ, Blaschuk OW, Doherty P. A novel family of cyclic peptide antagonists suggests that N-cadherin specificity is determined by amino acids that flank the HAV motif. J Biol Chem. 2000;275(6):4007–4012. doi: 10.1074/jbc.275.6.4007. [DOI] [PubMed] [Google Scholar]
- 37.Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176(12):7666–7675. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- 38.Bogdanowich-Knipp SJ, Jois DS, Siahaan TJ. The effect of conformation on the solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53(5):523–529. doi: 10.1034/j.1399-3011.1999.00055.x. [DOI] [PubMed] [Google Scholar]
- 39.Pollaro L, Heinis C. Strategies to prolong the plasma residence time of peptide drugs. MedChemComm. 2010;1(5):319–324. [Google Scholar]