Abstract
Small interfering RNAs (siRNAs), which downregulate gene expression guided by sequence complementarity, can be used therapeutically to block the synthesis of disease-causing proteins. The main obstacle to siRNA drugs — their delivery into the target cell cytosol — has been overcome to allow suppression of liver gene expression. Here, we review the results of recent clinical trials of siRNA therapeutics, which show efficient and durable gene knockdown in the liver, with signs of promising clinical outcomes and little toxicity. We also discuss the barriers to more widespread applications that target tissues besides the liver and the most promising avenues to overcome them.
Since the discovery in 1978 that a 13-mer DNA oligonucleotide could inhibit Rous sarcoma virus translation and proliferation in a sequence-specific manner1,2, substantial efforts have been devoted to harnessing antisense oligonucleotides (ASOs) for therapy3. ASOs that bind by complementary base pairing to intracellular mRNAs were initially designed to inhibit translation or to modify splicing. The challenges in turning ASOs into drugs were their degradation within body fluids full of nucleases, the potential to trigger innate immune nucleic acid sensors and, the most difficult, their delivery across the cell membrane into the cytosol (and also into the nucleus for some applications). Alterations in the chemistry of the basic nucleotide building blocks led to the development of nucleic acid analogues that are more stable, bind to their target with higher specificity and have improved cell penetration (reviewed in REFS 3–5), culminating in the first ASO-approved drug (fomivirsen) to treat cytomegalovirus retinitis in 1998 (at that time, a declining complication of HIV infection). More recently, mipomersen targeting APOB, a gene encoding the apolipoprotein B-100 in low-density lipoprotein (LDL)-cholesterol particles, was approved for the treatment of familial hypercholesterolaemia6.
The discovery of RNA interference (RNAi)7 approximately 20 years ago opened up a new mechanism for ASO therapeutics: gene silencing (FIG. 1). Transfection of short double-stranded RNAs — namely, small interfering RNAs (siRNAs), which are 21–23 nucleotides in length and contain an mRNA sequence (sense strand) and its complement (antisense active strand) —harnesses this ubiquitous pathway to degrade target gene mRNA and suppress its expression with high specificity8. The potential of siRNA therapeutics was first demonstrated just 12 years ago when injection of Fas siRNAs protected mice from autoimmune hepatitis9. Drug development since then has been rapid. The obstacles to turning siRNAs into drugs are similar to those faced with ASO drugs (see BOX 1 for a summary of the major strategies currently being evaluated to exploit the therapeutic potential of oligonucleotides). Although intracellular delivery of double-stranded RNAs is more challenging than that of mostly single-stranded ASOs, some of the ASO strategies could be adapted to siRNA therapeutics, thus accelerating siRNA preclinical drug development and clinical evaluation. Simple chemical modifications of the 2´-position of the ribose and substitution of phosphorothioate linkages protect siRNAs from nuclease digestion and thus prolong half-life in serum10,11 and other body fluids. 2´-modifications can also prevent recognition by innate immune receptors12,13 and limit off-target effects owing to suppression of partially complementary sequences14. Clinical Phase I and II studies of siRNA therapeutics in the past 2 years have demonstrated potent (as high as 98%) and durable (lasting for weeks) gene knockdown in the liver, with some signs of clinical improvement and without unacceptable toxicity. Two Phase III studies are in progress to treat familial neurodegenerative and cardiac syndromes caused by mutations in transthyretin (TTR).
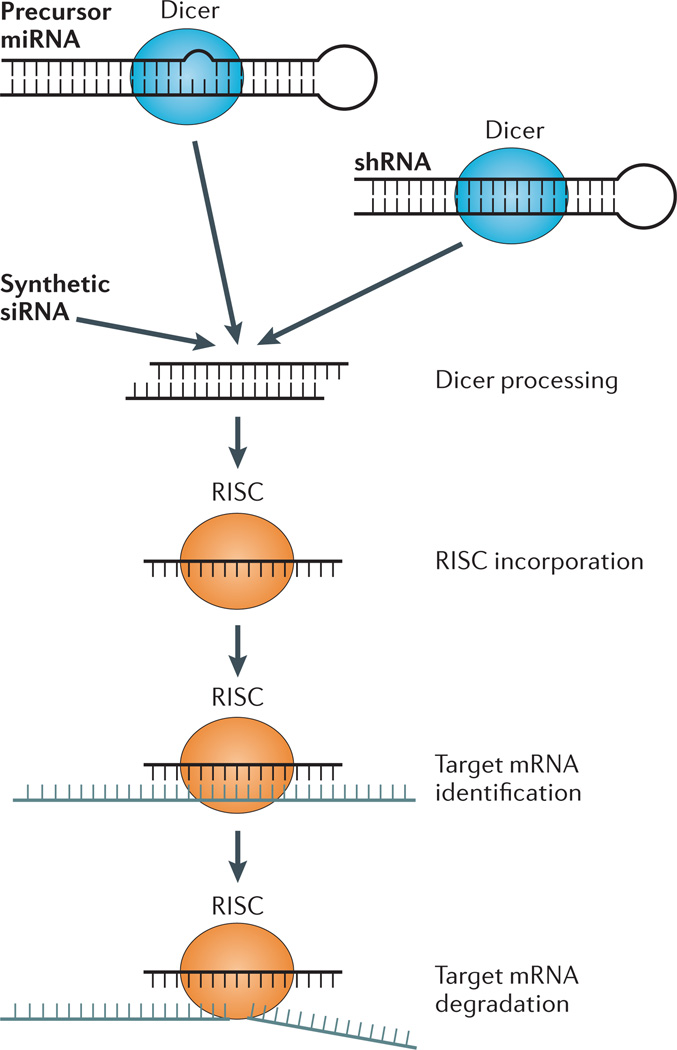
Figure 1. Mechanism of gene knockdown by siRNAs.
Exogenously administered small interfering RNAs (siRNAs; double-stranded ~22-nucleotide-long RNA molecules with 2-nucleotide overhangs at the 3´ ends) exploit the endogenous RNA interference (RNAi) machinery. In the endogenous pathway, Dicer generates microRNAs (miRNAs) from RNA hairpins encoded by miRNA genes. The Dicer machinery also processes exogenous double-stranded RNA (dsRNA) sequences containing loops called short hairpin RNAs (shRNAs). Synthetic siRNAs bypass Dicer processing and can directly associate with the RNA-induced silencing complex (RISC) to mediate recognition of target mRNAs through base-pair complementarity. Argonaute 2 in the RISC complex then enzymatically cleaves the target mRNA, leading to target gene knockdown.
Box 1. Classes of antisense oligonucleotide therapeutics and their mechanisms of action.
RNase H-dependent ASOs
RNase H-dependent antisense oligonucleotides (ASOs) are single-stranded, chemically modified oligonucleotides that bind to complementary sequences in target mRNAs and reduce gene expression both by RNase H-mediated cleavage of the target RNA and by inhibition of translation by steric blockade of ribosomes. The most clinically advanced ASOs are ‘gapmer’ ASOs that incorporate a 5-nucleotide-long or longer central DNA stretch between chemically modified RNA flanks4. RNase H cleaves within the central DNA stretch. Once cleaved, the target mRNA is rapidly degraded.
Exon-skipping ASOs
Exon-skipping ASOs are single-stranded, chemically modified ASOs that target intron–exon junctions (splice sites) or splicing-regulatory elements. Binding to the target site inhibits splicing at this site and forces the choice of an alternative splice site. Changing splice site leads to the translation of an alternative protein isoform that can restore stability or function to a mutated gene product88. Both 2´-O-methyl phosphorothioate and morpholino oligonucleotides have been evaluated clinically.
siRNAs
Small interfering RNAs (siRNAs) are 21–23-nucleotide-long, generally chemically modified, double-stranded RNAs with an antisense active strand that is exactly complementary to a sequence anywhere in the target mRNA. siRNAs are taken up by the cytosolic RNA-induced silencing complex (RISC), which ejects one strand, leaving the antisense strand to bind to the target mRNA and mediate its sequence-specific cleavage by the RISC RNase Argonaute 2 (REF. 8). Once cleaved, the target mRNA is rapidly degraded.
Anti-miRs and miRNA mimics
Oligonucleotides can be used to antagonize (in which case they are known as anti-miRs) or mimic the function of endogenous microRNAs (miRNAs). Native miRNAs are taken up by the RISC, which suppresses gene expression of RNAs containing partially complementary sequences by blocking their translation or accelerating their degradation. They suppress the expression of hundreds of transcripts, but less efficiently than siRNAs. Single-stranded, chemically modified (primarily with phosphorothioates or with morpholino or locked nucleic acid backbones) ASOs can bind to miRNAs to block their activity. Because miR-122 stabilizes the hepatitis C virus (HCV) genomic RNA, anti-miRs targeting miR-122 in the liver have been tested in early-phase trials as a therapy for HCV, with promising results89,90. miRNAs are globally depleted in many cancers with poor prognosis91. Therefore, their replacement could be used as a form of cancer therapy. Ionizable liposomes encapsulating a mimic of a tumour suppressor miRNA (miR-34a)92 are being evaluated in patients with primary and metastatic liver cancer.
In this Review, we discuss the advances in the development of siRNA-based therapy, recent clinical studies that apply this technology to target gene knockdown in the liver and promising approaches for developing siRNA drugs that knock down gene expression in target tissues beyond the liver. This Review will not cover other oligonucleotide therapeutics, such as antisense and exon-skipping oligonucleotides, which are reviewed elsewhere3,4.
Delivery and uptake of siRNA therapeutics
Efficient cellular delivery and uptake of large, negatively charged oligonucleotides are the main obstacles to widespread application of RNA therapeutics (BOX 2). The roadblocks involve getting across the cell membrane into endosomes and then across the endosomal membrane into the cytosol. Once inside a cell, one strand of an siRNA binds to the endogenous cytoplasmic RNA-induced silencing complex (RISC), which captures an mRNA bearing a complementary sequence, and then the RISC Argonaute RNase cuts the target mRNA to initiate its degradation (FIG. 1). The active (antisense) strand of the siRNA is stable within the RISC for weeks, but it is diluted with every cell division15. Thus, the same siRNA molecule can target multiple transcripts and knock down gene expression in slowly dividing or non-dividing cells for weeks. Because of the highly catalytic nature of RNAi, only a few hundred cytosolic siRNAs per cell are needed for efficient and sustained gene knockdown16,17. This low number makes overcoming the delivery obstacle less formidable, and the number is probably lower than what is required using other antisense mechanisms.
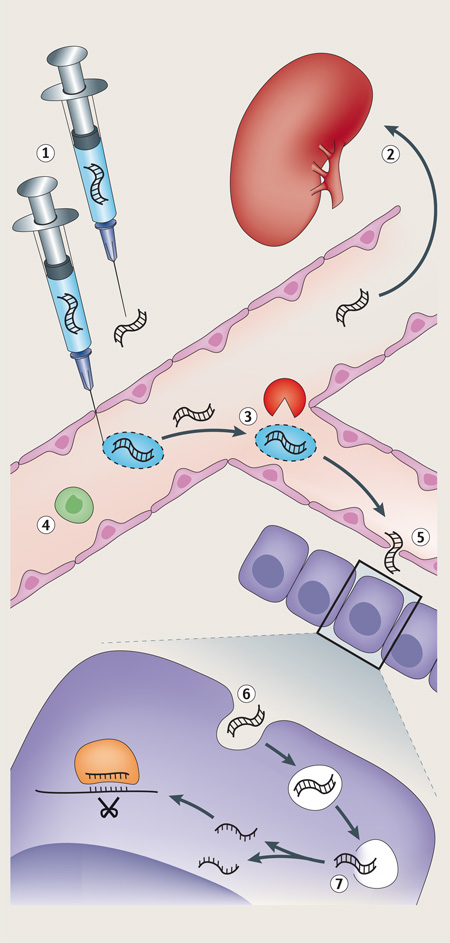
Box 2. Barriers to siRNA therapeutics and strategies to overcome them.
Small interfering RNA (siRNA) therapeutics face multiple barriers along the pathway from administration to delivery to the intracellular target site. The major barriers for both nanoparticle-formulated and targeting ligand-conjugated siRNAs are indicated with a number (see the figure). The table below provides a brief description of each barrier and suggests possible strategies to overcome them.
| Approach | Description |
|---|---|
| 1. Enter circulation or target tissue | |
| Parenteral administration | IV administration used for LNPs and other nanoparticles |
| Subcutaneous injection | GalNAc conjugates are administered subcutaneously and presumably reach the target tissue via lymphatics |
| Topical application | Used for targeting the skin, eye and mucosa |
| 2. Avoid excretion | |
| PEGylation | Increases the molecular weight of siRNA or delivery vehicle to avoid renal excretion |
| Cholesterol conjugation | Cholesterol-conjugated siRNAs bind to circulating lipoprotein particles |
| Nanoparticle formulation | Nanoparticles are above the renal filtration cut-off |
| 3. Avoid nuclease degradation | |
| Nucleic acid backbone modifications | Deoxynucleotides, phosphorothioate linkages and several other modifications of the ribonucleotides confer nuclease resistance |
| Nanoparticle formulation | Packaging within a delivery vehicle makes the RNA inaccessible to nucleases |
| 4. Avoid immune recognition | |
| Nucleic acid backbone modifications | 2´-O-methyl and 2´-fluoro modifications block innate immune stimulation |
| PEGylation | Surface charge minimizes binding to phagocytic cells and other cells |
| 5. Extravasation | |
| Target tissues with leaky vessels | The liver and spleen have a fenestrated endothelium. Tumours can have leaky vessels |
| Endothelial transcytosis | Theoretically attractive approach to gain access to any tissue |
| Target endothelial or blood cells | No need to exit the vasculature |
| 6. Cellular uptake | |
| Targeting ligand | Conjugate siRNA or delivery vehicle to receptor-targeting moiety (ligand, aptamer or antibody fragment) for cell-specific uptake |
| Association with endogenous ligand | Cholesterol-conjugated siRNAs and LNPs bind to serum apolipoproteins conferring uptake in hepatocytes |
| 7. Endosomal release | |
| Membrane-destabilizing lipids | Lipid nanoparticles contain lipid bilayer-disrupting lipids that are activated by low endosomal pH |
| Membrane-destabilizing peptides and polymers | Masked endosomolytic peptides or polymers become unmasked (positively charged) in acidic endosomes and enhance endosomal escape of siRNA |
| Increase endosomal accumulation | Even if endosomal release is inefficient, efficient uptake can compensate for poor release, as only a few hundred cytosolic siRNAs are needed for maximal knockdown |
IV, intravenous; GalNAc, N-acetylgalactosamine; LNP, lipid nanoparticle; PEG, polyethylene glycol.
Intracellular delivery of siRNAs is foiled by their large size (~12 kDa) and negative charge. Although they are too large to cross cell membranes, siRNAs are small enough to be filtered by the kidney. As a consequence, unless they are conjugated to other molecules or incorporated into complexes, intravenously injected siRNAs are rapidly excreted. Many different siRNA delivery approaches have been proposed (reviewed in REF. 18), and the clinically most advanced strategies (all liver-targeted) are described below and summarized in BOX 3.
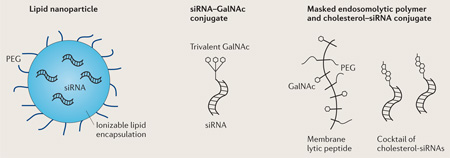
Box 3. Clinically advanced siRNA delivery methods for the liver.
There are several liver-targeted siRNA delivery strategies in the clinical development phase (see the figure).
Lipid nanoparticles
Lipid nanoparticles (LNPs) are ionizable particles that are 50–100 nm in diameter, and contain mixtures of polyethylene glycol-conjugated (PEGylated) lipids, cholesterol and nucleic acids12. Ionizable LNPs are neutral in the circulation, where they associate with apolipoproteins (in particular, apolipoprotein E3), which mediates their endocytosis, primarily by hepatocytes23. The lipids become protonated at low pH in endosomes, which triggers endosomal membrane destabilization, leading to cytosolic release of some of their nucleic acid cargo.
GalNAc conjugates
Trivalent (triantennary) N-acetylgalactosamine (GalNAc), positioned via a 20 Å spacer, is a high-affinity ligand37 for the asialoglycoprotein receptor, which is abundantly expressed on hepatocytes. The receptor is efficiently endocytosed and releases its cargo in acidic endosomes. Conjugation of triantennary GalNAc moieties to chemically stabilized small interfering RNAs (siRNAs)33, antisense oligonucleotides (ASOs)93 or anti-miRs90 enables gene targeting in hepatocytes.
Masked endosomolytic compounds
Masked endosomolytic compounds are polymeric multifunctional macromolecules that incorporate both targeting ligands and endosomal escape moieties. In the original version an amphipathic poly(vinyl-ether) was conjugated to GalNAc and PEG and to an siRNA via a reversible disulfide linkage43. The polymer becomes positively charged in the acidic environment of endosomes, facilitating cytosolic escape of the siRNA. The multifunctional endosomolytic polymer and cholesterol-conjugated siRNA can also be injected separately and both accumulate in hepatocytes to mediate gene knockdown34. Co-injection of a cholesterol-conjugated siRNA and melittin-like peptide94 (a reversibly masked amphipathic peptide polymer derived from bee venom conjugated to PEG and GalNAc) is being evaluated in clinical studies70.
Non-targeted delivery
Early strategies for solving the dual problems of intracellular delivery and rapid excretion involved incorporating siRNAs into lipid nanoparticles (LNPs) — smaller, more homogeneous analogues of lipoplexes used for laboratory transfection19–22. However, these complexes (and other nanoparticle strategies for siRNA delivery) accumulate in the liver and other filtering organs, which limits their effectiveness in penetrating other tissues. Neutral LNPs directly interact with apolipoprotein E3 in the circulation, which targets these particles for hepatocyte uptake23, whereas cationic LNPs also accumulate in the liver, independently of apolipoprotein E3 (REF. 22). As a consequence, most of the clinical focus of siRNA drug development has been on hepatic gene targets. Because the liver synthesizes many blood proteins and is a key metabolic hub, gene knockdown in the liver offers a broad array of therapeutic targets. So far, however, efforts have concentrated on treating rare genetic diseases. Accelerated drug approval for orphan diseases makes this an attractive drug development strategy. Readily accessible tissues, such as the skin, eye or mucosa, in which topical drug delivery is possible, are also attractive sites for siRNA drug development. LNPs and nanoparticles can also penetrate leaky or more permeable vasculature24,25. It remains uncertain whether the enhanced permeability and retention of tumour vasculature can lead to effective gene knockdown in solid cancers using nanoparticles, especially as poor tumour vascularization (and its consequence, hypoxia) are common.
Targeted delivery
siRNAs can also be targeted for uptake in selected tissues or cell types by taking advantage of high-affinity antibody or antibody fragments26–28, aptamers (nucleic acids selected for high-affinity binding)29–31, or receptor ligands32–36 to bind to cell surface receptors and to mediate cell-specific uptake. Targeted uptake has the advantages of being effective at a lower dose and reduced toxicity from knockdown in unintended tissues. These targeting moieties can be directly conjugated to siRNAs, bound non-covalently, or incorporated into LNPs or other nanoparticles. In particular, subcutaneous administration of siRNAs conjugated to trivalent N-acetylgalactosamine (GalNAc)33,37–39, which mediates hepatocyte uptake through the hepatocyte-restricted asialoglycoprotein receptor (ASGPR) causes whole-liver, durable gene knockdown without limiting toxicity40. These GalNAc–siRNA conjugates are currently being evaluated in a Phase III study for the treatment of a rare genetic neurodegenerative disease. It is likely that they will eventually replace most therapeutic LNP efforts for liver targets, as conjugated siRNAs seem to be better tolerated than LNPs, are simpler and cheaper to manufacture, and can be administered subcutaneously. The GalNAc–ASGPR ligand–receptor pair appears to be more effective than other siRNA conjugates that have been tried thus far, which are generally taken up into cells but do not result in efficient knockdown, possibly because of inefficient endocytosis or endocytic release.
Endocytosis and endosomal escape
Most methods of siRNA delivery involve endocytosis. To function, siRNAs need to escape to the cytosol, where the RISC works. Thus, release from the endosome is an important barrier. Understanding the mechanism (or mechanisms) that promotes and limits endosomal release, which is not currently well understood, should help to optimize this limiting step. We recently developed a high-resolution imaging technique that enabled us to identify releasing endosomes and to time and quantify siRNA endosomal release17. Only a small percentage of the siRNAs in LNPs that are taken up by the cell are released into the cytoplasm. siRNAs in LNPs are released only from maturing endosomes during a narrow time window: ~5–15 minutes after endocytosis. The releasing endosome is rapidly recognized by galectins, which are cytosolic glycoprotein sensors41 that target the damaged endosome for autophagy. However, inhibiting autophagy does not enhance cytosolic delivery, suggesting that another mechanism limits release. The prevailing theory of endosomal release, called the ‘proton sponge theory’ (REF. 42), is that a build-up of positive charge in the endosome is needed to disrupt the endosomal membrane for release. Indeed, endosomal release and gene knockdown are enhanced by co- or pre-transfection of cationic polymers or their incorporation into siRNA nanoparticles that become more positively charged as the endosome acidifies34,43. However, it is uncertain how well tolerated these compounds are in vivo.
Target selection
Target selection for therapeutic gene knockdown is guided by considerations that overlap with conventional drug target selection; however, because all cells have the RNAi machinery and any gene can be knocked down, in principle any disease-causing gene can be suppressed in any cell type. This greatly expands the world of potential drug targets beyond the enzymes and receptors that are druggable with conventional small molecules. As most gene products have non-redundant functions in normal physiology, efficient knockdown of any gene could lead to anticipated or unanticipated direct toxicity. Target selection is key to minimize these problems. Direct toxicity is less likely to occur if humans with null or hypomorphic mutations are asymptomatic or even protected. For example, individuals with rare nonsense mutations of the PCSK9 gene encoding proprotein convertase subtilisin/kexin type 9, an enzyme that degrades the LDL receptor, have extremely low blood cholesterol but are healthy44. Knockdown of some gene targets that are needed for health in otherwise normal individuals might be well tolerated and beneficial in certain disease backgrounds, but efficiently suppressing these targets is risky. One example is the antithrombin gene AT3, the mutation of which causes hypercoagulability. AT3 knockdown is being investigated in Phase I clinical trials for haemophilia, in which hypercoagulability is not likely to be a problem45. Other anticipated direct target side effects, such as splenic atrophy from knockdown of kinesin spindle protein (KSP)46 or non-alcoholic fatty liver disease from modulation of cholesterol metabolism, may be clinically well tolerated.
Potential toxicities
All oligonucleotide therapies have off-target effects that can cause unintended toxicity (BOX 4). To limit the therapeutic dose and potentially increase the therapeutic window for a therapeutic siRNA, the first step is to identify a highly potent siRNA sequence. Multiple siRNA sequences designed according to empirical rules derived from sequences that have shown high knockdown efficiency47,48 are usually screened in vitro, and siRNAs with low picomolar IC50 (half-maximal inhibitory concentration) can generally be identified.
Box 4. Sources of toxicity of siRNA therapeutics.
On-target effects
Knocking down any endogenous gene can lead to anticipated or unanticipated direct toxicity owing to non-redundant functions in the normal physiology of the target gene.
Sequence-specific off-target effects
Partial sequence complementarity of endogenous mRNAs with small interfering RNAs (siRNAs) can lead to their suppression via a microRNA (miRNA) mechanism49,51. Off-target effects are concentration dependent and can be reduced by chemical nucleotide modifications14, sequence selection and the use of cocktails or pools of oligonucleotides that dilute off-target effects by reducing the concentration of individual oligonucleotides52. Chemically modified siRNAs do not significantly alter the expression of more than a few non-targeted genes, and the reduction is rarely more than twofold14.
Innate immune activation
Double-stranded RNA can activate innate immune receptors, triggering inflammatory and interferon responses, through recognition by Toll-like receptor 3 (TLR3)54, TLR7 (REF. 55) and retinoic acid-inducible gene I protein (RIG-I; also known as DDX58). Specific chemical modifications that also reduce sequence-specific off-target effects largely abrogate binding and activation of these receptors13,24. However, signs of immune activation (injection reactions, cytokine induction, complement activation and flu-like symptoms) occur to some extent with many oligonucleotide therapies.
Toxicity of delivery vehicles
Endosomolytic components of delivery vehicles have to balance nonspecific cell membrane permeabilization with endosome-disrupting activity to minimize toxicity. Transient cytokine elevations are triggered by cell internalization of lipid-based vehicles, even in the absence of nucleic acid cargo58,59, but the mechanism by which this occurs is not known. Although most oligonucleotides on their own do not induce antibodies, protein–nucleic acid complexes are potentially highly immunogenic, and antibodies against polyethylene glycol (PEG) in PEG-conjugated RNAs can cause anaphylaxis61. Repeat dosing and accumulation of delivery agents that are not biodegradable in target organs could potentially lead to toxicity, which has prompted the development of biodegradable delivery materials60.
MicroRNA-like off-target effects
In addition to ontarget effects, siRNAs exhibit sequence-specific off-target effects, including downregulation of genes with partial sequence complementarity49. Primarily, this effect is mediated by inhibition of the translation of mRNAs complementary to the siRNA seed region (nucleotides at position 2–8 of the antisense strand), in a manner similar to microRNAs (miRNAs)50,51. Chemical modification of the siRNA antisense strand, in particular the 2´-O-methyl modification of position 2, can significantly reduce off-target effects14. Changing the sequence or using pools of oligonucleotides that reduce the concentration of any individual oligonucleotide52 can also limit off-target effects. In addition, siRNA cocktails could provide the added benefit of reducing mutational escape from viral or cancer targets. However, obtaining approval for cocktails may be more difficult.
Innate immune activation
siRNAs can activate innate immune receptors, triggering interferon and inflammatory responses53, through recognition by Toll-like receptor 3 (TLR3)54 and TLR7 (REF. 55). In fact, TLR activation was probably responsible for the promising early clinical trial data for ocular anti-angiogenic siRNAs54 and topically applied siRNAs against respiratory syncytial virus. Formulation of siRNAs in lipid-based delivery vehicles, which deliver siRNAs to endosomal compartments in which TLRs reside, seems to further enhance innate immune activation55. Limited chemical modification of the siRNA (selective 2-O-methyl modification of one strand), however, can block binding to these receptors12,13,24. There may be clinical situations, such as viral infection and cancer, in which these off-target effects could be beneficial56. However, signs of immune activation (injection reactions, complement activation and flu-like symptoms) occur to some extent with all oligonucleotide therapies and may be a particular problem for LNPs and phosphorothioate-modified oligonucleotides57. There is also some evidence that cellular uptake of LNPs or other nanoparticles, even those lacking nucleic acid cargo, can trigger innate immune responses58,59. Pre-medication can alleviate most of these side effects. Another potential source of toxicity, which has so far not been described, could result from the accumulation of non-biodegradable chemically modified nucleotides, unnatural lipid or lipidoid components of LNPs, or polymers used to construct nanoparticles. The design of suitable biodegradable analogues60 should address these problems if they occur.
Adaptive immune responses
Compared with protein-based therapeutics, nucleic acids have the advantage that they do not efficiently elicit antibodies, which would limit the effectiveness of repeated dosing. In fact, gene knockdown has not declined with chronic administration in the few clinical studies in which it has been tested. Although naked RNAs are poor immunogens, antibodies are easily generated against RNA–protein complexes. These are prominent in a variety of autoimmune diseases. So far, there have been no reports of antibodies against siRNAs bound to proteins, but it is unclear whether experiments to date have looked for such antibodies. Some strategies for siRNA delivery append polyethylene glycol (PEG) to the oligonucleotide to reduce excretion and improve circulating half-life. Recently, a large Phase III trial of a PEGylated RNA aptamer against factor IX, which is used as an anticoagulant during cardiac surgery, had to be terminated by Regado Biosciences because of severe anaphylactic reactions (some of which led to death) to the PEG component of the RNA61. This raises serious concerns about all siRNA delivery strategies that involve PEGylated oligonucleotides.
Clinical studies
Here, we discuss clinical trials of siRNA therapeutics, which have shown quantitative evidence of gene knockdown (TABLE 1).
Table 1.
RNAi therapeutics with demonstrated gene knockdown or efficacy in humans*
| Company | Drug | Target | Disease | Developmental phase |
Mechanism | Formulation or conjugation |
Efficacy | Refs |
|---|---|---|---|---|---|---|---|---|
| Alnylam Pharmaceuticals | ALN-TTRsc (also known as revusiran) | TTR | TTR-mediated amyloidosis | Phase III | siRNA | GalNAc conjugate | >90% serum TTR protein reduction | 67 |
| Alnylam Pharmaceuticals | ALN-PCS02 | PCSK9 | Hypercholesterolaemia | Phase I | siRNA | Second-generation LNP | ~70% reduction of plasma PCSK9 protein | 65 |
| Alnylam Pharmaceuticals | ALN-TTR02 | TTR | TTR-mediated amyloidosis | Phase III | siRNA | Second-generation LNP | >80% serum TTR protein reduction | 62 |
| Alnylam Pharmaceuticals | ALN-AT3 | AT3 | Haemophilia A and B, and rare bleeding disorders | Phase I | siRNA | GalNAc conjugate | >60% AT3 serum protein reduction | 68 |
| Alnylam Pharmaceuticals | ALN-TTR01 | TTR | TTR-mediated amyloidosis | Phase I (terminated) | siRNA | First-generation LNP | ~40% serum TTR protein reduction | 62 |
| Arrowhead Research | ARC-520 | HBV | HCV | Phase II | siRNA | Dynamic PolyConjugate | ~50% viral load reduction | 71 |
| Regulus Therapeutics | RG-101 | miR-122 | HCV | Phase II | Anti-miR | GalNAc conjugate | Viral load reductions of up to 4.8 log10 | 90 |
| Santaris Pharma/Roche | SPC3649 (also known as miravirsen) | miR-122 | HCV | Phase II | Anti-miR | Naked | Viral load reductions of up to 3.0 log10 | 89 |
AT3, anti-thrombin III; GalNAc, N-acetylgalactosamine; HBV, hepatitis B virus; HCV, hepatitis C virus; LNP, lipid nanoparticle; PCSK9, proprotein convertase subtilisin/kexin type 9; RNAi, RNA interference; siRNA, small interfering RNA; TTR, transthyretin.
This table summarizes RNAi therapeutics for which there is quantitative evidence of gene knockdown or inhibition.
First-generation LNPs
A first-generation LNP, 1,2-dilinoleyloxy-3-dimethylaminopropane (DLinDMA), which potently knocked down liver gene expression in rodents and non-human primates19, showed limited liver gene knockdown in initial clinical studies and caused some toxicity (complement and innate immune activation). ALN-VSP, an LNP formulation (DLinDMA) of siRNAs targeting KSP and vascular endothelial growth factor A (VEGFA)46, was tested in 41 patients with treatment-resistant liver metastases from a variety of tumours. Disease stabilized in three patients, and one heavily pretreated patient with endometrial carcinoma had a complete remission that lasted for the duration of the study (26 months and 50 ALN-VSP doses). However, mRNA knockdown was not convincingly shown. Administration of ALN-TTR01, which is designed to knockdown TTR, at a dose of 1 mg per kg knocked down TTR serum levels by 40%; TTR is expressed in the liver, and when mutated it causes familial amyloidotic neurodegeneration and cardiomyopathy62. Treatment was associated with infusion reactions, but none of these was serious. Ongoing Phase I/II studies of TKM-PLK1, a modified DLinDMA LNP with improved circulation time and enhanced tumour penetration and containing Polo-like kinase 1 (PLK1)-targeting siRNAs63, is being evaluated in select tumours, but so far it has failed to demonstrate unequivocal sequence-specific anti-tumour effects. A Phase I trial of a similar construct that targeted two Ebola virus genes (TKM-Ebola), which protected all non-human primates tested from a lethal challenge with Zaire ebolavirus64, was put on hold by the US Food and Drug Administration (FDA) in July 2014 because of flu-like symptoms associated with the infusion. Nonetheless, when the Ebola outbreak escalated during the summer of 2014, the FDA approved ‘expanded access’ to people with confirmed or suspected infections. A clinical study using a new construct designed to target the viral variant responsible for the recent West African outbreak was recently placed on hold for lack of efficacy.
Second-generation LNPs
Second-generation LNPs show substantially improved siRNA delivery and knockdown in the liver. Constructed with the anionic lipid dilinol eylmethyl-4-dimethylaminobutyrate (DLin-MC3-DMA), they mediate potent gene knockdown in humans at reduced doses compared with first-generation LNPs62,65. The furthest developed compound is ALN-TTR02 (also known as patisiran), which targets TTR and is being evaluated for familial amyloidotic polyneuropathy. Ttr-deficient mice have a mild phenotype, suggesting that TTR knockdown will be tolerated66. In a Phase I dose-escalation trial62, 32 patients and 17 healthy volunteers who were given single intravenous injections of ALN-TTR02 (0.15–0.3 mg per kg) showed ~85% knockdown of serum TTR protein at a nadir that was durable, remaining reduced to ~60% of pretreatment levels 4 weeks later. In an open-label extension Phase II study of ALN-TTR02, patients who were given 0.3 mg per kg ALN-TTR02 every 3 weeks for 6 months demonstrated ~80% sustained knockdown of serum TTR, with little toxicity (mild to moderate infusion reactions were observed in ~15% of patients). Neurological disease stabilized and even improved over 6 months, in contrast to the deterioration normally seen in historical control patients. A Phase III clinical trial was initiated in 2013 that should be completed in 2017.
In a Phase I study in 32 healthy volunteers, ALN-PCS02, a second-generation LNP targeting PCSK9 for the treatment of familial hypercholesterolaemia, was administered in a single dose of 0.4 mg per kg, and reduced serum PCSK9 by ~70% and serum LDL-cholesterol by ~40% at the nadir65. The treatment was given with corticosteroid pretreatment to suppress infusion reactions and was well tolerated. Thus, second-generation LNPs have shown impressive and durable gene knockdown in the liver at reduced doses compared with the first-generation products. Although infusion reactions are a potential problem, they can be mitigated by steroid pretreatment.
GalNAc conjugates
Although second-generation LNPs provide an acceptable delivery solution for the liver, in early-phase clinical trials GalNAc-conjugated siRNAs seem to provide robust knockdown. They also have several advantages — for instance, they can be given subcutaneously, appear to be better tolerated and do not require steroid pretreatment33. Moreover, conjugated siRNAs are much simpler to produce than LNP-formulated siRNAs. In a Phase I study in healthy volunteers, weekly injections of up to 10 mg per kg GalNAc-conjugated siRNA targeting TTR (ALN-TTRsc; also known as revusiran) resulted in sustained serum TTR protein knockdown of >90% and only mild injection-site erythemas in a minority of subjects as the main adverse reaction67. Similar levels of TTR knockdown and injection-site reactions were seen in preliminary data from a multi-dose (5 mg per kg and 7.5 mg per kg) Phase II trial of ALN-TTRsc in 2014. The study included 26 patients with TTR-mediated familial amyloid cardiomyopathy (FAC) and senile systemic amyloidosis (SSA) with mutated and wild-type TTR, respectively. Fifteen per cent of patients had minor (one patient had moderate) transient liver enzyme elevations that normalized with continued drug administration, indicating a favourable safety profile.
Recent proprietary siRNA modifications developed by Alnylam Pharmaceuticals, termed enhanced stabilization chemistry (ESC), improve gene knockdown potency by as much as 50-fold40. GalNAc-conjugated ESC-modified siRNAs are now in Phase I studies for haemophilia (ALN-AT3, targeting AT3), complement-mediated diseases (ALN-CC5, targeting complement component 5) and porphyrias (ALN-AS1, targeting 5-aminolevulinic acid synthase 1 (ALAS1), the first enzyme in haem biosynthesis). The haemophilia study takes a new approach to treating these bleeding disorders. Instead of supplying the missing clotting factors, it will seek to enhance thrombin generation and clotting by knocking down AT3. Preliminary data from the Phase I dose-escalation trial in patients with severe haemophilia showed >60% reduction in circulating AT3, increased thrombin generation and improved blood clotting using a dose as low as 45 µg per kg68. Clinical studies are planned to begin to evaluate this platform for the treatment of hypercholesterolaemia (ALN-PCSsc) and α1-antitrypsin deficiency (ALN-AAT) in 2015, and hepatitis B virus infection (ALN-HBV) in 2016 (REF. 69). Soon, clinical trials of GalNAc-conjugated ESC-siRNAs will expand from treating small numbers of patients with orphan diseases to addressing more common metabolic diseases.
Masked endosomolytic compounds
Arrowhead Research’s delivery strategy, referred to as Dynamic PolyConjugates (DPCs), seeks to improve endosomal release by using non-lipid reversibly masked endosome-disrupting peptides or polymers. The clinically most advanced DPC, called NAG–MLP, is based on a masked amphipathic melittin-like membranolytic peptide (MLP) covalently conjugated to GalNAc-targeting ligands, which bind to the ASGPR on hepatocytes and PEG (to reduce nonspecific cell adhesion and thereby improve retention in the circulation)70. siRNAs are conjugated to cholesterol, which facilitates cellular uptake, and injected separately. The peptide is unmasked as the endosome acidifies to enhance endosomal release. This platform is being evaluated in the clinic for treating chronic HBV infection (using separate injections of the targeted membranolytic polymer and the siRNA) and AAT deficiency. For HBV, a cocktail of two cholesterol-conjugated HBV-targeted siRNAs is co-injected with NAG–MLP (ARC-520). A Phase I dose-escalation trial in healthy volunteers and a Phase IIa dose-escalation trial in patients with chronic HBV infection have been initiated. Preliminary results71 suggest that all NAG–MLP doses tested (0.01–4 mg per kg) were well tolerated in 36 healthy volunteers receiving the active compound, with no changes in laboratory parameters or severe drug-related adverse reactions. However, a few cases of potential hypersensitivity reactions were noted, and the risk of immune reactions against the PEGylated endosomolytic agent — especially with repeat dosing — is a potential concern. In patients with chronic HBV infection, a modest ~50% reduction in HBsAg (HBV surface antigen; a measure of viral load) at the nadir was seen after a single dose of 2 mg per kg polymer, but further dose escalation is planned. A Phase I study testing ARC-AAT for the treatment of liver-associated disease in patients with AAT deficiency has just begun72. The two-molecule approach has the advantage of being easily adapted to new targets and facilitates the construction of RNA cocktails. However, requiring two different molecules to accumulate in the same endosome at the same time in functional amounts may limit efficacy and increase toxicity. A single-molecule DPC that is being developed and has been tested preclinically43 may be more practical.
In addition to the clinical studies described above, there are ongoing early-phase clinical studies by RXi Pharmaceuticals to reduce dermal scarring by targeting connective tissue growth factor73,74, by Dicerna Pharmaceuticals to treat primary and metastatic liver cancer by targeting MYC75,76, and by Silence Therapeutics to treat pancreatic cancer, with liposomes targeting protein kinase N3 in the tumour vasculature77.
Recent clinical trials targeting the liver have shown promising, durable and well-tolerated gene knockdown, with suggestions of clinical benefit, indicating that siRNA drugs, especially GalNAc-conjugated ESC-siRNAs, are well poised to become a new class of therapeutics within the next few years.
Moving beyond the liver
Although hepatocyte delivery seems to be a largely solved problem, treatment of most diseases requires developing clinically acceptable methods to deliver siRNAs to other tissues and organs. Tissues and organs that are readily accessible to topical application or injection, such as the skin, mucosa and eye, should be the easiest to target. Indeed, cholesterol-conjugated siRNAs may be suitable for delivery to many of these tissues78. Similarly, RXi Pharmaceuticals’ ‘self-delivering’ sd-rxRNAs, which are currently evaluated in clinical trials with intradermal administration, have shown dose-dependent (albeit rather modest) target gene knockdown in the eye following ocular administration in mice73.
However, many commercial and academic efforts for the development of delivery platforms are still devoted to improving the designs of LNPs and other nanoparticles. There are also some efforts to use lipoproteins79 or exosomes80 — naturally produced lipid vesicles that carry endogenous miRNAs which are taken up by nearby and distant cells — as siRNA carriers. Particle-based delivery is not ideal for targeting most internal organs and even tumours, as LNPs and particulates are mostly trapped in the liver and extravasation is limited in other tissues with non-fenestrated endothelium. Uniform and effective siRNA delivery and gene knockdown in solid tumours by particles will probably be difficult, given the heterogeneous and aberrant (albeit leaky) tumour vasculature.
The successes of relatively simply designed siRNA conjugates in the liver and the potential toxicity of LNPs and other complex particles (and their liver trapping, increased cost and regulatory complexity) suggest that more effort should be devoted to designing conjugated siRNAs for targeted uptake. However, no small-molecule ligand–receptor pair with similar delivery potency to the GalNAc–ASGPR pair has, so far, been identified. This ligand–receptor pair may have been an especially fortuitous choice, as the receptor is highly expressed and is recycled rapidly for new rounds of internalization40. Antibody fragments fused to protamine or to other nucleic acid-binding peptides have been used to deliver siRNAs and knock down gene expression in tumours, disseminated blood cells and organs such as the lungs26,27,81,82, but they are challenging to manufacture and develop into drugs. Antibodies can also be directly conjugated to siRNAs. However, a recent systematic analysis of covalently coupled siRNA–antibody conjugates with different internalization routes suggested that despite cellular internalization of the conjugates, only a subset were able to deliver functional amounts of siRNA intracellularly83. Thus, although internalizing siRNA–ligand conjugates can be constructed relatively easily, it may be necessary to select abundant receptors that efficiently internalize their ligands or to devote more effort to optimizing the ligand, the chemical modifications of the siRNA to enhance silencing activity and stability, or attaching modifying groups to enhance endosomal escape. Promising recent efforts to enhance the cytosolic bioavailability of siRNAs include various endosome-destabilizing compounds84 and chemical modifications that increase the hydrophobicity of the oligonucleotide73,85.
One especially attractive and flexible ‘conjugate’ platform for targeted delivery is RNA chimaeras composed of an RNA aptamer, selected for high-affinity binding to a cell surface receptor, and covalently linked to one strand of an siRNA29–31. These are taken up by receptor-mediated endocytosis and cleaved by Dicer to liberate an active siRNA. Recent work with breast cancer homing epithelial cell adhesion molecule (EpCAM) aptamer–siRNA chimaeras suggests that, like GalNAc conjugates, they concentrate in targeted tissues after subcutaneous injection95. Application of improved chemistries for nuclease stability and novel endosomal escape strategies may further improve this versatile siRNA delivery platform.
Conclusions
siRNA therapeutics are now well poised to enter the clinical formulary as a new class of drugs in the near future. Clinical studies conducted in the past 2 years indicate that safe and effective liver gene knockdown is achievable. Although targeting any individual gene might lead to unanticipated clinical toxicity that could stop the development of any individual siRNA drug, we anticipate a rapid expansion of clinical trials for multiple clinical indications. The first siRNA drug approval could occur in the next few years. The lessons learned about delivering siRNAs are likely to facilitate the development of ways to deliver the larger cargo that is being contemplated for therapeutics using modified mRNAs86 or CRISPR–Cas9 (clustered regularly interspaced short palindromic repeats–CRISPR-associated 9)-mediated87 gene editing. However, the delivery obstacles for these other approaches are greater than for siRNAs and should not be underestimated.
Acknowledgments
This work was supported by the Swedish Research Council (A.W.) and the US National Institutes of Health (NIH) grant CA139444 (to J.L.).
Glossary
- RNA interference (RNAi)
An endogenous gene silencing mechanism, present in virtually all eukaryotic cells, by which short double-stranded RNA molecules induce translational inhibition and/or degradation of mRNAs containing partially complementary sequences.
- Gene knockdown
An experimental technique used to reduce gene expression using sequence-specific oligonucleotides, typically by RNA interference (RNAi) or antisense mechanisms.
- RNA-induced silencing complex (RISC)
The catalytic effector complex of RNA interference (RNAi)-mediated gene silencing. The RISC is a multiprotein complex that incorporates one strand of a small interfering RNA (siRNA) or microRNA.
- Aptamers
Oligonucleotides (DNA or RNA) selected to bind with high affinity to defined structures.
- MicroRNAs (miRNAs)
Endogenous, ~21-nucleotide-long, imperfectly paired double-stranded RNA molecules present in both plants and animals that guide the silencing of a multitude of genes bearing partially complementary sequences.
- Endosomal escape
The process of cytosolic entry of small interfering RNAs (siRNAs) from a vesicular compartment, following initial endocytosis of the siRNA (and delivery vehicle) into the target cell.
Footnotes
Competing interests statement
The authors declare competing interests: see Web version for details.
References
- 1.Zamecnik PC, Stephenson ML. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl Acad. Sci. USA. 1978;75:280–284. doi: 10.1073/pnas.75.1.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stephenson ML, Zamecnik PC. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc. Natl Acad. Sci. USA. 1978;75:285–288. doi: 10.1073/pnas.75.1.285. First demonstration of the possibility of inhibiting gene expression using oligonucleotides.
- 3.Kole R, Krainer AR, Altman S. RNA therapeutics: beyond RNA interference and antisense oligonucleotides. Nat. Rev. Drug Discov. 2012;11:125–140. doi: 10.1038/nrd3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu. Rev. Pharmacol. Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 5.Sharma VK, Sharma RK, Singh SK. Antisense oligonucleotides: modifications and clinical trials. Med. Chem. Commun. 2014;5:1454–1471. [Google Scholar]
- 6.Raal FJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006. doi: 10.1016/S0140-6736(10)60284-X. [DOI] [PubMed] [Google Scholar]
- 7. Fire A, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. Initial report describing mediation of RNAi by double-stranded RNAs.
- 8. Elbashir SM, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. Demonstration that exogenously supplied short double-stranded RNAs can specifically and efficiently inhibit gene expression in mammalian cells.
- 9. Song E, et al. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 2003;9:347–351. doi: 10.1038/nm828. First proof of principle that siRNAs can be harnessed to treat disease in an in vivo model.
- 10.Morrissey DV, et al. Activity of stabilized short interfering RNA in a mouse model of hepatitis B virus replication. Hepatology. 2005;41:1349–1356. doi: 10.1002/hep.20702. [DOI] [PubMed] [Google Scholar]
- 11.Chiu Y-L, Rana TM. siRNA function in RNAi: a chemical modification analysis. RNA. 2003;9:1034–1048. doi: 10.1261/rna.5103703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morrissey DV, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. First demonstration that siRNAs can be chemically modified to attenuate nuclease degradation and immune stimulation to allow for efficient lipid-based systemic administration.
- 13.Judge AD, Bola G, Lee ACH, MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol. Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Jackson AL, et al. Position-specific chemical modification of siRNAs reduces ‘off-target’ transcript silencing. RNA. 2006;12:1197–1205. doi: 10.1261/rna.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett DW, Davis ME. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 2006;34:322–333. doi: 10.1093/nar/gkj439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilleron J, et al. Image-based analysis of lipid nanoparticle-mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013;31:638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- 17.Wittrup A, et al. Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat. Biotechnol. 2015 doi: 10.1038/nbt.3298. http://dx.doi.org/10.1038/nbt.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013;12:967–977. doi: 10.1038/nmat3765. [DOI] [PubMed] [Google Scholar]
- 19. Zimmermann TS, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. First report of potent RNAi-mediated gene silencing in non-human primates after systemic administration of siRNA formulated in LNPs.
- 20.Akinc A, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semple SC, et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 22.Shi B, et al. Biodistribution of small interfering RNA at the organ and cellular levels after lipid nanoparticle-mediated delivery. J. Histochem. Cytochem. 2011;59:727–740. doi: 10.1369/0022155411410885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akinc A, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Judge AD, et al. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J. Clin. Invest. 2009;119:661–673. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl Acad. Sci. USA. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song E, et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat. Biotechnol. 2005;23:709–717. doi: 10.1038/nbt1101. [DOI] [PubMed] [Google Scholar]
- 27.Peer D, Zhu P, Carman CV, Lieberman J, Shimaoka M. Selective gene silencing in activated leukocytes by targeting siRNAs to the integrin lymphocyte function-associated antigen-1. Proc. Natl Acad. Sci. USA. 2007;104:4095–4100. doi: 10.1073/pnas.0608491104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peer D, Park EJ, Morishita Y, Carman CV, Shimaoka M. Systemic leukocyte-directed siRNA delivery revealing cyclin D1 as an anti-inflammatory target. Science. 2008;319:627–630. doi: 10.1126/science.1149859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McNamara JO, et al. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 30.Berezhnoy A, Castro I, Levay A, Malek TR, Gilboa E. Aptamer-targeted inhibition of mTOR in T cells enhances antitumor immunity. J. Clin. Invest. 2014;124:188–197. doi: 10.1172/JCI69856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wheeler LA, et al. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. J. Clin. Invest. 2011;121:2401–2412. doi: 10.1172/JCI45876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis ME, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nair JK, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. Description of triantennary GalNAc-conjugated siRNA for liver-targeted gene knockdown.
- 34.Wong SC, et al. Co-injection of a targeted, reversibly masked endosomolytic polymer dramatically improves the efficacy of cholesterol-conjugated small interfering RNAs in vivo. Nucleic Acid Ther. 2012;22:380–390. doi: 10.1089/nat.2012.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kortylewski M, et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat. Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soutschek J, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 37.Biessen EA, et al. Synthesis of cluster galactosides with high affinity for the hepatic asialoglycoprotein receptor. J. Med. Chem. 1995;38:1538–1546. doi: 10.1021/jm00009a014. [DOI] [PubMed] [Google Scholar]
- 38.Matsuda S, et al. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem. Biol. 2015;10:1181–1187. doi: 10.1021/cb501028c. [DOI] [PubMed] [Google Scholar]
- 39.Rajeev KG, et al. Hepatocyte-specific delivery of siRNAs conjugated to novel non-nucleosidic trivalent N-acetylgalactosamine elicits robust gene silencing in vivo. ChemBioChem. 2015;16:903–908. doi: 10.1002/cbic.201500023. [DOI] [PubMed] [Google Scholar]
- 40.Manoharan M. GalNAc-siRNA with enhanced stabilization chemistry: ESC-GalNAc-siRNA. Alnylam. 2014 [online], http://www.alnylam.com/web/assets/ALNY-ESC-GalNAc-siRNA-TIDES-May2014-Capella.pdf. [Google Scholar]
- 41.Thurston TLM, Wandel MP, von Muhlinen N, Foeglein Á, Randow F. Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature. 2012;482:414–418. doi: 10.1038/nature10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boussif O, Zanta MA, Behr JP. Optimized galenics improve in vitro gene transfer with cationic molecules up to 1000-fold. Gene Ther. 1996;3:1074–1080. [PubMed] [Google Scholar]
- 43.Rozema DB, et al. Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes. Proc. Natl Acad. Sci. USA. 2007;104:12982–12987. doi: 10.1073/pnas.0703778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen B, et al. A subcutaneously administered RNAi therapeutic (ALN-AT3) targeting antithrombin for treatment of hemophilia: interim Phase 1 study results in healthy volunteers and patients with hemophilia A or B. Oral Poster Abstract 693. 56th American Society of Hematology. 2014 [online], https://ash.confex.com/ash/2014/webprogram/Paper75077.html. [Google Scholar]
- 46.Tabernero J, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3:406–417. doi: 10.1158/2159-8290.CD-12-0429. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds A, et al. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 48.Elbashir SM, Harborth J, Weber K, Tuschl T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods. 2002;26:199–213. doi: 10.1016/S1046-2023(02)00023-3. [DOI] [PubMed] [Google Scholar]
- 49.Jackson AL, et al. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 50.Jackson AL, et al. Widespread siRNA ‘off-target’ transcript silencing mediated by seed region sequence complementarity. RNA. 2006;12:1179–1187. doi: 10.1261/rna.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birmingham A, et al. 3´ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat. Methods. 2006;3:199–204. doi: 10.1038/nmeth854. [DOI] [PubMed] [Google Scholar]
- 52.Huang L, et al. Efficient and specific gene knockdown by small interfering RNAs produced in bacteria. Nat. Biotechnol. 2013;31:350–356. doi: 10.1038/nbt.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Judge AD, et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- 54.Kleinman ME, et al. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–597. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hornung V, et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat. Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- 56.Poeck H, et al. 5´-triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat. Med. 2008;14:1256–1263. doi: 10.1038/nm.1887. [DOI] [PubMed] [Google Scholar]
- 57.Roberts TL, Sweet MJ, Hume DA, Stacey KJ. Cutting edge: species-specific TLR9-mediated recognition of CpG and non-CpG phosphorothioate-modified oligonucleotides. J. Immunol. 2005;174:605–608. doi: 10.4049/jimmunol.174.2.605. [DOI] [PubMed] [Google Scholar]
- 58.Kedmi R, Ben-Arie N, Peer D. The systemic toxicity of positively charged lipid nanoparticles and the role of Toll-like receptor 4 in immune activation. Biomaterials. 2010;31:6867–6875. doi: 10.1016/j.biomaterials.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 59.Landesman-Milo D, Peer D. Toxicity profiling of several common RNAi-based nanomedicines: a comparative study. Drug Deliv. Transl. Res. 2014;4:96–103. doi: 10.1007/s13346-013-0158-7. [DOI] [PubMed] [Google Scholar]
- 60.Maier MA, et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013;21:1570–1578. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rusconi C. Phase 3 evaluation of revolixys kit: study summary and lessons learned. Celebrating the 25th Anniversary of Selex; ASGCT meeting; May 10–12, 2015; New Orleans. 2015. [Google Scholar]
- 62. Coelho T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. First demonstration of highly potent siRNA-mediated gene knockdown in humans.
- 63.Degenhardt Y, Lampkin T. Targeting Polo-like kinase in cancer therapy. Clin. Cancer Res. 2010;16:384–389. doi: 10.1158/1078-0432.CCR-09-1380. [DOI] [PubMed] [Google Scholar]
- 64.Geisbert TW, et al. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375:1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fitzgerald K, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–68. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adams D, et al. Phase 2 open-label extension study of patisiran, an RNAi therapeutic for the treatment of familial amyloidotic polyneuropathy. Alnylam. 2014 [online], http://www.alnylam.com/web/assets/Patisiran_Phase2_OLE_ANA_POSTER-3.pdf. [Google Scholar]
- 67.Zimmermann T, et al. Phase I first-in-human trial of ALN-TTRsc, a novel RNA interference therapeutic for the treatment of familial amyloidotic cardiomyopathy (FAC) Alnylam. 2013 [online], http://www.alnylam.com/web/wp-content/uploads/2013/09/ALN-TTRsc-PhI-HFSA-Poster-Sep2013.pdf. [Google Scholar]
- 68.Akinc AA. Subcutaneously administered investigational RNAi therapeutic (ALN-AT3) targeting antithrombin for treatment of hemophilia: interim Phase 1 study results in healthy volunteers and hemophilia A and B subjects. Alnylam. 2015 [online], http://www.alnylam.com/web/assets/2015-Goring-meeting-Akinc_Jan2015.pdf. [Google Scholar]
- 69.RNAi Roundtable: ALN-PCSsc for the treatment of hypercholesterolemia. Alnylam. 2014 [online], http://www.alnylam.com/web/assets/RNAi_Roundtable_PCSsc_081414.pdf. [Google Scholar]
- 70.Wooddell CI, et al. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol. Ther. 2013;21:973–985. doi: 10.1038/mt.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuen M-F, et al. Phase II, dose ranging study of ARC-520, a siRNA-based therapeutic, in patients with chronic hepatitis B virus infection. American Association for the Study of Liver Diseases. 2014 [online], http://files.shareholder.com/downloads/AMDA-2OTJP1/3374437865x0x793199/06940176-e88b-4bfd-9ded-aacd216c7e9a/AASLD%202014%20ARC-520.pdf. [Google Scholar]
- 72.Arrowhead begins Phase 1 trial of ARC-AAT for treatment of liver disease associated with alpha-1 antitrypsin deficiency. Arrowhead Research. 2015 [online], http://ir.arrowheadresearch.com/releasedetail.cfm?ReleaseID=897578. [Google Scholar]
- 73.Byrne M, et al. Novel hydrophobically modified asymmetric RNAi compounds (sd-rxRNA) demonstrate robust efficacy in the eye. J. Ocul. Pharmacol. Ther. 2013;29:855–864. doi: 10.1089/jop.2013.0148. [DOI] [PubMed] [Google Scholar]
- 74.Clinical trials — overview. RXI Pharmaceuticals. [online], http://www.rxipharma.com/clinical-trials/#additional. [Google Scholar]
- 75.Tolcher AW, et al. Safety and activity of DCR-MYC, a first-in-class Dicer-substrate small interfering RNA (DsiRNA) targeting MYC, in a phase I study in patients with advanced solid tumors. J. Clin. Oncol. 2015;33 915_suppl 11006. [Google Scholar]
- 76.Dudek H, et al. Knockdown of β-catenin with dicer-substrate siRNAs reduces liver tumor burden in vivo. Mol. Ther. 2014;22:92–101. doi: 10.1038/mt.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schultheis B, et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J. Clin. Oncol. 2014;32:4141–4148. doi: 10.1200/JCO.2013.55.0376. [DOI] [PubMed] [Google Scholar]
- 78.Wu Y, et al. Durable protection from herpes simplex virus-2 transmission following intravaginal application of siRNAs targeting both a viral and host gene. Cell Host Microbe. 2009;5:84–94. doi: 10.1016/j.chom.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakayama T, et al. Harnessing a physiologic mechanism for siRNA delivery with mimetic lipoprotein particles. Mol. Ther. 2012;20:1582–1589. doi: 10.1038/mt.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alvarez-Erviti L, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 81.Kumar P, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao Y-D, et al. Targeted delivery of PLK1-siRNA by ScFv suppresses Her2+ breast cancer growth and metastasis. Sci. Transl Med. 2012;4:130ra48. doi: 10.1126/scitranslmed.3003601. [DOI] [PubMed] [Google Scholar]
- 83.Cuellar TL, et al. Systematic evaluation of antibody-mediated siRNA delivery using an industrial platform of THIOMAB-siRNA conjugates. Nucleic Acids Res. 2015;43:1189–1203. doi: 10.1093/nar/gku1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rozema DB, et al. Protease-triggered siRNA delivery vehicles. J. Control. Release. 2015;209:57–66. doi: 10.1016/j.jconrel.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Meade BR, et al. Efficient delivery of RNAi prodrugs containing reversible charge-neutralizing phosphotriester backbone modifications. Nat. Biotechnol. 2014;32:1256–1261. doi: 10.1038/nbt.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin H, et al. Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype. Nat. Biotechnol. 2014;32:551–553. doi: 10.1038/nbt.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goemans NM, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. [DOI] [PubMed] [Google Scholar]
- 89.Janssen HLA, et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 90.Bhat B, et al. RG-101, a GalNAC-conjugated anti-miR employing aunique mechanism of action by targeting host factor microRNA-122 (miR-122), demonstrates potent activity and reduction of HCV in preclinical studies. Hepatology. 2013;58:1393A. [Google Scholar]
- 91.Hata A, Lieberman J. Dysregulation of microRNA biogenesis and gene silencing in cancer. Sci. Signal. 2015;8:re3. doi: 10.1126/scisignal.2005825. [DOI] [PubMed] [Google Scholar]
- 92.Daige CL, et al. Systemic delivery of a miR34a mimic as a potential therapeutic for liver cancer. Mol. Cancer Ther. 2014;13:2352–2360. doi: 10.1158/1535-7163.MCT-14-0209. [DOI] [PubMed] [Google Scholar]
- 93.Prakash TP, et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014;42:8796–8807. doi: 10.1093/nar/gku531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rozema DB, Ekena K, Lewis DL, Loomis AG, Wolff JA. Endosomolysis by masking of a membrane-active agent (EMMA) for cytoplasmic release of macromolecules. Bioconjug. Chem. 2003;14:51–57. doi: 10.1021/bc0255945. [DOI] [PubMed] [Google Scholar]
- 95.Gilboa-Geffen A, et al. Gene knockdown by EpCAM aptamer–siRNA chimeras suppresses epithelial breast cancers and their tumor-initiating cells. Mol. Cancer Ther. doi: 10.1158/1535-7163.MCT-15-0201-T. (in the press) [DOI] [PubMed] [Google Scholar]