SUMMARY
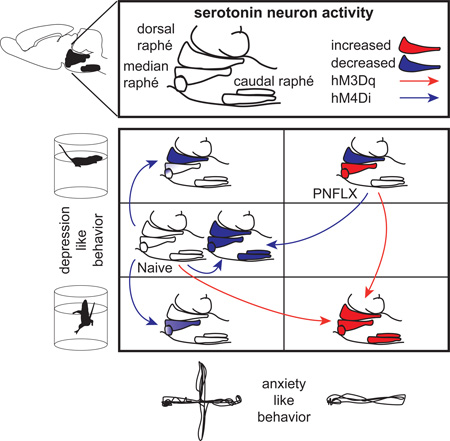
Despite the well-established role of serotonin signaling in mood regulation, causal relationships between serotonergic neuronal activity and behavior remain poorly understood. Using a pharmacogenetic approach, we find that selectively increasing serotonergic neuronal activity in wild-type mice is anxiogenic and reduces floating in the forced-swim-test, while inhibition has no effect on the same measures. In a developmental mouse model of altered emotional behavior, increased anxiety and depression-like behaviors correlate with reduced dorsal raphe and increased median raphe serotonergic activity. These mice display blunted responses to serotonergic stimulation and behavioral rescues through serotonergic inhibition. We furthermore identify opposing consequences of dorsal versus median raphe serotonergic neuron inhibition on floating behavior, together suggesting that median raphe hyperactivity increases anxiety, while a low dorsal/median raphe serotonergic activity ratio increases depression-like behavior. Thus we find a critical role of serotonergic neuronal activity in emotional regulation and uncover opposing roles of median and dorsal raphe function.
Graphical Abstract
INTRODUCTION
The involvement of serotonin (5-HT) in regulating emotional behavior is firmly established (Nutt, 2002; Suri et al., 2014). In fact, molecules that boost 5-HT signaling constitute the most frequently prescribed psycho-active drugs on the markets today, with a primary indication for the treatment of depression and anxiety disorders (Blier et al., 1990; Nutt, 2005). These drugs mainly increase 5-HT signaling by blocking peri-synaptic 5-HT reuptake or cytosolic 5-HT degradation, and proxies of decreased 5-HT signaling in patients further support the 5-HT deficiency theory of depression (Jacobsen et al., 2012). However, insight into the direct role of 5-HTergic neuronal activity on behavior remains scarce and conflicting. For example, several animal models of anxiety and depression-like behavior display reduced firing of 5-HTergic neurons, implying a causal relationship (Bambico et al., 2009; Lira et al., 2003), yet genetic blockade of 5-HTergic vesicular neurotransmission reduces anxiety (Kim et al., 2009; Narboux-Nême et al., 2011).
5-HTergic projections in the CNS arise from the brainstem raphé nuclei. The dorsal raphé nuclei (DR) and the median raphé nuclei (MR) harbor the vast majority of 5-HTergic neurons that innervate the forebrain, and as such are considered most relevant in modulating emotional behavior (Jacobs and Azmitia, 1992; Muzerelle et al., 2014). Despite this seemingly simple anatomical setup, DR and MR 5-HTergic neurons have different rhombomeric origins, functional specifications, and overlapping regions of axonal projection (Bang et al., 2012; Brust et al., 2014; Jensen et al., 2008; Muzerelle et al., 2014). Optogenetic stimulation of MR neurons produces rapid activation of hippocampal interneurons (Varga et al., 2009), and reduces the time spent in open arms of the elevated plus maze (Ohmura et al., 2014). Optogenetic stimulation of medial prefrontal cortex (mPFC) axons in the DR increases swimming behavior in the forced-swim test (Warden et al., 2012), and direct optogenetic stimulation of 5-HTergic DR neurons biases reward-associated behaviors (Liu et al., 2014). These studies establish a causal relationship between raphé activity and behavior but the frequent lack of serotonergic specificity, the super-acute and hyper-synchronous character of optogenetic stimulation protocols and the local activation of anatomically defined pathways provide an incomplete and biased picture of causal relationships between 5-HTergic neuronal activity and behavior. To advance insight, we studied the consequences of bidirectional pharmacogenetic manipulations of 5-HTergic neurons in naïve mice as well as in a developmental mouse model of increased anxiety and depression-like behavior, i.e. mice administered fluoxetine from postnatal day P2 to P11 (PNFLX) (Ansorge et al., 2004; Rebello et al., 2014).
RESULTS
Bidirectional manipulation of serotonergic neuronal activity via conditional expression of DREADDs in mice
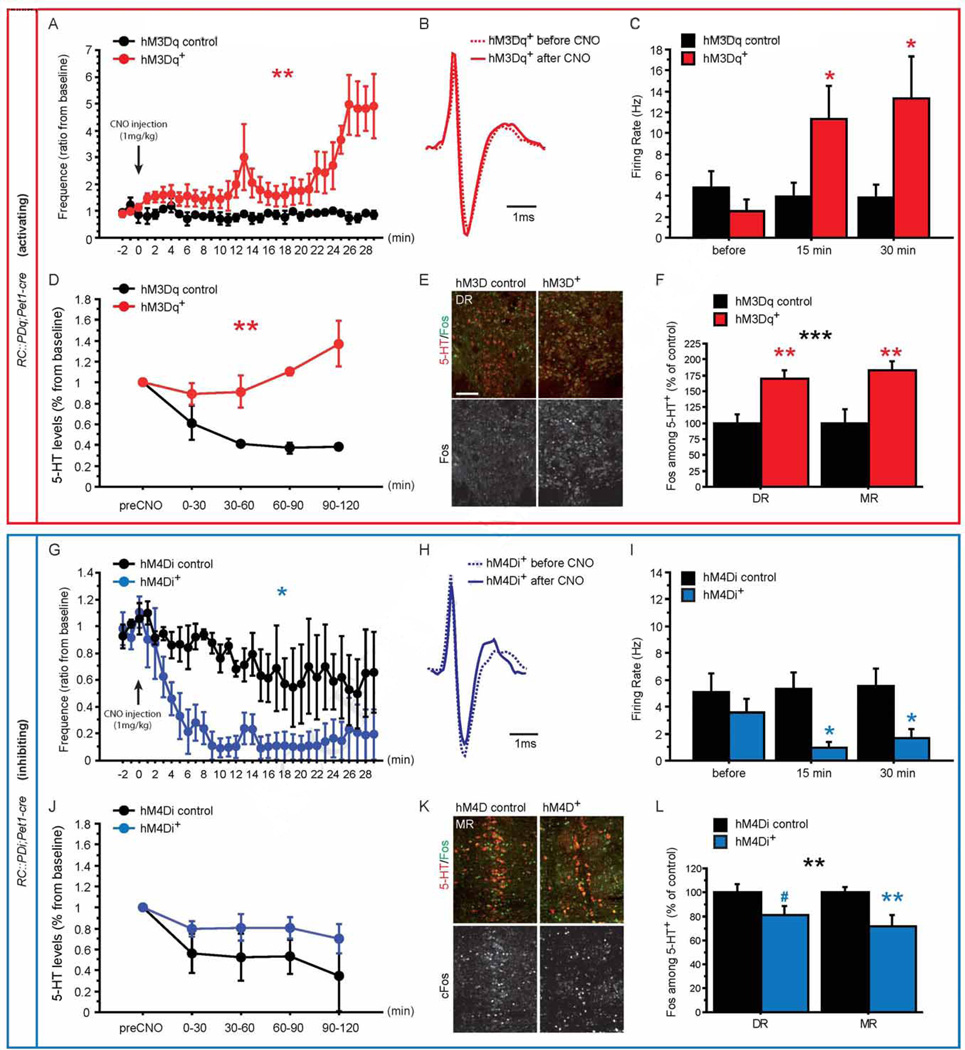
We first analyzed the consequence of manipulating 5-HT neurons in vivo using two conditional DREADD mouse lines, RC::PDq and RC::PDi. Both lines express the respective DREADD (hM3Dq coupling to Gq and hM4Di coupling to Gi/o) Cre-dependently under control of the Chicken β-actin promoter targeted to the (Gt)ROSA26Sor locus, and the RC::PDi line was previously described (Brust et al., 2014; Ray et al., 2011) (Figure S1). DREADD expression was directed to 5-HTergic neurons using the Pet1-cre line (Scott et al., 2005), and immunostaining against 5-HT and HA (with which both DREADDs are tagged) confirmed the specificity of DREADD expression in 5-HTergic neurons (Figure S2 A–F). Both DREADDs are specifically and selectively activated by clozapine-N-Oxide (CNO) (Armbruster et al., 2007). Hence, we next assessed the hM3Dq- and hM4Di-dependent impact of CNO on 5-HTergic neuronal activity by combining extracellular single unit recordings in the DR with micro-dialysis in the mPFC in anesthetized mice. Putative 5-HT neurons were identified based on a tri-phasic shape and long duration of spikes7 (Figure 1B, H). In DR neurons of RC::PDq;Pet1-cre mice, we observed a progressive increase in putative 5-HT neuron activity through longitudinal assessment during the first 30 min post CNO injection (Figure 1A, Genotype (G) X Time interaction, GXTime: F(31,124) = 4.314, p < 0.0001; G effect: F(1,124) = 27.934, p = 0.0061). Cross-sectional data analysis also revealed increased putative 5-HT neuron activity at 15 min and 30 min post-injection when compared to genetic controls lacking the Pet1-cre transgene (Figure 1C, GXTime: F(2,20) = 12.036, p = 0.0004) or pre-injection activity (Fisher's PLSD for hM3Dq+, p = 0.0149 for t15 and p = 0.0054 for t30). Micro dialysis in the mPFC revealed increased extracellular 5-HT levels in RC::PDq;Pet1-cre animals as compared to controls after CNO injection (GXTime interaction: F(4,16) = 3.528, p = 0.0302; G effect: F(1,16) = 36.463, p = 0.0038, Figure 1D). Next, we analyzed immediate early gene induction and found increased Fos+ 5-HT neurons after CNO treatment of RC::PDq;Pet1-cre mice as compared to their controls, in both the MR and DR (Figure 1E–F, G effect: F(1,26) = 24.265, p < 0.0001; Figure S2 G–I), indicating increased 5-HTergic neuronal activity. We did not find such an increase in non-5-HTergic neurons (F(1,9) = 0.486, p = 0.5033). In RC::PDi;Pet1-cre animals, we observed a decrease in putative 5-HT neuron activity through longitudinal assessment during the first 30 min post CNO injection (Figure 1G, GXTime: F(31,124)=1.195, p=0.2446; G effect: F(1,124)=7.976, p=0.0476), and in a cross sectional assessment at 15 min and 30 min post-injection when compared to genetic controls lacking the Pet1-cre transgene (Figure 1I, GXTime: F(2,20) = 4.094, p = 0.0323) or pre-injection activity (Fisher's PLSD p = 0.0390 for t15 and p = 0.1058 for t30; of note, we did not detect a correlation between spike width and change in firing rate after CNO, Figure S3). Through micro dialysis we could not detect an effect of CNO/hM4Di on extracellular 5-HT levels (Figure 1J), possibly due to mechanisms regulating local 5-HT homeostasis. However, immediate early gene analysis again confirmed reduced activation of 5-HT+ neurons in RC::PDi;Pet1-cre mice when compared to their controls, in both the MR and DR (Figure 1K–L; G effect: F(1,22) = 21.24, p = 0.0252; Figure S2 G–I). We did not find such a decrease in non-5-HTergic neurons (F(1,9) = 0.407, p = 0.5394). Together, these data demonstrate efficient bidirectional manipulation of 5-HTergic neuronal activity.
Figure 1. Pharmacogenetic modulation of 5-HTergic neuronal activity.
(A) Time course of in vivo firing rates of putative DR 5-HTergic neurons for 30min after CNO injection shows increased firing rates upon CNO injection in hM3Dq+ mice when compared to controls. Baseline is calculated using the last 3 minutes before CNO injection. n = 3–5 per genotype.
(B) Spike trace for a putative 5-HT neuron recorded in the DR of hM3Dq+ mice before (solid line) and 15 min after (dashed line) CNO injection.
(C) Firing rates of 5-HTergic neurons pooled by time before (−15 to 0 min) and after (0 to 15 min and 15 to 30 min) CNO injection demonstrate an increase in putative 5-HTergic neuronal firing rates in hM3Dq+ mice. n = 7 per genotype.
(D) Time course of changes in extracellular 5-HT levels in the mPFC of hM3Dq+ and respective control mice shows an hM3Dq+-dependent increase after CNO injection. n = 2–4 per genotype.
(E–F) Fos and 5-HT double immunostaining on raphé sections reveals increased Fos+ staining in 5-HT+ neurons in hM3Dq+ mice when compared to their controls in the DR and MR. n = 6–9 per genotype and region.
(G) Time course of in vivo firing rates of putative 5-HTergic neurons for 30min after CNO injection shows decreased firing rates upon CNO injection in hM4Di+ mice when compared to controls. Baseline is calculated using the last 3 minutes before CNO injection. n = 3–4 per genotype.
(H) Spike trace for a putative 5-HT neuron recorded in the DR of hM4Di+ mice before (solid line) and 15 min after (dashed line) CNO injection.
(I) Firing rates of 5-HTergic neurons pooled by time before (−15 to 0min) and after (0 to 15 min and 15 to 30 min) CNO injection demonstrate a decrease in putative 5-HTergic neuronal firing rates in hM4Di+ mice. n = 5–7 per genotype.
(J) Time course of changes in extracellular 5-HT levels in the mPFC of hM4Di+ and respective control mice. n = 2–5 per genotype.
(K–L) Fos and 5-HT double immunostaining on raphé sections reveals decreased Fos+ staining in 5-HT+ neurons of hM4Di+ mice when compared to their controls in the DR and MR. n = 6–9 per genotype and region.
#: p < 0.1; *: p < 0.05; **: p < 0.01; ***: p < 0.001. Scale bar is 100µm. DR: dorsal raphé, MR: median raphé.
Increased 5-HTergic neuronal activity increases levels of anxiety-related behaviors and swimming behavior, while decreased 5-HTergic neuronal activity does not impact emotional behavior
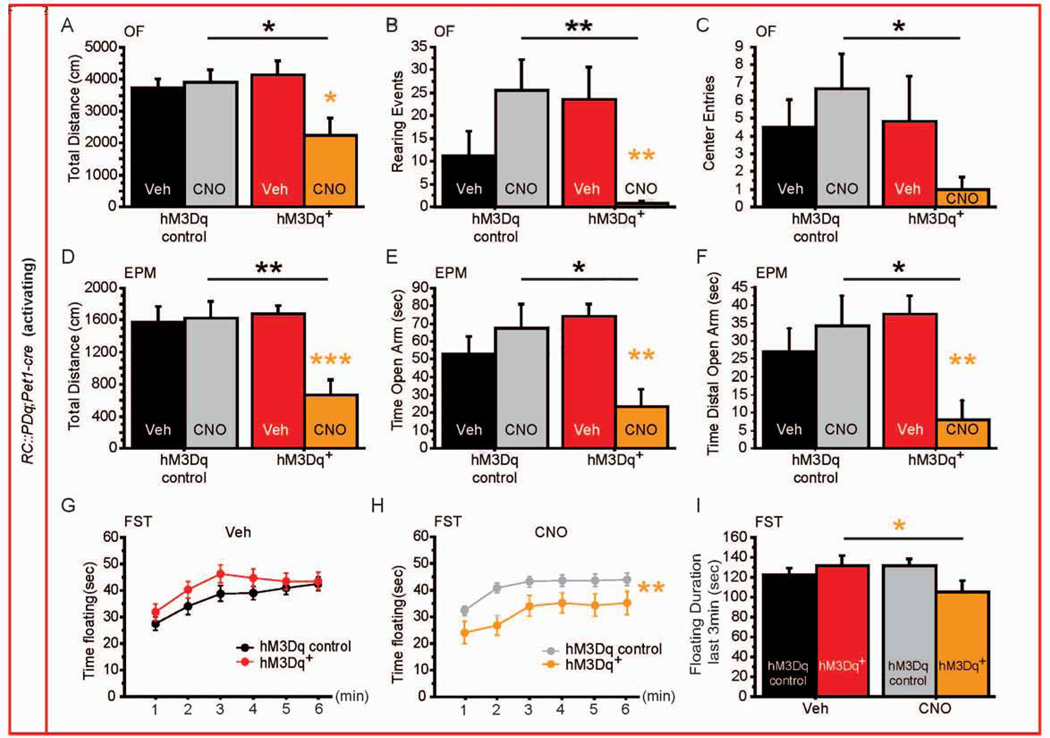
We next evaluated the effect of CNO administration to RC::PDq;Pet1-cre and RC::PDi;Pet1-cre mice on anxiety and depression-like behavior, controlling for treatment (Veh versus CNO, 1 mg/kg, ip) and genotype (littermate controls). For RC::PDq mice, we detected a genotype × treatment interaction (GXTx) on distance travelled in the open field (OF) (F(1,34) = 5.709, p = 0.0226, Figure 2A) and in the elevated-plus maze (EPM) (F(1,33) = 6.756, p = 0.0139, Figure 2D), suggesting a global decrease in exploration of a novel environment. Likewise, we also observed GXTx interactions for measures that are sensitive to anxiolytic treatment: time spent rearing in the OF, time spent in the open arm of the EPM, and time spent in the distal part of the open arm in the EPM (OF rearing: F(1,34) = 9.856, p = 0.0035; EPM open arm time: F(1,33) = 7.42, p = 0.0102; EPM distal open arm time: F(1,33) = 5.988, p = 0.0199; Figure 2B, E, F). The number of center entries in the OF did not reach significance (F(1,34) = 2.803, p = 0.1033) but displayed a similar pattern (Figure 2C). Post hoc analysis revealed that RC::PDq;Pet1-cre animals displayed an anxiogenic-like response to CNO/Gq-mediated manipulation of neuron activity (Figure 2B, E, F). In contrast, activity in the home-cage was not affected by genotype or treatment (Figure S4), supporting the interpretation of an anxiogenic effect of increased neuronal 5-HTergic activity.
Figure 2. Behavioral consequences of increasing 5-HTergic neuronal activity.
(A–C) hM3Dq+ mice exposed to CNO display decreased distance travelled (A), rearing events (B), and number of entries to the center (C) in the OF when compared with hM3Dq controls exposed to CNO or hM3Dq+ mice exposed to Veh. n = 6–12 per genotype and treatment.
(D–F) hM3Dq+ mice exposed to CNO display decreased distance travelled (D), time spent in the open arm (E) and time spent in the distal part of the open arm (F) in the EPM when compared with hM3Dq controls exposed to CNO or hM3Dq+ mice exposed to Veh. n = 6–12 per genotype and treatment.
(G, H) Time spent floating during 6min of the FST after Veh (G) and CNO (H) injections shows a reduction in floating specific to hM3Dq+ mice exposed to CNO. n = 15–25 per genotype and treatment.
(I) During the last 3 min of the FST, hM3Dq+ mice float less when exposed to CNO. n = 15–25 per genotype and treatment.
#: p < 0.1; *: p < 0.05; **: p < 0.01; ***: p < 0.001. EPM: elevated plus maze, OF: open field, FST: forced swim test.
We next assessed escape behavior in the Porsolt forced-swim test (FST), which serves as a proxy for behavioral despair and is sensitive to antidepressant treatment (Castagné et al., 2011; Porsolt et al., 1977). Because this test can be repeated (Mezadri et al., 2011), we used a cross-over design for treatment, allowing for within-animal repeated-measure analysis. In RC::PDq;Pet1-cre mice, we confirmed the absence of genotype effect upon Veh exposure (F(1,190) = 1.446, p = 0.2366, Figure 2G), and found an effect of treatment (F(1,190) = 7.420 p = 0.0097, Figure 2H). For minutes 4–6, we detected a GXTx interaction (F(1,38) = 9.492, p = 0.0038), where the mutant animals spent less time floating after exposure to CNO when compared to Veh, while control animals were unaffected by the treatment (Figure 2I).
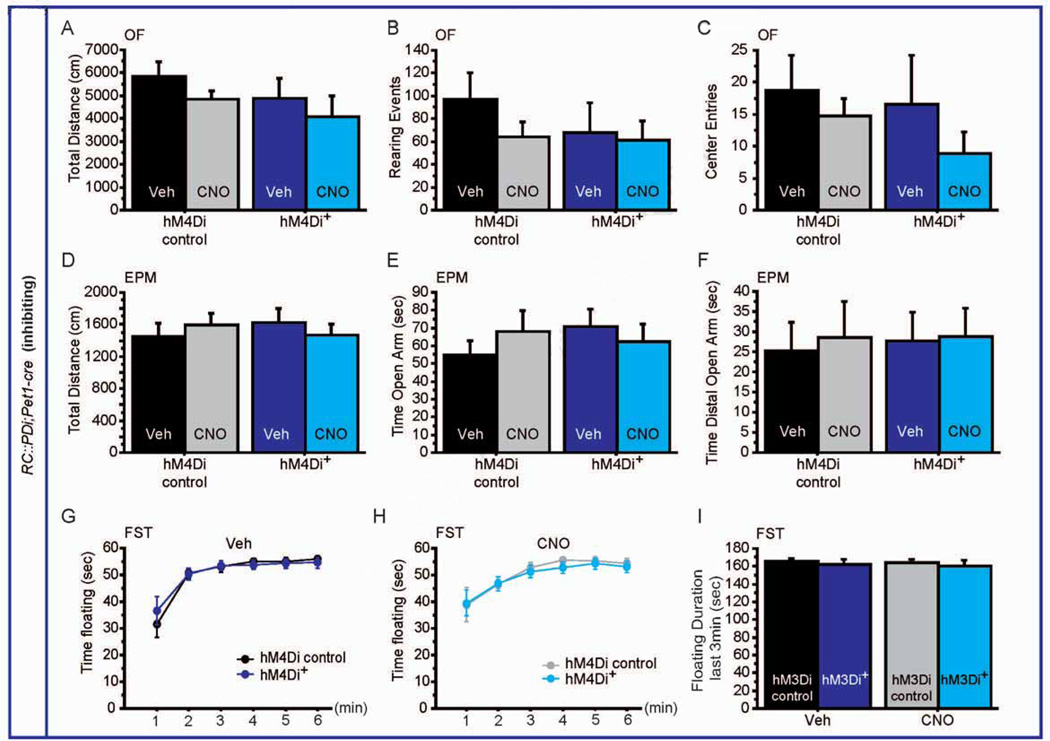
On the other hand, acute inhibition of 5-HTergic activity using RC::PDi;Pet1-cre mice altered neither exploration in the OF or EPM (GXTx: F(1,24) = 0.022, p = 0.8846 for OF; F(1,26) = 0.873, p = 0.3586 for EPM; Figure 3A, D), nor rearing activity, entries in the center of the OF or in the open arm of the EPM (rearing: F(1,24) = 0.457, p = 0.5055; entries: F(1,24) = 0.138, p = 0.714; F(1,26) = 1.155, p = 0.2924 for EPM; Figure 3B, C, E, F). Finally, no effect of CNO was observed in RC::PDi;Pet1-cre mice for time floating in the FST (GXTx: F(1,145) = 0.087, p = 0.9942; Figure 3G–I).
Figure 3. Behavioral consequences of decreasing 5-HTergic neuronal activity.
(A–C) hM4Di+ mice exposed to CNO display no phenotype in distance travelled (A), rearing events (B), and number of entries to the center (C) in the OF. n = 6–11 per genotype and treatment.
(D–F) Similarly, in the EPM, no difference could be detected for CNO exposed hM4Di+ mice in the distance travelled (D), time spent in the open arm (E) and time spent in the distal part of the open arm (F). n = 6–11 per genotype and treatment.
(G, H) Time spent floating during 6min of the FST after Veh (G) and CNO (H) injections shows no difference between genotypes. n = 13–18 per genotype and treatment.
(I) Likewise, time spent floating during the last 3 min of the test also shows no difference between hM4Di+ mice and controls upon Veh or CNO exposure. n = 13–18 per genotype and treatment.
EPM: elevated plus maze, OF: open field, FST: forced swim test.
Altered behavioral response to pharmacogenetic modulation of 5-HTergic neuronal activity in PNFLX mice
PNFLX animals (P2-P11) display a behavioral phenotype, which closely phenocopies that of 5-HT transporter-null mutants (Slc6a4−/−), including increased anxiety and depressive-like behaviors as well as altered social, sexual and sleep behaviors (Ansorge et al., 2004; Rebello et al., 2014; Silva et al., 2010). As such, studying PNFLX mice provides insight into the developmental origins of affective dysfunction. Here we investigated the behavioral consequences of altering 5-HTergic neuronal activity in PNFLX mice to gage global functional 5-HTergic status and to test the hypothesis that altered 5-HTergic activity contributes to the behavioral phenotype (Veerakumar et al., 2014).
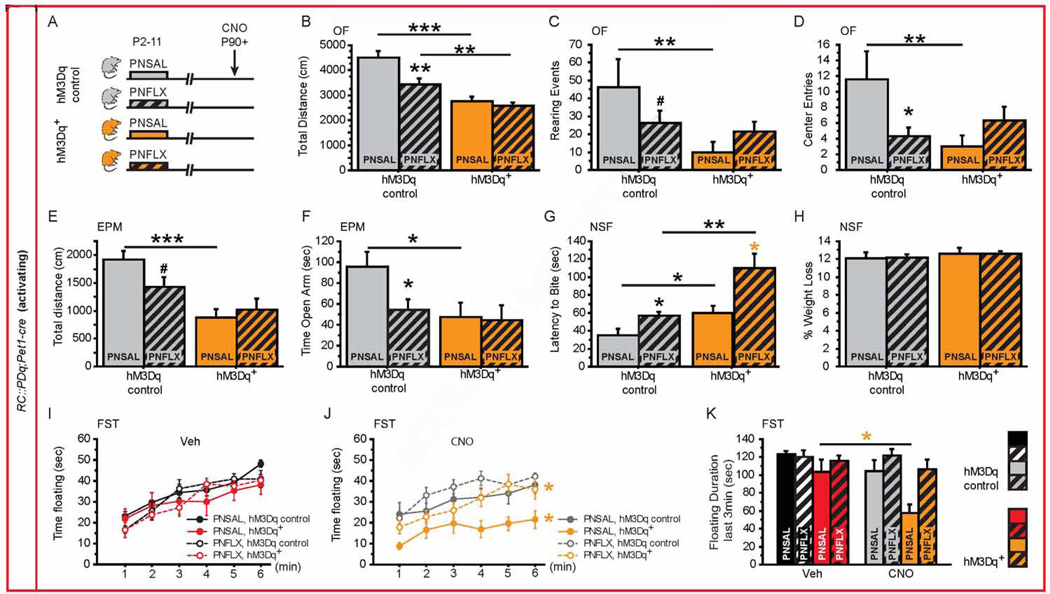
Mice were injected from P2-P11 with fluoxetine (10 mg/kg/day, PNFLX) or saline vehicle (PNSAL) (Figure 4A, 5A). Because we did not detect an effect of CNO in naïve RC::PDq and RC::PDi control mice (Figures 2 and 3), all PNFLX and PNSAL animals were injected with CNO 30 min before the onset of tests. In RC::PDq;Pet1-cre mice, we found interaction between post-natal treatment (PNT) and genotype (PNTXG) in OF parameters, demonstrating a differential response of CNO exposure in RC::PDq;Pet1-cre mice as a function of post-natal treatment (OF distance F(1,70) = 4.547, p = 0.0365; OF rearing: F(1,70) = 6.024, p = 0.0166; OF center entries: F(1,70) = 5.890, p = 0.0178). For the EPM and NSF, we detected genotype effects in distance (F(1,50) = 163987, p = 0.0001), time in the open arm (F(1,50) = 5.065, p = 0.0288) and latency to feed (F(1,58) = 12.62, p = 0.0008). Post-hoc analysis showed that PNFLX control animals explore less in the OF and EPM than PNSAL controls (Figure 4B, E). PNFLX animals also enter less frequently into the center of the OF, spend less time in the open arm of the EPM, and take a longer time to eat in a new environment (Novelty Suppressed Feeding test, NSF) without any difference in their weight loss (Figure 4D, F–H). We next confirmed that PNSAL RC::PDq;Pet1-cre mice display the same behavioral CNO response profile as identified for naïve RC::PDq;Pet1-cre mice (Figure 1 and 4). By contrast, PNFLX RC::PDq;Pet1-cre mice only displayed a significantly decreased distance travelled in the OF and increased latency to bite the food pellet in the NSF (Figure 4B, G). These data reveal a blunted behavioral response to 5-HT neuronal activation in PNFLX animals when compared to the effect of CNO in naïve and PNSAL mice. In the FST, we confirmed an absence of G effect upon Veh treatment (Figure 4I, repeated ANOVA F(1,150) = 1.728, p = 0.1986), while we observed a G effect and a PNT effect upon CNO exposure (G: F(1,110) = 15.188, p = 0.0008; PNT: F(1,110) = 6.788, p = 0.0162 Figure 4J). Finally, for floating behavior during min 4 to 6 we observed a PNTXTx interaction (F(1,53) = 4.989, p = 0.0297) and GXTx interaction (F(1,53) = 4.143, p = 0.0468). Post hoc analyses revealed decreased floating in PNSAL RC::PDq;Pet1-cre mice exposed to CNO when compared to Veh exposure or controls but not in PNFLX RC::PDq;Pet1-cre mice, again supporting a blunted response in PNFLX animals (Figure 4J). In summary, our experiments demonstrate anxiogenic and antidepressant-like effects of increasing 5-HTergic neuronal activity, with PNFLX animals displaying a blunted response.
Figure 4. Behavioral consequences of increasing 5-HTergic neuronal activity in PNFLX mice.
(A) Schematic of experimental groups: hM3Dq+ mice (orange bars) and hM3Dq controls (grey bars) were injected with saline (PNSAL, plain bars) or FLX (PNFLX, dashed bars) from P2 to P11 and were submitted to behavioral tests upon CNO injections starting after P90.
(B–D) In the OF, PNSAL hM3Dq+ mice display decreased distance travelled (B), rearing events (C) and number of entries to the center (D) when compared to hM3Dq controls, while this response is blunted in PNFLX hM3Dq+ mice as compared with PNFLX hM3Dq controls. n = 7–11 per genotype and post-natal treatment.
(E–F) In the EPM, PNSAL hM3Dq+ mice display decreased distance travelled (E) and open arm time (F). n = 11–16 per genotype and post-natal treatment.
(G–H) Latency to bite the pellet in the NSF test is significantly increased in both PNSAL and PNFLX hM3Dq+ mice as compared with their controls (H). No change in the % weight loss was observed between PNSAL or PNFLX hM3Dq+ mice and their respective controls (H). n = 13–15 per genotype and post-natal treatment.
(I, J) In the FST, PNSAL and PNFLX hM3Dq+ mice spent less time floating during 6min of the test upon CNO (J) but not Veh (I) injections when compared to their respective hM3Dq controls. n = 13–16 per genotype and post-natal treatment.
(K) During the last 3 min of the FST, PNSAL hM3Dq+ mice float less after CNO exposure when compared to Veh treatment. n = 13–16 per genotype and post-natal treatment.
p < 0.05; ** p < 0.01; *** p < 0.001. EPM: elevated plus maze, NSF: novelty-suppressed feeding, OF: open field, FST: forced swim test.
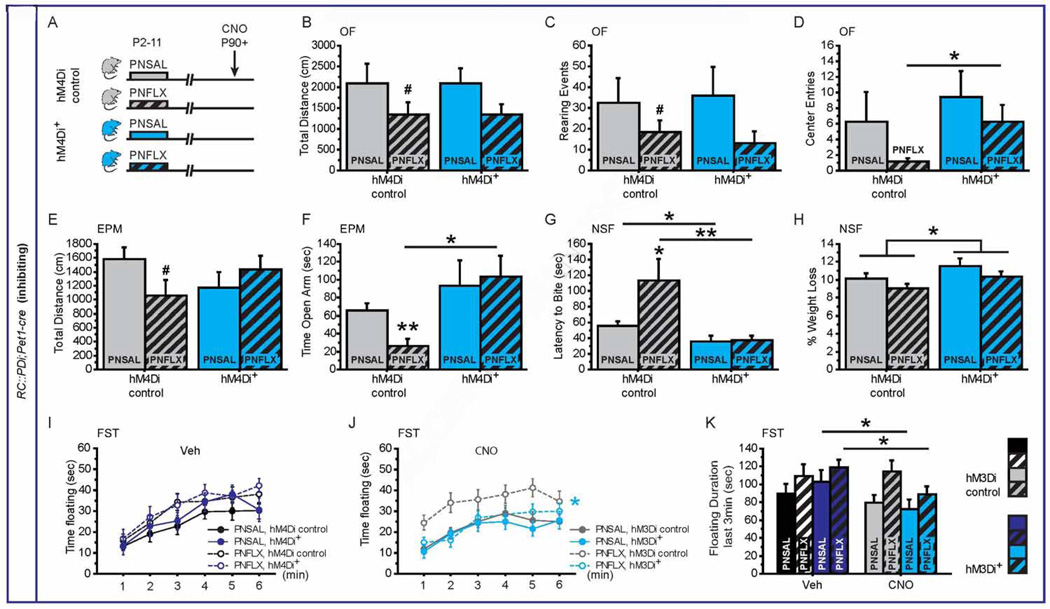
Figure 5. Behavioral consequences of decreasing 5-HTergic neuronal activity in PNFLX mice.
(A) Schematic of experimental groups: hM4Di+ mice (blue bars) and hM4Di controls (grey bars) were injected with saline (PNSAL, plain bars) or FLX (PNFLX, dashed bars) from P2 to P11 and were submitted to behavioral tests upon CNO injections starting after P90.
(B–D) In the OF, PNSAL and PNFLX hM4Di+ mice have similar distance travelled (B) and rearing events (C) when compared to their respective hM4Di controls, but PNFLX hM4Di+ mice display an increased number of entries to the center (D) when compared to PNFLX hM4Di controls. n = 10–13 per genotype and post-natal treatment.
(E–F) In the EPM, no significant difference between PNSAL and PNFLX hM4Di+ mice and their respective controls could be observed in the distance travelled (E), but PNFLX hM4Di+ mice display increased time spent in the open arm when compared to PNFLX hM4Di controls (F). n = 11–16 per genotype and post-natal treatment.
(G–H) In the NSF, the latency to bite the pellet is significantly decreased in both PNSAL and PNFLX hM4Di+ mice as compared with their controls (H). An increase in the % weight loss was observed for hM4Di+ mice and as compared to hM4Di controls (H). n = 9–16 per genotype and post-natal treatment.
(I, J) In the FST, time spent floating during 6min is significantly decreased upon CNO (J) but not Veh (I) injections only in PNFLX hM4Di+ mice as compared to their hM4Di littermate controls. n = 9–14 per genotype and post-natal treatment.
(K) Time spent floating during the last 3 min of the test shows significant decrease in floating for both PNSAL and PNFLX hM4Di+ mice exposed to CNO when compared to Veh injection. n = 13–16 per genotype and post-natal treatment.
* p < 0.05; ** p < 0.01; *** p < 0.001. EPM: elevated plus maze, NSF: novelty-suppressed feeding, OF: open field, FST: forced swim test.
These data support a general model in which early-life increase in 5-HT signaling impacts the maturation of the 5-HT system to leave the adult brain with altered global 5-HT function. The anxiogenic effect of stimulating 5-HTergic neuronal activity furthermore is suggestive of a hyperfunction in the 5-HT system of PNFLX animals. Hence we next assessed the consequences of transiently decreasing 5-HTergic neuronal activity in PNFLX animals and PNSAL controls. We detected a GXPNT interaction and a G effect for the latency to feed in the NSF (GXPNT: F(1,44) = 4.592, p = 0.0377; G effect: F(1,44) = 13.481, p = 0.0006; Figure 5G), and a G effect for EPM open-arm time (F(1,40) = 7.193, p = 0.0106; Figure 5F). We also detected a PNT effect for distance travelled in the OF (F(1, 41) = 4.111, p = 0.0491) and the time to feed in the NSF (F(1,44) = 4.53, p = 0.0292). In post hoc analyses, no differences were detected between PNSAL and PNFLX RC::PDi;Pet1-cre mice and their respective controls in distance travelled in the OF or the EPM, or in the number of rearing events (Figure 5B, C, E). However, PNFLX RC::PDi;Pet1-cre mice displayed increased number of entries into the center of the OF (Figure 5D), increased time spent in the open arm of the EPM (Figure 5F) and the decreased time to bite the pellet in the NSF, as compared to PNFLX control animals (Figure 5G). Even though we detected a genotype effect on the control measure weight loss in the NSF (F(1,44) = 4.612, p = 0.0373, Figure 5H), this effect is unlikely to account for the interaction effect on latency to bite.
In summary, CNO binding to hM4Di in 5-HTergic neurons does not impact anxiety-related behaviors in naïve or PNSAL mice, while it reduces respective measures of the OF, EPM and NSF in PNFLX mice. Moreover, upon CNO injections, PNFLX RC::PDi;Pet1-cre mice exhibit anxiety levels very similar to PNSAL controls, suggesting a rescue of their anxiety phenotype upon decreasing 5-HTergic neuronal activity. Behavioral sensitivity of PNFLX mice to reducing 5-HTergic neuronal activity indicates that their baseline behavioral phenotype is in part determined by an altered balance in 5-HTergic pathways regulating such behavior and that by reducing overall 5-HTergic neuronal activity, normal balance is restored. In the FST we detected for floating over time a post-natal treatment effect (F(1,190) = 6.360, p = 0.0160) and a trend for a genotype effect (F(1,190) = 3.79, p = 0.059) upon CNO exposure (Figure 5I, J), and a GXTx interaction as well as a post-natal treatment effect for floating during min 4–6 (F(1,38) = 5.810, p = 0.0.0209; F(1,38) = 4.774, p = 0.0351, Figure 5K). Furthermore, post hoc analysis revealed decreased floating duration upon CNO injections affecting both PNSAL and PNFLX RC::PDi;Pet1-cre mice during min 4–6, and only PNFLX RC::PDi;Pet1-cre mice for repeated assessment over min 1–6 (Figure 4J). These results are surprising in that they demonstrate that reducing and activating 5-HTergic neuronal activity can have similar effects on behavior. However, in the context of an underlying model of unbalanced pathways, both reducing and increasing 5-HTergic neuronal activity can normalize balance, further supporting such a mechanism.
Altered balance in DR versus MR raphé activity underlies PNFLX induced behavioral phenotype
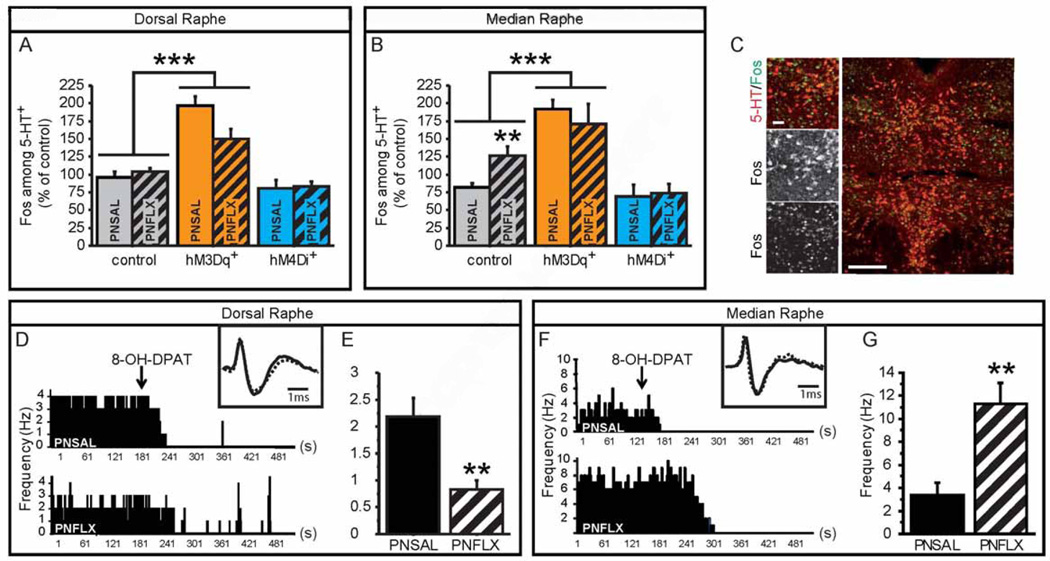
To further dissect how PNFLX animals respond to pharmacogenetic manipulation of 5-HT neurons, we performed co-labeling of 5-HT and Fos on raphé sections of PNFLX and PNSAL RC::PDq;Pet1-cre and RC::PDiPet1-cre mice and their respective controls, sacrificed 2 hours after CNO injection (Figure 6A–C). We counted the number of Fos+ nuclei among 5-HT+ neurons in both DR (Figure 6A, C) and MR (Figure 6B), as identified by their anatomic location. We found an overall GXPNT interaction (F(2,45) = 6.836, p = 0.0026). Notably, PNSAL RC::PDq;Pet1-cre mice show a more than 2-fold increase in %Fos+/5-HT+ (p < 0.001) while PNFLX RC::PDq;Pet1-cre mice only show a 1.3-fold increase (p = 0.0177), suggesting a blunted response in PNFLX RC::PDq;Pet1-cre mice (GXTx: F(1,36) = 12.342, p = 0.0012). Conversely, 5-HTergic neuronal activity in RC::PDi;Pet1-cre mice showed a 34% decrease in PNFLX mutants (p = 0.0339) but only a 14% decrease in PNSAL mutants (p = 0.2350), with a trend for GXPNT interaction (F(1,31) = 2.955, p = 0.0956). These results are paralleling the behavioral responses in anxiety tests, where PNSAL animals were more sensitive to increasing 5-HTergic neuronal activity while PNFLX animals were more sensitive to decreasing 5-HTergic neuronal activity. Lastly, Fos analysis also indicates that MR 5-HTergic neurons of PNFLX mice are more active at baseline than those of PNSAL mice (controls in Figure 6B, Fisher's PLSD p = 0.0049). To further study this effect of PNFLX at baseline, we recorded putative 5-HTergic neurons in vivo. Specifically, we performed extracellular recordings within the DR and MR of PNFLX and PNSAL animals. Neurons were classified as putatively 5-HTergic based on their AP length and shape, as well as by the efficient shut down of neuronal activity upon injection of the 5-HT1A receptor agonist, 8-OH-DPAT (1 mg/kg, i.p.) (Figure 6D, F). As previously reported for mouse models of increased depression-like behavior, we observed decreased activity of putative 5-HTergic neurons in the DR of PNFLX animals when compared to PNSAL controls (Figure 6E and S5, F(1,15) = 11.750, p = 0.0037). Conversely, and in line with our Fos data, we observed increased activity of putative 5-HTergic neurons in the MR of PNFLX animals when compared to PNSAL controls (Figure 6G and S5, F(1,45) = 9.835, p = 0.003). These results demonstrate an altered balance of raphé nuclei activity in PNFLX animals and support a role of MR hyperfunction in the anxiety phenotype of PNFLX mice. Furthermore, since activation of 5-HT neurons in naïve and PNSAL animals reduced floating behavior while inhibition of 5-HT neurons in PNFLX animals reduced floating behavior, these results suggest that relatively increased MR 5-HTergic activity over DR 5-HTergic activity is causing the depression-like phenotype in PNFLX mice. Such a relational model for depression-like behavior in the FST implies opposing effects of DR versus MR 5-HTergic activity on floating behavior.
Figure 6. Altered balance between DR and MR 5-HTergic neuronal activity in PNFLX mice.
(A–C) Fos and 5-HT immunostaining on hM3Dq+ and hM4Di+ mice and controls in DR (A) and MR (B) demonstrates an increase of in %Fos+/5-HT+ neurons in hM3Dq+ mice when compared to control animals. Moreover, PNFLX control animals display an increase in %Fos+/5-HT+ neurons in the MR (B), but not the DR (A) when compared to PNSAL control animals. n = 3–9 per genotype, post-natal treatment and region.
(D–G) The anatomical location of in vivo electrophysiological recordings of putative 5-HTergic raphe neurons was validated at the end of each recording session through responsiveness to 5-HT1A receptor mediated inhibition of neuronal firing, achieved by 8-OH-DPAT administration (ip, 1 mg/kg) (D, F). Insets show examples of single extracellularly recorded spikes from PNSAL (plain line) and PNFLX (dashed lines) animals. The firing frequency of putative 5-HTergic neurons of PNFLX mice were reduced in the DR (E) but increased in the MR (G) when compared to PNSAL animals. n = 8–9 animals.
* p < 0.05; ** p < 0.01; *** p < 0.005. Scale bars are 20µm and 100µm. DR: dorsal raphé, MR: median raphé.
Antagonistic effect of rhombomere 1 (r1) or rhombomere 2 (r2) derived 5-HT neuronal inhibition on swimming behavior
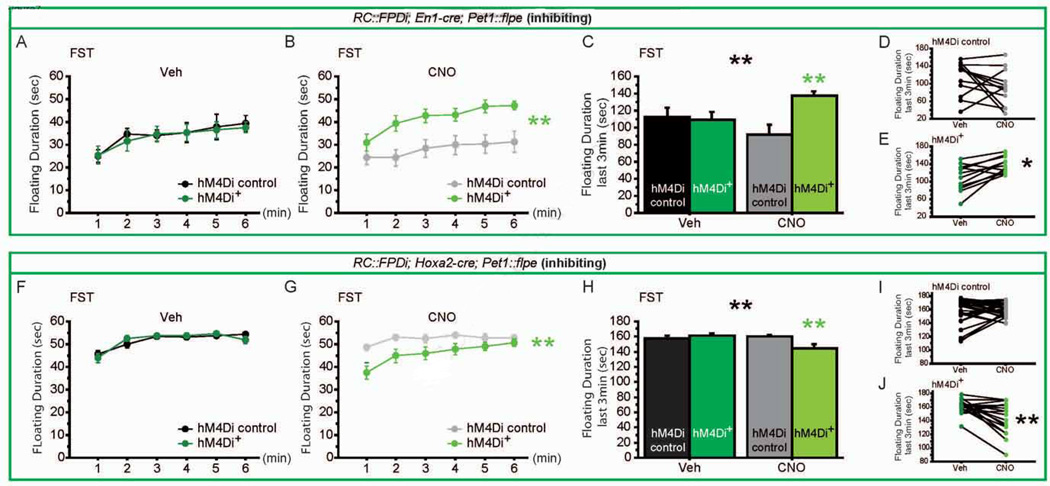
To test if DR and MR 5-HTergic activity exert opposing effects on behavior in the FST, we took advantage of the double conditional allele RC::FPDi, which allows for hM4Di expression only after both Cre-dependent and Flpe-dependent recombination events have occurred in a given cell (Brust et al., 2014; Ray et al., 2011). Using Pet1::Flpe and En1-cre, we targeted hM4Di expression to r1-derived 5-HTergic neurons in En1-cre,Pet1::Flpe,RC::FPDi mice; using Pet1::Flpe and Hoxa2-cre, we targeted hM4Di expression r2-derived 5-HTergic neurons in Hoxa2-cre,Pet1::Flpe,RC::FPDi mice (Brust et al., 2014). 5-HTergic neurons generated in r1 contribute to DR and a portion of MR, while a large fraction of MR 5-HTergic neurons are generated in r2 and a further small fraction in r3 (Bang et al., 2012; Jensen et al., 2008). r1- and r2-hM4Di expressing triple transgenic animals and their respective sibling controls (which did not carry the respective cre allele) were tested in the OF, EPM, NSF and FST. Of note, the En1-cre line is a knock-in (Kimmel et al., 2000) but we did not detect an effect of genotype in any test performed (Figure 7, S6, S7). We also did not detect a GXTx interaction on anxiety behaviors (Figure S6, S7). But as predicted by our model, we observed opposing effects of r1 versus r2 5-HTergic neuron inhibition on floating behavior in the FST paradigm. Specifically, inhibition of r1-derived 5-HT neurons (En1-cre; Pet1::Flpe; RC::FPDi) increased floating behavior (Figure 7A, B, C, D, GXTx for min 4–6: F(1,22) = 6.931, p = 0.0152). Conversely, CNO-driven inhibition of r2-derived 5-HT neurons (Hoxa2-cre; Pet1::Flpe; RC::FPDI) resulted in decreased floating (Figure 7E, F, G, H, GXTx for min 4–6: F(1,43) = 9.789, p = 0.0031).
Figure 7. Opposing consequences of r1 and r2 5-HTergic neuronal inhibition on forced swim test behavior.
(A–E) In the FST, we assessed floating duration for r1-hM4Di+ mice (green lines) and their controls (black and grey lines) throughout the 6min of FST upon Veh (A) and CNO (B) injections, and for the last 3 min of the test (C) upon Veh (D) and CNO (E). r1-hM4Di+ mice show increased floating behavior as compared to controls when exposed to CNO (B, C), but not when exposed to Veh (A, C). r1-hM4Di+ mice but not controls show increased floating behavior after CNO exposure, when compared to Veh exposure (D, E). n = 11 per genotype.
(F–J) In the FST, we assessed floating duration for r2-hM4Di+ mice (green lines) and their controls (black and grey lines) throughout the 6min of FST upon Veh (F) and CNO (G) injections, and for the last 3 min of the test (H) upon Veh (I) and CNO (J). r2-hM4Di+ mice show decreased floating behavior as compared to controls when exposed to CNO (G, H), but not when exposed to Veh (F, H). r2-hM4Di+ mice but not controls show decreased floating behavior after CNO exposure, when compared to Veh exposure (I, J). n = 18–25 per genotype.
* p < 0.05; ** p < 0.01; *** p < 0.005. FST: forced swim test.
DISCUSSION
We find that pharmacogenetic activation of 5-HTergic neurons, elicits anxiogenic and antidepressant-like behavioral consequences in naïve animals. Emotional behavior however appeared unchanged upon pharmacogenetic inhibition of 5-HTergic neuronal activity in naïve mice. When tested in PNFLX mice, anxiogenic and antidepressant-like behavioral efficacy of 5-HTergic neuron activation is blunted while 5-HTergic neuron inhibition has anxiolytic and antidepressant-like behavioral consequences. As a potential underlying neural mechanism, we identified reduced activity in DR 5-HTergic neurons but increased activity in MR 5-HTergic neurons of PNFLX mice. Behavioral and physiological data converge to suggest that MR hyperactivity drives the anxiety phenotype, while a low DR/MR 5-HTergic activity ratio increases depression-like behavior. In support of this model, we identify opposing consequences of DR and MR 5-HTergic neuron inhibition in naïve mice, promoting and decreasing FST immobility, respectively.
Pharmacogenetic increase of 5-HT neuronal activity has anxiogenic and antidepressant-like behavioral consequences
Increasing activity of 5-HTergic raphé neurons in both naïve and PNSAL animals decreased exploration, increased anxiety and decreased FST-immobility. The co-occurrence of anxiogenic and antidepressant-like behavioral responses is congruent with the effect of acute SSRI administration in rodents (Kurt et al., 2000) and humans (Sinclair et al., 2009). Moreover, FST results align with the antidepressant like effect of increasing DR neuronal activity optogenetically (Warden et al., 2012), while increased anxiety mirrors the consequences of local optogenetic stimulation of MR 5-HT neurons (Ohmura et al., 2014). Elucidating means to specifically and separately target these behavioral consequences will be important to guide drug development and improve therapeutic efficacy and safety.
Decreasing activity of 5-HTergic raphé neurons in RC::PDi;Pet1-cre mice did not produce behavioral effects. This finding is surprising considering decreased DR 5-HTergic activity in mouse models of depressive-like behavior (Bambico et al., 2009; Lira et al., 2003; Veerakumar et al., 2014) and suggestive evidence for decreased 5-HT signaling in depressed patients (Blier et al., 1990; Gos et al., 2008). Furthermore, genetic models with a life-long decrease in 5-HT levels display increased depression-like behaviors and decreased anxiety (Kim et al., 2009; Kiyasova et al., 2011; Mosienko et al., 2012; Narboux-Nême et al., 2011). The chronic versus acute nature of manipulations may contribute to differences in behavioral consequences. However, 5,7-DHT induced 5-HT lesions also have no effect on anxiety and depression-like behavior, respectively (Borsini, 1995; Lieben et al., 2006), indicating that development specific factors play a dominant role in behavioral consequences of many constitutive genetic manipulations (Ansorge et al., 2004; Donaldson et al., 2014; Gross et al., 2002; Suri et al., 2014). Importantly, our pharmacogenetic inhibition protocol did alter behavior in PNFLX mice, as well as in naïve mice with r1 and r2 directed inhibition, demonstrating that the behavioral consequences of decreasing 5-HTergic neuronal activity in adulthood vary drastically depending on the nature, specificity and localization of the manipulation.
PNFLX animals display a blunted response to 5-HTergic neuronal activation and show behavioral normalization after 5-HTergic neuronal inhibition
We find increased MR activity and decreased DR activity in PNFLX mice. Furthermore, increasing 5-HT neuronal activity produced blunted behavioral and physiological consequences, while decreasing 5-HT neuronal activity restores normal anxiety and depression-like phenotypes in PNFLX animals. Together with the antidepressant effect of increased raphe activity in naïve and PNSAL animals, these results provide strong functional evidence that an altered balance of raphé nuclei activity plays a role in the etiology of mood disorders.
Our findings are consistent with anxiogenic consequences of increased MR activity (Ohmura et al., 2014) and anxiolytic effects of MR lesions (Andrade et al., 1999; Pezzato et al., 2014). Of note, one pharmacologic study suggests an anti-depressant effect of MR inhibition (Almeida et al., 2013). Furthermore, increasing DR activity has an anti-depressant effect (Veerakumar et al., 2014; Warden et al., 2012), however the consequence of decreasing DR activity and the contribution of DR 5-HTergic neuronal activity to anxiety behavior still remains controversial (Spoida et al., 2014; Warden et al., 2012).
Several converging mechanisms may play a role in differentially impacting set points of MR and DR 5-HTergic neuronal activity. First, between P2 and P21, DR but not MR 5-HTergic neurons undergo a molecular, morphological and physiological transition which appears crucial for its proper maturation, including the establishment of 5-HT1a-dependent auto-inhibition (Donaldson et al., 2014; Gross et al., 2002; Rood et al., 2014). Hence, a postnatal increase in extracellular 5-HT levels might preferentially affect DR 5-HTergic neurons maturation. Second, developmental SSRI exposure may differentially alter the establishment of specific inputs onto DR and MR 5-HT neurons (Dorocic et al., 2014; Lechin et al., 2006), including those from local GABAergic interneurons (Challis et al., 2013; Rood et al., 2014). Finally, MR and DR send reciprocal projections to each other (Bang et al., 2012; Muzerelle et al., 2014; Vertes et al., 1999), and developmental interference with the establishment of interconnectivity can lead to permanently altered relative 5-HTergic activity. Together these mechanisms might converge on permanently altering the balance between DR and MR 5-HTergic activity and provide a pathway for environmental and genetic factors to guide, influence or perturb 5-HTergic system development.
r1- and r2-derived 5-HTergic neurons display opposing roles on swimming behavior
We observed that inhibition of r1-derived or r2-derived 5-HTergic neurons has opposing effects on floating behavior in the FST, while not affecting anxiety behavior. r1-derived 5-HT neurons include DR neurons and the rostral part of the MR, while r2-derived 5-HT neurons include the caudal part of the MR (Alonso et al., 2013; Bang et al., 2012). The functional distinction of r1- and r2-derived 5-HT neurons highlights the critical importance of developmental origin for 5-HT neuron diversity, as previously reported for 5-HT-dependent respiratory control (Brust et al., 2014).
Increased floating behavior in the FST upon decreasing r1 5-HTergic activity is in line with decreased floating behavior after increasing DR 5-HT activity (Veerakumar et al., 2014; Warden et al., 2012) and supports a causal relationship of low DR 5-HTergic neuronal activity with depression-like behavior (Bambico et al., 2009; Gos et al., 2008; Jacobsen et al., 2012; Lira et al., 2003). In addition, decreased floating behavior upon decreasing r2 5-HTergic activity also confirms a direct causal relationship that was previously only suggested (Almeida et al., 2013). This r1 versus r2 antagonism provides an explanation for the lack of behavioral response in the FST upon global 5-HT neuronal inhibition in WT mice. Interestingly though, if the normal balance of 5-HTergic activity is altered, global inhibition of 5-HTergic neuronal activity can restore balance and normalize behavior, as we found in PNFLX mice.
Several anatomical, physiological and behavioral experiments have previously indicated potential antagonistic effects of DR and MR in psychopathologies (Lechin et al., 2006). Yet, most preclinical studies focus on DR neurons and tend to generalize to all 5-HTergic neurons. Going forward, it will be critical to incorporate a much more differentiated view and investigate how this opposing design and an imbalance of such can impact the regulation of mood and behavior and mechanistically underlie the etiology of psychiatric disorders, such as major depression and bipolar disorders.
EXPERIMENTAL PROCEDURES
Subjects
Mice (129SvEv/Tac) were bred at Columbia Psychiatry, New York State Psychiatric Institute. Mice used for experiments were born to litters containing 4–8 pups. Mice were separated by sex and weaned into groups of five mice per cage at P22. Animals were maintained on a 12-hour light–dark cycle (lights on at 8 am) and provided with food and water ad libitum. Animal testing was conducted in accordance with the Principles of Laboratory Animal Care National Institute of Health (NIH) guidelines and the institutional animal committee guidelines. For more information about behavioral tests and drug treatments, see Supplemental Experimental Procedures.
Generation of the RC::PDq line
The targeting constructs for RC::PDq has a cassette including: the cytomegalovirus-chicken β-actin (CBA) promoter followed by a flox’d Pgk-Neomycin resistance gene, a hemagglutanin (HA, 3 copies)-tagged DREADD receptor, an internal ribosome entry site (ires), and enhanced green fluorescent protein (EGFP), targeted to the transcriptional start site of the mouse Gt(ROSA)26 Sor locus (see also Figure S1).
Statistics
Data were analyzed using Student’s t test, one-way ANOVA or two-ways ANOVA, with repeated measures when appropriate (Statview). Post hoc analyses were conducted using Fisher’s PLSD. Datasets containing male and female mice were analyzed for main effect and/or interaction of sex with the dependent variables under study. Since none were found, male and female data were combined. The criterion for significance was p < 0.05. All data are presented as mean ± SEM. Numbers of animals are mentioned in the figure legends. Supplemental Experimental Procedures contains detailed description of the generation of the RC::PDq mouse line, drugs administration, behavioral assays, electrophysiological recordings, microdialysis surgery, 5-HT levels analysis and immunohistochemistry.
Supplementary Material
ACKNOWLEGMENTS
M.A., A.T., H.M., B.I., R.R., R.P. and S.D. conceived the experiments. A.T., A.C., B.I. and S.B. performed the experiments. A.T., S.D. and M.A. wrote the paper. We thank Dr. Gaspar for her hospitality during the end of this project. We thank Dr. Roth for providing constructs to make the RC::PDq mouse line. We thank Dr. Roth and the NIH for providing CNO. We thank Cátia Teixeira for critical reviews of the manuscript.
This work has been supported by the National Institute of Mental health (R00MH083044, M.S.A), the Sackler Institute for developmental Psychobiology (M.S.A.) and the NARSAD Young investigator grant 2013 (#21501, A.T).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interest.
REFERENCES
- Almeida PVG, Trovo MC, Tokumoto AM, Pereira AC, Padovan CM. Role of serotonin 1A receptors in the median raphe nucleus on the behavioral consequences of forced swim stress. J. Psychopharmacol. 2013;27:1134–1140. doi: 10.1177/0269881113508829. [DOI] [PubMed] [Google Scholar]
- Alonso A, Merchán P, Sandoval JE, Sánchez-Arrones L, Garcia-Cazorla A, Artuch R, Ferrán JL, Martínez-De-La-Torre M, Puelles L. Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains. Brain Struct. Funct. 2013;218:1229–1277. doi: 10.1007/s00429-012-0456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade TG, Silva AM, Silva CL, Graeff FG. Effect of electrolytic lesion of the median raphe nucleus on behavioral and physiological measures of stress. Acta Physiol. Pharmacol. Ther. Latinoam. 1999;49:279–289. [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich Ja. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Nguyen N-T, Gobbi G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur. Neuropsychopharmacol. 2009;19:215–228. doi: 10.1016/j.euroneuro.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Jensen P, Dymecki SM, Commons KG. Projections and interconnections of genetically defined serotonin neurons in mice. Eur. J. Neurosci. 2012;35:85–96. doi: 10.1111/j.1460-9568.2011.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, de Montigny C, Chaput Y. A role for the serotonin system in the mechanism of action of antidepressant treatments: preclinical evidence. J. Clin. Psychiatry. 1990;51(Suppl):14–20. discussion 21. [PubMed] [Google Scholar]
- Borsini F. Role of the serotonergic system in the forced swimming test. Neurosci. Biobehav. Rev. 1995;19:377–395. doi: 10.1016/0149-7634(94)00050-b. [DOI] [PubMed] [Google Scholar]
- Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM. Functional and Developmental Identification of a Molecular Subtype of Brain Serotonergic Neuron Specialized to Regulate Breathing Dynamics. Cell Rep. 2014;9:2152–2165. doi: 10.1016/j.celrep.2014.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. 2011;Chapter 8(Unit 8.10A) doi: 10.1002/0471142301.ns0810as55. [DOI] [PubMed] [Google Scholar]
- Challis C, Boulden J, Veerakumar A, Espallergues J, Vassoler FM, Pierce RC, Beck SG, Berton O. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J. Neurosci. 2013;33:13978–13988. 13988a. doi: 10.1523/JNEUROSCI.2383-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Piel Da, Santos TL, Richardson-Jones J, Leonardo ED, Beck SG, Champagne Fa, Hen R. Developmental effects of serotonin 1A autoreceptors on anxiety and social behavior. Neuropsychopharmacology. 2014;39:291–302. doi: 10.1038/npp.2013.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorocic IP, Fu D, Xuan Y, Johansson Y, Pozzi L, Silberberg G, Carle M. Article A Whole-Brain Atlas of Inputs to Serotonergic Neurons of the Dorsal and Median Raphe Nuclei. 2014:663–678. doi: 10.1016/j.neuron.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Gos T, Krell D, Brisch R, Bielau H, Trübner K, Steiner J, Bernstein H-G, Bogerts B. Demonstration of decreased activity of dorsal raphe nucleus neurons in depressed suicidal patients by the AgNOR staining method. J. Affect. Disord. 2008;111:251–260. doi: 10.1016/j.jad.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, Santarelli L, Beck S, Hen R. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol. Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobsen JPR, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos. Trans. R. Soc. B Biol. Sci. 2012;367:2444–2459. doi: 10.1098/rstb.2012.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat. Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, Dymecki SM. Linking Genetically Defined Neurons to Behavior through a Broadly Applicable Silencing Allele. Neuron. 2009;63:305–315. doi: 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel RA, Turnbull DH, Blanquet V, Wurst W, Loomis CA, Joyner AL. Two lineage boundaries coordinate vertebrate apical ectodermal ridge formation. Genes Dev. 2000;14:1377–1389. [PMC free article] [PubMed] [Google Scholar]
- Kiyasova V, Fernandez SP, Laine J, Stankovski L, Muzerelle A, Doly S, Gaspar P. A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J. Neurosci. 2011;31:2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt M, Arik AC, Celik S. The effects of sertraline and fluoxetine on anxiety in the elevated plus-maze test in mice. J. Basic Clin. Physiol. Pharmacol. 2000;11:173–180. doi: 10.1515/jbcpp.2000.11.2.173. [DOI] [PubMed] [Google Scholar]
- Lechin F, van der Dijs B, Hernández-Adrián G. Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidences: relevance for neuropharmacological therapy. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:565–585. doi: 10.1016/j.pnpbp.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Lieben CKJ, Steinbusch HWM, Blokland A. 5,7-DHT lesion of the dorsal raphe nuclei impairs object recognition but not affective behavior and corticosterone response to stressor in the rat. Behav. Brain Res. 2006;168:197–207. doi: 10.1016/j.bbr.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge MS, Gordon Ja, Francis JH, Bradley-Moore M, Lira J, Underwood MD, Arango V, et al. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol. Psychiatry. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M, Feng Q, Zhang JE, Wang D, Zeng J, et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron. 2014;81:1360–1374. doi: 10.1016/j.neuron.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezadri TJ, Batista GM, Portes AC, Marino-Neto J, Lino-de-Oliveira C. Repeated rat-forced swim test: reducing the number of animals to evaluate gradual effects of antidepressants. J. Neurosci. Methods. 2011;195:200–205. doi: 10.1016/j.jneumeth.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Mosienko V, Bert B, Beis D, Matthes S, Fink H, Bader M, Alenina N. Exaggerated aggression and decreased anxiety in mice deficient in brain serotonin. Transl. Psychiatry. 2012;2:e122. doi: 10.1038/tp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzerelle A, Scotto-Lomassese S, Bernard JF, Soiza-Reilly M, Gaspar P. Conditional anterograde tracing reveals distinct targeting of individual serotonin cell groups (B5–B9) to the forebrain and brainstem. Brain Struct. Funct. 2014 doi: 10.1007/s00429-014-0924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narboux-Nême N, Sagné C, Doly S, Diaz SL, Martin CBP, Angenard G, Martres M-P, Giros B, Hamon M, Lanfumey L, et al. Severe Serotonin Depletion after Conditional Deletion of the Vesicular Monoamine Transporter 2 Gene in Serotonin Neurons: Neural and Behavioral Consequences. Neuropsychopharmacology. 2011;36:2538–2550. doi: 10.1038/npp.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ. The neuropharmacology of serotonin and noradrenaline in depression. Int. Clin. Psychopharmacol. 2002;17(Suppl 1):S1–S12. doi: 10.1097/00004850-200206001-00002. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. Overview of Diagnosis and Drug Treatments of Anxiety Disorders. CNS Spectr. 2005;10:49–56. doi: 10.1017/s1092852900009901. [DOI] [PubMed] [Google Scholar]
- Ohmura Y, Tanaka KF, Tsunematsu T, Yamanaka A, Yoshioka M. Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice. Int. J. Neuropsychopharmacol. 2014;17:1777–1783. doi: 10.1017/S1461145714000637. [DOI] [PubMed] [Google Scholar]
- Pezzato FA, Can A, Hoshino K, Horta J, de AC, Mijares MG, Gould TD. Effect of lithium on behavioral disinhibition induced by electrolytic lesion of the median raphe nucleus. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Thérapie. 1977;229:327–336. [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello TJ, Yu Q, Goodfellow NM, Caffrey Cagliostro MK, Teissier a, Morelli E, Demireva EY, Chemiakine a, Rosoklija GB, Dwork aJ, et al. Postnatal Day 2 to 11 Constitutes a 5-HT-Sensitive Period Impacting Adult mPFC Function. J. Neurosci. 2014;34:12379–12393. doi: 10.1523/JNEUROSCI.1020-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Calizo LH, Piel D, Spangler ZP, Campbell K, Beck SG. Dorsal raphe serotonin neurons in mice: immature hyperexcitability transitions to adult state during first three postnatal weeks suggesting sensitive period for environmental perturbation. J. Neurosci. 2014;34:4809–4821. doi: 10.1523/JNEUROSCI.1498-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MM, Wylie CJ, Lerch JK, Murphy R, Lobur K, Herlitze S, Jiang W, Conlon RA, Strowbridge BW, Deneris ES. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc. Natl. Acad. Sci. 2005;102:16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CM Da, Gonçalves L, Manhaes-de-Castro R, Nogueira MI. Postnatal fluoxetine treatment affects the development of serotonergic neurons in rats. Neurosci. Lett. 2010;483:179–183. doi: 10.1016/j.neulet.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Sinclair LI, Christmas DM, Hood SD, Potokar JP, Robertson A, Isaac A, Srivastava S, Nutt DJ, Davies SJC. Antidepressant-induced jitteriness/anxiety syndrome: systematic review. Br. J. Psychiatry. 2009;194:483–490. doi: 10.1192/bjp.bp.107.048371. [DOI] [PubMed] [Google Scholar]
- Spoida K, Masseck Oa, Deneris ES, Herlitze S. Gq/5-HT2c receptor signals activate a local GABAergic inhibitory feedback circuit to modulate serotonergic firing and anxiety in mice. Pnas. 2014;111:6479–6484. doi: 10.1073/pnas.1321576111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri D, Teixeira CM, Cagliostro MKC, Mahadevia D, Ansorge MS. Monoamine-Sensitive Developmental Periods Impacting Adult Emotional and Cognitive Behaviors. Neuropsychopharmacology. 2014;40:88–112. doi: 10.1038/npp.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- Veerakumar A, Challis C, Gupta P, Da J, Upadhyay A, Beck SG, Berton O. Antidepressant-like effects of cortical deep brain stimulation coincide with pro-neuroplastic adaptations of serotonin systems. Biol. Psychiatry. 2014;76:203–212. doi: 10.1016/j.biopsych.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Fortin WJ, Crane AM. Projections of the median raphe nucleus in the rat. J. Comp. Neurol. 1999;407:555–582. [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim S-Y, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.