Abstract
Background
The approved dose of rituximab (RTX) in rheumatoid arthritis is 1000 mg × 2, but some data have suggested similar clinical efficacy with 500 mg × 2. The purpose of this study was to compare the effectiveness of the regular and low doses given as first treatment course.
Methods
Twelve European registries participating in the CERERRA collaboration (The European Collaborative Registries for the Evaluation of Rituximab in Rheumatoid Arthritis) submitted anonymized datasets with demographic, efficacy and treatment data for patients who had started RTX. Treatment effectiveness was assessed by DAS28 reductions and EULAR responses after 6 months.
Results
Data on RTX dose were available for 2,873 patients, of whom 2,625 (91.4 %) and 248 (8.6 %) received 1000 mg × 2 and 500 mg × 2, respectively. Patients treated with 500 mg × 2 were significantly older, had longer disease duration, higher number of prior DMARDs, but lower number of prior biologics and lower baseline DAS28 than those treated with 1000 mg × 2. Fewer patients in the low-dose group received concomitant DMARDs but more frequently received concomitant corticosteroids.
Both doses led to significant clinical improvements at 6 months. DAS28 reductions at 6 months were comparable in the 2 dose regimens [mean DeltaDAS28 ± SD -2.0 ± 1.3 (high dose) vs. -1.7 ± 1.4 (low dose), p = 0.23 adjusted for baseline differences]. Similar percentages of patients achieved EULAR good response in the two dose groups, 18.4 % vs. 17.3 %, respectively (p = 0.36).
Conclusions
In this large observational cohort initial treatment with RTX at 500 mg × 2 and 1000 mg × 2 led to comparable clinical outcomes at 6 months.
Keywords: Rituximab, Rheumatoid arthritis, Observational
Background
Rituximab (MabThera, Rituxan) is a chimeric, monoclonal anti-CD20 antibody approved for the treatment of rheumatoid arthritis (RA) in combination with methotrexate in patients with active RA who have not responded to at least one tumor necrosis factor (TNF) inhibitor. The efficacy and acceptable safety profile of rituximab (RTX) have been demonstrated in randomized controlled trials [1, 2] and in large observational cohorts [3, 4]. The approved dose is 1000 mg × 2 (with a 2-week interval) per treatment course.
There is, however, evidence suggesting that a lower dose of RTX, 500 mg × 2, is also effective, although not approved. In the SERENE trial both 500 mg × 2 and 1000 mg × 2 of RTX significantly improved clinical outcomes (based on criteria of the American College of Rheumatology (ACR) responses, the European League Against Rheumatism (EULAR) responses, and improvement in the Disease Activity Score in 28 joints (DAS28) and in the Health Assessment Questionnaire (HAQ)) compared to placebo in a biologic-agent-naïve population of patients with RA [5]. The MIRROR, DANCER and IMAGE trials yielded similar results [6–8]. In all the above trials no significant difference was detected between the different doses in almost all clinical outcomes.
The purpose of this study was to assess and compare the effectiveness at 6 months of the higher (1000 mg × 2) and lower (500 mg × 2) dose of RTX given as the first treatment course in a merged dataset from observational cohorts.
Methods
The European Collaborative Registries for the Evaluation of Rituximab in Rheumatoid Arthritis (CERERRA) is an investigator-led initiative aiming to evaluate clinical aspects of RTX use in patients with RA [4, 9]. Twelve participating European registries (from the Czech Republic, Denmark, Finland, The Netherlands, Norway, Portugal, Romania, Russia, Slovenia, Spain, Sweden and Switzerland) submitted fully anonymized datasets with baseline demographic and disease characteristics, including age, gender, disease duration, number of previous synthetic and biological disease-modifying anti-rheumatic drugs (DMARDs), rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibody (anti-CCP) status of all patients with an established diagnosis of RA who started treatment with RTX. Data are collected prospectively in each register. Ethical approval for the use of register data from each register was obtained by local authorities of each country. The Regional Ethical Review Board in Stockholm approved the collection and analysis of anonymized data from the twelve participating registers. Informed consent was obtained from each patient before inclusion in each register, according to local regulations. Disease activity markers at baseline and after 3 and 6 months were also provided (number of swollen and tender joints, visual analog scales (VAS) for pain, patient’s and physician’s global assessment, DAS28 and erythrocyte sedimentation rate (DAS28-ESR), and HAQ). Information about RA treatment, such as doses of RTX and use of concomitant DMARDs and glucocorticoids was also included in the dataset. Effectiveness of RTX in the higher (1000 mg × 2) and the lower dose (500 mg × 2) was assessed by DAS28 and HAQ status at 3 and 6 months, by the improvement of DAS28 and HAQ at 3 and 6 months, by disease activity at 3 and 6 months based on DAS28 status and by EULAR responses at 6 months. A small number of patients were treated with other than the above doses (e.g., 750 mg) or did not provide information on the RTX dose and were therefore not included in the analysis.
Statistical analysis
Baseline characteristics of the two groups were analyzed by means of descriptive statistics. For normally distributed variables mean ± standard deviation (SD) and the independent samples t test was used, and median (interquartile range (IQR)) and the Mann–Whitney U test were used for the non-normally distributed variables. The chi-square test was used for comparison of categorical data.
Changes in DAS28 and HAQ were first compared in unadjusted analysis using the independent samples t test. Comparative adjusted analysis with correction for baseline group differences was subsequently performed by analysis of covariance (ANCOVA). In the ANCOVA we adjusted for baseline variables found to differ significantly between the two groups (age, disease duration, number of prior biologic agents used, baseline DAS28, and concomitant use of DMARDs) and for those thought to be clinically significant (concomitant use of corticosteroids). The number of prior DMARDs used was not included in the ANCOVA even though it was significantly different between groups, because of the small number of patients with available information in the 500-mg group and because of the high correlation with the number of prior biologic agents. Multivariate logistic regression analysis was performed with EULAR response as the dependent variable (first, analysis of good vs. moderate/no EULAR response and second, analysis of good/moderate vs. no EULAR response) and RTX dose (500 vs. 1000 mg), and several baseline variables as explanatory variables (age, gender, RA disease duration, use of previous biologic agents, baseline DAS28, anti-CCP status, and concomitant use of DMARDs and corticosteroids). Country was included in the model in an additional analysis. All statistical tests were evaluated at the 0.05 significance level. P values and 95 % confidence intervals are presented. The statistical analysis was performed with IBM SPSS Statistics version 20.
Results
The total number of patients included in the cohort was 3,266, and 2,873 patients (88 %) were eligible for analysis. The large majority of patients ([n = 2,625, 91.4 %) received 1000 mg × 2 (higher dose), and 248 patients (8.6 %) received 500 mg × 2 (lower dose). The demographics and baseline disease characteristics for the two treatment groups are shown in Table 1. Patients who were treated with the lower RTX dose were older, had longer disease duration, prior use of fewer biologic agents but more DMARDs, and lower baseline DAS28 than those treated with the higher dose. Additionally, fewer patients in the low-dose group received concomitant DMARDs but more frequently received concomitant corticosteroids. Baseline characteristics for those patients with available DAS28-ESR at 6 months are also shown in Table 1. No significant differences between the two populations (all patients at baseline and patients with available response data at 6 months) were observed, so missingness of data was not informative.
Table 1.
Baseline demographics, disease and treatment characteristics of patients treated with rituximab 500 mg × 2 or 1000 mg × 2 for all patients in the cohort at baseline and for those with available response data (DAS28-ESR) at 6 months
| All patients | Patients with available response data at 6 months* | |||||
|---|---|---|---|---|---|---|
| RTX 500 mg × 2 n = 248 |
RTX 1000 mg × 2 n = 2625 |
P value (t test, chi-square test) | RTX 500 mg × 2 n = 109 |
RTX 1000 mg × 2 n = 1385 |
P value (t test, chi-square test) | |
| Sex,% female | 83.9 % (248) | 80.3 % (2,625) | 0.17 | 88.1 % (109) | 81.2 % (1,125) | 0.08 |
| Age, years | 55.2 ± 15.8 (247) | 52.6 ± 12.6 (2,615) | 0.002 | 55.5 ± 15.1 (109) | 51.1 ± 11.8 (1,380) | <0.0001 |
| Disease duration, years | 10.5 (5-18) (240) | 8.1 (5-14) (2,360) | 0.02 | 10 (5-16.8) (108) | 7.5 (5.1-12) (1,329) | 0.02 |
| RF, % positive | 81.7 % (241) | 81.2 % (2,031) | 0.84 | 83 % (106) | 81.5 % (876) | 0.7 |
| Anti-CCP, % positive | 71.3 % (101) | 73.4 % (806) | 0.64 | 68 % (50) | 74.9 % (363) | 0.29 |
| Number of prior biologic agents | 0 (0-1) (207) | 1 (0-2) (2,560) | <0.0001 | 0 (0-1) (102) | 1 (0-1) (1,371) | 0.003 |
| Anti-TNF-naive (%) | 58 % (207) | 37.5 % (2,560) | <0.0001 | 58.8 % (102) | 37.1 % (1,371) | <0.0001 |
| Number of prior DMARDs | 2.6 ± 1.3 (126) | 2.4 ± 1.4 (2,248) | 0.04 | 2.7 ± 1.3 (55) | 2.3 ± 1.1 (1,256) | 0.02 |
| Baseline DAS28-ESR | 5.7 ± 1.3 (215) | 6.1 ± 1.3 (2,069) | <0.0001 | 5.9 ± 1.3 (100) | 6.3 ± 1.2 (1,344) | 0.002 |
| Baseline HAQ score | 1.6 ± 0.7 (212) | 1.7 ± 0.7 (1,584) | 0.48 | 1.6 ± 0.7 (100) | 1.8 ± 0.7 (695) | 0.001 |
| Concomitant medication: | ||||||
| – Any DMARD | 72.6 % (248) | 83.1 % (2,625) | <0.0001 | 75.2 % (109) | 87.5 % (1,385) | <0.0001 |
| MTX | 46.4 % (248) | 63.4 % (2,625) | <0.0001 | 50.5 % (109) | 75.1 % (1,385) | <0.0001 |
| Glucocorticoids | 65.7 % (248) | 59.3 % (2,221) | 0.06 | 71.6 % (109) | 64.7 % (983) | 0.15 |
The number of patients with available information for each variable is included in brackets. RTX rituximab, RF rheumatoid factor, anti-CCP anti-cyclic citrullinated peptide antibodies, DMARDs disease modifying anti-rheumatic drugs, DAS28-ESR Disease Activity Score based on 28 joints and erythrocyte sedimentation rate, HAQ health assessment questionnaire, MTX methotrexate
In the unadjusted analysis, the mean DAS28 improvement at 3 months was greater for patients treated with the higher dose than for those treated with the lower dose (1.9 ± 1.4 (n = 991) vs. 1.3 ± 1.3 (n = 125), p <0.0001) and it remained significant in the ANCOVA (p = 0.004) (Table 2). The difference in mean DAS28 improvement was also significant at 6 months (2.0 ± 1.3 (n = 1344) vs. 1.7 ± 1.4 (n = 100), p = 0.02) in the unadjusted analysis. However, there was no significant difference after adjustment (p = 0.23). Inclusion of country as a random variable in the ANCOVA did not change the results. Improvements in function as assessed by the HAQ were also similar between the groups both at 3 and 6 months (Table 2). The proportion of patients with high, moderate and low disease activity and remission based on the DAS28 was similar in the two groups at baseline, 3 and 6 months (Fig. 1a), as was the proportion of EULAR good responders, moderate responders and non-responders at 6 months (Fig. 1b).
Table 2.
Effectiveness of treatment across the two treatment groups as assessed by DAS28 and HAQ status and changes at 3 and 6 months
| RTX 500 mg × 2 | RTX 1000 mg × 2 | Unadjusted p values (ANOVA) | Adjusted p values** | |
|---|---|---|---|---|
| DAS28 baseline | 5.7 ± 1.3 (215) | 6.1 ± 1.3 (2069) | <0.0001 | |
| DAS28 3 m | 4.4 ± 1.2 (138) | 4.2 ± 1.3 (1046) | 0.15 | |
| DAS28 6 m | 4.3 ± 1.3 (109) | 4.3 ± 1.2 (1385) | 0.99 | |
| DeltaDAS28 3 m | –1.3 ± 1.3 (125) | –1.9 ± 1.4 (991) | <0.0001 | 0.004 |
| DeltaDAS28 6 m | –1.7 ± 1.4 (100) | –2.0 ± 1.3 (1344) | 0.02 | 0.23 |
| HAQ baseline | 1.6 ± 0.7 (212) | 1.6 ± 0.7 (1584) | 0.48 | |
| HAQ 3 m | 1.3 ± 0.7 (127) | 1.3 ± 0.7 (957) | 0.83 | |
| HAQ 6 m | 1.2 ± 0.7 (109) | 1.3 ± 0.7 (912) | 0.21 | |
| DeltaHAQ 3 m | –0.3 ± 0.5 (115) | –0.5 ± 0.6 (859) | 0.02 | 0.10 |
| DeltaHAQ 6 m | -0.4 ± 0.6 (103) | –0.5 ± 0.7 (826) | 0.13 | 0.27 |
Crude and adjusted p valued are presented. **Analysis of covariance (ANCOVA) adjusted for age, sex, disease duration, number of prior biologic agents used, baseline Disease Activity Score in 28 joints (DAS28), concomitant use of disease-modifying anti-rheumatic drugs and glucocorticoids. RTX rituximab, HAQ Health Assessment Questionnaire, m months
Fig. 1.
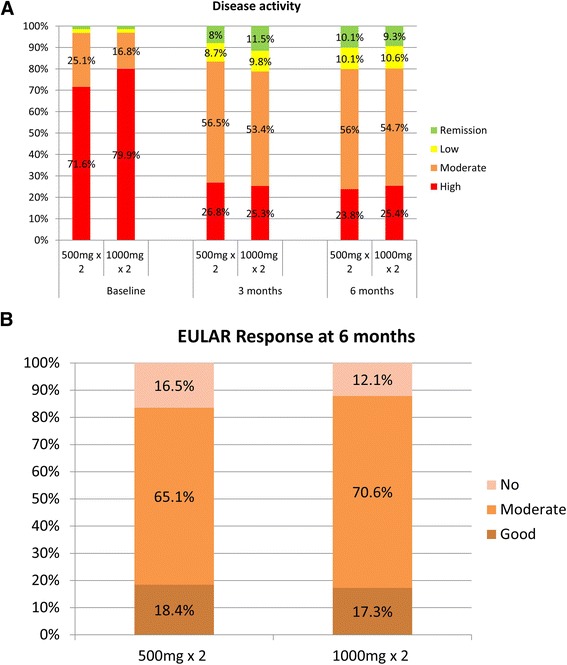
a Disease activity based on Disease Activity Score in 28 joints (DAS28)-erythrocyte sedimentation rate (ESR) at baseline, 3 and 6 months in the two treatment groups (rituximab (RTX) 500 mg × 2 and RTX 1000 mg × 2). No significant differences were observed: remission, DAS28 <2.6; low disease activity, 2.6≤ DAS28 ≤3.2; moderate disease activity, 3.2< DAS28 ≤5.1; high disease activity, DAS28 >5.1. b. Good, moderate or no European League Against Rheumatism (EULAR) response at 6 months for the two treatment groups (RTX 500 mg × 2 and RTX 1000 mg × 2). No significant differences were observed
Multivariate logistic regression analysis was performed to examine the possible association between RTX dose and good (vs. moderate/no) and good/moderate (vs. no) EULAR response, with adjustment for possible confounders, such as age, gender, RA disease duration, previous use of biologic agents, baseline DAS28, anti-CCP status, and use of concomitant DMARDs and corticosteroids. RTX dose (lower dose vs. higher dose) was not a statistically significant predictor of achieving a good EULAR response (odds ratio (OR) 1.08, 95 % CI 0.40, 2.94, p = 0.88) or good/moderate response EULAR (OR 1.22, 95 % CI 0.37, 4.09, p = 0.74). Similar results were observed when country was introduced into the model.
Discussion
In this study based on data from the CERERRA collaboration RTX provided significant clinical improvement at 3 and 6 months in patients with active RA. On comparison of the higher (1000 mg × 2) and lower dose (500 mg × 2) of RTX there was a significant difference in DAS28 improvement at 3 months but not in clinical effectiveness, as assessed by change in DAS28 and HAQ score at 6 months, after adjusting for baseline characteristics. EULAR response rates and remission rates were also similar between groups. The results of our study are thus consistent with those from the SERENE, IMAGE and MIRROR trials [5, 6, 8]. In the MIRROR trial the percentage of patients with good/moderate EULAR responses was borderline significantly higher in the 1000 mg × 2 (89 %) compared to the 500 mg × 2 group (73 %) (p = 0.05). The overall conclusions of the MIRROR trial was that the two RTX doses could not be clearly differentiated, although some clinical outcomes were in favor of the higher dose [6]. A recent systematic review and meta-analysis made a similar conclusion [10]. The similar effectiveness of lower dose RTX in patients with RA in clinical practice might have important pharmaco-economic implications for health systems globally.
Hitherto there have been no proper dose-response studies for RTX in RA. What is considered the “autoimmune dose and protocol” was developed by Professor Jo Edwards based on a small number of patients [11]. Our study provides some additional evidence about the lack of any striking difference and perhaps no clinically significant difference between the two different doses of RTX used in clinical practice. Recently interesting data from the UK Leeds group showed that response to RTX may be more dependent on the B-cell depletion rather than the dose. Although in the small number of patients studied “incomplete” peripheral blood depletion was more often seen in the patients treated with the lower dose, “complete” depletion was seen in both groups and correlated better with response than dose itself [12]. The depletion of B cells with anti-CD20 treatment varies between individuals, even with the same dose, as shown in several animal and human studies, but tends to be consistent in the same individuals [13–15]. This suggests that individual factors are important in determining the final extent of depletion.
There are several limitations that should be addressed: the observational character of the study, the different size of the two treatment groups under comparison (only 248 patients treated with the lower dose), and the fact that the two groups compared were not balanced for all baseline characteristics. Hence, there is a risk of channeling bias, as patients treated with the lower dose were older, had longer disease duration, lower disease activity at baseline and less prior use of biologic agents, and were more often treated with corticosteroids and less often with concomitant DMARDs. The lower-dose group may represent a population of patients with more comorbidities, for whom the treating rheumatologist chose the lower dose of RTX. However, such a population would be more prone to have a worse response to therapy, and therefore confounding by indication would bias the results against the lower dose of RTX. On assessing the potential influence of corticosteroids on response, the percentage of patients with concomitant use of corticosteroids in the two groups was quite similar and not statistically significant (Table 1). Additionally, in the ANCOVA we adjusted for concomitant use of corticosteroids (Table 2) as it is clinically significant.
The lack of radiological data is an additional limitation of the study. The golden triad of current treatment guidelines in RA is remission (or low disease activity when remission is not possible), preservation of functional ability, and prevention of structural damage. Tak et al. showed in the IMAGE study that the 1000 mg × 2 RTX, but not the 500 mg × 2 dose, significantly inhibited progression of joint damage during the first 6 months, but inhibition of structural progression was similar from 6 months onwards [8, 16]. However, the IMAGE trial included MTX-naïve patients of whom the majority had early RA, and its population was thus different from the population in our study. It would be interesting to further evaluate the ability of the lower RTX dose to prevent radiological progression in an established RA population that is more consistent with the routine use of RTX. The length of sustained response was not examined in the present study. The risk that the lower dose might be associated with shorter response cannot be ruled out and should be assessed in future studies. The large number of patients included in the cohort, which made the comparison of the different doses of RTX possible, and the real-life character of the study are important strengths of the study.
Conclusions
In this large observational cohort initial treatment with RTX at 500 mg × 2 and 1000 mg × 2 led to comparable clinical outcomes after 6 months. This result may have some important cost implications in the treatment of patients with RA.
Abbreviations
- ACR
American College of Rheumatology
- ANCOVA
analysis of covariance
- Anti-CCP
anti-cyclic citrullinated peptide
- CERERRA
European Collaborative Registries for the Evaluation of Rituximab in Rheumatoid Arthritis
- CRP
C-reactive protein
- DAS
Disease Activity Score
- DMARDs
disease modifying anti-rheumatic drugs
- ESR
erythrocyte sedimentation rate
- EULAR
European League Against Rheumatism
- HAQ
health assessment questionnaire
- MTX
methotrexate
- OR
odds ratio
- RA
rheumatoid arthritis
- RF
rheumatoid factor
- RTX
rituximab
- TNF
tumor necrosis factor
Footnotes
Competing interests
KC has received speaking and consulting fees from Roche and Prizer; EL has received fees for speaking and/or consulting from AbbVie, Bristol-Myers Squibb, Hospira, Pfizer, Roche, and UCB; GL has received fees for consulting from BMS, Roche, MSD, AbbVie, and Pfizer. The ARBITER registry is supported by a non-commercial partnership with “Equal rights to life”;
MH has received fees for consulting and/or research grants from BMS, MSD, Pfizer, Abbott, UCB, and Roche. DANBIO is funded by hospital authorities in Denmark, and has received unrestricted grants from AbbVie, BMS, Hospira, MSD, Pfizer, Roche, and UCB; IA has received fees for speaking and/or consulting from MSD, AbbVie, Roche, Pfizer, BMS, and UCB; KP has received fees for speaking and/or consulting from AbbVie, Roche, Amgen, MSD, BMS, UCB, and Egis. ATTRA was partially supported by the project from the Czech Ministry of Health for conceptual development of research organization 023728 (Institute of Rheumatology); DN has received fees for speaking and/or consulting from Abbvie, BMS, MSD, Roche, UCB, and Pfizer. ROB-FIN is funded by AbbVie, Hospira, BMS, MSD, Pfizer, Roche, and UCB; CG has received fees for speaking and/or consulting from AbbVie, BMS, Roche, Pfizer, Celgene, MSD, Janssen Cilag, Amgen, and UCB, and received research funding from Roche, AbbVie, MSD, and Pfizer. The SCQM Foundation is funded by the Swiss Society of Rheumatology, and by Abbvie, BMS, MSD, Pfizer, Roche, UCB, and Janssen. In addition, SCQM has received project-based financial supports from various institutions and companies (e.g., Arco Foundation, Switzerland, or Schweizerischer Verein Balgrist, Switzerland). HC has received fees for consulting from Roche and Pfizer. Reuma.pt is supported by unrestricted grants from Abbvie, MSD, Roche, and Pfizer. MT has received fees for speaking and/or consulting from Abbvie, Roche, MSD, and Pfizer paid to Revmatic d.o.o. BioRx.si has received funding for clinical research paid to Društvo za razvoj revmatologije from AbbVie, Roche, Medis, MSD, and Pfizer. PvR has received consulting fees and research grants from AbbVie, Pfizer, Roche, Eli Lilly, BMS, and UCB. DREAM-RA is funded by AbbVie, Roche, Pfizer, UCB, and BMS.
TK has received fees for speaking and/or consulting from AbbVie, BMS, Celgene, Celltrion, Eli Lilly, Hospira, Merck-Serono, MSD, Orion Pharma, Pfizer, Roche, Sandoz, and UCB, and received research funding for Diakonhjemmet Hospital from AbbVie, BMS, MSD, Pfizer, Roche, and UCB. NOR-DMARD was previously supported with research funding to Diakonhjemmet Hospital from AbbVie, BMS, MSD/Schering-Plough, Pfizer/Wyeth, Roche, and UCB. RvV has received fees for speaking and/or consulting from AbbVie, Biotest, BMS, Crescendo, GSK, Janssen, Lilly, Merck, Pfizer, Roche, UCB, and Vertex, and research grants from AbbVie, BMS, GSK, Pfizer, Roche, and UCB. No non-financial conflicts of interest exist for any of the authors.
Authors’ contributions
KC, EL, EN, GL, MH, UT, IA, KP, DN, CG, HC, MT, PvR, JG-R, TK, and RvV contributed equally and significantly in the design and coordination of the study, collection and analysis of data, and in the preparation of the manuscript. All authors have read and approved the final manuscript.
Contributor Information
Katerina Chatzidionysiou, Phone: 0046 762119018, Email: Aikaterini.chatzidionysiou@karolinska.se.
Elisabeth Lie, Email: Elisabeth_lie@yahoo.no.
Evgeny Nasonov, Email: nasonov@irramn.ru.
Galina Lukina, Email: gvl3@yandex.ru.
Merete Lund Hetland, Email: merete.hetland@dadlnet.dk.
Ulrik Tarp, Email: ulrik.tarp@ki.au.dk.
Ioan Ancuta, Email: iancuta@hotmail.com.
Karel Pavelka, Email: pavelka@revma.cz.
Dan C. Nordström, Email: dan.nordstrom@hus.fi
Cem Gabay, Email: Cem.Gabay@hcuge.ch.
Helene Canhão, Email: helenacanhao@gmail.com.
Matija Tomsic, Email: matija.tomsic@kclj.si.
Piet L. C. M. van Riel, Email: p.vanriel@reuma.umcn.nl
Juan Gomez-Reino, Email: Juan.Jesus.Gomez-Reino.Carnota@sergas.es.
Tore K. Kvien, Email: t.k.kvien@medisin.uio.no
Ronald F. van Vollenhoven, Email: ronald.van.vollenhoven@ki.se
References
- 1.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 3.Vander Cruyssen B, Durez P, Westhovens R, Kaiser MJ, Hoffman I, De Keyser F, et al. The Belgian MIRA (MabThera In Rheumatoid Arthritis) registry: clues for the optimization of rituximab treatment strategies. Arthritis Res Ther. 2010;12:R169. doi: 10.1186/ar3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, et al. Highest clinical effectiveness of rituximab in autoantibody-positive patients with rheumatoid arthritis and in those for whom no more than one previous TNF antagonist has failed: pooled data from 10 European registries. Ann Rheum Dis. 2011;70:1575–80. doi: 10.1136/ard.2010.148759. [DOI] [PubMed] [Google Scholar]
- 5.Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)) Ann Rheum Dis. 2010;69:1629–35. doi: 10.1136/ard.2009.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubbert-Roth A, Tak PP, Zerbini C, Tremblay JL, Carreno L, Armstrong G, et al. Efficacy and safety of various repeat treatment dosing regimens of rituximab in patients with active rheumatoid arthritis: results of a Phase III randomized study (MIRROR) Rheumatology (Oxford) 2010;49:1683–93. doi: 10.1093/rheumatology/keq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 2006;54:1390–400. doi: 10.1002/art.21778. [DOI] [PubMed] [Google Scholar]
- 8.Tak PP, Rigby WF, Rubbert-Roth A, Peterfy CG, van Vollenhoven RF, Stohl W, et al. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann Rheum Dis. 2011;70:39–46. doi: 10.1136/ard.2010.137703. [DOI] [PubMed] [Google Scholar]
- 9.Chatzidionysiou K, Lie E, Nasonov E, Lukina G, Hetland ML, Tarp U, et al. Effectiveness of disease-modifying antirheumatic drug co-therapy with methotrexate and leflunomide in rituximab-treated rheumatoid arthritis patients: results of a 1-year follow-up study from the CERERRA collaboration. Ann Rheum Dis. 2012;71:374–7. doi: 10.1136/annrheumdis-2011-200003. [DOI] [PubMed] [Google Scholar]
- 10.Bredemeier M, de Oliveira FK, Rocha CM. Low- versus high-dose rituximab for rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2014;66:228–35. doi: 10.1002/acr.22116. [DOI] [PubMed] [Google Scholar]
- 11.Leandro MJ, Edwards JC, Cambridge G. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann Rheum Dis. 2002;61:883–8. doi: 10.1136/ard.61.10.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vital EM, Rawstron AC, Dass S, Henshaw K, Madden J, Emery P, et al. Reduced-dose rituximab in rheumatoid arthritis: efficacy depends on degree of B cell depletion. Arthritis Rheum. 2011;63:603–8. doi: 10.1002/art.30152. [DOI] [PubMed] [Google Scholar]
- 13.Leandro MJ. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res Ther. 2013;15 Suppl 1:S3. doi: 10.1186/ar3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 15.Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–66. [PubMed] [Google Scholar]
- 16.Tak PP, Rigby W, Rubbert-Roth A, Peterfy C, van Vollenhoven RF, Stohl W, et al. Sustained inhibition of progressive joint damage with rituximab plus methotrexate in early active rheumatoid arthritis: 2-year results from the randomised controlled trial IMAGE. Ann Rheum Dis. 2012;71:351–7. doi: 10.1136/annrheumdis-2011-200170. [DOI] [PMC free article] [PubMed] [Google Scholar]


