Abstract
Introduction:
Chronic orchialgia is historically and currently a challenging disease to treat. It is a diagnostic and therapeutic challenge for physicians. Conservative therapy has served as the first line of treatment. For those who fail conservative therapy, surgical intervention may be required. We aim to provide a review of currently available surgical options and novel surgical treatment options.
Methods:
A review of current literature was performed using PubMed. Literature discussing treatment options for chronic orchialgia were identified. The following search terms were used to identify literature that was relevant to this review: Chronic orchialgia, testicular pain, scrotal content pain, and microsurgical denervation of the spermatic cord (MDSC).
Results:
The incidence of chronic orchialgia has been increasing over time. In the USA, it affects up to 100,000 men per year due to varying etiologies. The etiology of chronic orchialgia can be a confounding problem. Conservative therapy should be viewed as the first line therapy. Studies have reported poor success rates. Current surgical options for those who fail conservative options include varicocelectomy, MDSC, epididymectomy, and orchiectomy. Novel treatment options include microcryoablation of the peri-spermatic cord, botox injection, and amniofix injection.
Conclusion:
Chronic orchialgia has been and will continue to be a challenging disease to treat due to its multiple etiologies and variable treatment outcomes. Further studies are needed to better understand the problem. Treatment options for patients with chronic orchialgia are improving. Additional studies are warranted to better understand the long-term durability of this treatment options.
Key words: Chronic orchialgia, chronic scrotal pain, testicular pain
INTRODUCTION
Chronic orchialgia is historically and currently a challenging disease to treat. It provides a diagnostic and therapeutic challenge for physicians. It has been defined as intermittent or constant unilateral or bilateral scrotal pain, which lasts at least 3 months, that leads to a loss in normal activities of daily living. For many patients, return to daily activity is a goal of treatment. Conservative therapy (i.e. – nonsteroidal anti-inflammatory drugs, analgesic, antidepressants, etc.) has served as the first line of treatment. For those, who fail conservative therapy, surgical intervention may be required. This article provides an overview of chronic orchialgia with a specific focus on current new surgical therapies to aid in the management of this difficult problem.
METHODS
A review of current literature was performed using PubMed. Literature discussing treatment options for chronic orchialgia were identified. The following search terms were used to identify literature that was relevant to this review: Chronic orchialgia, testicular pain, scrotal content pain, and microsurgical denervation of the spermatic cord (MDSC).
EPIDEMIOLOGY
The incidence of chronic orchialgia has been increasing over time. This may be due to increased sensitivity amongst providers to address this problem. Though the incidence varies geographically, it is a common problem that has led to increase time and cost in diagnosing and treating these patients. In The Netherlands, the incidence is estimated at 350–400 cases per 100,000 men per year.[1] In the USA, it affects up to 100,000 men per year due to varying etiologies.[2] It has also been found to be the most common urologic reason for medical discharge from the USA army.[3] In the United Kingdom, the incidence has been estimated around 1%.[1]
ETIOLOGY/PATHOPHYSIOLOGY
The etiology of chronic orchialgia can be a confounding problem. Possible causes of chronic orchialgia can vary from infectious to iatrogenic, to idiopathic. The pain may be the result of prior vasectomy, inguinal hernia surgery, recurrent epididymitis, varicoceles, hydroceles, prior scrotal/abdominal surgery, and trauma or idiopathic in nature. Less common causes are diabetic neuropathy, retroperitoneal tumors, abdominal aortic aneurysms, and polyarteritis nodosa.[4,5,6,7,8,9,10,11,12,13,14,15,16] Men with chronic pelvic pain syndromes (CPPS) may suffer from orchialgia. These two entities may, in fact, be on the same spectrum of disease. Intraprostatic reflux, which can incite CPPS, may be a link to chronic orchialgia. The exposure of the reproductive tract to the bacterial source could be an inciting source. Pelvic floor spasms play a role in CPPS and may also play a role in chronic orchialgia. The etiology of chronic orchialgia, as with CPPS, is most likely multifactorial.
The definite pathophysiology of pain is not clearly understood. A commonly accepted theory is that there is a hypersensitivity of the pain sensory fibers in the peripheral neural pathways. The ilioinguinal, genital branch of the genitofemoral, or pudendal nerves are the most commonly affected nerves.[12,17,18,19,20,21] Hypersensitivity of pain sensory fibers may occur due to neural plasticity. The ability of our nervous system, both centrally and peripherally, to adapt when damaged or inflamed by altering gene expression can change the structure of the nerve or adapt the chemical and/or receptor profile. This can lead to lower threshold potentials in the neuropathic pathways. Furthermore, branches of the pelvic plexus decussate to the contralateral pelvic plexus, which may create contralateral pain phenomena.[22]
Another possible explanation for hypersensitivity in these fibers is Wallerian degeneration in these peripheral nerves. Wallerian degeneration is the auto-destruction of the proximal and distal axon, leading to an environment clear of inhibitory debris, and supportive of axon regrowth and functional recovery. aAn immune cell response initiated by neutrophils, cytokines, and macrophages is subsequently activated. This inflammatory change leads to neural hypersensitivity.[23,24,25]
Parekattil et al. found a high density of nerves with wallerian degeneration in three primary locations in the spermatic cord of patients with chronic orchialgia.[2] The three areas were the cremasteric muscle fibers, perivasal tissues/vasal sheath, and posterior peri-arterial lipomatous tissue. They termed these areas the “trifecta nerve complex.” Ablation of this complex is postulated as the possible basis for the success of MDSC as a treatment for chronic orchialgia.
DIAGNOSIS
A detailed history discussing past medical, surgical, traumatic, and sexual history is very important in assessing a patient with testicular pain. Parekattil et al. suggested a pain distribution classification system for patients with chronic orchialgia in order to better identify the location of pain.[2] Patient description of pain and location will aid in ruling out treatable causes such as referred pain from a ureteral stone.
Visual Analog scale is the most commonly employed tool use to quantify patient's pain level. This provides an avenue to compare pre- and post-treatment pain levels. A more objective tool for measuring pain level is the PIQ-6. The PIQ-6 is a validated questionnaire developed by RAND Corporation that can be used for monitoring patient pain levels throughout the treatment regimen.
The genitals are the main focus of the physical exam. An examination of the patient in a standing and supine position is best. The examination should begin on the nonpainful side. Palpation of the testis, epididymis, and vas deferens should be performed. A digital rectal exam may provide further information on patients that may be in the spectrum of CPPS with pelvic floor muscle tension. Laboratory evaluation should include a urinalysis and urine culture. A scrotal ultrasound remains the primary imaging study. Consideration should be given to performing computed tomography scan of abdomen and pelvis due to the possibility of ureteral stones leading to referred pain or inguinal hernias. Magnetic resonance imaging of the spine may be beneficial in patients with a history of back or hip pain. Studies report patients undergo an average of 4.7–7.2 diagnostic procedures and 1.6 operative procedures.[3,26] In addition, a spermatic cord block can serve as both diagnostic and therapeutic for patients with chronic orchialgia. Spermatic cord block is recommended prior to performing invasive surgical procedures.
TREATMENT
Conservative therapy
Current conservative therapy for chronic orchialgia include heat, ice, scrotal elevation, antibiotics with objective evidence of infection, analgesics, α-adrenergic antagonists, anti-inflammatory agents, antidepressants with doxepin, and amitriptyline seeming to have better efficacy than others, anticonvulsants, regional and local nerve blocks, physical therapy, biofeedback, acupuncture and psychotherapy for at least 3 months.[21,27,28,29] Conservative therapy should be viewed as the first line therapy for these patients. Though it is considered the first line, conservative therapies are frequently ineffective. Studies have reported on poor success rate with two studies showing a 15.2% and 4.2% success rate, respectively.[30,31]
Surgical options
For those patients who fail conservative therapy, surgical treatment should be considered.
The possible cause of pain will help guide surgical options [Figure 1]. Patients who have undergone vasectomy should consider vasectomy reversal if pain appears congestive in nature with the fullness of epididymis and association of pain with sexual intercourse. Nangia et al. reported 69% success rate for patients with postvasectomy pain who underwent vasectomy reversal.[16]
Figure 1.
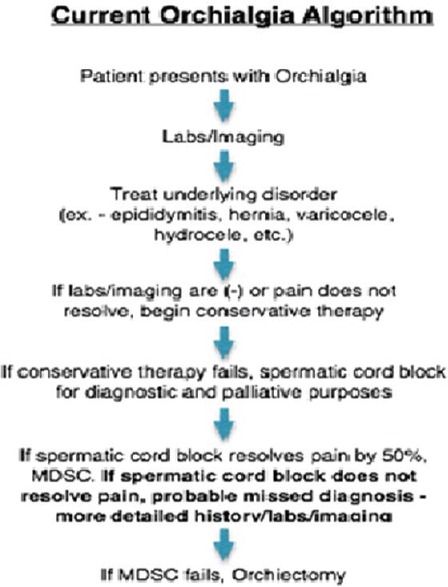
Old chronic orchialgia treatment algorithm
Diagnosis of a varicocele should lead to a varicocelectomy in patients with chronic orchialgia. Studies have shown that varicoceles are present in 2–10% men with orchialgia.[32] Maghraby reported 84.5% complete response rate and 10.3% partial response with only 3/58 patients having persistent symptoms after laparoscopic varicocelectomy.[33] Another study reported 114 patients with painful varicoceles were operated by the microsurgical inguinal approach. The overall response was 91.2% while 8.8% had pain postoperatively.[34] In another study, 72.4% responded while 27.6 did not respond to microsurgical sub-inguinal varicocelectomy[30] Park et al. used microsurgical inguinal or sub-inguinal approach and reported complete, partial, and no response in 52.8, 41.5, and 5.7%, respectively.[35]
For patients with idiopathic pain or who have failed other surgical options, MDSC has been shown to have very good outcomes. MDSC was spearheaded Devine and Schellhammer in 1978.[7] Levine et al. initially described total pain relief following MDSC in 6 out of 7 patients with chronic orchialgia.[17] Strom and Levine reported complete pain relief in 71%, partial pain relief in 17%, and no relief in 11% of men in a larger follow-up study of 95 testicular units (79 men).[21] Oliveira et al. reported similar results in a cohort of 60 men in a recent international, multi-institutional study. They reported 70% complete resolution of pain and 20% partial resolution of pain.[20] Complications include testicular atrophy, wound infection, hydrocele, and incisional hematoma.
Traditional MDSC is performed with an operating microscope. Parekattil and Gudeloglu reported on a series of patients undergoing Robot-assisted MDSC. The da Vinci robotic system with high definition digital visual magnification is used place of an operating microscope. They reported similar results with 72% of patients experiencing complete resolution of pain and 14% with >50% reduction in pain out of a cohort of 401 patients.[36]
Epididymectomy and orchiectomy are last resort surgical options with mixed results. One study showed greater outcomes (92% patient satisfaction) in those patients with pain from epididymal cysts rather than patients with epididymitis or epididymalgia (43% patient satisfaction).[37] Another long-term study showed a 90% patient satisfaction from epididymectomy in patients with postvasectomy epididymal pain or obstructive pain from previous hernia repair.[38] Chen and Ball performed epididymectomy to treat chronic orchialgia with a 50% success rate.[5] Inguinal orchiectomy relieved the pain 73% of men when compared to 55% in men who underwent scrotal orchiectomy for postvasectomy pain syndrome.[27] Men who elect to proceed with orchiectomy must be counseled on the risk of continued pain and hypogonadism.[39]
NOVEL THERAPIES
Ultrasound guided targeted microcryoablation of the peri-spermatic cord
Studies have shown that nerves are sensitive to freezing injury. Nerves can be completely desensitized when the nerve is exposed to −15°C to −20°C.[40,41,42,43,44] Men who fail MDSC may have residual nerve fibers around the spermatic cord as the source of persistent pain. Freezing these nerves is a noninvasive alternative treatment for patient [Figure 2].
Figure 2.
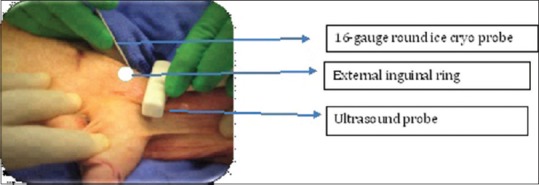
Under percutaneous ultrasound guidance determine left spermatic cord from lateral side at the level of external inguinal ring
Procedure
Ultrasound guided targeted microcryoablation (UTM) is performed under general anesthesia. The patient is placed in a supine position with the inguinal and genital region prepared for the procedure. A spermatic cord block is performed. An ultrasound probe is used to identify the spermatic cord at the external inguinal ring. An Endocare™ 1.7 mm round ice Cryoprobe is percutaneously placed into the peri-spermatic tissue on the medial and lateral aspect of the spermatic cord under ultrasound guidance at the external inguinal ring. The tissue is cooled to −40°C for 90 s. The cryoprobe is passively thawed and removed.
Outcomes
A cohort of 60 patients underwent UTM from November 2012 to August 2014. All patients had failed prior MDSC. 74% of patients experience a significant reduction in pain (9% complete resolution, 65% reported a >50% reduction in pain) at a median follow-up of 11 months. At 6 months and 1 year follow-ups, objective PIQ-6 was completed with the following outcomes: Significant reduction in pain in 59% of patients at 6 months and 56% at 1 year postoperative. The only complications were a wound infection and one patient developed penile pain.
Botox injection
Botulinum-A toxin has been shown to modulate the release of neuropeptides (substancePand calcitonin gene-related peptide) leading to inhibition of neurogenic inflammation and chronic pain.[45] Neurogenic inflammation leading to Wallerian degeneration of the neural fibers in the spermatic cord has been discussed as a possible pathologic cause of pain in patients with chronic orchialgia. The inhibitory effect of Botox on neurogenic inflammation provides could provide an antinociceptive effect. Botox has been used in orthopedics to treat intra-articular pain and in neurology for migraine headaches.
Procedure
100 units of botox were diluted in 10cc of saline. The mixture was injected medial and lateral to the spermatic cord at the external inguinal ring [Figure 3]. This was performed with the patient under conscious sedation.
Figure 3.
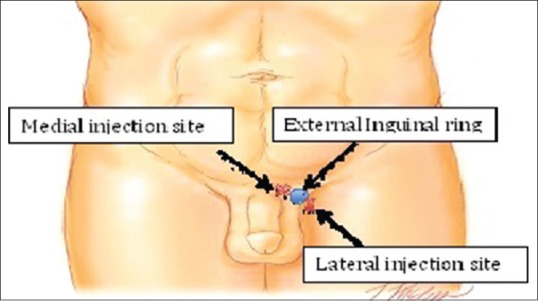
Injection sites for botox and amniofix injections
Outcomes
A cohort of 25 patients underwent botox injection. Based on visual analog scale, 70% of patients experienced a significant reduction in pain (14% complete resolution, 56% reported a >50% reduction in pain) with a median follow-up of 8 months. Analysis with the more objective PIQ-6 questionnaire revealed a significant reduction in pain in 40% of patients at 6 months and 20% at 1 year postoperative. There were no complications in our small cohort.
Amniofix injection
Injectable dehydrated amniotic/chorionic membrane allograft (AmnioFix®) is substance derived from human amniotic membrane. Amniofix has been shown to reduce scar tissue formation, reduce inflammation, and enhance healing. Patients with orchialgia have been shown to have neurogenic inflammation around the spermatic cord. Because of its anti-inflammatory properties, it may offer another noninvasive treatment option for difficult orchialgia patients.
Procedure
Amniofix injectable form is injected medial and lateral to the spermatic cord at the level of the external inguinal ring [Figure 3].
Outcomes
A cohort of 14 patients underwent treatment with amniofix injection over a mean follow-up period of 6 months. Amniofix was injected medial and lateral to cord at the external inguinal ring. The injection was done under ultrasound guidance. Over this period, 50% of patients expressed a significant reduction in their pain after treatment with amniofix. All patients had undergone conservative treatments, spermatic cord blocks, and microsurgical neurolysis prior to attempt with amniofix. There were no complications in this cohort of patients.
CONCLUSION
Chronic orchialgia has been and will continue to be a challenging disease to treat due to its multiple etiologies and variable treatment outcomes. Further studies are needed to better understand the problem. Treatment options for patients with chronic orchialgia are improving [Figure 4]. Additional studies are warranted to better understand the long-term durability of this treatment options.
Figure 4.
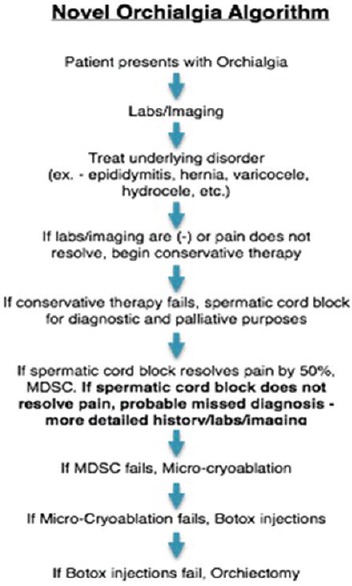
Proposed new treatment algorithm for chronic orchialgia
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Strebel RT, Leippold T, Luginbuehl T, Muentener M, Praz V, Hauri D. Chronic scrotal pain syndrome: Management among urologists in Switzerland. Eur Urol. 2005;47:812–6. doi: 10.1016/j.eururo.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Parekattil SJ, Gudeloglu A, Brahmbhatt JV, Priola KB, Vieweg J, Allan RW. Trifecta nerve complex: Potential anatomical basis for microsurgical denervation of the spermatic cord for chronic orchialgia. J Urol. 2013;190:265–70. doi: 10.1016/j.juro.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 3.Costabile RA, Hahn M, McLeod DG. Chronic orchialgia in the pain prone patient: The clinical perspective. J Urol. 1991;146:1571–4. doi: 10.1016/s0022-5347(17)38169-7. [DOI] [PubMed] [Google Scholar]
- 4.Bruning CO., 3rd Re: Vasectomy reversal for treatment of the post-vasectomy pain syndrome. J Urol. 1997;158:1528. doi: 10.1016/s0022-5347(01)64267-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen TF, Ball RY. Epididymectomy for post-vasectomy pain: Histological review. Br J Urol. 1991:407–13. doi: 10.1111/j.1464-410x.1991.tb15362.x. [DOI] [PubMed] [Google Scholar]
- 6.Choe JM, Kirkemo AK. Questionnaire-based outcomes study of nononcological post-vasectomy complications. J Urol. 1996;155:1284–6. [PubMed] [Google Scholar]
- 7.Devine CJ, Jr, Schellhammer PF. The use of microsurgical denervation of the spermatic cord for orchialgia. Trans Am Assoc Genitourin Surg. 1978;70:149–51. [PubMed] [Google Scholar]
- 8.Dickinson KJ, Thomas M, Fawole AS, Lyndon PJ, White CM. Predicting chronic post-operative pain following laparoscopic inguinal hernia repair. Hernia. 2008;12:597–601. doi: 10.1007/s10029-008-0408-7. [DOI] [PubMed] [Google Scholar]
- 9.Edwards IS. Vasectomy reversal for treatment of the post-vasectomy pain syndrome. J Urol. 1997;158:2252. doi: 10.1016/s0022-5347(01)68223-5. [DOI] [PubMed] [Google Scholar]
- 10.Loos MJ, Roumen RM, Scheltinga MR. Classifying post-herniorrhaphy pain syndromes following elective inguinal hernia repair. World J Surg. 2007;31:1760–5. doi: 10.1007/s00268-007-9121-4. [DOI] [PubMed] [Google Scholar]
- 11.Massaron S, Bona S, Fumagalli U, Battafarano F, Elmore U, Rosati R. Analysis of post-surgical pain after inguinal hernia repair: A prospective study of 1,440 operations. Hernia. 2007;11:517–25. doi: 10.1007/s10029-007-0267-7. [DOI] [PubMed] [Google Scholar]
- 12.McLoughlin J, Kelley CJ. Study of the effectiveness of bupivicaine infiltration of the ilioinguinal nerve at the time of hernia repair for post-operative pain relief. Br J Clin Pract. 1989;43:281–3. [PubMed] [Google Scholar]
- 13.McMahon AJ, Buckley J, Taylor A, Lloyd SN, Deane RF, Kirk D. Chronic testicular pain following vasectomy. Br J Urol. 1992;69:188–91. doi: 10.1111/j.1464-410x.1992.tb15494.x. [DOI] [PubMed] [Google Scholar]
- 14.Morris C, Mishra K, Kirkman RJ. A study to assess the prevalence of chronic testicular pain in post-vasectomy men compared to non-vasectomised men. J Fam Plann Reprod Health Care. 2002;28:142–4. doi: 10.1783/147118902101196298. [DOI] [PubMed] [Google Scholar]
- 15.Myers SA, Mershon CE, Fuchs EF. Vasectomy reversal for treatment of the post-vasectomy pain syndrome. J Urol. 1997;157:518–20. [PubMed] [Google Scholar]
- 16.Nangia AK, Myles JL, Thomas AL., JR Vasectomy reversal for the post-vasectomy pain syndrome: A clinical and histological evaluation. J Urol. 2000;164:1939–42. [PubMed] [Google Scholar]
- 17.Levine LA, Matkov TG, Lubenow TR. Microsurgical denervation of the spermatic cord: A surgical alternative in the treatment of chronic orchialgia. J Urol. 1996;155:1005–7. doi: 10.1016/s0022-5347(01)66369-9. [DOI] [PubMed] [Google Scholar]
- 18.Levine LA, Matkov TG. Microsurgical denervation of the spermatic cord as primary surgical treatment of chronic orchialgia. J Urol. 2001;165(6 Pt 1):1927–9. doi: 10.1097/00005392-200106000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Levine LA. Microsurgical denervation of the spermatic cord. J Sex Med. 2008;5:526–9. doi: 10.1111/j.1743-6109.2007.00762.x. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira RG, Camara C, Alves Jde M, Coelho RF, Lucon AM, Srougi M. Microsurgical testicular denervation for the treatment of chronic testicular pain initial results. Clinics (Sao Paulo) 2009;64:393–6. doi: 10.1590/S1807-59322009000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strom KH, Levine LA. Microsurgical denervation of the spermatic cord for chronic orchialgia: Long-term results from a single center. J Urol. 2008;180:949–53. doi: 10.1016/j.juro.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Taguchi K, Tsukamoto T, Murakami G. Anatomical studies of the autonomic nervous system in the human pelvis by the whole-mount staining method: Left-right communicating nerves between bilateral pelvic plexuses. J Urol. 1999;161:320–5. [PubMed] [Google Scholar]
- 23.Dubový P. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann Anat. 2011;193:267–75. doi: 10.1016/j.aanat.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granitsiotis P, Kirk D. Chronic testicular pain: An overview. Eur Urol. 2003;45:430–6. doi: 10.1016/j.eururo.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Heidenreich A, Olbert P, Engelmann UH. Management of chronic testalgia by microsurgical testicular denervation. Eur Urol. 2002;41:392–7. doi: 10.1016/s0302-2838(02)00023-4. [DOI] [PubMed] [Google Scholar]
- 27.Davis BE, Noble MJ, Weigel JW, Foret JD, Mebust WK. Analysis and management of chronic testicular pain. J Urol. 1990;143:936–9. doi: 10.1016/s0022-5347(17)40143-1. [DOI] [PubMed] [Google Scholar]
- 28.Nickel JC, Siemens DR, Nickel KR, Downey J. The patient with chronic epididymitis: Characterization of an enigmatic syndrome. J Urol. 2002;167:1701–4. [PubMed] [Google Scholar]
- 29.Tracy CR, Steers WD, Costabile R. Diagnosis and management of epididymitis. Urol Clin North Am. 2008;35:101–8; vii. doi: 10.1016/j.ucl.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Chen SS. Factors predicting symptomatic relief by varicocelectomy in patients with normospermia and painful varicocele nonresponsive to conservative treatment. Urology. 2012;80:585–9. doi: 10.1016/j.urology.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Yaman O, Ozdiler E, Anafarta K, Gögüs O. Effect of microsurgical subinguinal varicocele ligation to treat pain. Urology. 2000;55:107–8. doi: 10.1016/s0090-4295(99)00374-x. [DOI] [PubMed] [Google Scholar]
- 32.Abrol N, Panda A, Kekre NS. Painful varicoceles: Role of varicocelectomy. Indian J Urol. 2014;30:369–73. doi: 10.4103/0970-1591.128497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maghraby HA. Laparoscopic varicocelectomy for painful varicoceles: Merits and outcomes. J Endourol. 2002;16:107–10. doi: 10.1089/089277902753619627. [DOI] [PubMed] [Google Scholar]
- 34.Kim HT, Song PH, Moon KH. Microsurgical ligation for painful varicocele: Effectiveness and predictors of pain resolution. Yonsei Med J. 2012;53:145–50. doi: 10.3349/ymj.2012.53.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park HJ, Lee SS, Park NC. Predictors of pain resolution after varicocelectomy for painful varicocele. Asian J Androl. 2011;13:754–8. doi: 10.1038/aja.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parekattil SJ, Gudeloglu A. Robotic assisted andrological surgery. Asian J Androl. 2013;15:67–74. doi: 10.1038/aja.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padmore DE, Norman RW, Millard OH. Analyses of indications for and outcomes of epididymectomy. J Urol. 1996;156:95–6. [PubMed] [Google Scholar]
- 38.West AF, Leung HY, Powell PH. Epididymectomy is an effective treatment for scrotal pain after vasectomy. BJU Int. 2000;85:1097–9. doi: 10.1046/j.1464-410x.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 39.Parvis KK, Costabile RA. Orchialgia and the chronic pelvic pain syndrome. World J Urol. 2013;31:773–8. doi: 10.1007/s00345-013-1092-5. [DOI] [PubMed] [Google Scholar]
- 40.Beazley RM, Bagley DH, Ketcham AS. The effect of cryosurgery on peripheral nerves. J Surg Res. 1974;16:231–4. doi: 10.1016/0022-4804(74)90036-5. [DOI] [PubMed] [Google Scholar]
- 41.Breidenach LM, Thomford N, Pace WG. Cryosurgery of tumors involving the facial nerve. Arch Surg. 1972;105:306–7. doi: 10.1001/archsurg.1972.04180080154025. [DOI] [PubMed] [Google Scholar]
- 42.Carter DC, Lee PW, Gill W, Johnston RJ. The effect of cryosurgery on peripheral nerve function. J R Coll Surg Edinb. 1972;17:25–31. [PubMed] [Google Scholar]
- 43.Mandeville AF, McCabe BF. Some observations on the cryobiology of blood vessels. Laryngoscope. 1967;77:1328–50. doi: 10.1288/00005537-196708000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Whittaker DK. Degeneration and regeneration of nerves following cryosurgery. Br J Exp Pathol. 1974;55:595–600. [PMC free article] [PubMed] [Google Scholar]
- 45.Morré HH, Keizer SB, van Os JJ. Treatment of chronic tennis elbow with botulinum toxin. Lancet. 1997;349:1746. doi: 10.1016/s0140-6736(05)62958-3. [DOI] [PubMed] [Google Scholar]


