Abstract
Introduction:
Development of regional surveillance programs is necessary for the development of community-acquired urinary tract infection (UTI) guidelines, especially for sub-urban and rural areas where empirical treatment is the mainstay in the absence of proper diagnostic modalities. Our aim was to evaluate the bacteriological profile and antibiotic sensitivity patterns in children with UTI prospectively from a tertiary care center.
Methods:
A total of 800 children up to 18 years of age with suspected UTI attending our center were included. For all suspected cases urine microscopy, gram staining, and culture were done. Antibiotic sensitivity was performed on selected antimicrobials using disk diffusion method following Clinical Laboratory Standards Institute guidelines.
Results:
Majority of pathogens were isolated from female (54.2%) patients. Pre-teens (52.1%) and teens (27.1%) were most commonly affected age group. The most common presentation in culture-proven UTI was fever with urinary symptoms (33.3%). In a group of 192 patients 26.7% had proven UTI. Escherichia coli (42.3%) was the most common aetiological agent, followed by Enterococcus fecalis (13.5%), Klebsiella spp. (11.5%) and Staphylococcus aureus (11.5%). Most active antibiotics against Gram-negative isolates were nitrofurantoin, cefotaxime, and amikacin. Gram-positive isolates were sensitive to nitrofurantoin, cotrimoxazole, and novobiocin.
Conclusion:
E. coli was the commonest isolate. The organisms grown in significant numbers were E. fecalis, Klebsiella spp. and S. aureus, causing UTI in 0–18 years of age group. Gram-negative isolates were sensitive to nitrofurantoin, amikacin, and cefotaxime. Gram-positive isolates were sensitive to nitrofurantoin, cotrimoxazole, and novobiocin. Prospective, regional studies are ensured periodically to explain bacteriological profile and antibiotic sensitivity patterns to be applicable for children with UTI over that geographic area.
Key words: Antibiotic sensitivity, antimicrobial agents, bacteriological profile, community acquired urinary tract infection, pediatric urinary tract infections
INTRODUCTION
Urinary tract infections (UTIs) are common bacterial infections in children. The diagnosis of UTI is very often missed in young children due to minimal and nonspecific symptoms. The developing renal cortex in young children is vulnerable to renal scarring resulting in hypertension and chronic renal failure. These morbidities in adults often have their origin in childhood. A clinically suspected case of UTI should be defined and documented with urine culture report. After the diagnosis of UTI, its category should be defined. This helps in guiding a clinician about the appropriate radio/nuclear imaging evaluation, choice of antimicrobial agent, duration of treatment and need of chemoprophylaxis. Even a single confirmed UTI should be taken seriously.[1]
Etiological agents of UTI are variable and usually depend on time, geographical location and age of patients. However, Escherichia coli, Proteus mirabilis, Enterobacter agglomerans, Citrobacter frreundii and Klebsiella pneumonia account for over 70% of cases.[2,3]
The exact information on etiology and resistance pattern of community-acquired pediatric UTIs in a region is usually not available, and if available it is outdated as antimicrobial sensitivity patterns are bound to change over a period of time. This study aims to facilitate the empiric treatment of patients with symptoms of UTIs. Moreover, the data would also help authorities to formulate antibiotic prescription policies, at least for a region.[4]
METHODS
A prospective study was performed on 800 children with suspected UTI attending pediatric outdoor patient clinic, admitted to pediatric ward, Paediatric Intensive Care Unit (PICU) and Neonatal Intensive Care Unit (NICU) of a tertiary care centre (1st day admission) from January 2012 to July 2014. The study was conducted in accordance with the guidelines of the International Conference on Harmonization/World Health Organization Good Clinical Practice Standards and the principles of the Declaration of Helsinki. An Ethical Review Board approved the study protocol for this site, and the patients granted written informed consent before any study procedures.
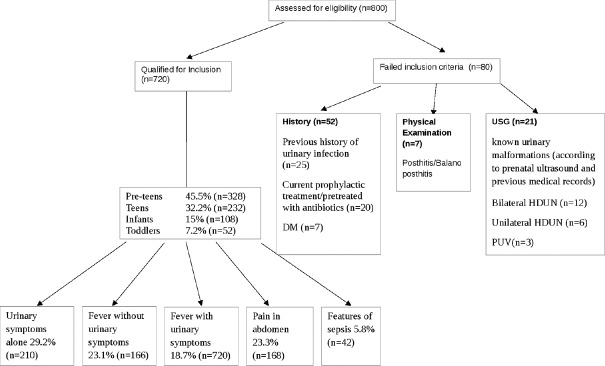
All children up to 18 years of age with urinary symptoms alone (frequency, dysuria, suprapubic pain), fever with urinary symptoms, fever without urinary symptoms, pain in abdomen with no previous history of UTI were included. Neonates with features of sepsis (i.e., poor feeding, jaundice or altered sensorium) were also included. The following cases were excluded: Previous history of urinary infection (documented previous urinary culture sensitivity report), known urinary malformations (according to prenatal ultrasound and previous medical records), chronic illness, or current prophylactic treatment/pretreated with antibiotics (within 4 weeks of presentation) [Figure 1].
Figure 1.
Flowchart showing patients’ enrolment in study
For all suspected cases of UTI urine microscopy, gram staining and culture were done for the patients admitted to the pediatric ward, PICU or NICU on 1st day and on the same day on OPD basis. Urine sample (10 ml) was collected by suprapubic aspiration in neonates, mid-stream clean-catch and at the time of first insertion/in and out sampling in the rest of children. The processing of urinary samples consisted of urine microscopy, gram staining, and culture. Wet mount microscopy was done on a well-mixed uncentrifuged urine sample to detect white blood cells (WBCs), red blood cells, yeast and epithelial cells. Isolates were identified by gram stain and biochemical reactions. The presence of at least 1 organism per oil immersion field in uncentrifuged urine corresponds to a colony of 105 CFU/ml. Urine culture was done on Cysteine Lactose Electrolyte Deficient agar (CLED media [Hi-Media, Mumbai, India] [Semi-Quantitative method]) and colony count done after overnight incubation at 37°C. Numbers of colonies obtained were multiplied by 1000 to obtain the colony forming units (CFU)/ml. Guidelines by Hellerstein[5] were strictly adhered to for the diagnosis of pediatric UTI. Antibiotic sensitivity was performed using Kirby-Baurer disk diffusion method following the Clinical Laboratory Standards Institute guidelines.[6]
E. coli ATCC 25922, Staphylococcus aureus ATCC 29213, Pseudomonas aeruginosa ATCC 27853, E. faecalis ATCC 29212 strains were tested for cefotaxime (30 mcg), cefpirome (30 mcg), amikacin (30 mcg), cotrimoxazole (25 mcg), norfloxacin (10 mcg), nitrofurantoin (300 mcg), sparfloxacin (5 mcg), nalidixic acid (30 mcg) and novobiocin (30 mcg). Diagnostic threshold for significant bacteriuria was considered as 105 CFU/ml for clean-catch voiding in girls (repeat testing if 10,000–100,000 CFU/ml), 104 CFU for clean-catch voiding in boys and catheter sampling (repeat testing if 1000–10,000 CFU/ml), any number of colonies for Gram-negative bacilli and >104 CFU/ml for Gram-positive cocci in suprapubic aspiration. Pyuria was defined as ≥5 WBCs/hpf of unspun urine, which corresponds to a colony count of >105 organisms/ml in fresh uncentrifuged urine.
Data were analyzed separately for four age groups: Infants (0–1 year), toddlers (>1–5 year), preteens (>5–12 year) and teens (>12–18 year). Data management and statistical analysis were performed using SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). The variables were analyzed using Chi-square test.
RESULTS
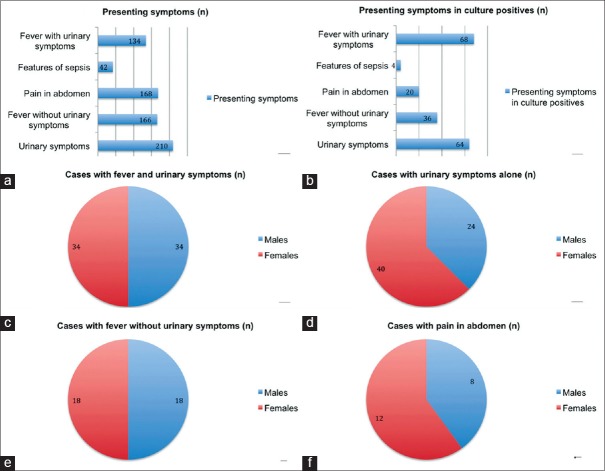
A total of 800 children with suspected UTI were evaluated, out of which 720 were enrolled in this study. Eighty cases did not meet the inclusion criteria. The most common age group presented with suspected UTI was preteens 45.5% (328/720), followed by teens 32.2% (232/720), infants 15% (108/720) and toddlers 7.2% (52/720), respectively. The presenting symptoms were urinary symptoms alone 29.2% (210/720), fever without urinary symptoms 23.1% (166/720), fever with urinary symptoms 18.7% (134/720), pain in abdomen 23.3% (168/720), and features of sepsis 5.8% (42/720) [Figure 2a].
Figure 2.
(a) Distribution of various presenting symptoms in cases recruited, (b) distribution of various presenting symptoms in culture positive cases, (c) sex wise distribution of culture proven cases presented with fever and urinary symptoms, (d) sex wise distribution of culture proven cases presented with urinary symptoms alone, (e) sex wise distribution of culture proven cases presented with fever without urinary symptoms, (f) sex wise distribution of culture proven cases presented with pain in abdomen
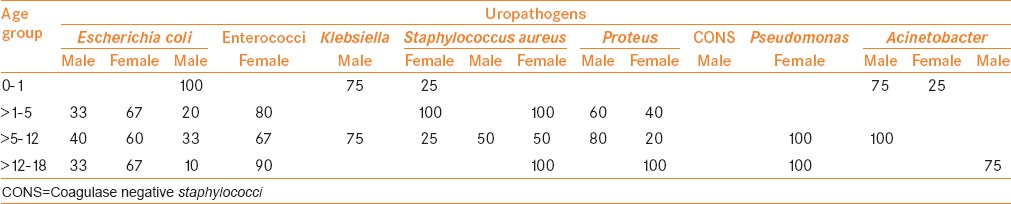
A total of 192 cases (26.7%) were culture positive. The most common age group was preteens 52.1% (100/192), followed by teens 27.1% (52/192), infants 8.33% (16/192) and toddlers 12.5% (24/192), respectively. Age and gender-wise distribution of uropathogenes was ascertained [Table 1]. Most culture proven cases presented with “fever with urinary symptoms” (n = 68, 35.4%) (category-5, Figure 2b). Fever with urinary symptoms was equally seen in males and females (34 in each) [Figure 2c]. Other symptom categories were urinary symptoms alone (n = 64; M:24, F:40), fever without urinary symptoms (n = 36; M:18, F:18) and pain abdomen (n = 20; M:8, F:12) [Figure 2d–f].
Table 1.
Age and gender wise distribution and frequency of uropathogens isolated from community-acquired infection
A total of 312 cases showed pyuria and culture negativity, 180 cases showed pyuria (on direct microscopy) and culture positivity, 12 cases had positive cultures with no pyuria and 23 cases of candiduria. A significant difference of direct microscopy and culture positivity is gained on Chi-square test with P ≤ 0.001.
Out of 192 culture positive cases, 16 (8.3%) had an infection with 2 types of bacteria whereas 176 (91.7%) had an infection with single organism [Figure 3a]. Of the 192 cases, UTI was more frequently found in females 104 (54.2%) as compared to males 88 (45.8%) [Figure 3b]. Out of total cases, we isolated a sum of 208 organisms.
Figure 3.
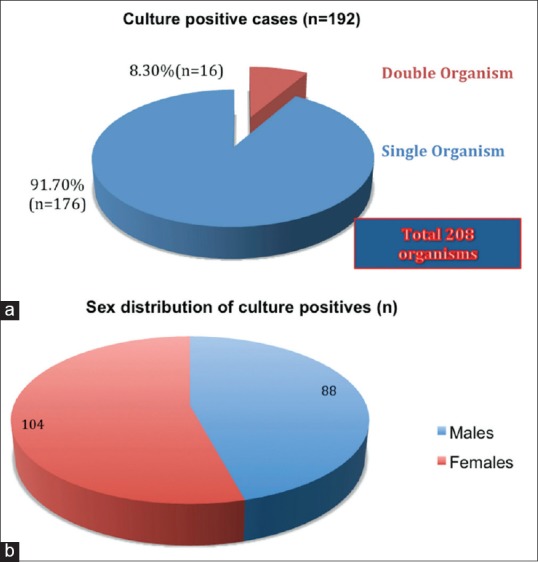
(a) Percentage distribution of number of organisms in culture proven urinary tract infection, (b) sex-wise distribution of culture positive cases
The predominant isolates were E. coli (42.3%), which showed significance (χ2 = 46.46, P < 0.01) [Table 1]. The predominant isolates were E. coli 42.3% (88/208) followed by enterococci 13.5% (28/208), S. aureus 11.5% (24/208) and Klebsiella spp. 11.5% (24/208), respectively. Proteus spp., coagulase negative staphylococci (CONS), P. aeruginosa and Acinetobacter spp. were the other organisms isolated.
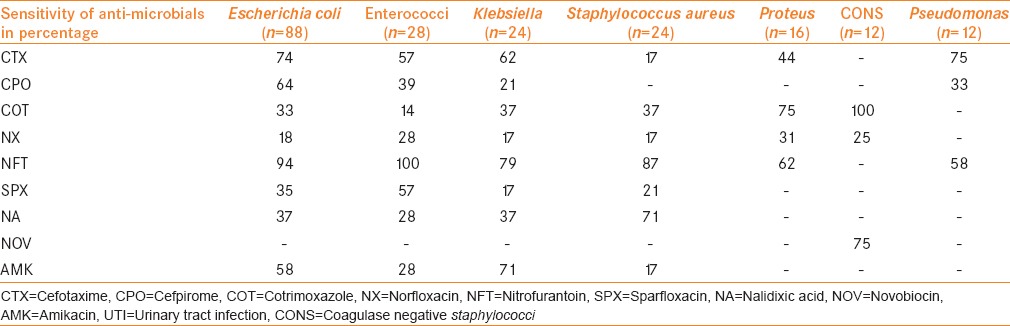
A panel of selected drugs on the most commonly found organisms confirmed antimicrobial sensitivity patterns [Table 2]. Among all the antimicrobials used nitrofurantoin had a widest coverage against E. coli Enterococcus fecalis, Klebsiella spp., S. aureus and Proteus spp. cefotaxime and amikacin showed high potency against Gram-negative organisms. Cotrimoxazole showed 100% coverage against CONS and good coverage against other organisms except Psedomonas auroginosa and Acenitobacter spp. Acenitobacter spp. were 100% sensitive to fluroquinolones (norfloxacin and sparfloxacin). Novobiocin showed sensitivity to CONS only.
Table 2.
Frequency and sensitivity pattern of UTI pathogens against nine selected antimicrobial agents
DISCUSSION
This study demonstrates the distribution and antibiotic susceptibility pattern of microbial species isolated from pediatric patients with suspected UTI from a tertiary care center. Among the children attending our center with symptoms suggestive of UTI, a significant percentage (26.7%) had culture positive, similar to study by Taneja et al., also from a tertiary center of northern India.[7] Mashouf et al. in a cross-sectional study performed on 912 children with UTI admitted to the pediatric department of a hospital in Iran had 34.2% cases with culture-proven UTI. This study included only admitted patients and had sicker patients. This appears to be the reason for higher positivity rates in their cultures.[3]
A majority of pathogens were isolated from female subjects (54.2%) in our study. T Akram et al. in their study analyzed age and gender-wise data of the prevalence of uropathogens in community-acquired urinary infections. They found that all the organisms were more common in females than males.[4] Data from other international studies on pediatric patients also report that UTIs are more common in females, which is similar to our finding.[8,9] However unlike to our study, Kalantar et al. in his prospective study of 1696 children aged up to 5 years reported male to female ratio of 1.07:1.[10] Taneja et al. in their study included children up to 12 years of age and found that UTI is more common in males (77.8%).[7]
In our study, population majority of cases presented with “fever with urinary symptoms” (35.4%) with equal male and female ratio. This finding is in contrast to other studies of Sharma et al.[11] where fever and abdominal pain and Brkic et al.,[9] however a retrospective study, where fever were the most common presenting features.
Sterile pyuria was found in significant numbers (43.3%) in our study. In the past international studies, pyuria has sometimes been dismissed as a nonspecific response to fevers in children. It is indicative of an inflammatory response to infection. There are three possible explanations for this:[12] (i) Specimen collection postantibacterial therapy, (ii) any inflammatory condition of bladder, (iii) infection by a fastidious organism not detected by overnight incubation on a primary isolation medium. Tuberculosis (TB) which is common in our country for adults but seldom described in children could be a possible etiology.[13] Sterile pyuria due to genitourinary TB is reported from Singapore, in a population where TB is uncommon and genitourinary TB is a rarity.[14] Hence, a detailed evaluation and a proper follow-up are required to address sterile pyuria. TB should also be actively looked for in isolated sterile pyuria in our country. Some of them could be having other diseases with vasculitis like Kawasaki's disease, which are known to have sterile pyuria.[15]
E. coli (42.3%) was the leading etiology of pediatric UTI at our center. This is consistent with studies reported by Mashouf et al.[3] (57.4%) in Iran, Kalantar et al.[10] (54.8%) in Iran, Brad et al.[16] (58%) in Romania and Gupta et al.[17] (64.0%) in India. Data from the above studies showed that E. coli are consistently found predominant uropathogen irrespective of country, community or hospital setting. Our study demonstrated Klebsiella spp. in 11.5% subjects. Akram et al. in a study from North India, showed similar data with Klebsiella spp. being detected in 22.0%[4] cases and in various parts of the world as 14.0%,[8] 14.5%[7] and 21.0%[16] cases.
P. aeruginosa was found in 5.8%, whereas Acinetobacter spp. In 1.9% subjects. Their low incidence could be justified by the studies like Akram et al. also from North India, where no such microorganism was found in children from 0 to 19 years[4] and Abdulhadi et al. they isolated P. aeruginosa from only 2% of cases.[8] However, in contrast to our findings, Taneja et al.[7] demonstrated P. aeruginosa (10.9%) and Acinetobacter spp. 6.6% as the major pathogens isolated from the PICU isolates. Inclusions of surgical cases with more chances of indwelling catheters may be responsible for the higher incidence.
Unlike to prospective studies where Enterobacter aerogenes 3.8% and streptococci spp. 1.7% from Northern India[7] and retrospective analysis where 3.8% and 2.3% respectively from a multi-speciality hospital in South-Africa,[18] these microbes were not found in samples from our centre. Variations in pathogens are known to occur with changing geographical areas and ethnicity.[4]
Gram-positive organisms have received more attention recently as a cause for bacteriuria and UTI. Coagulase negative Staphylococcus, S. aureus, streptococci, and enterococci have been reported in small numbers by various authors, but they are recognized as important causes of UTI.[3,10,19] We found similar occurrence rate as 13.5%, 11.5% and 5.8% for enterococci, S. aureus, and coagulase-negative Staphylococcus, respectively.
We found a valuable laboratory data on antibiotic susceptibilities of uropathogens and allows comparison of the situation in our area with that in other countries and other regions of our country. We found that the most active antibiotics against all the Gram-negative isolates were nitrofurantoin, amikacin, and cefotaxime. These organisms showed more than 50% sensitivity to all these three drugs. These findings are contrary to studies from Africa, South-Asia, and some Middle East countries which showed that these drugs are less potent against Gram-negative organisms.[4,18,20] Biswas et al. in a prospective study conducted on a total of 524 subjects have shown lower resistance rates which corresponds to results of our study.[21] Similar susceptibility patterns of nitrofurantoin and cefotaxime have been found in a study by Sharmin et al.[22]
Our study, similar to Kalantar et al.[10] and Mashouf et al.[3] demonstrated the extremely low susceptibility of Gram-negative organisms to the first-line agents like fluoroquinolones and cotrimoxazole, which are frequently used antibiotics in the settings of UTI in our population. Nalidixic acid showed high resistance to all isolates especially Gram-negative organisms analogous to studies from different parts of the world.[3,4,18,20]
We observed a significant degree of antibiotic resistance among the uropathogens isolated. Among the Gram-negative organisms, there was a tendency towards multidrug-resistance defined as resistance to one or more of the extended-spectrum cephalosporins and fluoroquinolones. Acinetobacter spp. in our study was sensitive to sparfloxacin, norfloxacin, and cefpirome. However, the numbers of this isolate are very less in our study (only 4). The possible reason of high level of antibiotic resistance among the uropathogens isolated could be due to high level of antibiotics used in our region.
Coagulase-negative Staphylococcus was the only organism susceptible (66.7%) to novobiocin whereas other Gram-positive organisms were highly resistant at our center. Novobiocin-resistant micrococci were rarely found in other studies.[23] It may be worthwhile using this drug in our country as other centers are not using it.
This study is not placebo controlled as only patients with symptoms included and that only from a single center. Furthermore, the quantitative methods of culture sensitivity were not used. The worldwide trend of empirically treating community-acquired UTI may not apply for specific geographical regions, where decreased susceptibility rates are documented for common urinary pathogens. International guidelines are no longer applicable for treating community-acquired UTI in a region, which have always the tendency of changing antimicrobial sensitivity over a period of time. The development of specific guidelines based on local susceptibility patterns is necessary to serve as a guide for empirical treatment before or in the absence of urine culture report when proper diagnostic modalities are limited in rural and sub-rural areas. However, it's very important to mention here that clinical use of any antibiotic for UTI cannot be recommended based on this study as further studies are warranted showing its effectiveness clinically on pediatric UTI patients. Development of regional surveillance programs is necessary periodically to enable the development of community-acquired UTI guidelines.[24]
CONCLUSION
E. coli was the commonest isolate in pediatric patients with UTI. Other organisms grown in significant numbers were E. fecalis, Klebsiella spp. and S. aureus. Gram-negative isolates were sensitive to nitrofurantoin, amikacin, and cefotaxime. Gram-positive isolates were sensitive to nitrofurantoin, cotrimoxazole, and novobiocin. Prospective, regional studies should be ensured periodically to identify bacteriological profile and antibiotic sensitivity patterns to be applicable for children with UTI for that geographic area at that particular period of time.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Prajapati BS, Prajapati RB, Patel PS. Advances in management of urinary tract infections. Indian J Pediatr. 2008;75:809–14. doi: 10.1007/s12098-008-0152-0. [DOI] [PubMed] [Google Scholar]
- 2.Wald ER, Feigin RD, Chery JD, Demmier GJ, Kapian SL. 5th ed. Philadelphia: Saunders; 2004. Cystitis and pyelonephritis. Textbook of Pediatric Infectious Diseases; pp. 541–53. [Google Scholar]
- 3.Mashouf RY, Babalhavaeji H, Yousef J. Urinary tract infections: Bacteriology and antibiotic resistance patterns. Indian Pediatr. 2009;46:617–20. [PubMed] [Google Scholar]
- 4.Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in J N M C Hospital Aligarh, India. Ann Clin Microbiol Antimicrob. 2007;6:4. doi: 10.1186/1476-0711-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellerstein S. Recurrent urinary tract infections in children. Pediatr Infect Dis. 1982;1:271–81. doi: 10.1097/00006454-198207000-00018. [DOI] [PubMed] [Google Scholar]
- 6.7th ed. American Society of Microbiology National Committee for Clinical Laboratory Standards; 2000. National Committee for Clinical Laboratory Standards. Methods for Disk Susceptibility Tests for Bacteria that Grow Aerobically. NCCLS Document M2-A7. [Google Scholar]
- 7.Taneja N, Chatterjee SS, Singh M, Singh S, Sharma M. Pediatric urinary tract infections in a tertiary care center from north India. Indian J Med Res. 2010;131:101–5. [PubMed] [Google Scholar]
- 8.Abdulhadi SK, Yashua AH, Uba A. Organisms causing urinary tract infection in paediatric patients at Murtala Muhammad Specialist Hospital, Kano, Nigeria. Int J Biomed Health Sci. 2008;4:165–7. [Google Scholar]
- 9.Brkic S, Mustafic S, Nuhbegovic S, Ljuca F, Gavran L. Clinical and epidemiology characteristics of urinary tract infections in childhood. Med Arh. 2010;64:135–8. [PubMed] [Google Scholar]
- 10.Kalantar E, Motlagh ME, Lornejad H, Reshadmanesh N. Prevalence of urinary tract pathogens and antimicrobial susceptibility patterns in children at hospitals in Iran. Iran J Clin Infect Dis. 2008;3:149–53. [Google Scholar]
- 11.Sharma A, Shrestha S, Upadhyay S, Rijal P. Clinical and bacteriological profile of urinary tract infection in children at Nepal Medical College Teaching Hospital. Nepal Med Coll J. 2011;13:24–6. [PubMed] [Google Scholar]
- 12.Pead L, Maskell R. Study of urinary tract infection in children in one health district. BMJ. 1994;309:631–4. doi: 10.1136/bmj.309.6955.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chattopadhyay A, Bhatnagar V, Agarwala S, Mitra DK. Genitourinary tuberculosis in pediatric surgical practice. J Pediatr Surg. 1997;32:1283–6. doi: 10.1016/s0022-3468(97)90302-x. [DOI] [PubMed] [Google Scholar]
- 14.Chiang LW, Jacobsen AS, Ong CL, Huang WS. Persistent sterile pyuria in children? Don’t forget tuberculosis! Singapore Med J. 2010;51:e48–50. [PubMed] [Google Scholar]
- 15.Avner ED, Harmon WE, Niaudet P. 5th ed. Springers; 2004. Urinary Tract Disorders. Pediatric Nephrology; pp. 1008–9. [Google Scholar]
- 16.Brad GF, Sabau I, Marcovici T, Maris I, Daescu C, Belei O, Vetesi T, Nilima K, Hodut A, Popoiu CM. Antibiotic resistance in urinary tract infections in children. Jurnalul Pediatrului. 2010;13(51-52):73–77. [Google Scholar]
- 17.Gupta V, Yadav A, Joshi RM. Antibiotic resistance pattern in uropathogens. Indian J Med Microbiol. 2002;20:96–8. [PubMed] [Google Scholar]
- 18.Yüksel S, Oztürk B, Kavaz A, Ozçakar ZB, Acar B, Güriz H, et al. Antibiotic resistance of urinary tract pathogens and evaluation of empirical treatment in Turkish children with urinary tract infections. Int J Antimicrob Agents. 2006;28:413–6. doi: 10.1016/j.ijantimicag.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Habte TM, Dube S, Ismail N, Hoosen AA. Hospital and community isolates of uropathogens at a tertiary hospital in South Africa. S Afr Med J. 2009;99:584–7. [PubMed] [Google Scholar]
- 20.Yildiz B, Kural N, Durmaz G, Yarar C, Ak I, Akcar N. Antibiotic resistance in children with complicated urinary tract infection. Saudi Med J. 2007;28:1850–4. [PubMed] [Google Scholar]
- 21.Biswas D, Gupta P, Prasad R, Singh V, Arya M, Kumar A. Choice of antibiotic for empirical therapy of acute cystitis in a setting of high antimicrobial resistance. Indian J Med Sci. 2006;60:53–8. [PubMed] [Google Scholar]
- 22.Sharmin S, Alamgir F, Fahmida, Saleh A. Antimicrobial sensitivity pattern of uropathogens in children. Bangladesh J Med Microbiol. 2009;03:18–22. [Google Scholar]
- 23.Pead L, Crump J, Maskell R. Staphylococci as urinary pathogens. J Clin Pathol. 1977;30:427–31. doi: 10.1136/jcp.30.5.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kothari A, Sagar V. Antibiotic resistance in pathogens causing community-acquired urinary tract infections in India: A Multicenter study. J Infect Dev Ctries. 2008;2:354–8. doi: 10.3855/jidc.196. [DOI] [PubMed] [Google Scholar]