Abstract
Introduction:
Peyronie's disease (PD) is a fibrotic diathesis of the tunica albuginea that results in penile plaque formation and penile deformity, negatively affecting sexual and psychosocial function of both patients and their partners. In this review, we discuss the PD literature and PD treatment options, with special emphasis on potential future therapies.
Methods:
The PD literature was reviewed, and articles of interest were identified using keyword search in PubMed. Articles evaluating investigational and novel PD treatments were emphasized.
Results:
Existing PD treatment modalities are diverse and include oral, topical, intralesional, mechanical, and surgical therapies. Surgical treatment has high success rates and is indicated in men with significant, stable deformity. The United States Food and Drug Administration-approved intralesional collagenase Clostridium histolyticum injection therapy is a minimally invasive option with demonstrated efficacy in PD. Other nonsurgical therapies have been reported, including Botox and stem cell therapy, but these currently have little or equivocal evidence to support their efficacy.
Conclusions:
Further research is essential to develop novel, safe, and effective minimally invasive PD treatment options. This work is ongoing, with the promise of specific, targeted, and highly effective therapies on the horizon.
Key words: Peyronie's disease, plaque incision and grafting, tunical plication
INTRODUCTION
Peyronie's disease (PD) is a fibrotic diathesis of the penis often leading to penile deformity that can be associated with pain, impaired ability to have sexual intercourse, shame, depression and/or anxiety, and decreased quality of life.[1,2,3] PD typically presents during the fifth decade of life, with a mean age of presentation of 52–57 years.[4,5,6,7] The estimated overall prevalence of PD varies widely, with rates ranging from 0.39% to 13.1%,[4,5,8] and even higher in certain sub-populations. For example, up to 16% of men after radical prostatectomy may develop PD.[9] François Gigot de la Peyronie, the French physician and surgeon to King Louis XV, is credited with first describing PD in 1743.[10] However, the first report of PD may date back even further, to Theodoric Borgognoni of Bologne during the 13th century.[11] Despite PD's long and storied history, consensus regarding its management is lacking, and relatively few therapies with definitive evidence of efficacy exist. In light of PD's high prevalence and its significant impact on affected men and their partners, a better understanding of this disease process, as well as more effective treatment options, are essential. This review addresses the etiology, diagnosis, and treatment of PD, with special emphasis on potential future PD therapies.
ETIOLOGY, PATIENT ASSESSMENT, AND TREATMENT: WHAT WE KNOW SO FAR
Although the etiology of PD is multifactorial and incompletely understood, penile trauma is widely believed to be an important contributing factor.[12,13] Penile trauma resulting in PD may either be acute and severe (e.g., sustained during an accident or surgical procedure), or repetitive microtrauma such as that which commonly occurs during sexual intercourse. While all sexually active men are exposed to some level of penile trauma during sexual activity, few develop PD, suggesting that other factors, including a man's genetics, likely contribute to PD pathogenesis. A personal history of nongonococcal urethritis[13,14] and smoking[13,15] are potential risk factors for PD, as is having a sexual partner with a history of inflammatory diseases of the genital tract,[13] fibromatous lesions of the genital tract,[13] or a history of genital tract surgery.[13,14]
Hypogonadism is prevalent in men with PD, suggesting that low testosterone (T) may also represent a PD risk factor. A recent study evaluating 121 men with PD reported hypogonadal T levels (<300 ng/dL) in 74.4% of the cohort.[16] It has also been suggested that hypogonadal men with PD may have greater penile curvature than eugonadal PD patients.[16,17] Similarly, high rates of low T were observed in men with PD and erectile dysfunction (ED) in a recent study, although low T and degree of penile curvature were not correlated.[18] Another study failed to show a difference between T levels in men with PD compared to controls, although the study was likely underpowered.[19] Interestingly, adrenal androgens were significantly lower in the PD group when compared with controls, suggesting that androgen deficiency may play a role in PD pathogenesis because androgens modulate the matrix metalloproteinases (MMP) that are essential in normal wound healing. There is no direct evidence for a causal relationship between hypogonadism and PD, nor is there evidence that testosterone therapy ameliorates PD symptoms. As such, T is not currently considered a treatment for PD. However, the literature does support a relationship between androgen levels and PD, and screening for low T should be considered in men with PD, with initiation of testosterone therapy for symptomatic hypogonadism.
Management of PD may include counseling, nonsurgical therapies, and surgical intervention. The Peyronie's Disease Questionnaire (PDQ) is a recently developed and validated tool that measures psychosexual impact of PD in men, and represents the first PD-specific self-reported assessment tool.[20] The PDQ is highly reproducible[21] and sensitive to changes in men with PD,[22] making it an invaluable tool for baseline assessments in new PD patients and for measuring PD treatment outcomes.
Scores of nonoperative treatments for PD have been utilized over the centuries since PD was first reported, the vast majority with minimal or equivocal success based on small retrospective or single-arm prospective studies. A list of these agents, proposed mechanism of action, and the level of evidence available for each is provided in Table 1.[23] Interferon (IFN) α2a and α2b have been evaluated in multiple observational studies for potential benefit in PD patients with stable, noncalcified plaques,[24,25,26,27,28,29,30,31] as well as in one randomized controlled trial (RCT) demonstrating efficacy[32,33] and one RCT showing no benefit, which was likely underpowered.[34] Guidelines for treatment of PD from the European Association of Urology conclude that intralesional IFNα2b is potentially effective in PD treatment.[35] The 2015 AUA guidelines also conclude that IFNα2b is effective for some PD symptoms and may be administered to patients with PD (moderate recommendation; evidence strength grade C), after appropriate counseling regarding potential adverse events, such as flu-like symptoms and penile swelling. However, further evidence in the form of another adequately powered RCT is necessary before IFNα2b can be definitively recommended for PD treatment.
Table 1.
PD treatment options[23]
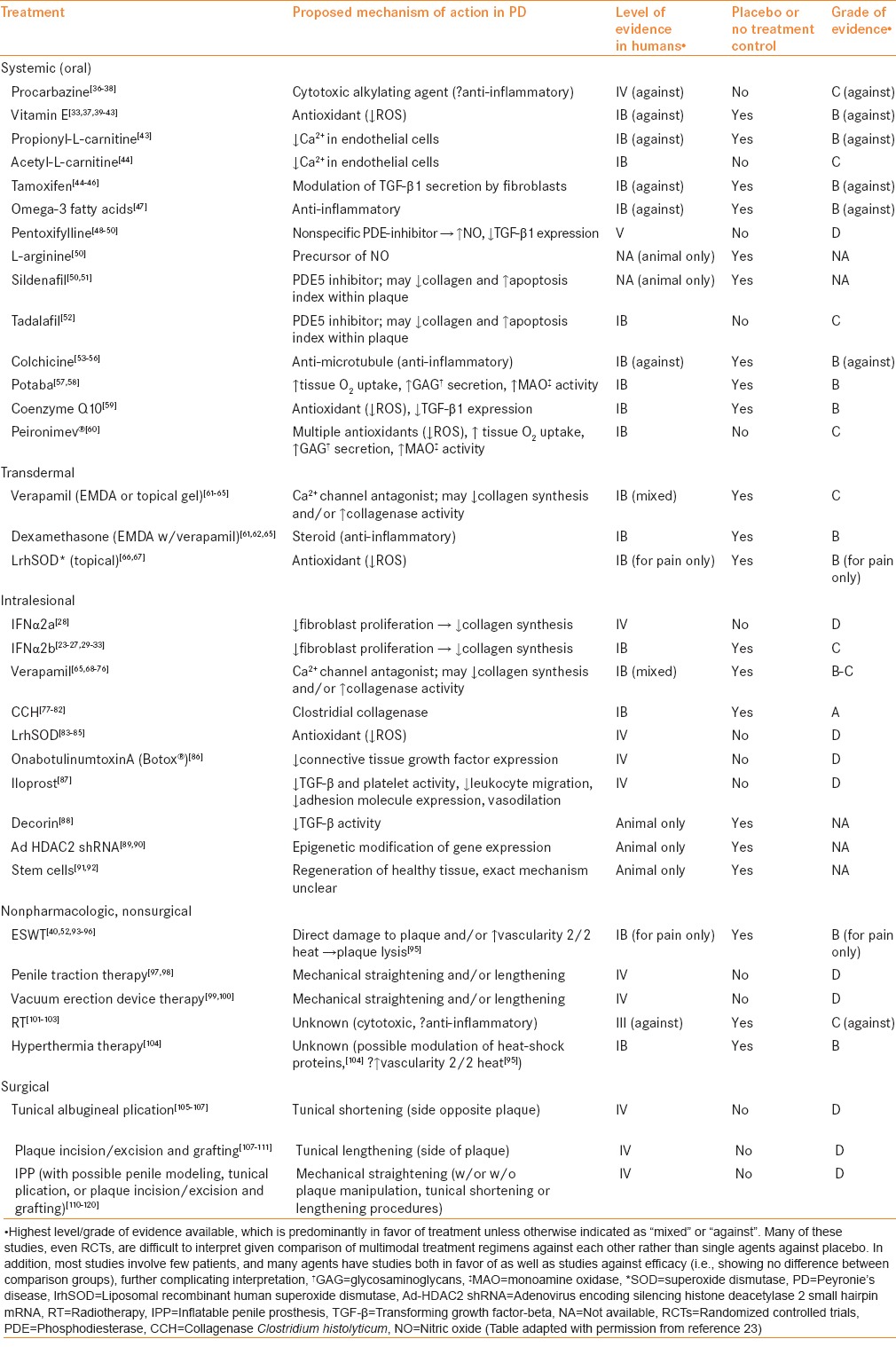
Intralesional collagenase Clostridium histolyticum (CCH) is a relatively recent addition to the PD treatment armamentarium, and is unique among nonsurgical options in that its safety and efficacy are supported by rigorous evidence from several RCTs.[77,78,79,80,81] These studies demonstrated a significantly greater improvement in penile curvature and PD symptom bother in CCH-treated men compared to placebo-treated men, while effects on pain and erectile function were similar in both groups. CCH is the only pharmacologic agent currently approved by the United States Food and Drug Administration (US FDA) for the treatment of PD. The recent AUA guidelines support CCH administration in combination with modeling for the reduction of curvature in patients with stable PD with curvature between 30° and 90° (moderate recommendation; evidence strength B).
Surgery represents an excellent treatment option when penile deformity is severe enough to interfere with sexual intercourse and has been stable for 3–6 months.[121] Ideally, any associated pain should resolve prior to operative intervention,[112] as pain tends to reflect active disease with ongoing inflammation and may limit surgical success. Surgical intervention may involve: (1) Tunical plication alone when there is adequate penile length and curvature <60°, (2) plaque incision/excision with or without grafting when penile length is inadequate and/or curvature is more severe or associated with deformities including hourglass or hinging, or (3) placement of inflatable penile prosthesis with or without adjuvant maneuvers, such as penile modeling, in the setting of concomitant ED that is unresponsive to treatment.[34] Surgery is safe in appropriately selected patients, with efficacy rates approaching 100% in some series.[108,109] However, there remains considerable interest in identifying effective nonoperative treatments for PD, as these would limit adverse events associated with surgery and may allow treatment during the active phase of disease, potentially modifying and attenuating the overall disease course. To develop such treatments, however, a comprehensive molecular understanding of PD pathogenesis and its natural history is required. An algorithm for the treatment of PD is provided in Figure 1.[23]
Figure 1.
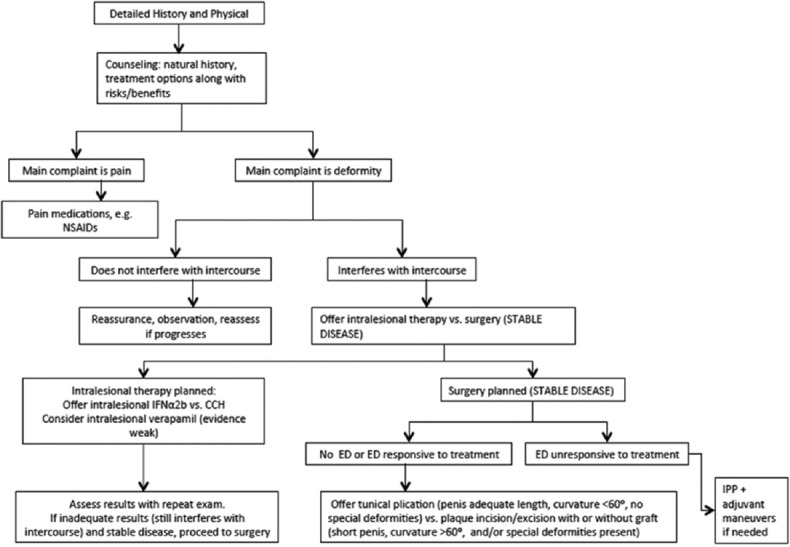
Peyronie's disease treatment algorithm[23] (Figure adapted with permission from reference 23)
FUTURE DIRECTIONS
Considerable interest remains in identifying novel minimally invasive PD treatment options. OnabotulinumtoxinA (Botox®) can reduce fibrosis in cell culture and in animal models of hypertrophic scars/keloids,[122,123,124,125] prompting a prospective cohort study to evaluate its potential in the setting of PD.[86] Following a single intralesional injection of 100U Botox®, investigators reported a significant decrease in penile plaque size and curvature, as well as a significant improvement in International Index of Erectile Function-5 (IIEF-5) score. However, this study only evaluated a small number of patients (n = 22) and lacked a placebo control group, limiting the conclusions that could be drawn.
Additional investigational agents for PD treatment include liposomal recombinant human superoxide dismutase (lrhSOD, also known as orgotein),[66,67,83,84,85] iloprost,[87] and Peironimev-Plus®.[60] The use of intralesional lrhSOD has only been described in observational studies,[83,84,85] while topical lrhSOD has been evaluated in one observational study[66] and one crossover RCT with promising results.[67] In the RCT, penile pain improved significantly in the treatment as compared to the placebo group. Decreases in penile curvature and plaque size were also observed, although these outcomes were not evaluated until after crossover, limiting a true efficacy comparison against placebo. Iloprost is a prostacyclin analog recently tested as a potential intralesional PD therapy, chosen because of its anti-transforming growth factor (TGF)-β activity in fibroblasts.[87] Additional potentially beneficial effects of prostacyclin include vasodilation, activation of fibrinolysis, and inhibition of platelet function, leukocyte migration, and cell adhesion molecule expression. Improvement in penile curvature was observed in 29% of 38 treated patients, but these data are difficult to interpret given the lack of a placebo comparison group. Peironimev-plus® is an oral supplement containing multiple antioxidants (Vitamin E, para-aminobenzoic acid, propolis, blueberry anthocyanins, soja isoflavones, muira puama, damiana, and Persea americana), several of which have been individually reported as possibly effective in PD treatment in small retrospective case series. A single study evaluating the efficacy of Peironimev-plus® included 64 men randomly assigned to one of the two treatment arms: (1) Perilesional verapamil injection + verapamil iontophoresis + Peironimev-plus® or (2) verapamil injection + verapamil iontophoresis.[60] The group receiving daily Peironimev-plus® and verapamil had significant improvements in penile plaque size, curvature, and IIEF score as compared to patients receiving verapamil alone. However, the absence of a placebo control group and lack of strong evidence supporting verapamil's efficacy in PD treatment limit the ability to interpret these data. Future well-designed, adequately powered RCTs are needed to determine the efficacy of lrhSOD, iloprost, and Peironimev-Plus® in PD. As such, these agents cannot be recommended for routine clinical use at this time.
Basic science research probing the molecular causes of PD has expanded in recent years, leading to an improved understanding of the underlying pathophysiology of PD that can inform future therapeutic efforts and improve clinical outcomes. A recent study characterized and compared the transcriptional signatures of human PD plaque, unaffected tunica albuginea, and corpora cavernosa cells in culture.[126] Several genes were found to be expressed at significantly higher levels (2.7–29.8-fold above baseline) in PD cells, including insulin-like growth factor-1, smooth muscle actin γ-2, myogenic factor 5, α-cardiac muscle actin 1 (ACTC1), periostin (POSTN), type III collagen (COL3A1), and MMP3, thereby identifying these as potential therapeutic targets. Genetic evaluation of PD plaque-derived fibroblasts in comparison with unaffected tunica albuginea demonstrated karyotype defects preferentially in plaque-derived fibroblasts, supporting a potential genetic etiology in affected men.[127] However, no specific genes have been causally linked to PD to date, despite the fact that PD can be inherited in autosomal dominant fashion.[128] HS-173, a novel imidazo 12-a] pyridine derivative that inhibits phosphoinositide 3-kinase (a downstream effector of TGF-β), may represent a candidate therapy for treating PD and other fibrotic diseases.[129] This small molecule inhibits growth and induces apoptosis in PD plaque-derived fibroblasts. Further investigation is warranted, with future work to include animal studies to demonstrate a benefit beyond the cell culture dish.
Animal models of PD using intra-tunical injection of profibrotic agents such as fibrin TGF-β[130] permit more rigorous testing of potential PD treatments prior to investigation in humans. PD animal models have been employed to study the possible therapeutic benefits of decorin, a proteoglycan with anti-TGF-β activity,[88] and adenovirus encoding silencing histone deacetylase 2 small hairpin mRNA (Ad-HDAC2 shRNA).[89] Intracavernous treatment with decorin prevents tunical thickening and collagen disorganization seen in the untreated PD model rats.[88] Maximal intracavernosal pressure and mean duration of erection were significantly increased in the treatment group. Epigenetic modifications, including histone acetylation and deacetylation, are known to play a role in fibrotic diatheses,[131] and small interfering RNA against HDAC2 has been shown to have antifibrotic effects on PD cells in culture.[90] In vivo, rats treated with Ad-HDAC2 shRNA exhibited regression of plaque and decreased inflammatory cell infiltration when compared to controls (untreated or treated with adenovirus encoding scrambled shRNA).[89]
Stem cells represent another potential future PD therapy. Stem cell use in a TGF-β-induced animal model of PD was first reported in 2013.[91] The investigators performed intralesional injection of either human adipocyte-derived stem cells (ADSCs) or buffer vehicle as control, demonstrating a significant objective improvement in erectile function (as measured by intracavernosal pressure over mean arterial pressure) in the treatment group as compared to control. Decreased fibrosis was apparent in the treatment group by histological and immunohistochemical examination, and was quantified using Western blot of the fibrotic markers collagen III (decreased 69.9% in treatment animals compared to control) and elastin (decreased 47.7%). A subsequent study confirmed the benefits of ADSCs in a PD rat model,[92] with improved erectile function and downregulation of fibrotic change in the ADSC groups, both for what they termed prevention (injection of ADSCs vs. vehicle together with TGF-β1) and treatment (injection of ADSCs vs. vehicle 30 days after injection of TGF-β1). While these studies suggest a potential benefit for ADSCs during the active phase of PD, further research is needed to elucidate the exact therapeutic mechanisms of ADSCs, and human trials are essential prior to widespread clinical application. Subcutaneous fat may be harvested from patients with active PD to permit autologous stem cell derivation for investigational intralesional treatment.
Ultimately, evidence of the efficacy and long-term safety of stem cell therapies, together with the other molecular or genetic therapies discussed above, will be required, particularly in light of the possibility of neoplastic risk with some of these treatment approaches.[132] Nevertheless, recent advances in our understanding of the molecular basis of PD and the application of novel molecular therapies herald the beginning of a new era in PD treatment – one of precision medicine. Future advances will enable not only the identification of additional specific treatment targets, but also of susceptible men to which specific, effective therapies can be targeted. In more completely understanding PD pathogenesis, as well as the relationship of PD with environmental factors and other comorbidities, an individualized, holistic, highly effective approach to treatment and prevention of further morbidity can be undertaken. While additional basic science and clinical research are required to completely understand all facets of PD, recent progress is encouraging and suggests a bright future for medicine's approach to this potentially devastating condition.
CONCLUSIONS
PD has a long history in the literature, and myriad minimally invasive therapies have been used, albeit few have demonstrable efficacy. CCH is the only nonsurgical treatment option rigorously shown to be safe and effective in the treatment of PD, and is thus currently the only US FDA-approved nonsurgical treatment for PD. Ongoing research may yield additional therapeutic options, although a more complete understanding of PD pathogenesis and genetic predisposition is needed. Numerous therapies have been tried in humans, including IFN-α2, Botox®, lrhSOD, iloprost, and Peironimev-Plus®, among others. However, rigorous clinical trial data are currently lacking to demonstrate true efficacy of the majority of these therapies. Other potential cell-based and small molecule therapies have been studied in vivo (decorin, HDAC2 shRNA, stem cells) and in vitro (HS-173), and recent studies have identified several novel potential therapeutic targets. Our expanding knowledge regarding the molecular pathophysiology of PD will inform future clinical and translational research, leading to minimally invasive treatment options that can be targeted to appropriately selected men. Ultimately, this will lead to improved outcomes for our patients, ushering in an era of precision medicine in the treatment of PD.
Financial support and sponsorship
A.W.P. is a National Institutes of Health (NIH) K12 Scholar supported by a Male Reproductive Health Research Career (MHRH) Development Physician-Scientist Award (HD073917-01) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Program (to Dolores J. Lamb).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nelson CJ, Diblasio C, Kendirci M, Hellstrom W, Guhring P, Mulhall JP. The chronology of depression and distress in men with Peyronie's disease. J Sex Med. 2008;5:1985–90. doi: 10.1111/j.1743-6109.2008.00895.x. [DOI] [PubMed] [Google Scholar]
- 2.Nelson CJ, Mulhall JP. Psychological impact of Peyronie's disease: A review. J Sex Med. 2013;10:653–60. doi: 10.1111/j.1743-6109.2012.02999.x. [DOI] [PubMed] [Google Scholar]
- 3.Hartzell R. Psychosexual symptoms and treatment of peyronie's disease within a collaborative care model. Sex Med. 2014;2:168–77. doi: 10.1002/sm2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsay MB, Schain DM, Grambsch P, Benson RC, Beard CM, Kurland LT. The incidence of peyronie's disease in Rochester, Minnesota, 1950 through 1984. J Urol. 1991;146:1007–9. doi: 10.1016/s0022-5347(17)37988-0. [DOI] [PubMed] [Google Scholar]
- 5.Schwarzer U, Sommer F, Klotz T, Braun M, Reifenrath B, Engelmann U. The prevalence of Peyronie's disease: Results of a large survey. BJU Int. 2001;88:727–30. doi: 10.1046/j.1464-4096.2001.02436.x. [DOI] [PubMed] [Google Scholar]
- 6.El-Sakka AI. Prevalence of Peyronie's disease among patients with erectile dysfunction. Eur Urol. 2006;49:564–9. doi: 10.1016/j.eururo.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Kadioglu A, Sanli O, Akman T, Canguven O, Aydin M, Akbulut F, et al. Factors affecting the degree of penile deformity in Peyronie disease: An analysis of 1001 patients. J Androl. 2011;32:502–8. doi: 10.2164/jandrol.110.011031. [DOI] [PubMed] [Google Scholar]
- 8.Dibenedetti DB, Nguyen D, Zografos L, Ziemiecki R, Zhou X. A population-based study of peyronie's disease: Prevalence and treatment patterns in the United States. Adv Urol 2011. 2011:282503. doi: 10.1155/2011/282503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tal R, Heck M, Teloken P, Siegrist T, Nelson CJ, Mulhall JP. Peyronie's disease following radical prostatectomy: Incidence and predictors. J Sex Med. 2010;7:1254–61. doi: 10.1111/j.1743-6109.2009.01655.x. [DOI] [PubMed] [Google Scholar]
- 10.de la Peyronie F. Sur quelques obstacles qui s‘opposent a l’ejaculation naturelle de la semence. Mem Acad Roy Chir. 1743;1:425–39. [Google Scholar]
- 11.Borgognoni T. Cyrugia edita et compilata. Venice 1498 (written 1265-1275) [Google Scholar]
- 12.Devine CJ, Jr, Somers KD, Jordan SG, Schlossberg SM. Proposal: Trauma as the cause of the Peyronie's lesion. J Urol. 1997;157:285–90. doi: 10.1016/s0022-5347(01)65361-8. [DOI] [PubMed] [Google Scholar]
- 13.Bjekic MD, Vlajinac HD, Sipetic SB, Marinkovic JM. Risk factors for Peyronie's disease: A case-control study. BJU Int. 2006;97:570–4. doi: 10.1111/j.1464-410X.2006.05969.x. [DOI] [PubMed] [Google Scholar]
- 14.Carrieri MP, Serraino D, Palmiotto F, Nucci G, Sasso F. A case-control study on risk factors for Peyronie's disease. J Clin Epidemiol. 1998;51:511–5. doi: 10.1016/s0895-4356(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 15.La Pera G, Pescatori ES, Calabrese M, Boffini A, Colombo F, Andriani E, et al. Peyronie's disease: Prevalence and association with cigarette smoking. A multicenter population-based study in men aged 50-69 years. Eur Urol. 2001;40:525–30. doi: 10.1159/000049830. [DOI] [PubMed] [Google Scholar]
- 16.Moreno SA, Morgentaler A. Testosterone deficiency and Peyronie's disease: Pilot data suggesting a significant relationship. J Sex Med. 2009;6:1729–35. doi: 10.1111/j.1743-6109.2009.01250.x. [DOI] [PubMed] [Google Scholar]
- 17.Nam HJ, Park HJ, Park NC. Does testosterone deficiency exaggerate the clinical symptoms of Peyronie's disease? Int J Urol. 2011;18:796–800. doi: 10.1111/j.1442-2042.2011.02842.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirby EW, Verges D, Matthews J, Carson CC, Coward RM. Low testosterone has a similar prevalence among men with sexual dysfunction due to either Peyronie's disease or erectile dysfunction and does not correlate with Peyronie's disease severity. J Sex Med. 2015;12:690–6. doi: 10.1111/jsm.12805. [DOI] [PubMed] [Google Scholar]
- 19.Karavitakis M, Komninos C, Simaioforidis V, Kontos S, Lefakis G, Politis V, et al. The relationship between androgens, regulators of collagen metabolism, and Peyronie's disease: A case control study. J Sex Med. 2010;7:4011–7. doi: 10.1111/j.1743-6109.2010.01915.x. [DOI] [PubMed] [Google Scholar]
- 20.Hellstrom WJ, Feldman R, Rosen RC, Smith T, Kaufman G, Tursi J. Bother and distress associated with Peyronie's disease: Validation of the Peyronie's disease questionnaire. J Urol. 2013;190:627–34. doi: 10.1016/j.juro.2013.01.090. [DOI] [PubMed] [Google Scholar]
- 21.Coyne KS, Currie BM, Thompson CL, Smith TM. The test-retest reliability of the Peyronie's disease questionnaire. J Sex Med. 2015;12:543–8. doi: 10.1111/jsm.12769. [DOI] [PubMed] [Google Scholar]
- 22.Coyne KS, Currie BM, Thompson CL, Smith TM. Responsiveness of the Peyronie's Disease Questionnaire (PDQ) J Sex Med. 2015;12:1072–9. doi: 10.1111/jsm.12838. [DOI] [PubMed] [Google Scholar]
- 23.Bilgutay AN, Pastuszak AW. Peyronie's disease: A review of etiology, diagnosis, and management. Curr Sex Health Rep. 2015;7:117–131. doi: 10.1007/s11930-015-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahuja S, Bivalacqua TJ, Case J, Vincent M, Sikka SC, Hellstrom WJ. A pilot study demonstrating clinical benefit from intralesional interferon alpha 2B in the treatment of Peyronie's disease. J Androl. 1999;20:444–8. [PubMed] [Google Scholar]
- 25.Astorga R, Cantero O, Contreras D, del Río-Martín A, Labarta-Beceiro V, Gutiérrez-Elvírez A, et al. Intralesional recombinant interferon alpha-2b in Peyronie's disease. Arch Esp Urol. 2000;53:665–71. [PubMed] [Google Scholar]
- 26.Brake M, Loertzer H, Horsch R, Keller H. Treatment of peyronie's disease with local interferon-alpha 2b. BJU Int. 2001;87:654–7. doi: 10.1046/j.1464-410x.2001.02139.x. [DOI] [PubMed] [Google Scholar]
- 27.Dang G, Matern R, Bivalacqua TJ, Sikka S, Hellstrom WJ. Intralesional interferon-alpha-2B injections for the treatment of Peyronie's disease. South Med J. 2004;97:42–6. doi: 10.1097/01.smj.0000056658.60032.d3. [DOI] [PubMed] [Google Scholar]
- 28.Judge IS, Wisniewski ZS. Intralesional interferon in the treatment of Peyronie's disease: A pilot study. Br J Urol. 1997;79:40–2. doi: 10.1046/j.1464-410x.1997.02849.x. [DOI] [PubMed] [Google Scholar]
- 29.Polat O, Gül O, Ozbey I, Ozdikici M, Bayraktar Y. Peyronie's disease: Intralesional treatment with interferon alpha-2A and evaluation of the results by magnetic resonance imaging. Int Urol Nephrol. 1997;29:465–71. doi: 10.1007/BF02551115. [DOI] [PubMed] [Google Scholar]
- 30.Trost LW, Ates E, Powers M, Sikka S, Hellstrom WJ. Outcomes of intralesional interferon-a2B for the treatment of Peyronie disease. J Urol. 2013;190:2194–9. doi: 10.1016/j.juro.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Wegner HE, Andresen R, Knipsel HH, Miller K. Treatment of peyronie's disease with local interferon-alpha 2b. Eur Urol. 1995;28:236–40. doi: 10.1159/000475057. [DOI] [PubMed] [Google Scholar]
- 32.Hellstrom WJ, Kendirci M, Matern R, Cockerham Y, Myers L, Sikka SC, et al. Single-blind, multicenter, placebo controlled, parallel study to assess the safety and efficacy of intralesional interferon alpha-2B for minimally invasive treatment for Peyronie's disease. J Urol. 2006;176:394–8. doi: 10.1016/S0022-5347(06)00517-9. [DOI] [PubMed] [Google Scholar]
- 33.Kendirci M, Usta MF, Matern RV, Nowfar S, Sikka SC, Hellstrom WJ. The impact of intralesional interferon alpha-2b injection therapy on penile hemodynamics in men with Peyronie's disease. J Sex Med. 2005;2:709–15. doi: 10.1111/j.1743-6109.2005.00110.x. [DOI] [PubMed] [Google Scholar]
- 34.Inal T, Tokatli Z, Akand M, Ozdiler E, Yaman O. Effect of intralesional interferon-alpha 2b combined with oral vitamin E for treatment of early stage Peyronie's disease: A randomized and prospective study. Urology. 2006;67:1038–42. doi: 10.1016/j.urology.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Hatzimouratidis K, Eardley I, Giuliano F, Hatzichristou D, Moncada I, Salonia A, et al. EAU guidelines on penile curvature. Eur Urol. 2012;62:543–52. doi: 10.1016/j.eururo.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 36.Byström J. Induration penis plastica. Experience of treatment with procarbazine Natulan. Scand J Urol Nephrol. 1976;10:21–5. doi: 10.3109/00365597609179649. [DOI] [PubMed] [Google Scholar]
- 37.Morgan RJ, Pryor JP. Procarbazine (Natulan) in the treatment of Peyronie's disease. Br J Urol. 1978;50:111–3. doi: 10.1111/j.1464-410x.1978.tb03038.x. [DOI] [PubMed] [Google Scholar]
- 38.Oosterlinck W, Renders G. Treatment of Peyronie's disease with procarbazine. Br J Urol. 1975;47:219–20. doi: 10.1111/j.1464-410x.1975.tb03951.x. [DOI] [PubMed] [Google Scholar]
- 39.Ahuja SK, Sikka SC, Hellstrom WJ. Stimulation of collagen production in an in vitro model for Peyronie's disease. Int J Impot Res. 1999;11:207–12. doi: 10.1038/sj.ijir.3900414. [DOI] [PubMed] [Google Scholar]
- 40.Claro JA, Passerotti CC, Figueiredo Neto AC, Nardozza A, Jr, Ortiz V, Srougi M. An alternative non-invasive treatment for Peyronie's disease. Int Braz J Urol. 2004;30:199–204. doi: 10.1590/s1677-55382004000300004. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto K, Hisasue S, Kato R, Kobayashi K, Shimizu T, Tsukamoto T. Outcome analysis for conservative management of Peyronie's disease. Int J Urol. 2006;13:244–7. doi: 10.1111/j.1442-2042.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 42.Paulis G, Brancato T, D’Ascenzo R, De Giorgio G, Nupieri P, Orsolini G, et al. Efficacy of vitamin E in the conservative treatment of Peyronie's disease: Legend or reality?. A controlled study of 70 cases. Andrology. 2013;1:120–8. doi: 10.1111/j.2047-2927.2012.00007.x. [DOI] [PubMed] [Google Scholar]
- 43.Safarinejad MR, Hosseini SY, Kolahi AA. Comparison of Vitamin E and propionyl-L-carnitine, separately or in combination, in patients with early chronic Peyronie's disease: A double-blind, placebo controlled, randomized study. J Urol. 2007;178:1398–403. doi: 10.1016/j.juro.2007.05.162. [DOI] [PubMed] [Google Scholar]
- 44.Biagiotti G, Cavallini G. Acetyl-L-carnitine vs tamoxifen in the oral therapy of Peyronie's disease: A preliminary report. BJU Int. 2001;88:63–7. doi: 10.1046/j.1464-410x.2001.02241.x. [DOI] [PubMed] [Google Scholar]
- 45.Ralph DJ, Brooks MD, Bottazzo GF, Pryor JP. The treatment of Peyronie's disease with tamoxifen. Br J Urol. 1992;70:648–51. doi: 10.1111/j.1464-410x.1992.tb15836.x. [DOI] [PubMed] [Google Scholar]
- 46.Teloken C, Rhoden EL, Grazziotin TM, Ros CT, Sogari PR, Souto CA. Tamoxifen versus placebo in the treatment of Peyronie's disease. J Urol. 1999;162:2003–5. doi: 10.1016/S0022-5347(05)68087-1. [DOI] [PubMed] [Google Scholar]
- 47.Safarinejad MR. Efficacy and safety of omega-3 for treatment of early-stage Peyronie's disease: A prospective, randomized, double-blind placebo-controlled study. J Sex Med. 2009;6:1743–54. doi: 10.1111/j.1743-6109.2009.01235.x. [DOI] [PubMed] [Google Scholar]
- 48.Brant WO, Dean RC, Lue TF. Treatment of Peyronie's disease with oral pentoxifylline. Nat Clin Pract Urol. 2006;3:111–5. doi: 10.1038/ncpuro0409. [DOI] [PubMed] [Google Scholar]
- 49.Shindel AW, Lin G, Ning H, Banie L, Huang YC, Liu G, et al. Pentoxifylline attenuates transforming growth factor-ß1-stimulated collagen deposition and elastogenesis in human tunica albuginea-derived fibroblasts part 1: Impact on extracellular matrix. J Sex Med. 2010;7:2077–85. doi: 10.1111/j.1743-6109.2010.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valente EG, Vernet D, Ferrini MG, Qian A, Rajfer J, Gonzalez-Cadavid NF. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie's fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–44. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 51.Ferrini MG, Kovanecz I, Sanchez S, Vernet D, Davila HH, Rajfer J, et al. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol Reprod. 2007;76:915–23. doi: 10.1095/biolreprod.106.059642. [DOI] [PubMed] [Google Scholar]
- 52.Palmieri A, Imbimbo C, Creta M, Verze P, Fusco F, Mirone V. Tadalafil once daily and extracorporeal shock wave therapy in the management of patients with Peyronie's disease and erectile dysfunction: Results from a prospective randomized trial. Int J Androl. 2012;35:190–5. doi: 10.1111/j.1365-2605.2011.01226.x. [DOI] [PubMed] [Google Scholar]
- 53.Akkus E, Carrier S, Rehman J, Breza J, Kadioglu A, Lue TF. Is colchicine effective in Peyronie's disease?. A pilot study. Urology. 1994;44:291–5. doi: 10.1016/s0090-4295(94)80155-x. [DOI] [PubMed] [Google Scholar]
- 54.El-Sakka AI, Bakircioglu ME, Bhatnagar RS, Yen TS, Dahiya R, Lue TF. The effects of colchicine on a Peyronie's-like condition in an animal model. J Urol. 1999;161:1980–3. [PubMed] [Google Scholar]
- 55.Kadioglu A, Tefekli A, Köksal T, Usta M, Erol H. Treatment of Peyronie's disease with oral colchicine: Long-term results and predictive parameters of successful outcome. Int J Impot Res. 2000;12:169–75. doi: 10.1038/sj.ijir.3900519. [DOI] [PubMed] [Google Scholar]
- 56.Safarinejad MR. Therapeutic effects of colchicine in the management of Peyronie's disease: A randomized double-blind, placebo-controlled study. Int J Impot Res. 2004;16:238–43. doi: 10.1038/sj.ijir.3901185. [DOI] [PubMed] [Google Scholar]
- 57.Weidner W, Hauck EW, Schnitker J. Peyronie's Disease Study Group of Andrological Group of German Urologists. Potassium paraaminobenzoate (POTABA) in the treatment of Peyronie's disease: A prospective, placebo-controlled, randomized study. Eur Urol. 2005;47:530–5. doi: 10.1016/j.eururo.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 58.Zarafonetis CJ, Horrax TM. Treatment of Peyronie's disease with potassium para-aminobenzoate (potaba) J Urol. 1959;81:770–2. doi: 10.1016/S0022-5347(17)66108-1. [DOI] [PubMed] [Google Scholar]
- 59.Safarinejad MR. Safety and efficacy of coenzyme Q10 supplementation in early chronic Peyronie's disease: A double-blind, placebo-controlled randomized study. Int J Impot Res. 2010;22:298–309. doi: 10.1038/ijir.2010.20. [DOI] [PubMed] [Google Scholar]
- 60.Paulis G, Cavallini G, Brancato T, Alvaro R. Peironimev-Plus® in the treatment of chronic inflammation of tunica albuginea (Peyronie's disease). results of a controlled study. Inflamm Allergy Drug Targets. 2013;12:61–7. doi: 10.2174/1871528111312010009. [DOI] [PubMed] [Google Scholar]
- 61.Di Stasi SM, Giannantoni A, Capelli G, Jannini EA, Virgili G, Storti L, et al. Transdermal electromotive administration of verapamil and dexamethasone for Peyronie's disease. BJU Int. 2003;91:825–9. doi: 10.1046/j.1464-410x.2003.04242.x. [DOI] [PubMed] [Google Scholar]
- 62.Di Stasi SM, Giannantoni A, Stephen RL, Capelli G, Giurioli A, Jannini EA, et al. A prospective, randomized study using transdermal electromotive administration of verapamil and dexamethasone for Peyronie's disease. J Urol. 2004;171:1605–8. doi: 10.1097/01.ju.0000116450.82816.2c. [DOI] [PubMed] [Google Scholar]
- 63.Fitch WP, 3rd, Easterling WJ, Talbert RL, Bordovsky MJ, Mosier M. Topical verapamil HCl, topical trifluoperazine, and topical magnesium sulfate for the treatment of Peyronie's disease – A placebo-controlled pilot study. J Sex Med. 2007;4:477–84. doi: 10.1111/j.1743-6109.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 64.Greenfield JM, Shah SJ, Levine LA. Verapamil versus saline in electromotive drug administration for Peyronie's disease: A double-blind, placebo controlled trial. J Urol. 2007;177:972–5. doi: 10.1016/j.juro.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 65.Mehrsai AR, Namdari F, Salavati A, Dehghani S, Allameh F, Pourmand G. Comparison of transdermal electromotive administration of verapamil and dexamethasone versus intra-lesional injection for Peyronie's disease. Andrology. 2013;1:129–32. doi: 10.1111/j.2047-2927.2012.00018.x. [DOI] [PubMed] [Google Scholar]
- 66.Riedl CR, Plas E, Vorauer K, Vcelar B, Wagner A, Pflüger H. Pilot study on liposomal recombinant human superoxide dismutase for the treatment of Peyronie's disease. Eur Urol. 2001;40:343–8. doi: 10.1159/000049797. [DOI] [PubMed] [Google Scholar]
- 67.Riedl CR, Sternig P, Gallé G, Langmann F, Vcelar B, Vorauer K, et al. Liposomal recombinant human superoxide dismutase for the treatment of Peyronie's disease: A randomized placebo-controlled double-blind prospective clinical study. Eur Urol. 2005;48:656–61. doi: 10.1016/j.eururo.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 68.Bennett NE, Guhring P, Mulhall JP. Intralesional verapamil prevents the progression of Peyronie's disease. Urology. 2007;69:1181–4. doi: 10.1016/j.urology.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 69.Cavallini G, Modenini F, Vitali G. Open preliminary randomized prospective clinical trial of efficacy and safety of three different verapamil dilutions for intraplaque therapy of Peyronie's disease. Urology. 2007;69:950–4. doi: 10.1016/j.urology.2007.01.080. [DOI] [PubMed] [Google Scholar]
- 70.Chung E, Garcia F, Young LD, Solomon M, Brock GB. A comparative study of the efficacy of intralesional verapamil versus normal saline injection in a novel Peyronie disease animal model: Assessment of immunohistopathological changes and erectile function outcome. J Urol. 2013;189:380–4. doi: 10.1016/j.juro.2012.08.191. [DOI] [PubMed] [Google Scholar]
- 71.Heidari M, Nejadi JR, Ghate A, Delfan B, Iran-Pour E. Evaluation of intralesional injection of verapamil in treatment of Peyronie's disease. J Pak Med Assoc. 2010;60:291–3. [PubMed] [Google Scholar]
- 72.Levine LA, Goldman KE, Greenfield JM. Experience with intraplaque injection of verapamil for Peyronie's disease. J Urol. 2002;168:621–5. doi: 10.1016/s0022-5347(05)64691-5. [DOI] [PubMed] [Google Scholar]
- 73.Levine LA, Merrick PF, Lee RC. Intralesional verapamil injection for the treatment of Peyronie's disease. J Urol. 1994;151:1522–4. doi: 10.1016/s0022-5347(17)35291-6. [DOI] [PubMed] [Google Scholar]
- 74.Moskovic DJ, Alex B, Choi JM, Nelson CJ, Mulhall JP. Defining predictors of response to intralesional verapamil injection therapy for Peyronie's disease. BJU Int. 2011;108:1485–9. doi: 10.1111/j.1464-410X.2010.10029.x. [DOI] [PubMed] [Google Scholar]
- 75.Rehman J, Benet A, Melman A. Use of intralesional verapamil to dissolve Peyronie's disease plaque: A long-term single-blind study. Urology. 1998;51:620–6. doi: 10.1016/s0090-4295(97)00700-0. [DOI] [PubMed] [Google Scholar]
- 76.Shirazi M, Haghpanah AR, Badiee M, Afrasiabi MA, Haghpanah S. Effect of intralesional verapamil for treatment of Peyronie's disease: A randomized single-blind, placebo-controlled study. Int Urol Nephrol. 2009;41:467–71. doi: 10.1007/s11255-009-9522-4. [DOI] [PubMed] [Google Scholar]
- 77.Gelbard M, Goldstein I, Hellstrom WJ, McMahon CG, Smith T, Tursi J, et al. Clinical efficacy, safety and tolerability of collagenase Clostridium histolyticum for the treatment of peyronie disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013;190:199–207. doi: 10.1016/j.juro.2013.01.087. [DOI] [PubMed] [Google Scholar]
- 78.Gelbard M, Lipshultz LI, Tursi J, Smith T, Kaufman G, Levine LA. Phase 2b study of the clinical efficacy and safety of collagenase Clostridium histolyticum in patients with Peyronie disease. J Urol. 2012;187:2268–74. doi: 10.1016/j.juro.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 79.Gelbard MK, James K, Riach P, Dorey F. Collagenase versus placebo in the treatment of Peyronie's disease: A double-blind study. J Urol. 1993;149:56–8. doi: 10.1016/s0022-5347(17)35998-0. [DOI] [PubMed] [Google Scholar]
- 80.Levine LA, Cuzin B, Mark S, Gelbard MK, Jones NA, Liu G, et al. Clinical safety and effectiveness of collagenase Clostridium histolyticum injection in patients with Peyronie's disease: A phase 3 open-label study. J Sex Med. 2015;12:248–58. doi: 10.1111/jsm.12731. [DOI] [PubMed] [Google Scholar]
- 81.Lipshultz LI, Goldstein I, Seftel AD, Kaufman GJ, Smith TM, Tursi JP, et al. Clinical efficacy of collagenase Clostridium histolyticum in the treatment of Peyronie's disease by subgroup: Results from two large, double-blind, randomized, placebo-controlled, phase III studies. BJU Int. 2015;116:650–6. doi: 10.1111/bju.13096. [DOI] [PubMed] [Google Scholar]
- 82.Egui Rojo MA, Moncada Iribarren I, Carballido Rodriguez J, Martinez-Salamanca JI. Experience in the use of collagenase Clostridium histolyticum in the management of Peyronie's disease: Current data and future prospects. Ther Adv Urol. 2014;6:192–7. doi: 10.1177/1756287214537331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bartsch G, Menander-Huber KB, Huber W, Marberger H. Orgotein, a new drug for the treatment of Peyronie's disease. Eur J Rheumatol Inflamm. 1981;4:250–9. [PubMed] [Google Scholar]
- 84.Gustafson H, Johansson B, Edsmyr F. Peyronie's disease: Experience of local treatment with Orgotein. Eur Urol. 1981;7:346–8. doi: 10.1159/000473262. [DOI] [PubMed] [Google Scholar]
- 85.Lenk S, Schönberger B. Penile deviation – Our therapeutic concept. Acta Chir Hung. 1994;34:189–94. [PubMed] [Google Scholar]
- 86.Muñoz-Rangel CA, Fernandez-Vivar E, Bañuelos-Gallo RA, Gonzalez-Ojeda A, Macias-Amezcua MD, Chavez-Tostado M, et al. Minimally invasive therapy using intralesional onabotulinumtoxina in peyronie's disease. Urol J. 2015;12:2105–10. [PubMed] [Google Scholar]
- 87.Pavone C, Napoli G, Caruana G, Alonge V, Usala M, Abbadessa D. Safety and tolerability of local treatment with iloprost, a prostacyclin analogue, in patients with Peyronie's disease: A phase I study. BJU Int. 2012;110:117–21. doi: 10.1111/j.1464-410X.2011.10733.x. [DOI] [PubMed] [Google Scholar]
- 88.Akman T, Tefekli A, Armagan A, Kiliçaslan I, Özerman B, Tepeler A, et al. Decorin as a new treatment alternative in Peyronie's disease: Preliminary results in the rat model. Andrologia. 2013;45:101–6. doi: 10.1111/j.1439-0272.2012.01318.x. [DOI] [PubMed] [Google Scholar]
- 89.Kwon KD, Choi MJ, Park JM, Song KM, Kwon MH, Batbold D, et al. Silencing histone deacetylase 2 using small hairpin RNA induces regression of fibrotic plaque in a rat model of Peyronie's disease. BJU Int. 2014;114:926–36. doi: 10.1111/bju.12812. [DOI] [PubMed] [Google Scholar]
- 90.Ryu JK, Kim WJ, Choi MJ, Park JM, Song KM, Kwon MH, et al. Inhibition of histone deacetylase 2 mitigates profibrotic TGF-ß1 responses in fibroblasts derived from Peyronie's plaque. Asian J Androl. 2013;15:640–5. doi: 10.1038/aja.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Castiglione F, Hedlund P, Van der Aa F, Bivalacqua TJ, Rigatti P, Van Poppel H, et al. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie's disease. Eur Urol. 2013;63:551–60. doi: 10.1016/j.eururo.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gokce A, Abd Elmageed ZY, Lasker GF, Bouljihad M, Kim H, Trost LW, et al. Adipose tissue-derived stem cell therapy for prevention and treatment of erectile dysfunction in a rat model of Peyronie's disease. Andrology. 2014;2:244–51. doi: 10.1111/j.2047-2927.2013.00181.x. [DOI] [PubMed] [Google Scholar]
- 93.Chitale S, Morsey M, Swift L, Sethia K. Limited shock wave therapy vs sham treatment in men with Peyronie's disease: Results of a prospective randomized controlled double-blind trial. BJU Int. 2010;106:1352–6. doi: 10.1111/j.1464-410X.2010.09331.x. [DOI] [PubMed] [Google Scholar]
- 94.Hatzichristodoulou G, Meisner C, Gschwend JE, Stenzl A, Lahme S. Extracorporeal shock wave therapy in Peyronie's disease: Results of a placebo-controlled, prospective, randomized, single-blind study. J Sex Med. 2013;10:2815–21. doi: 10.1111/jsm.12275. [DOI] [PubMed] [Google Scholar]
- 95.Husain J, Lynn NN, Jones DK, Collins GN, O’Reilly PH. Extracorporeal shock wave therapy in the management of Peyronie's disease: Initial experience. BJU Int. 2000;86:466–8. doi: 10.1046/j.1464-410x.2000.00827.x. [DOI] [PubMed] [Google Scholar]
- 96.Palmieri A, Imbimbo C, Longo N, Fusco F, Verze P, Mangiapia F, et al. A first prospective, randomized, double-blind, placebo-controlled clinical trial evaluating extracorporeal shock wave therapy for the treatment of Peyronie's disease. Eur Urol. 2009;56:363–9. doi: 10.1016/j.eururo.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 97.Gontero P, Di Marco M, Giubilei G, Bartoletti R, Pappagallo G, Tizzani A, et al. Use of penile extender device in the treatment of penile curvature as a result of Peyronie's disease. Results of a phase II prospective study. J Sex Med. 2009;6:558–66. doi: 10.1111/j.1743-6109.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 98.Levine LA, Newell M, Taylor FL. Penile traction therapy for treatment of Peyronie's disease: A single-center pilot study. J Sex Med. 2008;5:1468–73. doi: 10.1111/j.1743-6109.2008.00814.x. [DOI] [PubMed] [Google Scholar]
- 99.Lue TF, El-Sakka AI. Lengthening shortened penis caused by Peyronie's disease using circular venous grafting and daily stretching with a vacuum erection device. J Urol. 1999;161:1141–4. [PubMed] [Google Scholar]
- 100.Raheem AA, Garaffa G, Raheem TA, Dixon M, Kayes A, Christopher N, et al. The role of vacuum pump therapy to mechanically straighten the penis in Peyronie's disease. BJU Int. 2010;106:1178–80. doi: 10.1111/j.1464-410X.2010.09365.x. [DOI] [PubMed] [Google Scholar]
- 101.Furlow WL, Swenson HE, Jr, Lee RE. Peyronie's disease: A study of its natural history and treatment with orthovoltage radiotherapy. J Urol. 1975;114:69–71. doi: 10.1016/s0022-5347(17)66945-3. [DOI] [PubMed] [Google Scholar]
- 102.Incrocci L, Hop WC, Slob AK. Current sexual functioning in 106 patients with Peyronie's disease treated with radiotherapy 9 years earlier. Urology. 2000;56:1030–4. doi: 10.1016/s0090-4295(00)00805-0. [DOI] [PubMed] [Google Scholar]
- 103.Niewald M, Wenzlawowicz KV, Fleckenstein J, Wisser L, Derouet H, Rübe C. Results of radiotherapy for Peyronie's disease. Int J Radiat Oncol Biol Phys. 2006;64:258–62. doi: 10.1016/j.ijrobp.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 104.Perugia G, Liberti M, Vicini P, Colistro F, Gentile V. Role of hyperthermia in the treatment of Peyronie's disease: A preliminary study. Int J Hyperthermia. 2005;21:367–74. doi: 10.1080/02656730500133892. [DOI] [PubMed] [Google Scholar]
- 105.Adibi M, Hudak SJ, Morey AF. Penile plication without degloving enables effective correction of complex Peyronie's deformities. Urology. 2012;79:831–5. doi: 10.1016/j.urology.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 106.Greenfield JM, Lucas S, Levine LA. Factors affecting the loss of length associated with tunica albuginea plication for correction of penile curvature. J Urol. 2006;175:238–41. doi: 10.1016/S0022-5347(05)00063-7. [DOI] [PubMed] [Google Scholar]
- 107.Taylor FL, Levine LA. Surgical correction of Peyronie's disease via tunica albuginea plication or partial plaque excision with pericardial graft: Long-term follow up. J Sex Med. 2008;5:2221–8. doi: 10.1111/j.1743-6109.2008.00941.x. [DOI] [PubMed] [Google Scholar]
- 108.Cormio L, Zucchi A, Lorusso F, Selvaggio O, Fioretti F, Porena M, et al. Surgical treatment of Peyronie's disease by plaque incision and grafting with buccal mucosa. Eur Urol. 2009;55:1469–75. doi: 10.1016/j.eururo.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 109.Salem EA, Elkady EH, Sakr A, Maarouf AM, Bendary L, Khalil S, et al. Lingual mucosal graft in treatment of Peyronie disease. Urology. 2014;84:1374–7. doi: 10.1016/j.urology.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 110.Kadioglu A, Sanli O, Akman T, Cakan M, Erol B, Mamadov F. Surgical treatment of Peyronie's disease: A single center experience with 145 patients. Eur Urol. 2008;53:432–9. doi: 10.1016/j.eururo.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 111.Usta MF, Bivalacqua TJ, Sanabria J, Koksal IT, Moparty K, Hellstrom WJ. Patient and partner satisfaction and long-term results after surgical treatment for Peyronie's disease. Urology. 2003;62:105–9. doi: 10.1016/s0090-4295(03)00244-9. [DOI] [PubMed] [Google Scholar]
- 112.Wilson SK, Carson CC. Surgical straightening with penile prosthesis. In: Levine LA, editor. Peyronie's Disease: A Guide to Clinical Management. Totowa, NJ: Humana Press; 2007. pp. 249–58. [Google Scholar]
- 113.Carson CC. Penile prosthesis implantation in the treatment of Peyronie's disease and erectile dysfunction. Int J Impot Res. 2000;12(Suppl 4):S122–6. doi: 10.1038/sj.ijir.3900590. [DOI] [PubMed] [Google Scholar]
- 114.Chung PH, Scott JF, Morey AF. High patient satisfaction of inflatable penile prosthesis insertion with synchronous penile plication for erectile dysfunction and Peyronie's disease. J Sex Med. 2014;11:1593–8. doi: 10.1111/jsm.12530. [DOI] [PubMed] [Google Scholar]
- 115.Mulhall J, Ahmed A, Anderson M. Penile prosthetic surgery for Peyronie's disease: Defining the need for intraoperative adjuvant maneuvers. J Sex Med. 2004;1:318–21. doi: 10.1111/j.1743-6109.04046.x. [DOI] [PubMed] [Google Scholar]
- 116.Rolle L, Ceruti C, Timpano M, Sedigh O, Destefanis P, Galletto E, et al. A new, innovative, lengthening surgical procedure for Peyronie's disease by penile prosthesis implantation with double dorsal-ventral patch graft: The “sliding technique”. J Sex Med. 2012;9:2389–95. doi: 10.1111/j.1743-6109.2012.02675.x. [DOI] [PubMed] [Google Scholar]
- 117.Sansalone S, Garaffa G, Djinovic R, Antonini G, Vespasiani G, Ieria FP, et al. Simultaneous total corporal reconstruction and implantation of a penile prosthesis in patients with erectile dysfunction and severe fibrosis of the corpora cavernosa. J Sex Med. 2012;9:1937–44. doi: 10.1111/j.1743-6109.2012.02748.x. [DOI] [PubMed] [Google Scholar]
- 118.Sansalone S, Garaffa G, Djinovic R, Egydio P, Vespasiani G, Miano R, et al. Simultaneous penile lengthening and penile prosthesis implantation in patients with Peyronie's disease, refractory erectile dysfunction, and severe penile shortening. J Sex Med. 2012;9:316–21. doi: 10.1111/j.1743-6109.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 119.Yafi FA, Sangkum P, McCaslin IR, Hellstrom WJ. Strategies for penile prosthesis placement in Peyronie's disease and corporal fibrosis. Curr Urol Rep. 2015;16:21. doi: 10.1007/s11934-015-0491-0. [DOI] [PubMed] [Google Scholar]
- 120.Egydio PH, Kuehhas FE. Penile lengthening and widening without grafting according to a modified ‘sliding’ technique. BJU Int. 2015 doi: 10.1111/bju.13065. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 121.Jordan GH, McCammon KA. Peyronie's disease. In: Kavoussi LR, Novick AC, Peters CA, Wein AJ, editors. Campbell-Walsh Urology. 10th ed. Vol. 1. Philadelphia: Elsevier Saunders; 2012. pp. 792–809. [Google Scholar]
- 122.Lee BJ, Jeong JH, Wang SG, Lee JC, Goh EK, Kim HW. Effect of botulinum toxin type a on a rat surgical wound model. Clin Exp Otorhinolaryngol. 2009;2:20–7. doi: 10.3342/ceo.2009.2.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang L, Tai NZ, Fan ZH. Effect of botulinum toxin type A injection on hypertrophic scar in rabbit ear model. Zhonghua Zheng Xing Wai Ke Za Zhi. 2009;25:284–7. [PubMed] [Google Scholar]
- 124.Xiao Z, Zhang M, Liu Y, Ren L. Botulinum toxin type a inhibits connective tissue growth factor expression in fibroblasts derived from hypertrophic scar. Aesthetic Plast Surg. 2011;35:802–7. doi: 10.1007/s00266-011-9690-3. [DOI] [PubMed] [Google Scholar]
- 125.Xiaoxue W, Xi C, Zhibo X. Effects of botulinum toxin type A on expression of genes in keloid fibroblasts. Aesthet Surg J. 2014;34:154–9. doi: 10.1177/1090820X13482938. [DOI] [PubMed] [Google Scholar]
- 126.Gelfand RA, Vernet D, Kovanecz I, Rajfer J, Gonzalez-Cadavid NF. The transcriptional signatures of cells from the human Peyronie's disease plaque and the ability of these cells to generate a plaque in a rat model suggest potential therapeutic targets. J Sex Med. 2015;12:313–27. doi: 10.1111/jsm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mulhall JP, Nicholson B, Pierpaoli S, Lubrano T, Shankey TV. Chromosomal instability is demonstrated by fibroblasts derived from the tunica of men with Peyronie's disease. Int J Impot Res. 2004;16:288–93. doi: 10.1038/sj.ijir.3901170. [DOI] [PubMed] [Google Scholar]
- 128.Nyberg LM, Jr, Bias WB, Hochberg MC, Walsh PC. Identification of an inherited form of Peyronie's disease with autosomal dominant inheritance and association with Dupuytren's contracture and histocompatibility B7 cross-reacting antigens. J Urol. 1982;128:48–51. doi: 10.1016/s0022-5347(17)52751-2. [DOI] [PubMed] [Google Scholar]
- 129.Jung KH, Ryu YL, Lee HS, Lee H, Son MK, Yan HH, et al. A novel PI3K inhibitor alleviates fibrotic responses in fibroblasts derived from Peyronie's plaques. Int J Oncol. 2013;42:2001–8. doi: 10.3892/ijo.2013.1905. [DOI] [PubMed] [Google Scholar]
- 130.Gonzalez-Cadavid NF, Rajfer J. Experimental models of Peyronie's disease. Implications for new therapies. J Sex Med. 2009;6:303–13. doi: 10.1111/j.1743-6109.2008.01104.x. [DOI] [PubMed] [Google Scholar]
- 131.Pang M, Zhuang S. Histone deacetylase: A potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther. 2010;335:266–72. doi: 10.1124/jpet.110.168385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shindel AW. Sexual dysfunction: The potential of stem cell therapy for Peyronie disease. Nat Rev Urol. 2013;10:8–9. doi: 10.1038/nrurol.2012.221. [DOI] [PubMed] [Google Scholar]


