INTRODUCTION
Each year, over 70,000 Americans develop invasive melanoma, and roughly 9000 die with distant metastases.1 Overall survival for stage IV melanoma is poor, with one-year survival of 33-62% and median survival of 8-9 months.2 One in three patients undergo therapeutic metastasectomy,3 yet median survival after complete resection is only 2 to 4 years.4-7 New checkpoint blockade antibodies (to CTLA-4 or PD-1) and targeted therapies (inhibitors of mutated BRAF and MEK) promise to extend median survival of patients with stage IV melanoma. However, clinical responses with BRAF/MEK inhibition are usually transient,8 and although antibodies to CTLA-4 or PD-1 may produce more durable regressions, about 80% of treated patients still die of their disease within 5 years.9, 10 Thus, there is an urgent need for more effective treatment of advanced melanoma.
Cancer vaccines offer promise to augment specific antitumor immunity and to improve clinical outcomes, despite negative results in most phase III trials to date.11-16 In preclinical models, vaccines against cancer antigens can induce strong T cell responses and can be therapeutic alone or in combination with other immune therapies.17, 18 However, vaccine approaches in humans are not optimized, and there is no consensus regarding the best way to induce therapeutic immune responses. Many vaccines have been developed to induce CD8+ T cells, but there has been limited focus on vaccines targeting CD4+ helper T cells. We have previously reported the safety, immunogenicity, and clinical activity of a combination vaccine comprised of 6 HLA-DR restricted peptides that can be recognized by helper T cells (6 melanoma helper peptides, 6MHP).19-21 Within these studies, cytokine profiles in peripheral blood and sentinel immunized nodes of vaccinated patients indicate a Th1-biased CD4+ T-cell immune response. Moreover, the proportion of FoxP3-positive T-cells responding to 6MHP does not increase with vaccination. The 6MHP vaccine can also induce a CD8+ T cell response through epitope spreading. In a multicenter trial incorporating 6MHP vaccines administered to patients with measurable stage IV melanoma, there was a significant association between the development of specific immune responses to 6MHP and overall survival; furthermore, 1 year survival compared favorably to prior experience.22, 23
We now have the opportunity to evaluate long-term outcomes of stage IV patients vaccinated with 6MHP on two clinical trials, and to test whether the association observed between immune response and survival in the E1602 trial is reproducible in another patient population. We hypothesized that vaccination with 6MHP would be associated with better overall survival than in untreated control patients, and that helper T cell immune responses to vaccination would be associated with improved overall survival.
METHODS
Patient Selection
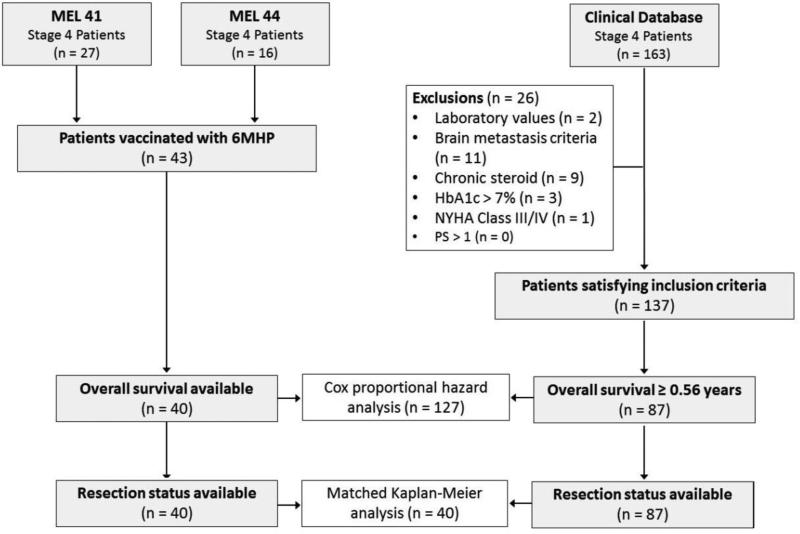
Patients with stage IV melanoma were vaccinated with 6MHP in two clinical trials: MEL41 (NCT00089219) and MEL44 (NCT00118274). All patients were required to express at least one of the five HLA-DR alleles by which CD4+ T-cell recognition for the 6 component peptides had previously been defined (HLA-DR1, -DR4, -DR11, -DR13, or -DR15; about 80% of patients). Other inclusion criteria were age 18 years or greater, Eastern Cooperative Oncology Group performance status 0 or 1, serum creatinine less than 1.5× the upper limit of normal, serum aspartate transaminase and alanine transaminase less than 2.5× the upper limit of normal, and hemoglobin A1c no greater than 7%. Exclusion criteria included ocular melanoma, greater than 3 brain metastases, untreated brain metastases, chronic corticosteroid use, and New York Heart Association class III or IV heart disease. Combining both trials, a total of 43 patients were identified, 40 of which had survival outcomes available.
Consecutive, unvaccinated patients with stage IV melanoma were identified from a prospectively-collected clinical database approved for research use (IRB #10803). Patients with an unknown date of initial distant metastasis were excluded. A detailed chart review was performed for the resulting 163 patients. Patients who did not satisfy all of the vaccine trials’ inclusion and exclusion criteria were excluded. Because HLA-DR typing was not routinely performed for unvaccinated patients, this was not an exclusion factor for the control group. Twenty-six patients were excluded from the control group due to ineligibility, leaving 137 who satisfied all of the aforementioned criteria.
We also corrected for the fact that some patients with stage IV melanoma progress and die rapidly, before being eligible for a clinical trial. Among MEL41 and MEL44 patients, the average interval from initial metastasis to first vaccine administration was 0.56 years, effectively excluding patients who did not survive to that time point. Therefore, we further excluded from the control patients all who survived less than 0.56 years (n = 50) in order to correct for lead-time bias that would otherwise favor the vaccinated group. Ultimately, 87 control patients were included for analysis (Figure 1). For control patients who developed their initial metastases during the enrollment period for MEL41 and MEL44, reasons for not enrolling on these trials were noted.
Figure 1.
Inclusion criteria and matching protocol for study patients. 6MHP: 6 melanoma helper peptide vaccine; HbA1c: hemoglobin A1c level; NYHA: New York Heart Association; PS: Eastern Cooperative Oncology Group performance status;
Clinical Trial Design
The 6MHP vaccine is comprised of 6 peptide epitopes each 14-23 amino acids in length: gp10044-59, tyrosinase56-70, tyrosinase386-406, Melan-A/MART-151-73, MAGE-A3281-295, and MAGE-A1, 2,3,6121-134. Clinical trial design and immune response assay protocols have been reported.19, 24 In brief, for MEL41, peptides were administered with 110 mcg GMCSF in a stable emulsion with 1 mL Montanide ISA-51 adjuvant (IFA, Seppic, Inc., Paris, France/Fairfield, NJ) at one of three dose levels per peptide (A: 200 mcg, n = 10; B: 400 mcg, n = 7; C: 800 mcg, n = 10) for each of 6 vaccine administrations. Using repeated peripheral blood samples during and after the vaccination protocol, specific immune responses were assessed in vitro by measuring CD4+ T-cell proliferation following antigen exposure. Negative controls included peripheral blood mononuclear cells in culture media alone, phytohemagglutinin, or bovine serum albumin (Sigma-Aldrich, St. Louis, MO).
For MEL44, 6MHP was administered in Montanide ISA-51 adjuvant over 10 doses (200 mcg each peptide per vaccine) in combination with a class I MHC-restricted multipeptide vaccine (12MP) with (Arm D, n = 6) or without (Arm C, n = 7) cyclophosphamide. Peptide-specific CD4+ T-cell responses were measured using interferon gamma ELISpot assays performed on peripheral blood directly ex vivo after cryopreservation.25 Negative controls included irrelevant peptides and phytohemagglutinin. For both trials, the immune response was based on the ratio of the T cell response to 6MHP versus the highest negative control value (Rvax). Rvax was also corrected for any pre-vaccine response.
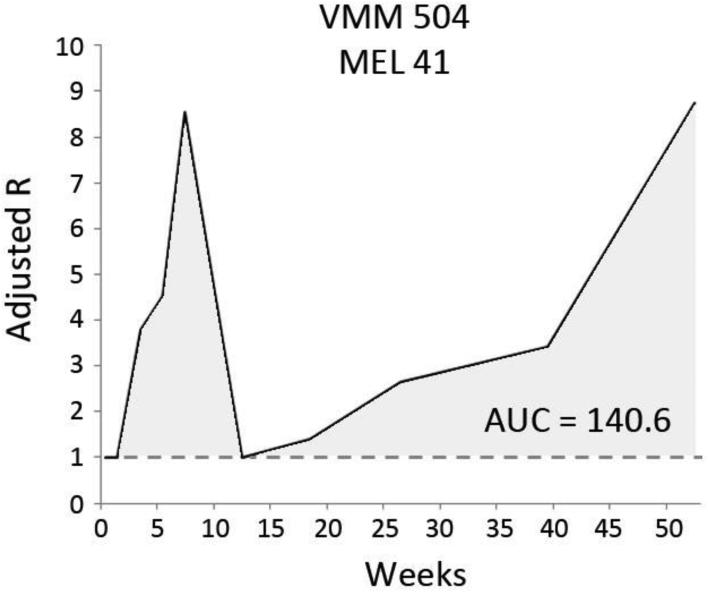
Prior reports of MEL41 and MEL44 data defined immune responses based on the maximal Rvax over time.25 MEL41 included immune responses in both peripheral blood samples and the sentinel immunized node, while MEL44 evaluated peripheral blood only. For consistency in the present study, immune responses were based on peripheral blood only, and we developed an immune response metric that also incorporates response durability. Rvax – 1 represents the magnitude of vaccine-specific stimulation beyond the highest negative control. By taking the area under the curve (AUC) of multiple Rvax – 1 measurements for each patient over time, the immune response metric incorporates both durability of response and multimodal response patterns (Figure 2). A high-sensitivity threshold of AUC > 1 (unit = Rvax x week) was used to indicate a positive immune response.
Figure 2.
Calculation of the area under the curve (AUC) measure of immune response. Data from patient VMM504, participant within MEL 41. Stimulation index (R) is the ratio of the number of T-cells responding to vaccine peptides versus negative control.
Clinical Endpoints
For all patients, the following clinical data were recorded: age at initial distant metastasis, initial metastasis category (M1a, M1b, and M1c),2 resection status, and date of death from any cause as assessed through the Social Security Death Index. For comparisons between vaccinated and control patients, the primary outcome was overall survival (OS) measured from the date of initial distant metastasis. Within the vaccinated group, predictors of clinical outcome were assessed using overall survival from the date of first vaccination. Progression-free survival (PFS), available among vaccinated patients with measurable disease, was defined as time from the first vaccine treatment to disease progression or death. Disease-free survival (DFS), available among vaccinated patients who underwent metastasectomy prior to enrollment, was defined as time from the first vaccine treatment to recurrence or death.
In a prior meta-analysis, Korn and colleagues collected data on 2100 patients with advanced melanoma and measurable disease who were enrolled in clinical trials in national cooperative groups.22 Similar to our trial group, patients in this study were treated before the era of checkpoint blockade antibodies and targeted therapies. To set survival benchmarks for future trials, this prior study reported predicted one-year survival rates based on age, performance status, and metastasis category. Using these predicted values, we computed expected one-year survival outcomes for our patient population.
Statistical Analyses
The relationship between immune response and OS from the date of first vaccination was measured for trial patients using Cox proportional hazards survival analysis, taking into account the following clinical risk factors: age, metastasis category, resection status, and immune response. Comparisons between vaccinated and control patients were performed in two methods. First, patients from both groups were pooled into one study sample, and Cox proportional hazards analysis was used to measure the impact of the following risk factors on OS from the date of initial distant metastasis: age, metastasis category, resection status, and vaccination. Second, vaccinated and control patients were matched using a greedy 1:1 algorithm based on identical metastasis category and resection status, and age within 10 years. In the matched sample, Kaplan-Meier survival analysis and the log-rank test were used to assess differences in OS. All statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
This study was conducted under the sanction of the Institutional Review Board of the University of Virginia (IRB #10524, 10464, 10803) and the US Food and Drug Administration (BB-IND #10825, #9847).
RESULTS
Patient populations
The study included 40 vaccinated patients (27 from MEL41, 13 from MEL44) and 87 control patients. Vaccinated patients were enrolled between September 2003 and July 2007 (diagnosed with stage IV July 2001 – July 2006). The control patients developed distant metastases (stage IV) during an overlapping time interval (July 1995 – January 2014), with 66% (57/87) diagnosed before or after the enrollment periods for MEL41 and MEL44. For control patients who were eligible during the trial enrollment periods, reasons for non-participation included opting for alternative treatments or no treatment (43%), HLA-DR mismatch (27%), failure to follow-up for a scheduled visit (20%), long travel distance (7%), and inability to provide informed consent (3%). Demographic characteristics are shown in Table 1. Just over half of all patients underwent complete metastasectomy, rendering them clinically free of disease at some point in their disease course. Data pertaining to non-vaccine systemic therapies were available for 28 vaccinated patients and 82 control patients. Vaccinated patients were treated with cytotoxic chemotherapy less frequently than controls (21%, 6/28 vs 51%, 42/82, p = 0.008); treatment rates of other systemic agents were not significantly different across groups (Table 1).
Table 1.
Clinical characteristics of vaccinated and control patients
| Unmatched | Matched | |||||
|---|---|---|---|---|---|---|
| Vaccinated Median (IQR) N (%) | Controls Median (IQR) N (%) | p-value | Vaccinated Median (IQR) N (%) | Controls Median (IQR) N (%) | p-value | |
| Total | 40 | 87 | 40 | 40 | ||
| Stage IV diagnosis date | 7/04 (7/03 – 3/05) | 3/05 (5/02 – 2/09) | 7/04 (7/03 – 3/05) | 2/05 (11/01 – 3/08) | ||
| Agea | 60.8 (53.3 – 66.3) | 57.3 (49.5 – 69.9) | 0.427 | 60.8 (53.3 – 66.3) | 58.3 (53.5 – 69.9) | 0.677 |
| Resected | 23 (58%) | 50 (57%) | 0.998 | 23 (58%) | 23 (58%) | 1.000 |
| Stage | 0.100 | 1.000 | ||||
| M1a | 11 (28%) | 26 (30%) | 11 (28%) | 11 (28%) | ||
| M1b | 19 (48%) | 26 (30%) | 19 (48%) | 19 (48%) | ||
| M1c | 10 (25%) | 35 (40%) | 10 (25%) | 10 (25%) | ||
| Other Therapies | ||||||
| Records available | 28 | 82 | 28 | 37 | ||
| Chemotherapy | 6 (21%) | 42 (51%) | 0.008 | 6 (21%) | 21 (57%) | 0.005 |
| Immune therapy | 6 (21%) | 31 (38%) | 0.164 | 6 (21%) | 10 (27%) | 0.773 |
| Targeted therapy | 1 (4%) | 3 (4%) | 1.000 | 1 (4%) | 1 (3%) | 1.000 |
IQR – Interquartile range
Age at earliest evidence of Stage 4 melanoma
Clinical outcome
The average time interval between initial distant metastasis and initiation of vaccine therapy was 0.56 years (interquartile range, IQR 0.40 – 1.56). Thirty-nine patients completed the vaccine treatment course (98%); one patient's vaccine course in MEL44 was truncated due to disease progression during treatment. Fourteen vaccinated patients (35%) were alive at the time of analysis. Measuring from the date of the first vaccine, median follow-up was 9.1 years (IQR 8.6 – 9.8), median OS was 4.6 years (IQR 1.5 – 7.1), and five-year OS was 41% and 68% for MEL41 and MEL44 patients, respectively (p = 0.069). Median PFS for vaccinated patients with unresected measurable disease was 0.21 years (IQR 0.17 – 0.24); median DFS for vaccinated patients who underwent metastasectomy was 1.54 years (IQR 0.74 – 3.62). Cox regression analysis of the 127 combined vaccinated and control patients identified independent predictors of improved overall survival to be resection status (HR 0.54, p = 0.004) and vaccination (HR 0.24, p < 0.001). Age and metastasis category were not significantly associated with survival.
Matched pair analysis
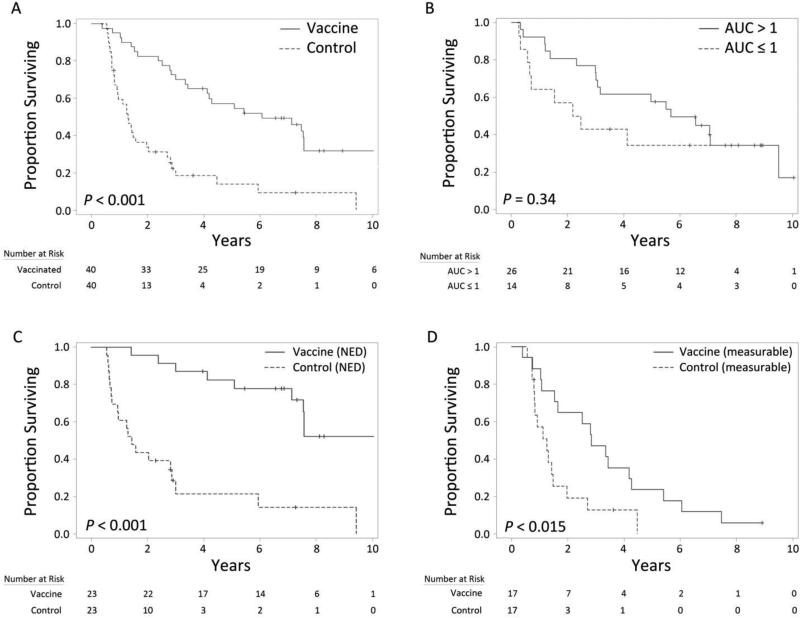
Forty pairs of vaccinated and control patients were matched by metastatic category (M stage), resection status, and by age (within 10 years, at diagnosis of stage IV). For 29 of the matched pairs (73%), age differed by less than five years (Table 1). Kaplan-Meier survival analysis within this matched population is shown in Figure 3A. Vaccinated patients experienced significantly superior overall survival compared to matched controls, with estimated one- and five-year survival rates of 95% and 57%, respectively, versus 57% and 16% (p < 0.001). Median OS from date of initial metastasis was 5.4 years for vaccinated patients (IQR 2.8 – 7.6), compared to 1.3 years (IQR 0.8 – 2.7) for controls.
Figure 3.
Kaplan-Meier survival analyses. Comparison of matched vaccinated and control patients (A). Stratification of vaccinated patients by immune response (B) and resection status (C, D). AUC: area under the immune response curve; NED: no evidence of disease at time of first vaccination.
Immune Response
Twenty-six vaccinated patients developed a specific immune response to 6MHP in peripheral blood (65%), with a median AUC of 41.1. Among immune responders (AUC > 1), one- and five-year survival rates were 92% and 58%, respectively, compared to 64% and 34% among non-responders (Figure 3B). In multivariable analysis, OS was significantly higher among patients who developed an immune response (HR 0.35, p = 0.040).
Resection Status
Among vaccinated patients who underwent resection prior to treatment (n = 23), one- and five-year OS were 96% and 74%, respectively, with median OS of 6.6 years (IQR 3.8 – 7.9) from the date of first vaccination. Among patients not considered resectable (n = 17) who had measurable distant disease, one- and five-year OS were 65% and 18%, respectively, with median OS of 1.4 years (IQR 0.7 – 3.2). The difference in survival between resected and non-resected vaccinated patients was significant on multivariable analysis (HR 0.09, p < 0.001). Vaccinated patients in both the resected (Figure 3C) and non-resected (Figure 3D) subpopulations had significantly superior overall survival when compared to non-vaccinated matched controls.
Comparison to expected outcomes
A large prior study reported clinical outcomes of patients with advanced melanoma (stage IV, unresected) enrolled on national cooperative group clinical trials.22 Correcting for gender (47% male), visceral disease (M1b/M1c 76% vs M1a 24%), and performance status (53% PS 0; 47% PS 1), the expected one-year OS rate for patients matching our vaccinated study population is 35%, compared to the 65% one-year survival observed in patients with advanced melanoma who were vaccinated with 6MHP.
DISCUSSION
When melanoma is identified early, long-term survival is likely. Even for patients with sentinel node metastasis (stage IIIA), 5-year survival is approximately 70% with appropriate surgical management.26 More problematic is the appearance of distant metastases (stage IV). Though some metastases are resectable, most of these patients have historically gone on to recur and to die of melanoma. During the years in which patients were treated with 6MHP in the present study (2003-2007), cytotoxic chemotherapy and high-dose interleukin-2 were the only approved systemic therapies for unresectable stage IV melanoma. Durable clinical response rates to cytotoxic chemotherapy are below 1%. High dose-IL-2 is too toxic for many patients, and induces durable complete regressions in only about 5%.27, 28
New FDA-approved therapies for advanced melanoma are revolutionizing management. Inhibitors of BRAF and MEK prolong survival, with median OS of 16 months and clinical response duration of 6-8 months.29, 30 Immune checkpoint blockade with antibody to CTLA-4 can induce durable clinical regressions, with median survival of 10 to 12 months and 1 and 4 year survival rates of approximately 50 and 20%, respectively. 9, 31 Checkpoint blockade with PD-1 antibody can induce durable clinical regressions in 30-40% of patients, with 1 and 2 year survival rates of 62% and 43%, respectively.10 In this study, we observed favorable clinical outcomes among metastatic patients treated using a multi-epitope helper peptide vaccine in the era before availability of targeted therapies and checkpoint blockade antibodies. Contrasted against a group of clinically-comparable historical controls, vaccinated patients experienced extended overall survival. Recent data suggest that 77% of patients vaccinated with 6MHP in the setting of stage III/IV disease develop antigen-specific antibody responses within the first two months.32 These responses often persist beyond 6 months after the last vaccination. Antibody responses are associated with clinical outcome, even after controlling for the CD4 T-cell response. Thus, the induction of durable immune memory may play a role in this association.
Strengths of the present study's control group are that detailed clinical data are available, and that they were managed in a period overlapping with the vaccine trial periods. This is important because a later date of stage IV diagnosis has been associated with better survival. Within the matched cohorts, median date of stage IV diagnosis in the vaccinated group was roughly 5 months earlier than the control group (Table 1). Thus, while date of stage IV diagnosis was not accounted for in multivariable analyses, it is unlikely to significantly alter the study outcomes. The matching protocol also accounted for several key confounding factors. Demographic comparisons revealed a higher rate of treatment with chemotherapy among control patients (51% vs 21%). Historically, chemotherapy for metastatic melanoma was associated with low objective response rates and no improvement in overall survival.33 Thus, we did not match patients based on this treatment factor.
Nevertheless, there may be unaccounted biases with comparisons between patients who do and do not enroll in clinical trials. Referral bias is one factor to consider, as clinicians may be more inclined to enroll patients with more indolent disease courses for experimental therapy. Thus, comparing outcomes of patients vaccinated with 6MHP against outcomes from other clinical trials is worthwhile. In our experience, patients with measurable stage IV melanoma treated with 6MHP vaccines experienced 65% one-year survival. By comparison, the expected one-year survival of our population—based on Korn and colleague's prior meta-analysis—is 35%. Similarly favorable outcomes were noted in the multi-institutional E1602 trial, in which patients vaccinated with 6MHP with or without 12MP were observed to have one-year OS exceeding the upper bound of the 95% confidence interval of expected outcomes.24
For patients with stage IV melanoma completely resected with surgery, other peptide vaccine trials have reported median survival of 3.8 years with 5-year survival of 45% 34. In a trial comparing resected stage IV patients treated with allogeneic whole cell vaccine and Bacille-Calmette Guerin (BCG) versus control patients treated with BCG only, median survival of the two groups were 2.7 and 3.3 years, respectively.35 In the present study, we observed even more favorable overall survival from initial vaccination among resected patients (median 6.6 years) using a different, peptide-based vaccine.
PD-1 blockade, CTLA-4 blockade, and BRAF inhibitor therapy are effective systemic therapies for advanced melanoma that were not available during the present study's enrollment periods. Treatment with each of those agents (with or without other vaccines) has resulted in survival data approaching our experience with 6MHP vaccines alone (Table 3). 9, 10, 15, 29, 31 Acknowledging limitations of any non-randomized comparisons, outcomes for both resected and advanced melanoma patients treated with 6MHP vaccines is similar to available outcomes with these modern agents in the adjuvant or advanced disease settings.
Table 3.
Cox regression analysis for overall survival across vaccinated patients (n = 40)
| Risk Factor | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Age | 0.98 | 0.94 – 1.02 | 0.340 |
| Stage (M1a/b/c) | 0.53 | 0.29 – 0.98 | 0.043 |
| Resection | 0.09 | 0.03 – 0.28 | < 0.001 |
| Immune Response | 0.35 | 0.13 – 0.96 | 0.040 |
CI – confidence interval
These promising findings incentivize the incorporation of 6MHP into combination treatment regimens for metastatic melanoma. At the time of this publication, phase II clinical trials of 6MHP plus ipilimumab (MEL62, NCT02385669) and 6MHP plus vemurafenib (MEL61, NCT02382549) are in active recruitment, while a trial of 6MHP plus pembrolizumab (MEL64) is under review for approval. Improving the immunogenicity of 6MHP is also worthwhile; a trial assessing the impact of two adjuvants—cyclophosphamide and polyICLC—on the immune response rate of 6MHP is currently undergoing recruitment (MEL63, NCT02425306). Ultimately, a phase III randomized trial comparing combination therapies for stage IV patients has the potential to influence standard of care; the present study provides preliminary data to help power such a trial.
This study's results are limited by potential biases inherent to comparisons of patients on clinical trials with those not enrolled on trials. Indeed, Morton and colleagues previously reported optimistic results for the Canvaxin vaccine in a matched cohort study, only to find no significant impact on survival in the subsequent randomized phase III trial.36 To adjust for potential confounders, we excluded control patients who would not have met trial inclusion criteria, matched patients on key clinical variables, and adjusted for lead-time bias. Despite these corrections, unmeasured biases may remain, and randomized trial data will be required to make definitive conclusions about the clinical benefit of 6MHP. Secondly, we did not select controls based on HLA-DR expression. In subsequent work, we found that CD4+ T cell responses to the 6MHP vaccine were not restricted by HLA-DR expression,21 and we are no longer limiting enrollment in current trials based on HLA-DR alleles. The HLA-DR11 molecule has been associated with poorer survival in two studies.37, 38 In the present study, 20% (8/40) of vaccinated patients expressed HLA-DR11; among control patients with known HLA type, HLA-DR11 prevalence was comparable (21%, 3/14). Third, because this study's patients were combined from two clinical trials, immune responses were measured using two different assays. Nevertheless, immune response rates in the blood were similar across the two trials.19, 25 A subset of patients also received 12MP vaccines and/or low-dose cyclophosphamide; however, neither of these have had impact on CD4+ T cell responses in randomized studies.24, 25 Despite these limitations, we have observed very encouraging clinical outcomes in stage IV melanoma patients vaccinated with 6MHP, and prior findings of association between immune response and survival are supported.
CONCLUSIONS
Survival outcomes of patients treated with the 6MHP vaccine are very favorable, exceeding those of matched institutional controls and previously-reported data from stage IV patients treated with other therapies. Encouraging outcomes were seen both in patients with measurable advanced melanoma and those rendered clinically free of disease with surgery. These early findings argue for reinvigorated efforts to study how the vaccine-primed immune system interacts with new metastases through collaborative, prospective randomized trials. Factors limiting the clinical success of cancer vaccines have included immunologic dysfunction in the tumor microenvironment and obstacles to T cell homing to the tumor. However, checkpoint blockade and BRAF inhibitor therapy can modulate the tumor microenvironment to support T cell infiltration and can reverse tumor-associated immune dysfunction. Thus, there are new opportunities to test whether combinations of these effective systemic therapies with 6MHP vaccines can generate durable tumor control and immunologic memory.
Table 2.
Comparison with Advanced Melanoma Trials
| Study | Treatment | Year | Stage | N | PS | M1b/M1c | Resection | Median OS | 1YS | 5YS |
|---|---|---|---|---|---|---|---|---|---|---|
| Present Study | 6MHP | IV | 23 | 0-1 | 70% | NED | 80 mos | 96% | 74% | |
| Gibney 15 | Nivolumab | 2014 | IIIC-IV | 33 | 0-1 | 73% | NED | NA | 87% | NA |
| Morton 35 | Vaccines (cell) + BCG | 2007 | IV | 246 | NA | NA | NED | 32 mos | 78%* | 40% |
| Morton 35 | BCG alone | 2007 | IV | 250 | NA | NA | NED | 39 mos | 80%* | 45% |
| Tagawa 34 | Vaccines (peptide) | 2006 | IV | 41 | 0 | 46% | NED | 45.6 mos | 90%* | 45% |
| Present Study | 6MHP | IV | 17 | 0-1 | 76% | Measurable | 16.5 mos | 65% | 18% | |
| Schadendorf 9 | Ipilimumab | 2015 | IIIC-IV | 1861 | 0-1 | NA | Measurable | 11.4 mos | 48%* | 20%* |
| Topalian 10 | Nivolumab | 2014 | IV | 107 | 0-2 | 78% | Measurable | 16.8 mos | 62% | NA |
| Sosman 29 | Vemurafenib | 2012 | IV | 132 | 0-1 | 75% | Measurable | 15.9 mos | 58% | NA |
| Hodi 31 | Ipilimumab +/− gp100 | 2010 | IIIC-IV | 540 | 0-1 | 90% | Measurable | 10.0 mos | 45% | NA |
| Korn 22 | Multiple | 2008 | IV | 2100 | 0-3 | 78% | Measurable | 6.2 mos | 26%† | NA |
estimated from published Kaplan-Meier survival curve
1-year survival for the whole study population listed; value is 35% when corrected for demographics in the present study.
PS: Eastern Cooperative Oncology Group Performance Status; OS: overall survival; NED: no evidence of disease on enrollment;
NA: not available
ACKNOWLEDGEMENTS
Financial Support: This study was funded by NIH/NCI grants NIH R01 CA057653 and CA118386, and NIH R21 CA103528 (to C.L.S), and NIH T32 CA163177 (to YH). Support was also provided by the University of Virginia Cancer Center Support Grant (NIH/NCI P30 CA44579, Clinical Trials Office, Tissue Procurement Facility, and Biomolecular Core Facility); and the UVA General Clinical Research Center (NIH M01 RR00847). Peptides used in the vaccines were prepared with philanthropic support from the Commonwealth Foundation for Cancer Research and Alice and Bill Goodwin. Additional philanthropic support was provided from Frank and Jane Batten, the James and Rebecca Craig Foundation, George S. Suddock, Richard and Sherry Sharp, and the Patients and Friends Research Fund of the University of Virginia Cancer Center.
ABREVIATION LIST
- AUC
Area under the curve
- BCG
Bacille-Calmette Guerin
- IQR
interquartile range
- OS
overall survival
- PFS
progression-free survival
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huo J, Du XL, Lairson DR, et al. Utilization of Surgery, Chemotherapy, Radiation Therapy, and Hospice at the End of Life for Patients Diagnosed With Metastatic Melanoma. Am J Clin Oncol. 2013 doi: 10.1097/COC.0b013e31829378f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Essner R, Lee JH, Wanek LA, et al. Contemporary surgical treatment of advanced-stage melanoma. Arch Surg. 2004;139:961–6. doi: 10.1001/archsurg.139.9.961. discussion 966-7. [DOI] [PubMed] [Google Scholar]
- 5.Rose DM, Essner R, Hughes TM, et al. Surgical resection for metastatic melanoma to the liver: the John Wayne Cancer Institute and Sydney Melanoma Unit experience. Arch Surg. 2001;136:950–955. doi: 10.1001/archsurg.136.8.950. [DOI] [PubMed] [Google Scholar]
- 6.Schuhan C, Muley T, Dienemann H, et al. Survival after pulmonary metastasectomy in patients with malignant melanoma. Thorac Cardiovasc Surg. 2011;59:158–162. doi: 10.1055/s-0030-1250669. [DOI] [PubMed] [Google Scholar]
- 7.Tarhini AA, Agarwala SS. Management of brain metastases in patients with melanoma. Curr Opin Oncol. 2004;16:161–166. doi: 10.1097/00001622-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 8.McDermott D, Lebbe C, Hodi FS, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40:1056–1064. doi: 10.1016/j.ctrv.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Schadendorf D, Hodi FS, Robert C, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol. 2015 doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hersey P, Coates AS, McCarthy WH, et al. Adjuvant immunotherapy of patients with high-risk melanoma using vaccinia viral lysates of melanoma: results of a randomized trial. J Clin Oncol. 2002;20:4181–4190. doi: 10.1200/JCO.2002.12.094. [DOI] [PubMed] [Google Scholar]
- 12.Suriano R, Rajoria S, George AL, et al. Follow-up analysis of a randomized phase III immunotherapeutic clinical trial on melanoma. Mol Clin Oncol. 2013;1:466–472. doi: 10.3892/mco.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sondak VK, Liu PY, Tuthill RJ, et al. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: overall results of a randomized trial of the Southwest Oncology Group. J Clin Oncol. 2002;20:2058–2066. doi: 10.1200/JCO.2002.08.071. [DOI] [PubMed] [Google Scholar]
- 14.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 15.Gibney GT, Kudchadkar RR, DeConti RC, et al. Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high-risk metastatic melanoma. Clin Cancer Res. 2015;21:712–720. doi: 10.1158/1078-0432.CCR-14-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber JS, Kudchadkar RR, Yu B, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31:4311–4318. doi: 10.1200/JCO.2013.51.4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrios K, Celis E. TriVax-HPV: an improved peptide-based therapeutic vaccination strategy against human papillomavirus-induced cancers. Cancer Immunol Immunother. 2012;61:1307–1317. doi: 10.1007/s00262-012-1259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein MJ, Varghese B, Brody JD, et al. A CpG-loaded tumor cell vaccine induces antitumor CD4+ T cells that are effective in adoptive therapy for large and established tumors. Blood. 2011;117:118–127. doi: 10.1182/blood-2010-06-288456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slingluff CL, Jr, Petroni GR, Olson W, et al. Helper T-cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. J Clin Oncol. 2008;26:4973–4980. doi: 10.1200/JCO.2008.17.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dillon PM, Olson WC, Czarkowski A, et al. A melanoma helper peptide vaccine increases Th1 cytokine production by leukocytes in peripheral blood and immunized lymph nodes. J Immunother Cancer. 2014;2:23–1426-2-23. doi: 10.1186/2051-1426-2-23. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Petroni GR, Olson WC, et al. Immunologic hierarchy, class II MHC promiscuity, and epitope spreading of a melanoma helper peptide vaccine. Cancer Immunol Immunother. 2014;63:779–786. doi: 10.1007/s00262-014-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 23.Slingluff CL, Jr, Lee S, Zhao F, et al. A Randomized Phase II Trial of Multiepitope Vaccination with Melanoma Peptides for Cytotoxic T Cells and Helper T Cells for Patients with Metastatic Melanoma (E1602). Clin Cancer Res. 2013;19:4228–4238. doi: 10.1158/1078-0432.CCR-13-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slingluff CL, Jr, Lee S, Zhao F, et al. A Randomized Phase II Trial of Multiepitope Vaccination with Melanoma Peptides for Cytotoxic T Cells and Helper T Cells for Patients with Metastatic Melanoma (E1602). Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, et al. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol. 2011;29:2924–2932. doi: 10.1200/JCO.2010.33.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599–609. doi: 10.1056/NEJMoa1310460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 28.Atkins MB, Kunkel L, Sznol M, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–4. [PubMed] [Google Scholar]
- 29.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed CM, Cresce ND, Mauldin IS, et al. Vaccination with Melanoma Helper Peptides Induces Antibody Responses Associated with Improved Overall Survival. Clin Cancer Res. 2015 doi: 10.1158/1078-0432.CCR-15-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatia S, Tykodi SS, Thompson JA. Treatment of metastatic melanoma: an overview. Oncology (Williston Park) 2009;23:488–496. [PMC free article] [PubMed] [Google Scholar]
- 34.Tagawa ST, Cheung E, Banta W, et al. Survival analysis after resection of metastatic disease followed by peptide vaccines in patients with Stage IV melanoma. Cancer. 2006;106:1353–1357. doi: 10.1002/cncr.21748. [DOI] [PubMed] [Google Scholar]
- 35.Morton DL, Mozzillo N, Thompson JF, et al. An international, randomized, phase III trial of bacillus Calmette-Guerin (BCG) plus allogeneic melanoma vaccine (MCV) or placebo after complete resection of melanoma metastatic to regional or distant sites. Journal of clinical oncology. 2007 [Google Scholar]
- 36.Morton DL, Hsueh EC, Essner R, et al. Prolonged survival of patients receiving active immunotherapy with Canvaxin therapeutic polyvalent vaccine after complete resection of melanoma metastatic to regional lymph nodes. Ann Surg. 2002;236:438–48. doi: 10.1097/00000658-200210000-00006. discussion 448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JE, Abdalla J, Porter GA, et al. Presence of the human leukocyte antigen class II gene DRB1*1101 predicts interferon gamma levels and disease recurrence in melanoma patients. Ann Surg Oncol. 2002;9:587–593. doi: 10.1007/BF02573896. [DOI] [PubMed] [Google Scholar]
- 38.Pollack MS, Livingston PO. HLA and DR antigen frequencies in melanoma patients: possible relation to disease prognosis. Tissue Antigens. 1985;26:262–265. doi: 10.1111/j.1399-0039.1985.tb00970.x. [DOI] [PubMed] [Google Scholar]