Abstract
Development of hyperglycemia during sepsis is associated with increased morbidity and mortality. Nutritional support is common practice in the intensive care unit, but the metabolic effects are not well understood. The purpose of this study is to determine the effect of early low-level calorie provision on the development of hyperglycemia in a clinically relevant murine model of sepsis. C57BL/6J mice underwent femoral arterial and venous catheterization followed by cecal ligation and puncture (CLP) or sham surgery and low-dose intravenous dextrose or saline infusion. Blood glucose, plasma insulin, and cytokines were measured after 24 h. Additional septic mice underwent hyperinsulinemic-euglycemic clamps or received intravenous insulin concurrent with dextrose to determine whole-body insulin sensitivity and test the efficacy of insulin to reverse hyperglycemia. Neither dextrose infusion nor CLP alone induced hyperglycemia. Early initiation of low-level dextrose in septic mice produced a variable glycemic response: 49% maintained euglycemia (blood glucose <200) and 27% developed severe hyperglycemia (blood glucose≥600). Hyperglycemia was associated with increased inflammation and reduced insulin secretion and sensitivity compared with control mice or CLP mice maintaining euglycemia. Insulin prevented the progression to severe hyperglycemia but was ineffective in reestablishing glycemic control once hyperglycemia had developed. In conclusion, early initiation of clinically relevant low-level dextrose (~20% daily caloric requirements) precipitated hyperglycemia akin to an acute diabetic phenotype in septic mice characterized by decreased insulin sensitivity, decreased insulin secretion, and an increased inflammatory response.
Keywords: dextrose, nutrition, sepsis, mouse, hyperglycemia, critical illness
Introduction
Sepsis is a significant cause of morbidity and mortality among critically ill patients and is characterized by overwhelming inflammation in response to infection leading to organ dysfunction. Metabolic dysfunction characterized by elevations in circulating blood glucose occurs in nearly 12% of cases and is associated with increased organ dysfunction, longer hospital stays, and higher mortality (Krinsley 2003; Martin et al. 2003; Leonidou et al. 2008; Kyle et al. 2010). Initial clinical trials in critically ill patients demonstrated mortality benefits with tight glycemic control using intravenous insulin, but subsequent trials failed to show the same benefit and suggested potential harm because of the increased risk of hypoglycemia with the use of insulin infusions to target intensive glucose control (van den Berghe et al. 2001; Brunkhorst et al. 2008; Finfer et al. 2009; Ling et al. 2012). Of note, septic patients maintaining euglycemia and not requiring supplemental insulin appear to experience improved mortality (Leonidou et al. 2008). Therefore, developing strategies to prevent the development of hyperglycemia rather than treat it once established may improve morbidity and mortality in sepsis.
Nutritional support, particularly early in sepsis, may contribute to glucose elevation and thus adversely impact patient outcomes. The large clinical trials of glucose control in critical illness all employed glucose infusion early in the course of the intervention and demonstrated variable effects on outcome (van den Berghe et al. 2001; Brunkhorst et al. 2008; Finfer et al. 2009). The EPaNIC trial showed that early administration of parenteral nutrition (as low as 20% of daily calorie needs during the first 24 h) was associated with increased organ dysfunction and increased length of stay in the intensive care unit in critically ill patients (Casaer et al. 2011). However, the recent CALORIES trial demonstrated that the early administration of parenteral nutritional support, at least in comparison with similar levels of enteral nutrition, was not harmful (Harvey et al. 2014). Based on conflicting data from large clinical trials, controversy remains regarding the risks and benefits of early caloric supplementation in septic patients, and the metabolic consequences of caloric supplementation at low levels have not been systematically evaluated either in human studies or in animal models.
We designed the current study to determine how metabolic dysfunction in sepsis is induced by low-level caloric supplementation. We examined the impact of early initiation of parenteral dextrose at 20% of daily caloric needs (equivalent to a maintenance infusion of 5% dextrose in humans) on inflammation, insulin sensitivity, and the development of hyperglycemia in the murine cecal ligation and puncture (CLP) model of sepsis. We hypothesized that early administration of low-level parenteral dextrose administration induces insulin resistance and hyperglycemia and propagates the inflammatory response in the acute phase of sepsis.
Materials and methods
Animal care
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh (protocol no. 12050422).
Vascular catheterization and cecal ligation and puncture
Technical descriptions relating to chronic catheterization of mice have been published previously in detail (Alonso et al. 2007). In brief, 10-week-old male C57BL/6J mice received femoral arterial and venous catheters (MRE-025; Braintree Scientific, Braintree, Mass., USA) for the purposes of hemodynamic monitoring, arterial blood sampling, and administration of intravenous fluids or insulin. Catheters were secured in the femoral region with sutures and surgical grade tissue glue, tunneled subcutaneously to the upper back, and connected to a dual channel swivel to allow free movement throughout the experimental period. Pulsatile blood pressure was monitored and recorded continuously using WinDaq Data Acquisition DI-7x0 Software (Akron, Ohio, USA). Catheter patency was maintained with a low-flow heparinized saline infusion (7 µL/h).
Mice underwent either CLP or sham surgery immediately after implantation of the femoral catheters (total surgical time ~60 min) as previously described (Watanabe et al. 2013). In brief, an abdominal vertical midline incision allowed for mobilization and ligation of the cecum 25% of the distance from the ileocecal valve to the tip of the cecum, a single puncture of the cecum with a 20-gauge needle, and expression of feces into the peritoneal space before closure. The sham surgery consisted of isolation and mobilization of the cecum without ligation or puncture.
Experimental protocol
In a block design study of 4 experimental groups, CLP and sham mice received either intravenous dextrose or intravenous saline infusion for 24 h immediately following completion of the surgery (Supplementary Fig. S11). Mice were administered either 25% dextrose (D25; Hospira, Lake Forest, Ill., USA) at 100 µL/h, approximately 20% of the daily caloric requirements for a 25-g mouse, or 0.9% normal saline at an equivalent rate. Animal behavior and food intake were monitored during the 24-h experimental protocol. Blood glucose, and plasma insulin, corticosterone, and cytokines were measured at 24 h. At the completion of the experimental protocol, mice were euthanized according to American Veterinary Medical Association guidelines and pancreatic tissue was harvested for histological analysis and serial dilutions of whole blood were plated, incubated at 37 °C for 24 h, and bacterial colonies counted.
In a second set of experiments, using 4 comparable experimental groups, hyperinsulinemic-euglycemic clamps were performed 22 h after surgery to assess whole-body insulin sensitivity as previously described (Iiyori et al. 2007). Briefly, insulin was continuously infused (20 mU/kg/min) and intravenous dextrose administered and adjusted to achieve and maintain euglycemia (100–110 mg/dL) over a 2-h period. Whole-body insulin sensitivity was determined as the average glucose infusion rate over the last 30 min of the clamp.
In a third set of experiments, insulin was administered continuously for 24 h from the time of surgery at rates of either 10 or 20 mU/kg/min in CLP mice receiving saline or dextrose infusion at the same dose and rate as detailed above. Blood glucose and plasma cytokines were measured at 24 h after surgery.
Biochemical assays
Blood glucose was measured using a Prodigy Autocode Glucometer (~1 µL whole blood; Diagnostic Devices, Charlotte, N.C., USA). Plasma insulin was measured using the Crystal Chem Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem Inc., Downers Grove, Ill., USA). Corticosterone was measured using the Mouse and Rat Corticosterone ELISA Assay (ALPCO, Salem, N.H., USA). Plasma cytokines were measured using BioRad 23-Plex Mouse Cytokine Multiplex Assay and a Bioplex 100 System (Bio-Rad Laboratories, Hercules, Calif., USA).
Immunohistochemistry
Pancreata were fixed with 4% formalin (v/v) at room temperature for 5 h and embedded in paraffin. Five to seven micron sections were stained with hematoxylin and eosin, or blocked and labeled with primary antibodies recognizing insulin (Abcam, Cambridge, Mass., USA) and activated Caspase 3 (Cell Signaling, Beverly, Mass., USA), and DyLight secondary antibodies (Jackson Immunochemical). Images were acquired using a Nikon microscope.
Statistical analyses
Mice were characterized as euglycemic (Eug; <200 mg/dL), hyperglycemic (Hyp; 200–599 mg/dL), or severely hyperglycemic (Sev; ≥600 mg/dL; the upper limit of detection of the glucometer) based on blood glucose levels at 22–24 h. Severely hyperglycemic animals were assigned a blood glucose of 600 mg/dL for the purposes of analysis.
Statistical analyses were performed using GraphPad PRISM 6 for Windows (GraphPad Software Inc., La Jolla, Calif., USA) and Stata version 13 (StataCorp LP, College Station, Tex., USA). Data are presented as means ± SE unless otherwise indicated. Differences between means were assessed with unpaired t tests and ANOVA as appropriate. Post hoc analyses were performed with Tukey’s test or Dunnett’s test where appropriate. Results were considered statistically different for p < 0.05. Sample sizes for groups are provided in figure legends.
Results
Exogenous low level dextrose infusion can induce severe hyperglycemia following cecal ligation and puncture
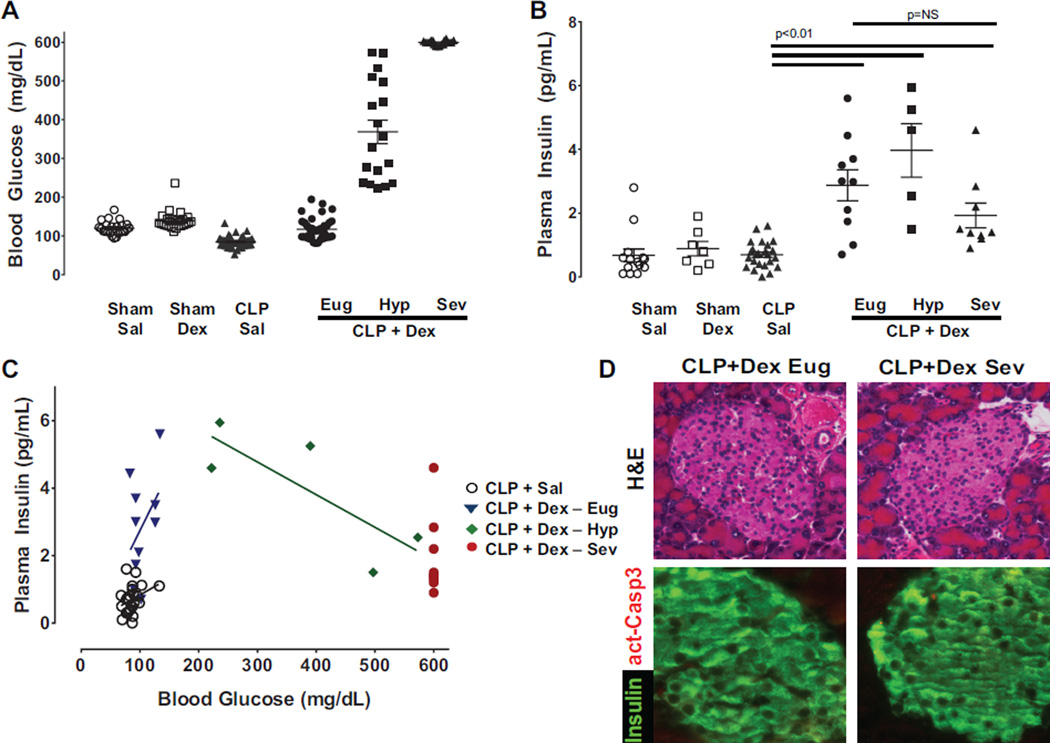
Mice undergoing sham surgery exhibited a small, but significant, increase in blood glucose in response to parenteral dextrose administration (140 ± 5 mg/dL; p < 0.005) compared with sham mice administered saline (120 ± 4 mg/dL; n ≥ 20 for sham groups, Fig. 1A). A relative hypoglycemia occurred in CLP mice administered saline (84 ± 2 mg/dL), associated with a reduction in food intake (0.36 ± 0.10 g/24 h) compared with sham mice administered saline (0.80 ± 0.08 g/24 h; p = 0.009). In CLP mice administered parenteral dextrose the blood glucose averaged (304 ± 25 mg/dL, n ≥ 40). Further inspection of the glucose response data revealed that CLP mice receiving dextrose exhibited 3 distinct phenotypes (Fig. 1A). The majority of animals tended to maintain blood glucose in a euglycemic range (<200 mg/dL, Eug 49%; 120 ± 4 mg/dL) or exhibit severe hyperglycemia (≥600 mg/dL, Sev 27%). An intermediate hyperglycemia group (Hyp 200–599 mg/dL; 24%, 368 ± 30 mg/dL) were captured amidst the transition to severe hyperglycemia. Thus, early initiation of low-level dextrose in septic animals produced an effectively dichotomous blood glucose response of maintained euglycemia or severe hyperglycemia, with a third group in transition between the 2 states.
Fig. 1.
Low-dose parenteral glucose induces hyperglycemia and pancreatic dysfunction in septic mice. (A) Mice underwent either sham surgery (Sham) or cecal ligation and puncture (CLP) and received intravenous infusion of saline (Sal) or dextrose (Dex). Blood glucose was measured at 24 h following surgery (n ≥ 20 in sham groups and n ≥ 16 in CLP groups). Mice undergoing CLP and receiving dextrose demonstrated a dichotomous response with half of the group maintaining euglycemia (CLP Dex Eug, <200 mg/dL; n = 75) and the other half developing either hyperglycemia (CLP Dex Hyp, 200–599 mg/dL; n = 18) or severe hyperglycemia (CLP Dex Sev, ≥600 mg/dL; n = 20). (B) Plasma insulin levels are similar 24 h after sham surgery (Sham Sal, Sham Dex, n ≥ 7) or after CLP in the setting of saline infusion (CLP Sal, n ≥ 6). In contrast, circulating insulin is significantly increased in all CLP mice receiving dextrose (CLP + Dex) compared with CLP Sal mice (p = 0.003 by 1-way ANOVA). Plasma insulin levels were similar between CLP + Dex mice in Eug, Hyp, and Sev groups (p > 0.05 by 1-way ANOVA). (C) Plasma insulin levels increase with elevations in blood glucose for CLP Sal and CLP + Dex Eug groups (Slope = 0.045 ± 0.01 (p < 0.001 compared with zero, r2 = 0.32)). However, for mice in CLP + Dex Hyp and CLP + Dex Sev groups, higher blood glucose levels do not result in corresponding increases in plasma insulin (Slope = –0.009 ± 0.004 (p = 0.1 compared with zero, r2 = 0.64)). (D) Paraffin sections of pancreas stained with hematoxylin and eosin (H&E) (top panels) demonstrate islets with normal morphology independent of development of hyperglycemia. Immunofluorescence for insulin (green) and activated caspase 3 (act-Casp3) (red) confirmed that very few beta cells showed signs of apoptotic cell death (bottom panels) in septic mice exposed to dextrose infusion.
Septic animals receiving early low-dose parenteral dextrose exhibiting hyperglycemia have impaired insulin secretion
Plasma insulin remained in the expected basal range in both sham groups and was not different in the CLP group that was administered saline (Fig. 1B). In CLP mice administered dextrose, plasma insulin was higher in all 3 glycemic groups (Eug, Hyp, and Sev) compared with CLP mice administered saline (CLP + saline 0.7 ± 0.1 ng/mL, Eug 2.9 ± 0.5, Hyp 4.0 ± 0.8, Sev 1.9 ± 0.4, n ≥ 7 each, p < 0.001). Despite large differences in circulating blood glucose among the 3 CLP groups receiving dextrose, no corresponding differences in plasma insulin were observed. When plasma insulin is plotted against blood glucose, there is no increase in plasma insulin once blood glucose surpasses 200 mg/dL (Fig. 1C). Immunohistochemical analyses of islets from CLP mice receiving dextrose that either maintained euglycemia or developed severe hyperglycemia reveal similar gross histology and no evidence of increased β-cell apoptosis (Fig. 1D). Taken together, the data demonstrate that the development of severe hyperglycemia in CLP mice receiving low-level dextrose administration is, at least in part, due to an inability of the pancreas to mount an appropriate insulin response that cannot be explained by an increase in β-cell death.
Profound insulin resistance contributes to development of severe hyperglycemia in septic animals receiving early low-dose parenteral dextrose
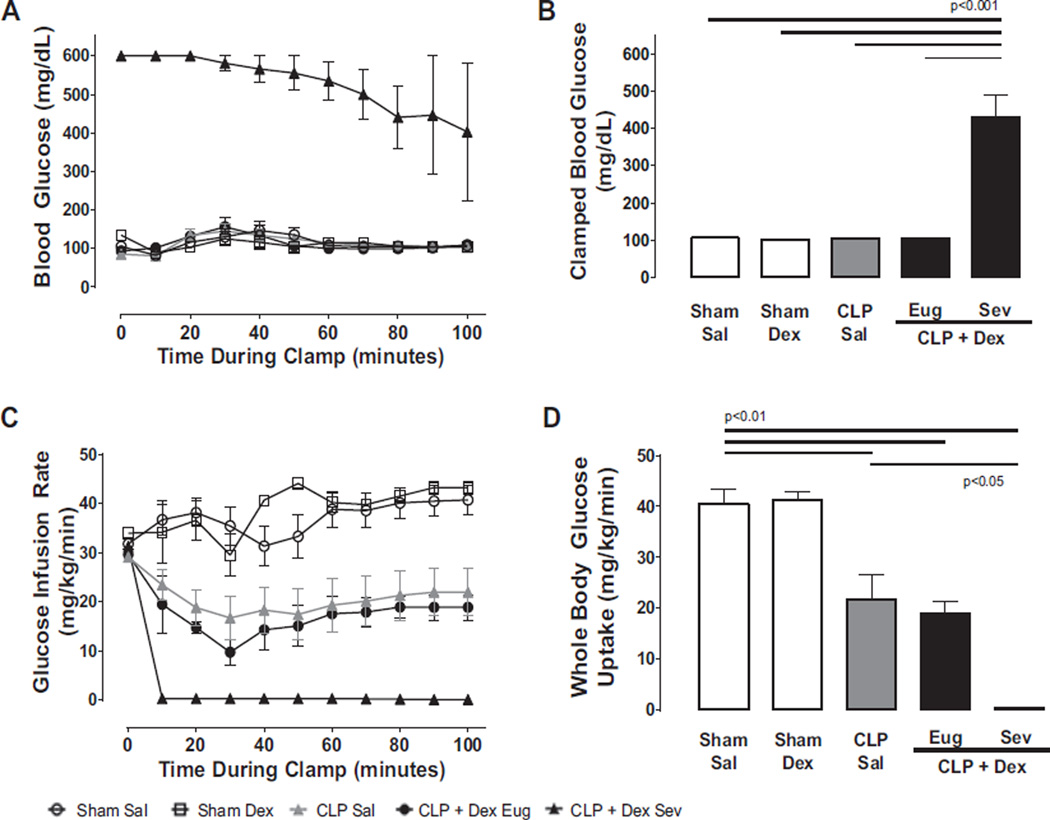
In sham mice administered either saline or dextrose, blood glucose was clamped between 100 and 110 mg/dL (Fig. 2A) and whole-body glucose uptake was in the normal range (~40 mg/kg/min, Fig. 2D); reflecting a level of insulin sensitivity comparable to previous studies in healthy C57BL/J6 mice (Iiyori et al. 2007). In contrast, CLP mice administered saline demonstrated a 50% reduction in whole-body glucose uptake compared with sham mice despite identically clamped blood glucose reflecting a 50% reduction in insulin sensitivity (Fig. 2C, third bar). A comparable degree of insulin resistance was also evident in CLP mice that were administered dextrose and were able to maintain euglycemia. However, in CLP mice administered dextrose that exhibited severe hyperglycemia, we were unable to clamp blood glucose in the euglycemic range despite 2 h of constant high-dose insulin administration (20 mU/kg/min, Fig. 2A), indicating the presence of profound insulin resistance (Fig. 2C, 2D).
Fig. 2.
Low-dose parenteral glucose induces marked insulin resistance in septic mice developing severe hyperglycemia. Blood glucose (A) and glucose infusion rates (C) were plotted during hyperinsulinemic-euglycemic clamp experiments performed 22 h after sham or cecal ligation and puncture (CLP) surgery and infusion of either saline or dextrose (Sal, Dex, n ≥ 4 each). (B) The average glucose infusion rate during the final 30 min of the clamp. In CLP + Dex mice developing severe hyperglycemia (Sev), blood glucose could not be reduced to the euglycemic range (100–110 mg/dL) despite continuous administration of 20 mU/kg/min of insulin. (D) Insulin sensitivity as measured by whole-body glucose uptake. Insulin sensitivity was approximately 50% of control in CLP Sal and CLP + Dex Eug mice. However, in CLP + Dex Sev mice, whole-body glucose uptake was reduced to zero, indicating a profoundly insulin resistant state. Data are presented as means ± SE. Differences determined by 1-way ANOVA with Tukey’s post hoc analysis.
Concurrent administration of insulin prevents severe hyperglycemia in septic animals receiving early low-dose parenteral dextrose
Given that we were unable to clamp blood glucose in the normal range in septic mice with hyperglycemia (i.e., we were unable to rescue the mice once metabolic decompensation occurred), we sought to determine whether we could prevent the elevation in blood glucose with insulin infusion started at the time of septic insult. Sham mice administered dextrose and insulin demonstrated mean blood glucose levels that were significantly lower (88 ± 8 mg/dL and 82 ± 11 mg/dL for low 10 and 20 mU/kg/min, respectively) than sham mice receiving dextrose alone (140 ± 5mg/ dL, p < 0.001). In CLP mice receiving dextrose, insulin infusion prevented the development of severe hyperglycemia; 3 out of 8 mice in the low-insulin group and only 1 mouse out of 7 in the high-insulin group developed hyperglycemia (blood glucose 200– 599 mg/dL). Thus, we were able to prevent the development of severe hyperglycemia with high-dose insulin infusion in septic mice receiving parenteral dextrose, but insulin was ineffective once hyperglycemia had developed.
Severe hyperglycemia in septic animals receiving early low-dose parenteral dextrose is not dependent on hemodynamic status, degree of bacteremia, or counter-regulatory hormone production
We next sought to explore the physiological mechanism(s) underlying the development of metabolic dysfunction in septic mice receiving low-level caloric support. Sham mice administered either saline or dextrose maintained normal mean systemic arterial blood pressure (BP) throughout the experimental protocol (Table 1). CLP reduced BP by approximately 20 mm Hg compared with sham surgery, independent of the development of hyperglycemia (Table 1). Corticosterone doubled in CLP mice receiving saline compared with either sham group. Importantly, however, CLP mice receiving dextrose displayed similar corticosterone levels to those receiving saline without significant difference in subsets of mice remaining euglycemic or developing severe hyperglycemia (Table 1). Similarly, CLP mice receiving dextrose experienced a similar degree of bacteremia compared with CLP mice receiving saline independent of the development of severe hyperglycemia as determined by colony forming units measured in the blood after sacrifice (Table 1). Thus, differences in the degree of hemodynamic compromise, activation of counter-regulatory hormones, or the severity of infection cannot account for the development of insulin resistance or impaired insulin secretion observed in septic mice receiving early low-dose parenteral dextrose that develop severe hyperglycemia.
Table 1.
Measures of illness severity and counter-regulatory hormone production.
| Variable | Sham-Sal | Sham-Dex | CLP-Sal | CLP-Dex-Eug | CLP-Dex-Hyp | CLP-Dex-Sev |
|---|---|---|---|---|---|---|
| Body weighta (g) | 27.6±0.5 | 27.9±0.4 | 28.0±0.3 | 27.7±0.4 | 26.8±0.5 | 27.2±0.5 |
| Food intake (g/d) | 0.8±0.1 | 0.8±0.1 | 0.4±0.1* | 0.3±0.1* | 0.2±0.03* | 0.2±0.03* |
| Mean arterial pressureb (mm Hg) | ||||||
| 0–8 h | 112±3 | 106±2 | 87±2* | 93±2* | 87±2* | 86±2* |
| 8–16 h | 104±2 | 105±3 | 79±3* | 83±3* | 79±3* | 83±4* |
| 16–24 h | 103±2 | 103±2 | 80±3* | 80±3* | 80±5* | 76±7* |
| Bacterial CFUc | 212±131 | 148±61 | 815±100* | 496±97* | 1000+* | 390±116* |
| Corticosterone (pg/mL) | 211±16 | 195±21 | 405±17* | 384±14* | 371±27* | 428±25* |
Note: Data are presented as means ± SE. Differences were assessed by 1-way ANOVA and post hoc analyses between groups were performed with Tukey’s test.
CFU, colony-forming unit; CLP, cecal ligation and puncture; Dex, dextrose; Eug, euglycemia; Hyp, hyperglycemia; Sal, saline; Sev, severe hyperglycemia.
, p < 0.05 compared to Sham-Sal.
Body weight recorded just prior to CLP and catheterization surgeries.
Mean arterial pressure averaged over each 8-h period following CLP surgery.
Bacterial CFUs recorded 24 h after culture plate inoculation. Bacterial CFU values were capped at 1000 per protocol.
Degree of systemic inflammation correlates with the development of hyperglycemia in septic mice receiving early low-level parenteral dextrose
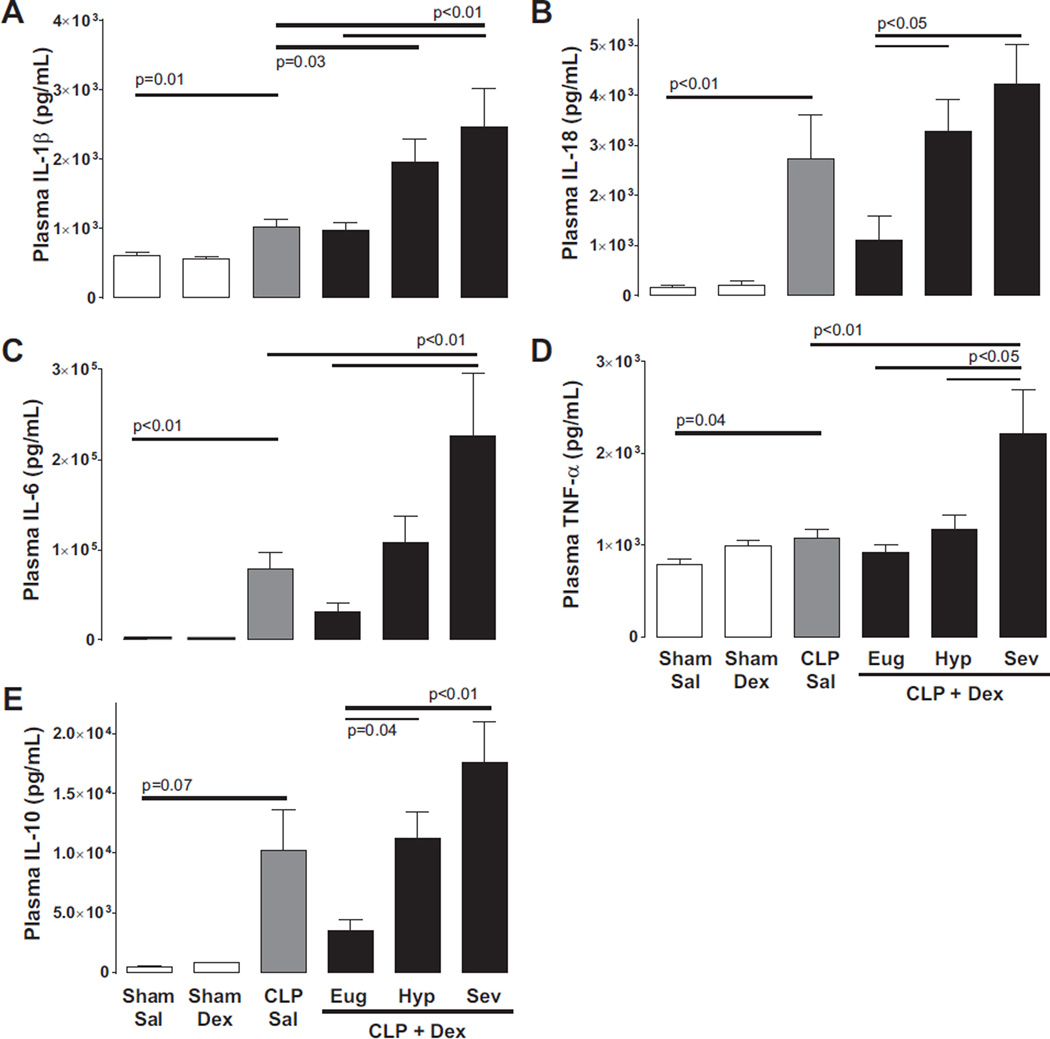
In sham operated animals, inflammatory cytokine production was unaffected by dextrose infusion (Fig. 3). In contrast, CLP mice receiving saline exhibited increases in circulating interleukin (IL)- 1β, IL-18, tumor necrosis factor alpha (TNF-α), and IL-6 compared with sham controls (Fig. 3). CLP mice receiving dextrose infusion demonstrated increased circulating cytokines that corresponded with blood glucose elevation. In mice maintaining euglycemia, the systemic inflammatory response was similar to CLP saline controls. In contrast, plasma IL-1β, IL-18, TNF-α, and IL-6 were markedly increased in mice developing severe hyperglycemia compared with those that maintained euglycemia and an intermediate cytokine response was evident in mice captured in the transition period (Fig. 3; note: the administration of insulin prior to the onset of metabolic dysfunction did not lower circulating cytokines relative to septic mice receiving dextrose alone, see Supplementary Table S11).
Fig. 3.
The development of hyperglycemia in septic mice exposed to dextrose infusion is associated with increased inflammatory cytokines. (A) Cecal ligation and puncture (CLP) increased pro-inflammatory interleukin (IL)-1β secretion compared with sham surgery (CLP saline (Sal) vs. Sham Sal, p = 0.01). IL-1β levels increased in mice developing hyperglycemia in the setting of CLP and dextrose infusion (CLP + Dex Hyp, blood glucose = 200–599 mg/dL and CLP + Dex Sev, blood glucose ≥ 600 mg/dL) compared with Sal controls or CLP + Dex mice maintaining euglycemia (Eug, blood glucose < 200 mg/dL). Similar patterns were noted for the cytokines IL-18, tumor necrosis factor alpha (TNF-α), IL-6, and IL-10 (B, C, D, and E). Data are presented as means ± SE (Sham n ≥ 9, CLP n ≥ 7). Differences determined by 1-way ANOVA with Tukey’s or Dunnett’s post hoc analyses as appropriate.
Discussion
Our study found that early administration of low levels of caloric support in a murine cecal ligation and puncture model of sepsis induced profound glucose intolerance. We observed rapid decompensation in glucose control (within 24 h) characterized by profound insulin resistance, deficient insulin secretion, and severe hyperglycemia in septic animals receiving low-level parenteral dextrose, which resulted in a phenotype that resembled an accelerated form of type 2 diabetes. This pattern mimicked that seen in prior experiments with septic mice receiving higher levels of caloric support (Watanabe et al. 2013). Thus, our data suggest, in contrast with the recently published CALORIES trial, that parenteral support (even at low levels) may in fact be detrimental in the early stages of critical illness.
Hyperinsulinemic-euglycemic clamp data demonstrate that once fulminant hyperglycemia ensues, even 2 h of high-dose continuous insulin is ineffective at reestablishing normal glucose homeostasis; none of the severely hyperglycemic mice lowered their blood glucose below 200 mg/dL during the clamp. In contrast, when insulin administration was initiated at the time of CLP, no animals developed severe hyperglycemia, and in the highdose insulin infusion group (20 mU/kg/min) the average blood glucose was ~150 mg/dL in septic animals receiving early low-dose parenteral dextrose. Thus, our data support a 2-hit mechanism underlying the development of metabolic dysfunction early in the evolution of sepsis whereby administration of low-level exogenous dextrose and systemic inflammation conspire to precipitate profound insulin resistance and worsening hyperglycemia. Furthermore, our early insulin administration data support the concept of a threshold or “tipping point” of decompensation as insulin administered at the time of septic insult interrupts the cycle of insulin resistance, β-cell dysfunction, and severe hyperglycemia, whereas insulin administered in high doses once hyperglycemia develops is ineffective at restoring glucose control.
The acuity of metabolic decompensation in our septic mice, and its relationship to activation of pro-inflammatory cytokines, implicates a potential role for the inflammasome in creating and propagating the “accelerated type 2 diabetic state”. Inflammasomes are cytosolic complexes built around specific intracellular pattern recognition (NOD-like) receptor proteins, which organize to coordinate and regulate the host response to microbial infection or other stress and damage signals (Strowig et al. 2012). Pathogen- or damage-associated molecular pattern recognition by the NOD-like receptor (NLRP) results in caspase-1 activation, which subsequently drives IL-1β and IL-18 production, both key regulators of the innate immune response. The NLRP3 inflammasome is activated in response to several bacterial pathogens (Franchi et al. 2012; Rathinam et al. 2012) and has been linked to the pathogenesis of sepsis (Cinel and Opal 2009). In clinical sepsis trials, IL-1β antagonism proved safe and demonstrated a nonsignificant trend toward improvement in mortality in all-comers (Fisher et al. 1994a, 1994b, 1994c; Opal et al. 1997). Inflammasome activation has also been linked to type 2 diabetes (Maedler et al. 2002; Sauter et al. 2008; Youm et al. 2011; Wen et al. 2012). IL-1β activation plays a role in the development of insulin resistance in peripheral glucose disposing tissue by direct inhibition of insulin receptor substrate-1 signaling and indirectly through the production of pro-inflammatory cytokines and generation of reactive oxygen species (Strowig et al. 2012). In the pancreas, the combination of low-dose IL-1β and TNF-α also suppresses insulin production by pancreatic islets (Mehta et al. 1994), and lipopolysaccharide-induced IL-1β has been shown to suppress pancreatic beta cell function in vitro (Cruzat et al. 2015). Thus, in sepsis, inflammasome activation may act to disrupt normal disposal of glucose in peripheral metabolic tissue and impair insulin secretion. Our cytokine response pattern suggests that inflammation in the setting of sepsis interacts with low-level parenteral dextrose support to hyperactivate the inflammasome, accentuating IL-1β production and impairing metabolic function. The ensuing cascade propagates the crossing of a metabolic threshold leading to development of severe and fulminant hyperglycemia.
Despite the demonstration of discrete physiologic phenotypes observed in our mice, 3 potential limitations of our study require acknowledgement. First, we used dextrose as our source of early nutritional support, and it is unclear if different outcomes would occur with low-level total parenteral nutrition. However, as discussed below, early administration of dextrose at low rates is an important and clinically relevant scenario. Second, we did not include a hyperglycemic group (200–599 mg/dL) in hyperinsulinemic-euglycemic clamp experiments. The progression from hyperglycemia to severe hyperglycemia occurs rapidly in CLP mice receiving dextrose and, therefore, would create significant challenges with timing the intervention and establishing a euglycemic steady-state during the clamp. Nevertheless, results from the severely hyperglycemic mice clearly demonstrate a state of profound insulin resistance, in which high-dose exogenous insulin administration was unable to induce euglycemia within a 2-h period of continuous infusion. Finally, we measured corticosterone as an indicator of counter-regulatory hormone activity as it is the predominant glucocorticoid in mice and an important stress hormone. Our data indicate that the development of severe hyperglycemia in septic mice administered dextrose was independent of increased corticosterone levels compared with septic mice that did not exhibit hyperglycemia. While other counter-regulatory hormones such as glucagon are likely important contributors to glucose control in sepsis and should not be dismissed, we were unable to measure them because of volume limitations of our plasma samples.
Clinical implications
The recent CALORIES trial (Harvey et al. 2014) suggests that low-level early parenteral nutritional support, in comparison with equivalent enteral nutrition, is a viable clinical approach in the initial stages of critical illness, thus renewing debate about whether early initiation of parenteral nutritional support is clinically contraindicated in critically ill patients. Clinically, 5% dextrose can be used as a carrier for other medications (most commonly intravenous bicarbonate) or as a maintenance intravenous fluid in patients unable to tolerate enteral nutrition. Administration of 5% dextrose at rates of 75–100 mL/h over 24 h represents approximately 20% of the daily caloric requirement of the average size adult. Our data would suggest that in a heterogeneous population of critically ill patients, susceptible individuals may have the potential for profound metabolic compromise with early initiation of even this low-level dextrose support and clinicians should consider this potential when prescribing caloric interventions.
Conclusions
The underlying pathophysiologic and metabolic consequences of low-level early parenteral calorie provision in sepsis are not well studied. In a clinically relevant model of sepsis, neither parenteral dextrose nor sepsis alone led to hyperglycemia, but in combination a dichotomous response was evident whereby half of the septic mice maintained euglycemia and half developed hyperglycemia. The development of severe hyperglycemia in septic mice receiving low-level parenteral dextrose was independent of bacterial load, hemodynamic status, or counter-regulatory hormone production. The degree of hyperglycemia in septic animals correlated with secretion of pro-inflammatory cytokines, in particular IL-1β, TNF-α, and IL-6, potentially implicating the inflammasome in a feed-forward pathogenic process of metabolic dysfunction induced by low-level parenteral dextrose administration in sepsis.
Supplementary Material
Acknowledgments
Funding for this project was provided by the National Heart, Lung, and Blood Institute (NHLBI) (P01HL114453 C.P.O., B.J.M.; 5T32HL007563-27 F.A.S.; R01DK095140 L.C.A.), the University of Pittsburgh Metabolic Center (C.P.O., B.J.M.), the University of Pittsburgh Medical Center Competitive Medical Research Fund (B.J.M.), and the University of Pittsburgh Vascular Medicine Institute in collaboration with the Institute for Transfusion Medicine (C.P.O.). Laura C. Alonso and Rohit Sharma have relocated to the Division of Diabetes at the University of Massachusetts Medical School, Worcester, Massachusetts, USA, since completion of the work reported in this study.
Footnotes
Supplementary data are available with the article through the journal Web site at http://nrcresearchpress.com/doi/suppl/10.1139/apnm-2015-0213.
Conflict of interest statement
The authors have nothing to disclose.
Authorship contributions: S.S. and F.A.S. shared primary data collection, data analysis, and manuscript preparation responsibilities. L.G., Y.W., S.M., R.S., and Y.Z. contributed to data collection. L.C.A. contributed to study design, data collection, data analysis, and revision of the manuscript. C.P.O. and B.J.M. oversaw design and performance of all studies, analysis of all data, and manuscript preparation and revision.
Contributor Information
Srikanth Singamsetty, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Faraaz Ali Shah, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Lanping Guo, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Yoshio Watanabe, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Sherie McDonald, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Rohit Sharma, Division of Endocrinology and Metabolism, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Yingze Zhang, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Laura C. Alonso, Division of Endocrinology and Metabolism, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA.
Christopher P. O’Donnell, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA
Bryan J. McVerry, Division of Pulmonary, Allergy, and Critical Care Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213, USA
References
- Alonso LC, Yokoe T, Zhang P, Scott DK, Kim SK, O’Donnell CP, Garcia-Ocana A. Glucose infusion in mice: a new model to induce β-cell replication. Diabetes. 2007;56(7):1792–1801. doi: 10.2337/db06-1513. PMID:17400928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N. Engl. J. Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. PMID:18184958. [DOI] [PubMed] [Google Scholar]
- Casaer MP, Mesotten D, Hermans G, Wouters J, Schetz M, Meyfroidt G, et al. Early versus late parenteral nutrition in critically ill adults. N. Engl. J. Med. 2011;365(6):506–517. doi: 10.1056/NEJMoa1102662. PMID:21714640. [DOI] [PubMed] [Google Scholar]
- Cinel I, Opal SM. Molecular biology of inflammation and sepsis: a primer. Crit. Care Med. 2009;37(1):291–304. doi: 10.1097/CCM.0b013e31819267fb. PMID:19050640. [DOI] [PubMed] [Google Scholar]
- Cruzat VF, Keane KN, Scheinpflug AL, Cordeiro R, Soares MJ, Newsholme P. Alanyl-glutamine improves pancreatic β-cell function following ex vivo inflammatory challenge. J. Endocrinol. 2015;224(3):261–271. doi: 10.1530/JOE-14-0677. PMID:25550445. [DOI] [PubMed] [Google Scholar]
- Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N. Engl. J. Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. PMID:19318384. [DOI] [PubMed] [Google Scholar]
- Fisher CJ, Jr, Slotman GJ, Opal SM, Pribble JP, Bone RC, Emmanuel G, et al. Initial evaluation of human recombinant interleukin-1 receptor antagonist in the treatment of sepsis syndrome: a randomized, open-label, placebo-controlled multicenter trial. Crit. Care Med. 1994a;22(1):12–21. doi: 10.1097/00003246-199401000-00008. PMID:8124953. [DOI] [PubMed] [Google Scholar]
- Fisher CJ, Jr, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994b;271(23):1836–1843. PMID:8196140. [PubMed] [Google Scholar]
- Fisher CJ, Jr, Opal SM, Lowry SF, Sadoff JC, LaBrecque JF, Donovan HC, et al. Role of interleukin-1 and the therapeutic potential of interleukin-1 receptor antagonist in sepsis. Circ. Shock. 1994c;44(1):1–8. PMID:7704933. [PubMed] [Google Scholar]
- Franchi L, Muñoz-Planillo R, Nuñez G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012;13(4):325–332. doi: 10.1038/ni.2231. PMID:22430785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, et al. Trial of the route of early nutritional support in critically ill adults. N. Engl. J. Med. 2014;371(18):1673–1684. doi: 10.1056/NEJMoa1409860. PMID:25271389. [DOI] [PubMed] [Google Scholar]
- Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O’Doherty RM, et al. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am. J. Respir. Crit. Care Med. 2007;175(8):851–857. doi: 10.1164/rccm.200610-1527OC. PMID:17272786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin. Proc. 2003;78(12):1471–1478. doi: 10.4065/78.12.1471. PMID:14661676. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Coss, Bu JA, Kennedy CE, Jefferson LS. Organ dysfunction is associated with hyperglycemia in critically ill children. Intensive Care Med. 2010;36(2):312–320. doi: 10.1007/s00134-009-1703-1. PMID:19882139. [DOI] [PubMed] [Google Scholar]
- Leonidou L, Michalaki M, Leonardou A, Polyzogopoulou E, Fouka K, Gerolymos M, et al. Stress-induced hyperglycemia in patients with severe sepsis: a compromising factor for survival. Am. J. Med. Sci. 2008;336(6):467–471. doi: 10.1097/MAJ.0b013e318176abb4. PMID:19092319. [DOI] [PubMed] [Google Scholar]
- Ling Y, Li X, Gao X. Intensive versus conventional glucose control in critically ill patients: a meta-analysis of randomized controlled trials. Eur. J. Intern. Med. 2012;23(6):564–574. doi: 10.1016/j.ejim.2012.02.013. PMID:22863436. [DOI] [PubMed] [Google Scholar]
- Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced β cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 2002;110(6):851–860. doi: 10.1172/JCI15318. PMID:12235117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. PMID:12700374. [DOI] [PubMed] [Google Scholar]
- Mehta VK, Hao W, Brooks-Worrell BM, Palmer JP. Low-dose interleukin 1 and tumor necrosis factor individually stimulate insulin release but in combination cause suppression. Eur. J. Endocrinol. 1994;130(2):208–214. doi: 10.1530/eje.0.1300208. PMID:8130898. [DOI] [PubMed] [Google Scholar]
- Opal SM, Fisher CJ, Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, et al. Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit. Care Med. 1997;25(7):1115–1124. doi: 10.1097/00003246-199707000-00010. PMID:9233735. [DOI] [PubMed] [Google Scholar]
- Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150(3):606–619. doi: 10.1016/j.cell.2012.07.007. PMID:22819539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149(5):2208–2218. doi: 10.1210/en.2007-1059. PMID:18239070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi: 10.1038/nature10759. PMID:22258606. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N. Engl. J. Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. PMID:11794168. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Singamsetty S, Zou B, Guo L, Stefanovski D, Alonso LC, et al. Exogenous glucose administration impairs glucose tolerance and pancreatic insulin secretion during acute sepsis in non-diabetic mice. PLoS ONE. 2013;8(6):e67716. doi: 10.1371/journal.pone.0067716. PMID:23826335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases–did Warburg miss inflammation? Nat. Immunol. 2012;13(4):352–357. doi: 10.1038/ni.2228. PMID:22430788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youm YH, Adijiang A, Vandanmagsar B, Burk D, Ravussin A, Dixit VD. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152(11):4039–4045. doi: 10.1210/en.2011-1326. PMID:21862613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.