Abstract
HIV testing and counseling (HTC) is an essential component of comprehensive HIV programs. Retrospective HTC program data from 2006 to 2010 were examined to determine patterns of re-testing and seroconversion in Lesotho. Among 104,662 initially negative clients, 6,777 (6.5%) were re-testers. Predictors of re-testing included being male, age ≥ 25 years, divorced/separated, having more than a high school education, being tested as a couple, testing in the year 2006, testing in the capital city, and awareness of partner’s recent testing behavior. Among re-testers who seroconverted (N = 259), predictors included being female and having less than a high school education. There is a critical need for more effectively targeting HIV retesting messages to align with WHO (2010) guidelines and identify persons at highest risk for HIV, to increase timely diagnoses and link persons to appropriate HIV prevention, care, and treatment services.
HIV testing and counseling (HTC) is essential to identify HIV-infected persons in need of care and treatment, and to provide targeted HIV prevention services. Worldwide, uptake of HTC has been steadily increasing, due to the increase in provider-initiated testing in health care facilities and continued growth in testing sought at fixed voluntary counseling and testing (VCT) sites and via mobile HTC services (World Health Organization, United Nations Program on HIV & AIDS, & UNICEF [UNAIDS], 2013). In some countries, repeat HIV testing, or re-testing (i.e., returning for a test after an initial HIV-negative test), has accounted for one-third to two-thirds of self-initiated HIV tests (Fernyak, Page-Shafer, Kellogg, McFarland, & Katz, 2002; Leaity, 2000; MacKellar et al., 2002; Matovu et al., 2007). Reports vary considerably on frequency of re-testing, characteristics of re-testers, and rates of HIV diagnoses among re-testing clients. In rural Tanzania, only 25% of those surveyed reported ever repeat tested (Cawley et al., 2013), and a similar rate (26%) was observed in outpatient HTC settings in South Africa (Regan et al., 2013). Among VCT clients re-tested in Namibia, most did so 6 months after their initial test despite being told to come back in 3 months, and approximately 1.5% of re-testers seroconverted between their first and last HIV test (Wolmarans & Koppenhaver, 2008).
In 2010, the World Health Organization (WHO; 2010) issued recommendations to encourage more targeted re-testing, and to identify situations where retesting is not necessary (WHO, 2010). These guidelines suggest at least annual retesting for populations at high risk of seroconversion; e.g., HIV-negative partners in serodiscordant relationships, men who have sex with men, persons who engage in sex work, and the general population in countries with high prevalence. Four-week repeat testing is recommended for persons with a known recent exposure or who are at risk of acute infection, which revises earlier recommendations for repeat testing at 3-months to rule out error due to the test’s window period (i.e., the time interval between HIV infection and the development of detectable HIV antibodies). These recommendations were revised as, in more recent years, HIV tests have become available that can detect HIV antigen and/or antibodies much sooner than three months following infection.
Despite WHO’s recommendations, HTC guidelines in many countries continue to recommend that persons at risk of infection should be re-tested three months after a negative test result (Government of Lesotho, 2009). In Lesotho, counselors at VCT sites are trained to recommend re-testing to persons with a recent exposure (i.e., within the last three months), but VCT counselors often give this recommendation to all clients, regardless of their risk or timing of their last potential exposure (Population Services International Lesotho, personal communication). The generic recommendation becomes diluted, clients may or may not return for re-testing, and often when they do return, more than three months has passed since their initial test. It is important that HTC counselors follow WHO recommendations regarding retesting within the appropriate recommended time frames in order to more accurately identify persons at highest risk of having acute HIV infection, and to minimize unnecessary re-testing (WHO, 2010).
The Kingdom of Lesotho is a small, landlocked country located within the borders of South Africa, with a population of 1.9 million people and an estimated 23% of adults aged 15–49 living with HIV (UNAIDS, 2013). The proportion of Basotho (persons from Lesotho) who have ever been tested for HIV and received their HIV test results increased between 2004 and 2009, from 15% to 69% among women and from 11% to 39% among men (Cawley et al., 2013; UNAIDS, 2013). However, rates and patterns associated with re-testing have not been examined among Basotho. To understand whether clients seeking re-testing services report higher risk behaviors, whether persons who seroconvert exhibit characteristics that can inform how programs target services, and whether the timeframe of re-testing is consistent with international guidance, we analyzed routinely-collected data from HTC sites in Lesotho.
METHODS
ETHICAL REVIEW
This protocol was reviewed and approved by U.S. Centers for Disease Control and Prevention (CDC) in Atlanta, and the Lesotho Ministry of Health and Social Welfare (MOHSW). This evaluation was determined to be research not involving human subjects, since only retrospective client intake records were analyzed. No personal identifiers were used, client identification numbers cannot be traced back to individual persons.
DESIGN
We conducted a retrospective analysis of client intake records collected as part of routine program monitoring in Lesotho HTC sites between January 2006 and June 2010. These records included all testing conducted at each site, including mobile and outreach services. Data from the intake records were entered into a central database at each site daily, and data cleaning and reporting was conducted monthly. Each client intake record was coded with a unique client identification (CID) number. Clients were provided with a copy of their CID and asked to bring the card back upon return visits. These forms did not link to client names or other identifying information. Therefore, if clients did not bring back their CID upon subsequent visits, they were issued a new CID.
SETTINGS
During the data collection period, Population Services International (PSI) managed five stand-alone VCT sites in Lesotho, located in Maseru (the capital city), Mafeteng, Maputsoe (Leribe), Butha Buthe, and Qachas Nek; these sites also offered mobile and outreach services. This program represented a large proportion of HTC provided at the time in Lesotho.
Participants
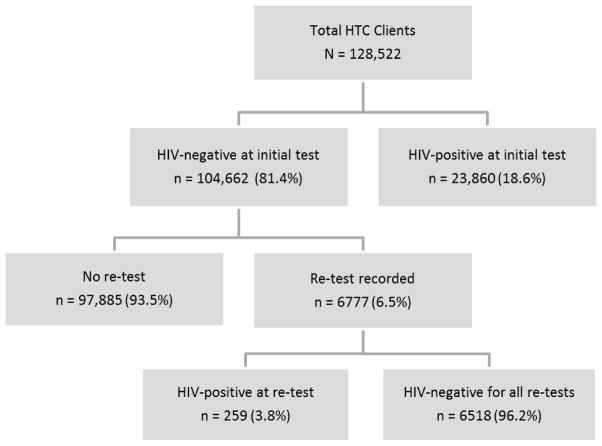
Records were reviewed for all clients aged 15 years and older for whom an intake form was completed, including assignment of a CID, and test results recorded at any of the HTC sites during the 4.5-year data collection period. A confirmed re-tester was defined as anyone who received an initial HIV test and at least one subsequent HIV test documented during the data collection period. Confirmation of the re-test was performed by matching CIDs recorded on the intake forms. Seroconverters were defined as individuals who initially tested HIV-negative and who subsequently tested HIV-positive within the data collection period. Figure 1 illustrates the flow of clients, including the total tested (N = 128,522), those who were HIV-negative at the initial test and did not have a subsequent test recorded (n = 97,885), and the serostatus of persons who had more than one recorded test result (n = 6,777).
FIGURE 1.
Flowchart of clients attending voluntary HIV testing and counseling (HTC) facilities in Lesotho, January 2006–June 2010.
Measures
As part of routine program activities, HTC providers completed an intake form for all clients attending an HTC session. In addition to recording the date, site, and type of visit, the form collected basic sociodemographic information, HIV testing history of the client and client’s partner, several items assessing behavioral risk for HIV, as well as HIV test results. Sociodemographic information, testing history and risk behavior were based on client self-report.
Forms were revised in October 2008, affecting the way several measures were collected. Where measures varied across the two versions of the intake form, categories were collapsed into comparable groupings.
STATISTICAL ANALYSIS
Univariate analyses were conducted to determine HIV prevalence among all clients tested. For clients with an initial negative test result, we used Pearson’s chi-square to compare re-testers and non-re-testers on demographic and behavioral characteristics. For re-testers who had an initial negative test result, we compared seroconverters to nonseroconverters in a similar fashion. Odds ratios (OR) and two-sided 95% confidence intervals (CIs) were calculated by logistic regression. All of the client characteristics with P value < .20 were included in multivariable models, and age was included because of its association with HIV prevalence. In the multivariable model to compare seroconverters to nonseroconverters, we assessed interaction between gender and other characteristics.
We plotted re-testing and seroconversion by time from initial HIV-negative test in order to assess patterns among these groups. The probability of remaining un-infected among re-testers was calculated using the nonparametric maximum likelihood estimator to account for interval-censored data for the seroconversions. All analyses were performed using SAS software version 9.3 (SAS Institute Inc, 2011). Statistical significance was assessed at the 0.05 level for all analyses.
RESULTS AND DISCUSSION
CLIENT CHARACTERISTICS
Client intake records were available for 157,947 nonduplicated records. Of these, approximately 11.5% were excluded for missing key elements (e.g., CID, testing date). Of the records that remained, 128,522 unique clients were identified for analyses. At their initial recorded visit, 27% self-reported previous testing for HIV. This could be over-reporting, or could include persons who could not be confirmed re-testers because of missing CID. The largest proportions of clients were tested in Maseru, the capital city (Table 1). More women than men tested for HIV, and a large proportion of persons who tested were over the age of 25. Higher prevalence of HIV was observed among first-time clients in Maseru and Leribe compared to clients from other sites. At initial test, a total of 18.6% (n = 23,860) of HTC clients were HIV-positive.
TABLE 1.
HIV Prevalence Rates in Clients at the First Recorded Visit at HIV Testing and Counseling Sites, and Re-testing Rates in Clients with HIV-Negative Test Results at the First Visit, by Selected Characteristics of Clients in Lesotho, January 2006 to June 2010
| Clients at first visit
|
Clients re-tested at subsequent visits
|
||||||
|---|---|---|---|---|---|---|---|
| Characteristic | n tested | n (%) HIV + | n (%) HIV − | n (%) re-tested | P-valuea | OR (95% CI) | aOR (95% CI)b |
| All clients | 128,522 | 23,860 (18.6) | 104,662 | 6,777 (6.5) | |||
| Site (n = 128,522) | |||||||
| Butha Buthe | 18,257 | 2,139 (11.7) | 16,118 (88.3) | 966 (6.0) | < 0.01 | 0.65 [0.60, 0.70] | 0.66 [0.60, 0.73] |
| Leribe | 24,061 | 5,283 (22.0) | 18,778 (78.0) | 1,259 (6.7) | 0.73 [0.69, 0.79] | 0.58 [0.53, 0.63] | |
| Mafeteng | 22,146 | 3,257 (14.7) | 18,889 (85.3) | 1,025 (5.4) | 0.59 [0.54, 0.63] | 0.43 [0.39, 0.48] | |
| Maseru (urban) | 46,682 | 11,609 (24.9) | 35,073 (75.1) | 3,126 (8.9) | 1.00 (Referent) | 1.00 (Referent) | |
| Qachas Nek | 17,376 | 1,572 (9.0) | 15,804 (91.0) | 401 (2.5) | 0.27 [0.24, 0.30] | 0.27 [0.23, 0.33] | |
| Initial year of visit (n = 128,522) | |||||||
| 2006 | 18,616 | 5,366 (28.8) | 13,250 (71.2) | 1,473 (11.1) | < 0.01 | 1.00 (Referent) | 1.00 (Referent) |
| 2007 | 33,007 | 7,572 (22.9) | 25,435 (77.1) | 2,339 (9.2) | 0.81 [0.76, 0.87] | 0.80 [0.72, 0.90] | |
| 2008 | 43,783 | 6,380 (14.6) | 37,403 (85.4) | 2,472 (6.6) | 0.57 [0.53, 0.61] | 0.62 [0.55, 0.69] | |
| 2009 | 26,182 | 3,439 (13.1) | 22,743 (86.9) | 465 (2.0) | 0.17 [0.15, 0.19] | 0.16 [0.14, 0.19] | |
| 2010 | 6,934 | 1,103 (15.9) | 5,831 (84.1) | 28 (0.5) | 0.04 [0.03, 0.06] | 0.02 [0.02 0.04] | |
| Gender (n = 128,515) | |||||||
| Male | 48,059 | 7,095 (14.8) | 40,964 (85.2) | 2,797 (6.8) | < 0.01 | 1.00 (Referent) | 1.00 (Referent) |
| Female | 80,456 | 16,764 (20.8) | 63,692 (79.2) | 3,980 (6.2) | 0.91 [0.86, 0.96] | 0.86 [0.80, 0.92] | |
| Age (n = 106,083) | |||||||
| 15–24 | 44,532 | 4,522 (10.2) | 40,010 (89.8) | 2,469 (6.2) | < 0.01 | 1.00 (Referent) | 1.00 (Referent) |
| 25+ | 61,551 | 17,790 (28.9) | 43,761 (71.1) | 3,599 (8.2) | 1.36 [1.29, 1.44] | 1.31 [1.20,1.43] | |
| Marital status (n = 118,613) | |||||||
| Never married | 58,418 | 4,589 (7.9) | 53,829 (92.1) | 2,959 (5.5) | < 0.01 | 1.00 (Referent) | 1.00 (Referent) |
| Married/cohabiting | 46,727 | 11,333 (24.2) | 35,394 (75.7) | 2,376 (6.7) | 1.24 [1.17, 1.31] | 0.80 [0.72, 0.88] | |
| Polygamous | 218 | 70 (32.1) | 148 (67.9) | 10 (6.8) | 1.25 [1.66, 2.37] | 0.99 [0.49, 2.00] | |
| Divorced/separated | 4,021 | 1,884 (46.8) | 2,137 (53.1) | 217 (10.2) | 1.94 [1.68, 2.25] | 1.23 [1.02, 1.48] | |
| Widowed | 8,961 | 2,909 (32.5) | 6,052 (67.5) | 339 (5.6) | 1.02 [0.91, 1.14] | 0.87 [0.74, 1.02] | |
| Other | 268 | 99 (36.9) | 169 (63.1) | 27 (16.0) | 3.27 [2.16, 4.94] | 1.20 [0.74, 1.96] | |
| Education (n = 127,930) | |||||||
| No formal education | 11,021 | 1,952 (17.7) | 9,069 (82.3) | 272 (3.0) | < 0.01 | 0.22 [0.19, 0.26] | 0.26 [0.22, 0.31] |
| Less than high school | 64,491 | 12,284 (19.0) | 52,207 (81.0) | 2,401 (4.6) | 0.35 [0.32, 0.38] | 0.41 [0.37, 0.46] | |
| High school | 42,517 | 8,168 (19.2) | 34,349 (80.8) | 3,021 (8.8) | 0.70 [0.64, 0.75] | 0.69 [0.63, 0.77] | |
| More than high school | 9,901 | 1,372 (13.9) | 8,529 (86.1) | 1,039 (12.2) | 1.00 (Referent) | 1.00 (Referent) | |
| Client type (n = 128,486) | |||||||
| Individual | 124,664 | 22,770 (18.3) | 101,894 (81.7) | 6,469 (6.4) | < 0.01 | 1.00 (Referent) | 1.00 (Referent) |
| Couple | 3,822 | 1,086 (28.4) | 2,736 (71.6) | 306 (11.2) | 1.86 [1.64, 2.10] | 1.30 [1.12, 1.51] | |
| Most recent partner tested in last 3 months (n = 103,226) | |||||||
| Yes | 17,501 | 3,521 (20.1) | 13,980 (79.9) | 1,309 (9.4) | < 0.01 | 2.73 [2.52, 2.96] | 1.73 [1.55, 1.95] |
| No | 47,324 | 12,253 (25.9) | 35,071 (74.1) | 2,927 (8.4) | 2.41 [2.25, 2.58] | 1.42 [1.28, 1.57] | |
| Don’t know | 38,401 | 3,363 (8.8) | 35,038 (91.2) | 1,276 (3.6) | 1.00 (Referent) | 1.00 (Referent) | |
| Self-Reported Riskc | |||||||
| Had sex while intoxicated/drunk (n = 128,364) | |||||||
| Yes | 8,717 | 3,163 (36.3) | 5,554 (63.7) | 490 (8.8) | < 0.01 | 1.43 [1.30, 1.57] | 0.89 [0.78, 1.02] |
| No | 119,647 | 20,671 (17.3) | 98,976 (82.7) | 6,280 (6.3) | 1.00 (Referent) | 1.00 (Referent) | |
| Exchanged money/materials/goods for sex (n = 128,401) | |||||||
| Yes | 1,393 | 592 (42.5) | 801 (57.5) | 67 (8.4) | 0.03 | 1.32 [1.03, 1.70] | 0.92 [0.64, 1.30] |
| No | 127,008 | 23,248 (18.3) | 103,760 (81.7) | 6,706 (6.5) | 1.00 (Referent) | 1.00 (Referent) | |
| Had unprotected sex (n = 128,321)d | |||||||
| Yes | 75,774 | 19,861 (26.2) | 55,913 (73.8) | 4,213 (7.5) | < 0.01 | 1.47 [1.40, 1.54] | 1.08 [0.99, 1.18] |
| No | 52,547 | 3,973 (7.6) | 48,574 (92.4) | 2,554 (5.3) | 1.00 (Referent) | 1.00 (Referent) | |
Pearson’s chi-square statistic for testing the null hypothesis of no association between the characteristic and re-testing.
The multivariable logistic regression model contains all variables listed in this table. The sample size for the model was 61,779 clients.
In the pre-2008 form, these questions were asked only for the past 12 months, whereas in the post-2008 form, these questions were asked as ever.
In the post-2008 form, two separate questions were asked, one for sex with a regular partner and another for sex with a nonregular partner; a composite variable was created and coded Yes if either question’s response was answered Yes, otherwise No.
In terms of HIV-related risk behavior, 17% of clients reported that their partner had been recently tested for HIV, i.e., in the past 3 months. While very small percentages of clients reported having sex while intoxicated or exchanging money or other goods for sex, HIV prevalence among clients who reported these behaviors was twice as high as it was among clients not reporting these behaviors. Having any unprotected sex was associated with more than three times as high a prevalence of HIV (Table 1).
RE-TESTING
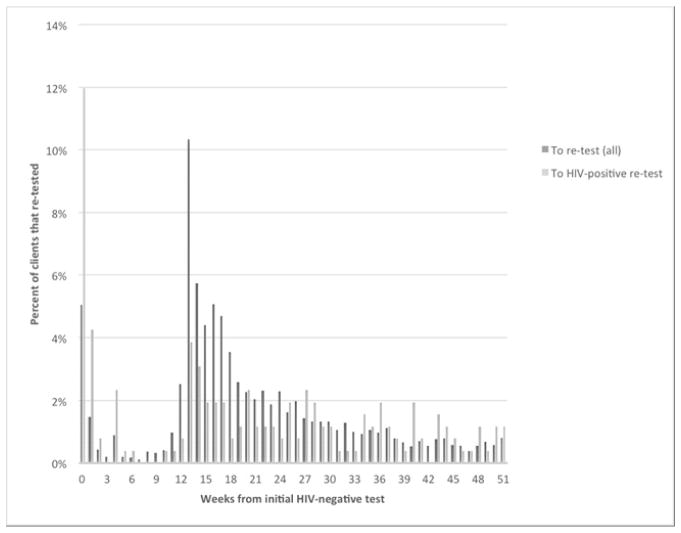
Of the 6.5% (n = 6,777) of HTC clients who were confirmed re-testers, 15.1% (n = 1,025) re-tested within 0–91 days, with a substantial proportion re-testing in weeks 1–2 (Figure 2). Most clients who re-tested did so within one year of the initial HIV-negative result (85.3%). The largest proportion re-tested within 4–6 months (47.4%), with a peak at 13 weeks, suggesting that many were heeding recommendations to return in 3 months. Of the 6,777 re-testers, most re-tested only once during the data collection period (76.6%, n = 5,191; Courtenay-Quirk, Grabbe, Baughman, Djomand, & Pederson 2013). A lesser number (16.1%, n = 1,090) re-tested twice following the initial HIV-negative result, with a median of 192 days between the last two tests (interquartile range = 126–308).
FIGURE 2.
Percentage of clients re-tested by weeks from initial HIV-negative test to re-test (n = 6,777) and percentage re-tested who seroconverted by weeks from initial HIV-negative test to HIV-positive re-test (n = 259).
Compared to HIV-negative persons who did not re-test, re-testers were more likely to have been tested at Maseru, tested in 2006, to be male, age ≥ 25, divorced or separated, and had more than a high school education (Table 1). HTC clients were also more likely to re-test if they had tested as a couple, were aware of whether their partner had recently, i.e., in the past 3 months, tested for HIV (compared to those who did not know if their partner had been tested), and reported unprotected sex.
SEROCONVERSION
Of the HIV-negative persons who returned for at least one re-test, 3.8% (259) tested HIV-positive at a subsequent test (Figure 2). Among all seroconverters, the temporal sequence indicates that a total of 54 persons (21%) tested HIV-positive within 12 weeks of the last HIV-negative test. Of these, 44 tested HIV-positive by week 4 (17% of all seroconverters and 81% of seroconverters within 12 weeks). A total of 130 (50%) persons tested HIV-positive between week 13 and week 52. Among seroconverters, the median time between the first HIV-negative and a subsequent HIV-positive result was 106 days (interquartile range, 48 to 226 days). In the cohort of all re-testers, 95.6% remained negative at 1 year, 93.4% at 2 years, and 87.5% at 3 years.
Women were twice as likely as men to seroconvert upon subsequent HIV testing (Table 2). Compared to persons who reported more than a high school education, persons with less education were more likely to seroconvert. Clients who knew that their partner had not recently tested for HIV were more likely than others to seroconvert in the bivariate analyses, but this association was not significant in the multivariable analysis.
TABLE 2.
Client Characteristics Associated With Seroconversion Among Re-testing Clients with HIV Negative Test Results at the First Visit, Lesotho, January 2006 to June 2010 (n = 6,777)
| Chatacteristic | Re-testers (n) | Seroconverted (%) | P-valuea | OR (95% CI) | aOR (95% CI)b |
|---|---|---|---|---|---|
| All clients | 6,777 (100.0) | 259 (3.8) | |||
| Site | |||||
| Butha Buthe | 966 | 24 (2.5) | < 0.01 | 0.58 [0.37, 0.90] | 0.72 [0.44, 1.20] |
| Leribe | 1,259 | 64 (5.1) | 1.22 [0.90, 1.65] | 1.20 [0.83, 1.72] | |
| Mafeteng | 1,025 | 27 (2.6) | 0.61 [0.40, 0.93] | 0.81 [0.49, 1.34] | |
| Maseru | 3,126 | 132 (4.2) | 1.00 (Referent) | 1.00 (Referent) | |
| Qachas Nek | 401 | 12 (3.0) | 0.70 [0.38, 1.28] | 0.50 [0.18, 1.37] | |
| Year of visit | |||||
| 2006 | 1,473 | 74 (5.0) | 0.04 | 1.00 (Referent) | 1.00 (Referent) |
| 2007 | 2,339 | 77 (3.3) | 0.64 [0.46, 0.89] | 0.69 [0.47, 1.01] | |
| 2008 | 2,472 | 92 (3.7) | 0.73 [0.53, 1.00] | 0.76 [0.52, 1.11] | |
| 2009–2010 | 493 | 16 (3.2) | 0.63 [0.37, 1.10] | 0.71 [0.34, 1.48] | |
| Gender | |||||
| Male | 2,797 | 90 (3.2) | 0.03 | 1.00 (Referent) | 1.00 (Referent) |
| Female | 3,980 | 169 (4.2) | 1.33 [1.03, 1.73] | 1.98 [1.07, 2.06] | |
| Age | |||||
| 15–24 | 2,469 | 99 (4.0) | 0.77 | 1.00 (Referent) | 1.00 (Referent) |
| 25+ | 3,599 | 139 (3.9) | 0.96 [0.74, 1.25] | 0.76 [0.56, 1.04] | |
| Marital status | |||||
| Never married | 2,959 | 103 (3.5) | 0.24 | 1.00 (Referent) | Not included |
| Married/cohab/poly | 2,386 | 90 (3.8) | 1.09 [0.82, 1.45] | ||
| Divorced/separated | 217 | 10 (4.6) | 1.34 [0.69, 2.60] | ||
| Widowed | 339 | 15 (4.4) | 1.28 [0.74, 2.23] | ||
| Other | 27 | 3 (11.1) | 3.47 [1.03, 11.7] | ||
| Education | |||||
| No formal education | 272 | 5 (1.8) | < 0.01 | 0.95 [0.36, 2.57] | 0.52 [0.12, 2.28] |
| Less than high school | 2,401 | 118 (4.9) | 2.63 [1.63, 4.25] | 2.41 [1.42, 4.10] | |
| High school | 3,021 | 115 (3.8) | 2.02 [1.25, 3.26] | 1.66 [0.98, 2.82] | |
| More than high school | 1,039 | 20 (1.9) | 1.00 (Referent) | 1.00 (Referent) | |
| Client type | |||||
| Individual | 6,469 | 250 (3.9) | 0.26 | 1.00 (Referent) | Not included |
| Couple | 306 | 8 (2.6) | 0.67 [0.33, 1.36] | ||
| Most recent partner tested in last 3 months | |||||
| Yes | 1,309 | 38 (2.9) | < 0.01 | 1.09 [0.68, 1.75] | 1.10 [0.62, 1.96] |
| No | 2,927 | 131 (4.5) | 1.71 [1.17, 2.51] | 1.60 [0.98, 2.63] | |
| Don’t know | 1,276 | 34 (2.7) | 1.00 (Referent) | 1.00 (Referent) | |
| Self-Reported Riskc | |||||
| Had sex while intoxicated/drunk | |||||
| Yes | 490 | 25 (5.1) | 1.39 [0.91, 2.12] | 1.40 [0.82, 2.38] | |
| No | 6,280 | 234 (3.7) | 0.13 | 1.00 (Referent) | 1.00 (Referent) |
| Exchanged money/materials/goods for sex | |||||
| Yes | 67 | 4 (6.0) | 1.61 [0.58, 4.45] | Not included | |
| No | 6,706 | 255 (3.8) | 0.36 | 1.00 (Referent) | |
| Had unprotected sexd | |||||
| Yes | 4,213 | 186 (4.4) | 1.57 [1.19, 2.07] | 1.18 [0.79, 1.76] | |
| No | 2,554 | 73 (2.9) | < 0.01 | 1.00 (Referent) | 1.00 (Referent) |
Pearson’s chi-square statistic for testing the null hypothesis of no association between the characteristic and serocon-version.
The multivariable logistic regression model contains all variables listed in this table, except marital status, client type, and exchanged money/materials/goods for sex. The sample size for the model was 4,994 clients.
In the pre-2008 form, these questions were asked only for the past 12 months, whereas in the post-2008 form, these questions were asked as ever.
In the post-2008 form, two separate questions were asked, one for sex with a regular partner and another for sex with a nonregular partner; a composite variable was created and coded Yes if either question’s response was answered Yes, otherwise No.
The HTC program in Lesotho may have attracted a slightly different set of repeat testers than other settings, as other studies have reported higher rates of repeat testing among women (Regan et al., 2013). Many other factors, such as attitudes and knowledge about HIV, the increase in mobile testing, availability of new rapid testing technology, background HIV prevalence and national prevention efforts may have influenced the patterns and motivations of re-testing in Lesotho. The models of testing also shifted over time to increase availability of mobile testing. Previous studies conducted among repeat testers have found mixed results as to the association between risk behavior and repeat testing (Bradley, Tsui, Kidanu, & Gillespie, 2011; Houdmont, Munir, & Grey, 2013; Leaity et al., 2000; Nascimento, 2004; Norton, Elford, Sherr, Miller, & Johnson, 1997; Ryder et al., 2005).
Over time, 3.8% of clients with a recorded re-test had seroconverted. Given the expected lag between an infection event and the point at which a person’s immune system has developed a detectable antibody response, it is likely that many of the 17% of seroconverters who tested HIV-positive within 4 weeks of their initial HIV-negative test were infected with HIV—or were in the acute HIV infection period—when previously tested (Patel, 2010; Pilcher, 2013). These are high risk clients who should receive targeted messages about acute infection, re-testing, and how to prevent transmission until diagnosis is confirmed or dismissed. The very few (4%) who seroconverted between weeks 4 and 12 supports the 2010 WHO guidelines to recommend re-testing within 4 weeks for a known exposure. The peak in re-testing between 12–18 weeks following the initial test, however, suggests that many clients may have been following a 3-month recommendation.
Predictors for HIV seroconversion included being female and having less than a high school education. Several explanations may account for these associations, including biological factors that put women at increased risk, socio-cultural reasons such as age-discordant mixing (Kelly et al., 2003; Ott, Barnighausen, Tanser, Lurie, & Newell, 2011) and gender norms that give women less power than men in marriage and sexual relationships (Harrison, Short, & Tuoane-Nkhasi, 2013). Lower educational attainment was independently associated with HIV risk, reflecting a trend observed throughout Africa (Hargreaves, 2008). Persons who are less educated may have lower awareness of HIV risk, and may have lower socio-economic status, leading them to participate in higher-risk behaviors such as transactional sex.
While the association with seroconversion of knowing the partner had not recently tested for HIV was not maintained in the multivariable analysis, it highlights the important counseling component of programs to encourage sexual partners to test and to be aware of one another’s HIV status. Couples HTC is one strategy for increasing partner testing and mutual disclosure, which should also be encouraged in order to dispel myths about serodiscordance and establish prevention plans based on both partners’ HIV status.
Our study has a number of limitations. Because we used retrospective and incomplete programmatic data, not all records could be used. Therefore these results may not represent all re-testers in Lesotho. Some variables, such as occupation, were also not evaluated because they were unable to be analyzed. Because our study involved VCT and mobile HTC clients, the results may not reflect patterns of HIV testing that occur elsewhere, e.g., public clinics, hospitals, or the private sector. The available data also did not allow us to compare between clients of mobile testing and VCT sites. Additionally, if clients did not return with their client ID card, the programs were not able to track previous tests for that individual. It is also possible that clients could have re-tested at a clinic not included in this study. Our counts of re-testers are therefore likely to be underestimates and are lower than those reported in other studies.
CONCLUSIONS
We examined patterns of re-testing and seroconversion in a large cohort of HTC clients from stand-alone and mobile HTC sites in Lesotho over a 4.5-year period. HIV testing offers a critical opportunity to diagnose HIV infection and link to HIV medical care and treatment services, and to identify high-risk HIV-negative clients who would benefit from targeted HIV prevention services, including re-testing. Among those who seroconverted in this study, 17% were probably already infected when last tested: these are persons for whom it is very important to target messages about acute infection, re-testing, and linkage to care. A substantial majority of persons who re-tested remained HIV-negative on their subsequent visits, and may not have needed re-testing sooner than the 1 year recommended by WHO.
There is a critical need for more targeted prevention services in Lesotho, particularly for women and persons with lower levels of education, and a need for increased emphasis on the importance of partner HIV testing and disclosure in preventing HIV transmission. Consistent with WHO recommendations, HTC programs should update policies to ensure that all counselors and health workers giving HIV test results are trained to encourage re-testing within 4 weeks if the client reports recent risk, and at least annually within populations at higher risk (UNAIDS, 2013). Tools and training should be developed to help counselors identify and provide tailored messages to higher-risk clients, and testing programs should track re-testers and those who seroconvert to ensure early enrollment in care.
Acknowledgments
This project was funded through the President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
The authors thank the following persons for their contributions to this project: Mosele Machitje, Karen Banda, formerly of Population Services, International (PSI) Lesotho. Hope Hempstone, PSI Lesotho, now USAID/Washington, A.D. McNaghten, formerly of the Centers for Disease Control and Prevention (CDC), now Emory University, and Ray Shiraishi, CDC.
Contributor Information
Kristina L. Grabbe, Division of Global HIV/AIDS, Centers for Disease Control and Prevention, Atlanta, Georgia.
Cari Courtenay-Quirk, Division of Global HIV/AIDS, Centers for Disease Control and Prevention, Atlanta, Georgia.
Andrew L. Baughman, Division of Global HIV/AIDS, Centers for Disease Control and Prevention, Atlanta, Georgia.
Gaston Djomand, Division of Global HIV/AIDS, Centers for Disease Control and Prevention, Atlanta, Georgia.
Brian Pedersen, Population Services, International (PSI) Lesotho.
Mankhala Lerotholi, Population Services, International (PSI) Lesotho.
John Nkonyana, Ministry of Health, Kingdom of Lesotho.
Puleng Ramphalla-Phatela, Ministry of Health, Kingdom of Lesotho.
Elizabeth Marum, Division of Global HIV/AIDS, Centers for Disease Control and Prevention, Atlanta, Georgia.
References
- Bradley H, Tsui A, Kidanu A, Gillespie D. Client characteristics and HIV risk associated with repeat HIV testing among women in Ethiopia. AIDS and Behavior. 2011;15:725–733. doi: 10.1007/s10461-010-9765-1. [DOI] [PubMed] [Google Scholar]
- Cawley C, Wringe A, Isingo R, Mtenga B, Clark B, Marston M, … Zaba B. Low rates of repeat HIV testing despite increased availability of antiretro-viral therapy in rural Tanzania: Findings from 2003–2010. PLoS One. 2013;8:e62212. doi: 10.1371/journal.pone.0062212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay-Quirk C, Grabbe K, Baughman A, Djomand G, Pederson B. Patterns of HIV retesting among clients of voluntary HIV counselling and testing facilities: Lesotho, January 2006–January 2010. Poster presented at the 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, Georgia. 2013. Mar, [Google Scholar]
- Fernyak SE, Page-Shafer K, Kellogg TA, Mc-Farland W, Katz MH. Risk behaviors and HIV incidence among repeat testers at publicly funded HIV testing sites in San Francisco. Journal of Acquired Immune Deficiency Syndromes. 2002;31:63–70. doi: 10.1097/00126334-200209010-00009. [DOI] [PubMed] [Google Scholar]
- Government of Lesotho. National guidelines for HIV counseling and testing (HTC) Maseru: Government of Lesotho; 2009. [Google Scholar]
- Hargreaves JR, Bonell CP, Boler T, Boccia D, Birdthistle I, Fletcher A, … Glynn JR. Systematic review exploring time trends in the association between educational attainment and risk of HIV infection in sub-Saharan Africa. AIDS (London, England) 2008;22:403–414. doi: 10.1097/QAD.0b013e3282f2aac3. [DOI] [PubMed] [Google Scholar]
- Harrison A, Short SE, Tuoane-Nkhasi M. Re-focusing the gender lens: Caregiving women, family roles and HIV/AIDS vulnerability in Lesotho. AIDS and Behavior. 2013;18:595–604. doi: 10.1007/s10461-013-0515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdmont J, Munir F, Grey M. Acceptance of repeat worksite HIV voluntary counselling and testing in a rural South African factory. AIDS Care. 2013;25:1199–202. doi: 10.1080/09540121.2013.764388. [DOI] [PubMed] [Google Scholar]
- Kelly RJ, Gray RH, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Wawer MJ. Age differences in sexual partners and risk of HIV-1 infection in rural Uganda. Journal of Acquired Immune Deficiency Syndromes. 2003;32:446–451. doi: 10.1097/00126334-200304010-00016. [DOI] [PubMed] [Google Scholar]
- Leaity S, Sherr L, Wells H, Evans A, Miller R, Johnson M, Elford J. Repeat HIV testing: High-risk behaviour or risk reduction strategy? AIDS (London, England) 2000;14:547–552. doi: 10.1097/00002030-200003310-00010. [DOI] [PubMed] [Google Scholar]
- MacKellar DA, Valleroy LA, Secura GM, Bartholow BN, McFarland W, Shehan D, … Janssen RS. Repeat HIV testing, risk behaviors, and HIV serocon-version among young men who have sex with men: A call to monitor and improve the practice of prevention. Journal of Acquired Immune Deficiency Syndromes. 2002;29:76–85. doi: 10.1097/00042560-200201010-00011. [DOI] [PubMed] [Google Scholar]
- Matovu JK, Gray RH, Kiwanuka N, Kigozi G, Wabwire-Mangen F, Nalugoda F, … Wawer MJ. Repeat voluntary HIV counseling and testing (VCT), sexual risk behavior and HIV incidence in Rakai, Uganda. AIDS and Behavior. 2007;11:71–78. doi: 10.1007/s10461-006-9170-y. [DOI] [PubMed] [Google Scholar]
- Nascimento CM, Casado MJ, Casabona J, Ros R, Sierra E, Zaragoza K, … Oliver C. Estimation of HIV incidence among repeat anonymous testers in Catalonia, Spain. AIDS Research and Human Retroviruses. 2004;20:1145–1147. doi: 10.1089/0889222042544956. [DOI] [PubMed] [Google Scholar]
- Norton J, Elford J, Sherr L, Miller R, Johnson MA. Repeat HIV testers at a London same-day testing clinic. AIDS (London, England) 1997;11:773–781. [PubMed] [Google Scholar]
- Ott MQ, Barnighausen T, Tanser F, Lurie MN, Newell ML. Age-gaps in sexual partnerships: Seeing beyond ‘sugar daddies’. AIDS (London, England) 2011;25:861–863. doi: 10.1097/QAD.0b013e32834344c9. doi:0.1097/QAD.0b013e32834344c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P, Mackellar D, Simmons P, Uniyal A, Gallagher K, Bennett B, Sullivan PS. Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA, 2006–2008. Archives of Internal Medicine. 2010;170:66–74. doi: 10.1001/archinternmed.2009.445. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Louie B, Facente S, Keating S, Hackett J, Jr, Vallari A, Pandori MW. Performance of rapid point-of-care and laboratory tests for acute and established HIV infection in San Francisco. PLoS One. 2013;8:e80629. doi: 10.1371/journal.pone.0080629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan S, Losina E, Chetty S, Giddy J, Walensky RP, Ross D, Bassett IV. Factors associated with self-reported repeat HIV testing after a negative result in Durban, South Africa. PLoS One. 2013;8:e62362. doi: 10.1371/journal.pone.0062362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K, Haubrich DJ, Calla D, Myers T, Burchell AN, Calzavara L. Psychosocial impact of repeat HIV-negative testing: A follow-up study. AIDS and Behavior. 2005;9:459–464. doi: 10.1007/s10461-005-9032-z. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT 9.3 User’s guide. Cary, NC: SAS Institute; 2011. [Google Scholar]
- Wolmarans LJD, Koppenhaver T. Behavior change among repeat testers in Namibia. Paper presented at the XVII International AIDS Conference; Mexico City, Mexico. 2008. Aug, Retrieved June 13, 2015, from https://www.aids2014.org/Abstracts/A200720864.aspx. [Google Scholar]
- World Health Organization. Delivering HIV test results and messages for re-testing and counseling in adults. 2010 Retrieved April 9, 2015, from http://whqlibdoc.who.int/publications/2010/9789241599115_eng.pdf. [PubMed]
- World Health Organization, United Nations Program on HIV AIDS & UNICEF. Global update on HIV treatment 2013: Results, impact and opportunities. 2013 Retrieved April 9, 2015, from http://apps.who.int/iris/bitstream/10665/85326/1/9789241505734_eng.pdf?ua=1.