Abstract
Background
Although lobar patterns of emphysema heterogeneity are indicative of optimal target sites for lung volume reduction (LVR) strategies, the presence of segmental, or sublobar, heterogeneity is often underappreciated.
Objective
The aim of this study was to understand lobar and segmental patterns of emphysema heterogeneity, which may more precisely indicate optimal target sites for LVR procedures.
Methods
Patterns of emphysema heterogeneity were evaluated in a representative cohort of 150 severe (GOLD stage III/IV) chronic obstructive pulmonary disease (COPD) patients from the COPDGene study. High-resolution computerized tomography analysis software was used to measure tissue destruction throughout the lungs to compute heterogeneity (≥ 15% difference in tissue destruction) between (inter-) and within (intra-) lobes for each patient. Emphysema tissue destruction was characterized segmentally to define patterns of heterogeneity.
Results
Segmental tissue destruction revealed interlobar heterogeneity in the left lung (57%) and right lung (52%). Intralobar heterogeneity was observed in at least one lobe of all patients. No patient presented true homogeneity at a segmental level. There was true homogeneity across both lungs in 3% of the cohort when defining heterogeneity as ≥ 30% difference in tissue destruction.
Conclusion
Many LVR technologies for treatment of emphysema have focused on interlobar heterogeneity and target an entire lobe per procedure. Our observations suggest that a high proportion of patients with emphysema are affected by interlobar as well as intralobar heterogeneity. These findings prompt the need for a segmental approach to LVR in the majority of patients to treat only the most diseased segments and preserve healthier ones.
Keywords: Heterogeneity, Lung volume reduction, Chronic obstructive pulmonary disease, Bronchoscopy, Computed tomography
Introduction
Emphysema is pathologically defined as an ‘abnormal, permanent enlargement of air spaces distal to the terminal bronchioles, accompanied by the destruction of alveolar walls’ [1]. The extent and distribution of these enlargements vary within the lung, resulting in a heterogeneous spatial distribution of emphysema. The patterns and degree of emphysema heterogeneity within the lung can be defined and characterized to quantify the most diseased portions of the lung with computed tomography (CT) density being used as an indicator for tissue destruction [2]. Once quantified, the most diseased portions of the lung can potentially be selectively reduced as part of a lung volume reduction (LVR) procedure with the aim of improving patient quality of life. There is some evidence that the degree of disease heterogeneity correlates with clinical efficacy after LVR [3–5].
There is currently no gold standard for defining heterogeneity. The National Emphysema Treatment Trial (NETT) quantified this by visually scoring CT scans. Each lung was divided into thirds to define three apical to basal zones. Each zone was compared against the remaining two ipsilateral zones to evaluate heterogeneity [5]. However, this method of visual scoring for emphysema severity is radiologist dependent and does not necessarily follow anatomical lobar boundaries. Computerized quantitative measurement tools have enabled more precise scoring of the lung. By doing so, the variability seen during the radiologist-dependent visual scoring method is minimized, allowing for a user-independent result [6].
Advances in computerized quantitative measurement tools have also enabled a better understanding of how emphysema is spatially distributed at a sublobar level by assessing tissue destruction for individual bronchopulmonary segments. Understanding which bronchopulmonary segments are most diseased within a lobe may lead to a more precise understanding of which regions (e.g. segments and lobes) of the lung are the best targets for LVR procedures.
The current study aims to evaluate the patterns of emphysema heterogeneity in GOLD stage III and IV patients using computerized quantitative measurement tools to objectively assess the pattern of emphysema by evaluating tissue destruction throughout regions of the lung. The analysis presented assesses heterogeneity between lobes as well as heterogeneity within lobes. We hypothesize that many patients have patterns of intralobar heterogeneity regardless of interlobar heterogeneity, and, therefore, many patients are not truly homogeneous at a segmental level.
Materials and Methods
Patient Selection and High-Resolution CT Analysis
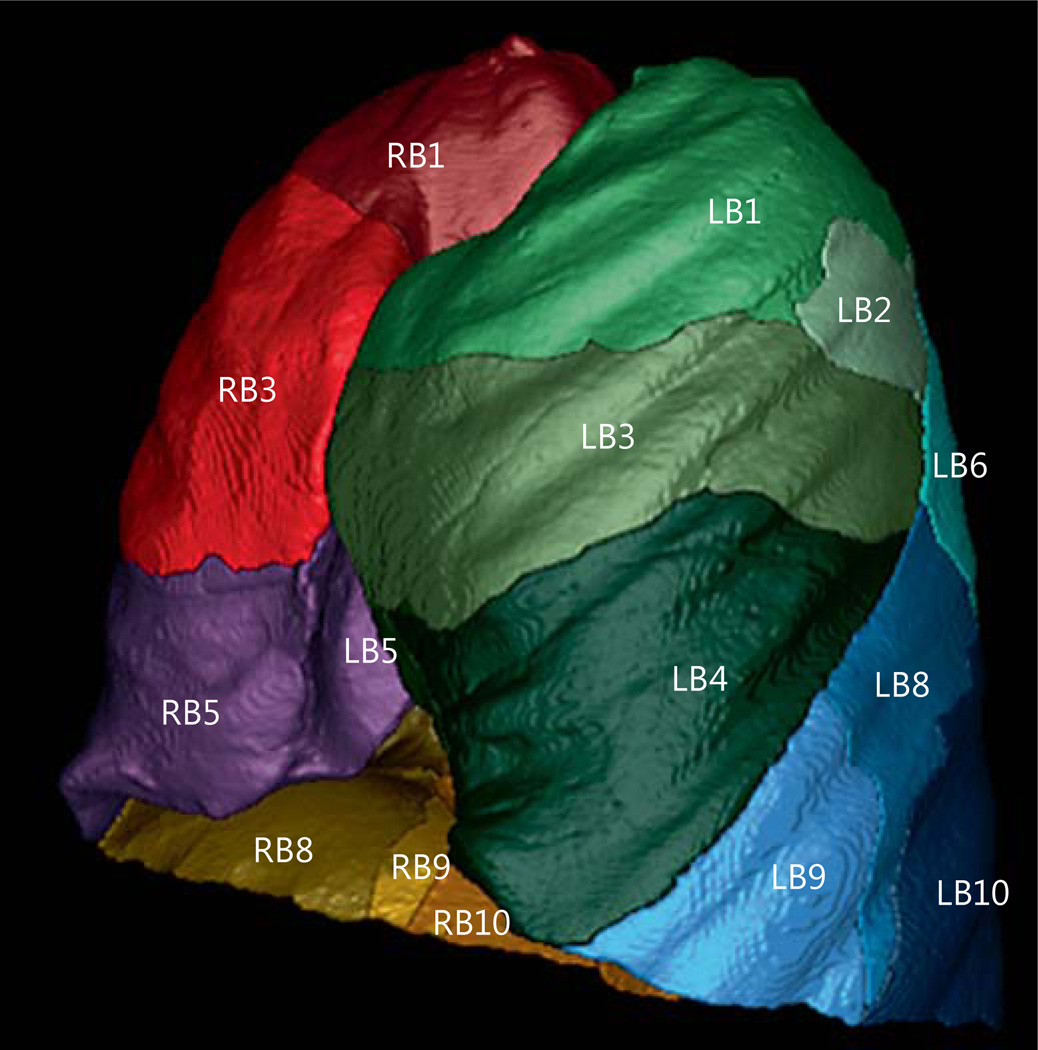
A representative sample [7–10] of 150 GOLD stage III/IV patients was selected from the COPDGene study, and their anonymized inspiratory high-resolution CT scans (HRCTs) were acquired. Permission to use this dataset was obtained from the COPDGene study committee, and institutional review board approval was obtained as part of the COPDGene study. Detailed inclusion criteria for the COPDGene study are discussed elsewhere [11]. Briefly, the inclusion criteria were: age between 45 and 80 years, minimum of a 10 pack-year smoking history, no previous surgical excision of at least one lung lobe (or LVR procedure), no active cancer under treatment, and no suspected lung cancer. The COPDGene study began enrolling patients in November 2007 and is expected to be completed by November 2017. The analysis of the subgroup presented in this paper was performed from May 2014 to January 2015. The HRCTs were quantitatively analyzed using the commercially available Apollo version 2.1 software (VIDA Diagnostics, Coralville, Iowa, USA). For this study, the software provided a sublobar characterization of air volume and tissue mass volume for each of the segmental branches (RB1–10, LB1–10). See figure 1 for a representation of sublobar segmentation from the VIDA Apollo software. An airway analysis was performed to extend airway paths to the sub-subsegmental generation, and manual edits were made to fissure boundaries as needed. The editing mechanism is described in more detail in a previous publication [12].
Fig. 1.
Representation of sublobar segmentation from the VIDA Apollo software. Density and heterogeneity measurements are computed at the lobar and segmental levels.
Defining Heterogeneity Indices
Relative low-density regions are indicative of high tissue destruction within the lung and/or the presence of hyperinflation [13]. There are different methods that can be used to quantify these relatively low-density regions, including characterizing air volume and tissue mass for regions across the lung [14]. In this study, air volume and tissue mass volumes were quantitatively assessed to compute an air volume to tissue mass ratio, a measure of hyperinflation and tissue destruction. The air volume to tissue mass ratio is the inverse of the previously published measure of lung density, the tissue to air ratio, and is directly correlated with disease severity [5, 14]. During HRCT analysis, the right lung and left lung were each divided into three lobar regions, and each lobar region was divided into bronchopulmonary segments as follows: right upper lobe (RB1, RB2, RB3), right middle lobe (RB4, RB5), and right lower lobe (RB6, RB7, RB8, RB9, RB10); left upper lobe (LB1, LB2, LB3), lingula (LB4, LB5), and left lower lobe (LB6, LB8, LB9, LB10).
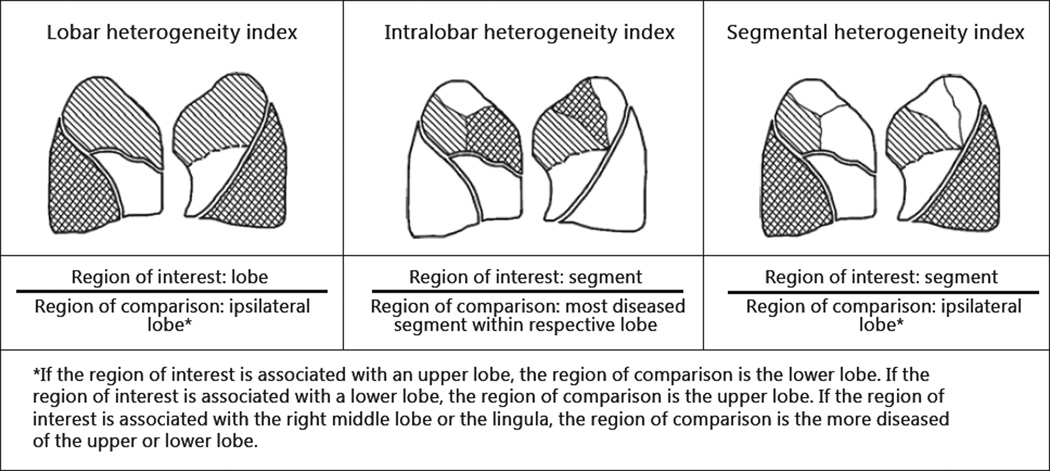
Tissue destruction was measured for each lobe and each bronchopulmonary segment. A ratio comparing the degree of tissue destruction for two regions of interest was compared to yield a heterogeneity index (equation 1). Depending on the type of regions being compared, three different heterogeneity indices can be computed: lobar heterogeneity index, intralobar heterogeneity index, and segmental heterogeneity index as outlined by figure 2. A lobar heterogeneity index compares the degree of tissue destruction between two lobes. An intralobar heterogeneity index compares the degree of tissue destruction between two bronchopulmonary segments of the same lobar region. A segmental heterogeneity index compares the degree of tissue destruction between a bronchopulmonary segment and an ipsilateral lobe. In the latter two instances, the pattern of emphysema heterogeneity was dependent on individual segments. Individual segments are found to vary in size relative to the lobe and can therefore represent a small percentage of their respective lobe. For this reason, segments in the 10th percentile of lobe size were removed from the intralobar and segmental heterogeneity index analyses to avoid concluding the existence of segmental heterogeneity based on a small portion of a given lobe.
| (1) |
Fig. 2.
Equations for three different heterogeneity indices.
Heterogeneity Index Thresholds
A heterogeneity index was established to differentiate between low and high levels of heterogeneity. A heterogeneity index equal to 1.0 indicates that the two regions being compared are equivalently diseased. Previous studies have denoted a difference of approximately 15% in tissue destruction to differentiate between low and high levels of heterogeneity [6, 15]. A 15% difference is equivalent to a heterogeneity index of 1.15, which is used as a threshold to differentiate heterogeneous disease from homogenous disease. Another, more stringent, heterogeneity index threshold of ≥ 1.30 was derived by doubling the commonly reported threshold of 15% and was used to verify a difference of at least 30% within a lobe.
Patterns of Emphysema Heterogeneity
Interlobar Heterogeneity
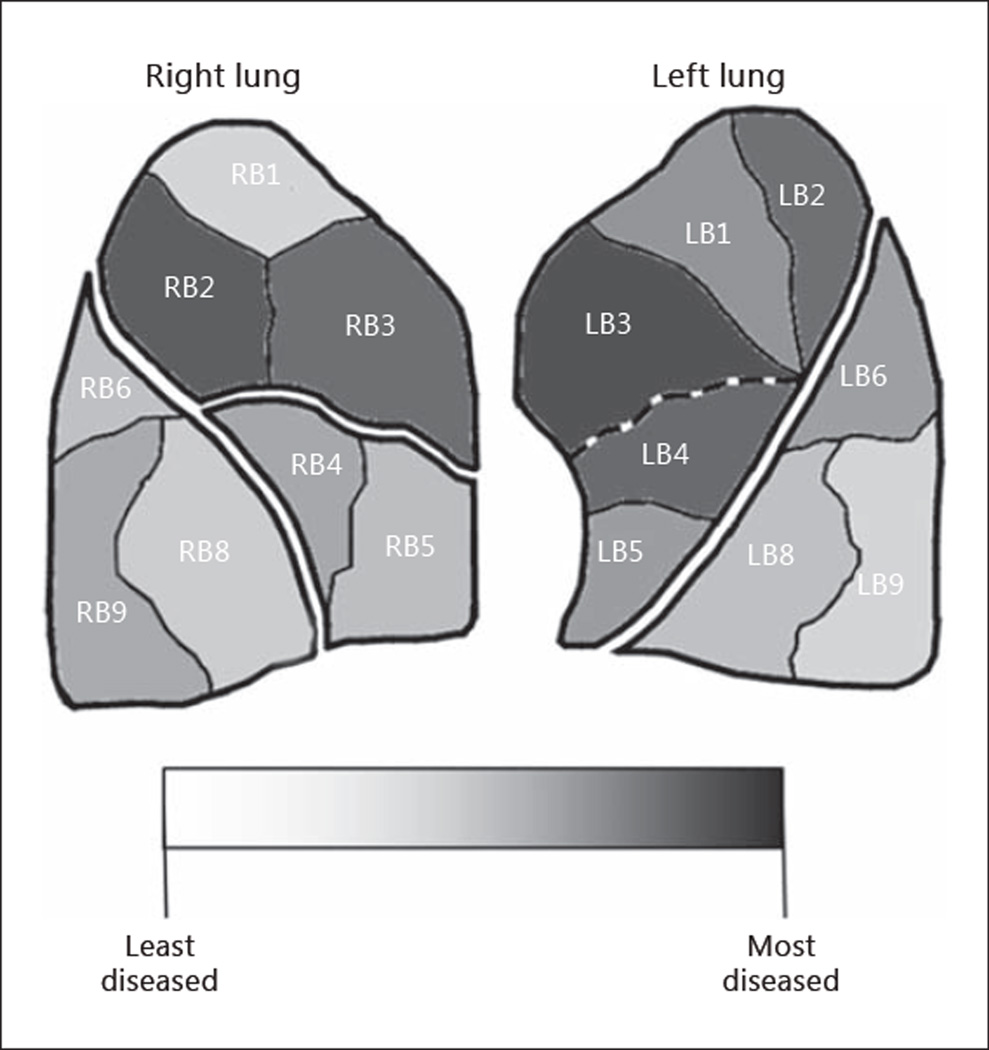
Interlobar heterogeneity indicates emphysema that is predominant at a lobar level. The interlobar heterogeneity index can be used to describe patterns where a lobe on average is more diseased than its ipsilateral lobe. However, this does not take individual segments within a lobe into account. The segmental heterogeneity index can be used to evaluate the disease state of each segment as compared to the ipsilateral lobe. An example emphysema case is presented in figure 3. The right lung is perceived to be upper lobe predominant, but a segmental heterogeneity assessment reveals that not all segments of the right upper lobe are more diseased than its ipsilateral lower lobe. The left lung is also perceived to be upper lobe predominant. However, in this case, a segmental heterogeneity assessment reveals that all segments of the left upper lobe are more diseased than its ipsilateral lower lobe. The left upper lobe is truly more diseased than its ipsilateral lower lobe. Therefore, interlobar heterogeneity can be grouped into two categories: perceived interlobar heterogeneity and true interlobar heterogeneity.
Fig. 3.
Example patient with intralobar heterogeneous emphysema. The right upper lobe is perceived to be interlobar heterogeneous but is not considered to be truly interlobar heterogeneous because not all right upper lobe segments are more diseased than the lower lobe. The left upper lobe is truly interlobar heterogeneous because all left upper lobe segments are more diseased than the lower lobe.
Perceived Interlobar Heterogeneity
The average disease state of the lobe of interest is greater than that of the ipsilateral lobe. In this case, the lobe of interest must meet the lobar heterogeneity index threshold of 1.15.
True Interlobar Heterogeneity
The disease state of each segment in the lobe of interest is greater than in the ipsilateral lobe. In this group, which is a subset of perceived interlobar heterogeneity, all segments within the lobe of interest must meet the segmental heterogeneity index threshold of 1.15.
Intralobar Heterogeneity: Intralobar Heterogeneous Emphysema
The disease state of a segment in the lobe of interest is greater than that of another segment in the lobe of interest. In this case, at least one segment within the lobe of interest will meet the intralobar heterogeneity index. Two analyses were performed to compute the level of heterogeneity. The initial analysis was performed using a heterogeneity index threshold of 1.15, and the second analysis was performed using a more stringent threshold of 1.30. Furthermore, each segment was assessed for how frequently it is the most diseased segment in its respective lobar region.
Homogeneous Emphysema
A minor or no regional difference in the severity of emphysema is present between regions of the lung. This is defined with all three heterogeneity indices. In true homogenous emphysema, there is no heterogeneity in disease between or within lobes. Two analyses were performed to compute the frequency of homogeneity. The initial analysis was performed using a heterogeneity index threshold of 1.15, and the second analysis was performed using a more stringent threshold of 1.30.
Statistical Analysis
Individual segments are found to vary in size relative to the lobe and can therefore represent a small percentage of their respective lobe. For this reason, segments in the 10th percentile of lobe size were removed from the intralobar and segmental heterogeneity index analyses to avoid concluding the existence of segmental heterogeneity based on a small portion of a given lobe. The patterns of heterogeneity were then assessed by calculating the percentage of the cohort that exhibited a heterogeneity index above a specified threshold for each lobe and lung using Microsoft Excel.
Results
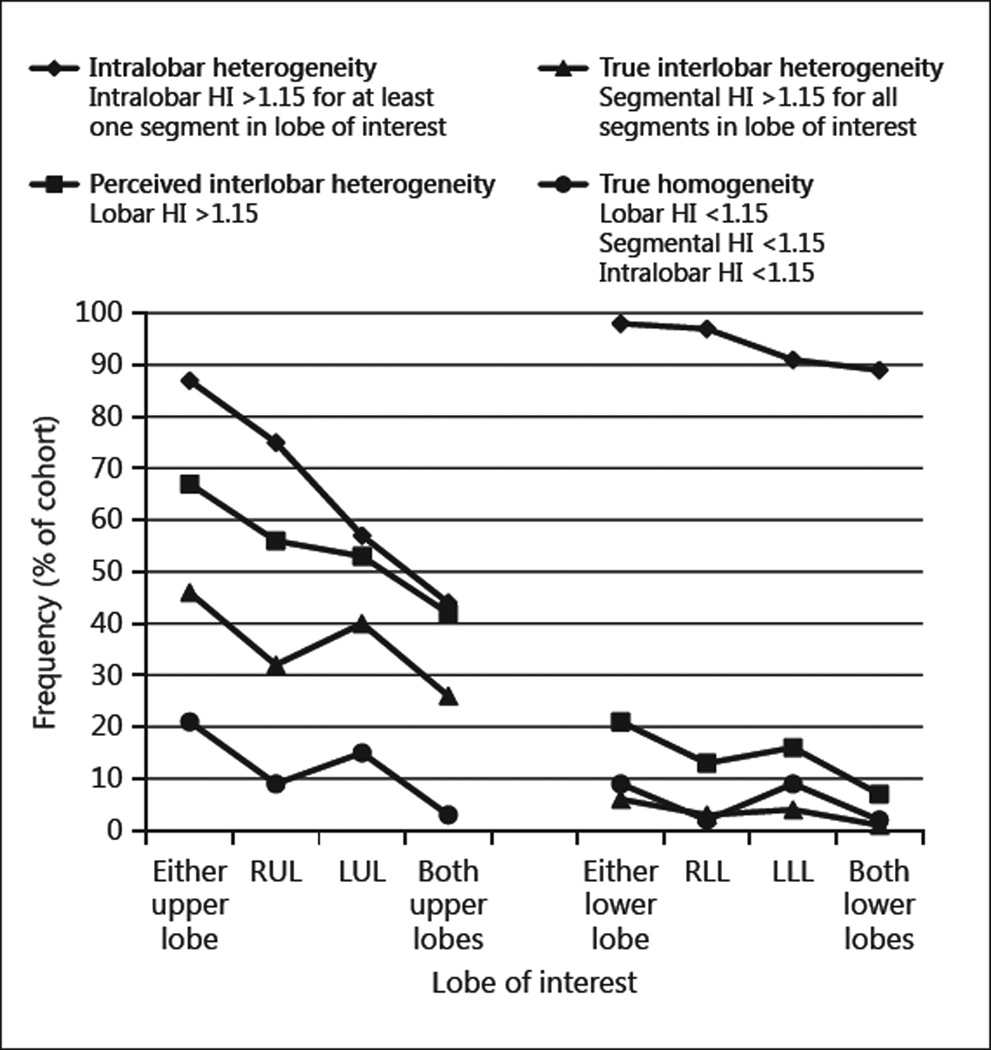
Demographic variables and lung function for all patients are reported in table 1. HRCTs of 75 patients with GOLD stage III emphysema and of 75 patients with GOLD stage IV emphysema were studied. All patients in the study cohort were current or former smokers, and a diagnosis of α1-antitrypsin deficiency was confirmed in 6 patients. Quantifying the disease state at the segmental level in the right lower lobe of 2 patients was not possible due to atypical anatomy. The frequency of interlobar heterogeneity, intralobar heterogeneity, and homogeneity in the study cohort is summarized in figure 4 and is detailed in the following sections.
Table 1.
Patient dataset characterization
| All patients (n = 150) |
GOLD stage III (n = 75) |
GOLD stage IV (n = 75) |
|
|---|---|---|---|
| Gender | |||
| Male | 88 | 43 | 45 |
| Female | 62 | 32 | 30 |
| Age, years | 65 (8) | 67 (8) | 64 (8) |
| FVC% pred. | 66 (16) | 75 (13) | 57 (15) |
| FEV1% pred. | 32 (11) | 40 (5) | 23 (5) |
| FEV1/FVC, % | 48 (13) | 56 (11) | 40 (8) |
Values are numbers or means (standard deviations). FVC% pred. = Forced vital capacity percent predicted; FEV1% pred. = forced expiratory volume in 1 s percent predicted.
Fig. 4.
Frequency of disease heterogeneity and homogeneity in the study cohort for each upper and lower lobe. HI = Heterogeneity index; RUL = right upper lobe; LUL = left upper lobe; RLL = right lower lobe; LLL = left lower lobe.
Interlobar Heterogeneous Emphysema
The frequency of interlobar heterogeneity in the study cohort is presented in table 2. Sixty-five percent of the cohort was perceived to have interlobar heterogeneous patterns bilaterally. However, only 39% of the cohort had true interlobar heterogeneous patterns bilaterally. A closer look at upper lobe predominant emphysema revealed that 42% of the cohort was perceived to be upper lobe predominant bilaterally, but only 26% of the cohort was truly upper lobe predominant bilaterally.
Table 2.
Frequency of interlobar heterogeneity in GOLD stage III/IV patients
| Frequency of interlobar heterogeneity, % | ||
|---|---|---|
| perceived as interlobar heterogeneitya |
true interlobar heterogeneityb |
|
| Upper lobe | ||
| Left | 53 | 40 |
| Right | 56 | 32 |
| Both | 42 | 26 |
| Middle lobe/lingula | ||
| Left | 47 | 35 |
| Right | 49 | 37 |
| Both | 31 | 19 |
| Lower lobe | ||
| Left | 16 | 4 |
| Right | 13 | 3 |
| Both | 7 | 1 |
| At least one lobe of the lung | ||
| Left | 78 | 57 |
| Right | 81 | 52 |
| Both | 65 | 39 |
HI = Heterogeneity index.
Lobar HI ≥1.15 for the lobe of interest.
Segmental HI ≥1.15 for all segments in the lobe of interest.
Intralobar Heterogeneous Emphysema
The frequency of intralobar heterogeneity in the study cohort is presented in table 3. In patients with intralobar heterogeneous emphysema, as defined by the intralobar heterogeneity index and a threshold of 1.15, it can be observed that heterogeneity exists within the lung for nearly all patients. Using a more stringent threshold of 1.30, intralobar heterogeneity was found bilaterally in 63% of the cohort.
Table 3.
Frequency of interlobar heterogeneity in GOLD stage III/IV patients
| Frequency of intralobar heterogeneity, % | ||
|---|---|---|
| emphysema is heterogeneous within a lobea |
emphysema is heterogeneous within a lobeb |
|
| Upper lobe | ||
| Left | 57 | 29 |
| Right | 75 | 40 |
| Both | 44 | 17 |
| Middle lobe/lingula | ||
| Left | 44 | 31 |
| Right | 35 | 12 |
| Both | 13 | 3 |
| Lower lobe | ||
| Left | 91 | 59 |
| Right | 97 | 78 |
| Both | 89 | 49 |
| At least one lobe of the lung | ||
| Left | 97 | 73 |
| Right | 100 | 83 |
| Both | 97 | 63 |
HI = Heterogeneity index.
Intralobar HI ≥1.15 for at least one segment in the lobe of interest.
Intralobar HI ≥1.30 for at least one segment in the lobe of interest.
A closer look at intralobar heterogeneous emphysema to determine how frequently each segment was the most diseased in its respective lobe indicates that LB3 and RB3 were the most diseased segments of the upper lobes, 56 and 43% of the time, respectively. LB4 an RB4 were the most diseased segments of the lingula and right middle lobe, 79 and 67% of the time, respectively. LB6 and RB8 were the most diseased segments of the lower lobe, 46 and 51% of the time, respectively.
Homogeneous Emphysema
The frequency of true homogeneity in the study cohort is presented in tables 4, 5. These patients did not exhibit high levels of heterogeneity as determined by the lobar, segmental, and intralobar heterogeneity indices. As defined by a threshold of 1.15, all patients in this cohort had a form of heterogeneity present within the right lung, and only 1% of the cohort was found to be truly homogeneous in the left lung. As defined by a more stringent threshold of 1.30, 16% of the cohort was truly homogeneous in the left lung, 8% of the cohort was truly homogeneous in the right lung, and only 3% of the cohort was truly homogeneous bilaterally.
Table 4.
Frequency of homogeneous emphysema in GOLD stage III/IV patients using a 15% heterogeneity threshold
| Frequency of homogeneous emphysema, % | |||||
|---|---|---|---|---|---|
| emphysema is not heterogeneous at lobar levela |
emphysema is not heterogeneous at segmental levelb |
emphysema is not heterogeneous within a lobec |
emphysema is truly homogeneousd |
||
| Upper lobe | Left | 47 | 33 | 43 | 15 |
| Right | 44 | 29 | 25 | 9 | |
| Both | 33 | 21 | 13 | 3 | |
| Middle lobe/lingula | Left | 53 | 33 | 56 | 23 |
| Right | 51 | 36 | 65 | 20 | |
| Both | 35 | 19 | 34 | 6 | |
| Lower lobe | Left | 84 | 70 | 9 | 9 |
| Right | 87 | 68 | 3 | 2 | |
| Both | 79 | 57 | 2 | 2 | |
| Entire lung | Left | 1 | |||
| Right | 0 | ||||
| Both | 0 | ||||
HI = Heterogeneity index.
Lobar HI <1.15.
Segmental HI <1.15 for all segments in the lobe of interest.
Intralobar HI <1.15 for all segments in the lobe of interest.
Lobar HI <1.15; segmental HI <1.15; intralobar HI <1.15.
Table 5.
Frequency of homogeneous emphysema in GOLD stage III/IV patients using a 30% heterogeneity threshold
| Frequency of homogeneous emphysema, % | |||||
|---|---|---|---|---|---|
| emphysema is not heterogeneous at lobar levela |
emphysema is not heterogeneous at segmental levelb |
emphysema is not heterogeneous within a lobec |
emphysema is truly homogeneousd |
||
| Upper lobe | Left | 65 | 54 | 71 | 42 |
| Right | 66 | 50 | 60 | 37 | |
| Both | 57 | 40 | 47 | 25 | |
| Middle lobe/lingula | Left | 81 | 63 | 69 | 52 |
| Right | 81 | 69 | 88 | 61 | |
| Both | 69 | 47 | 59 | 33 | |
| Lower lobe | Left | 93 | 81 | 41 | 39 |
| Right | 95 | 83 | 22 | 20 | |
| Both | 89 | 74 | 13 | 11 | |
| Entire lung | Left | 16 | |||
| Right | 8 | ||||
| Both | 3 | ||||
HI = Heterogeneity index.
Lobar HI <1.30.
Segmental HI <1.30 for all segments in the lobe of interest.
Intralobar HI <1.30 for all segments in the lobe of interest.
Lobar HI <1.30; segmental HI <1.30; intralobar HI <1.30.
Discussion
The current study quantifies the pattern of heterogeneity based on HRCT analysis and the spatial distribution of tissue destruction throughout the lungs in GOLD stage III and IV patients. The results of the analysis presented indicate that approximately half of GOLD stage III and IV patients have true interlobar heterogeneity in either lung. True interlobar heterogeneity was exhibited in either upper lobe in 46% of the cohort, and only 6% of the cohort exhibited true interlobar heterogeneity in either lower lobe. When considering intralobar heterogeneity, 97% of the cohort exhibited heterogeneity in both lungs. True homogeneity in GOLD stage III and IV patients is uncommon.
There are therapeutic reasons to characterize patterns of emphysema heterogeneity. LVR studies have found a correlation between the degree of heterogeneity and efficacy of treatment [2, 6]. The NETT study in particular demonstrated that patients with an upper lobe predominant pattern of emphysema are most likely to benefit from LVR surgery [15]. A study published by Higuchi et al. [16] also found that homogeneous patterns of emphysema may also indicate a risk for interlobar collateral ventilation. Based on these associations, defining the pattern and degree of emphysema heterogeneity can assist in treatment planning and play a large role in personalized therapy by indicating which regions of the lung to reduce during an LVR procedure.
Traditionally, LVR procedures have been performed via surgery. However, with advances in technology, endoscopic techniques are quickly becoming the most feasible interventions for treatment of emphysema. Each endoscopic LVR technique differs in its mechanistic target and, therefore, safety and efficacy capability. Accordingly, each technique assesses different patient and disease characteristics, such as fissure integrity and collateral ventilation, to determine patient eligibility and LVR technique compatibility [17–21]. Among these patient characteristics, disease heterogeneity is also assessed but, typically, is not evaluated within a lobe. We hypothesize that assessing heterogeneity within a lobe may provide a more precise indication of where tissue destruction is most pronounced in the lung and may therefore indicate which treatment approach and technique might theoretically benefit the patient most. Targeting only the most diseased segments during an LVR procedure allows less diseased segments of the lung to continue contributing to positive lung function after treatment, which may benefit patient efficacy. A randomized, controlled trial utilizing a segmental approach is currently ongoing and may assist in identifying the impact of heterogeneity within a lobe and segmental treatment planning [5].
There are currently four endoscopic LVR techniques being investigated for patients with emphysema: valves, coils, sealants, and vapor [17–21]. Due to intralobar collateral ventilation, valve LVR technologies are limited to a lobar approach where an entire lobe is targeted during an LVR procedure. Coils can be placed segmentally but are recommended for and typically used to reduce an entire lobe. These lobar-approach LVR interventions may be appropriate for patients with true interlobar heterogeneity, where the entire lobe is more diseased than its ipsilateral lobe. With a lobar treatment for truly interlobar heterogeneous disease, 46% of the patients could have unilateral upper lobe treatment, 26% of the patients could have bilateral upper lobe treatment, and less than 10% of the patients could have a unilateral or bilateral lower lobe treatment. Vapor and sealant technologies are able to use a segmental approach, where individual bronchopulmonary segments can be targeted during an LVR procedure. With a segmental treatment for segmental heterogeneous disease, 44% of the patients could have bilateral upper lobe treatment, and over 85% of the patients could have a unilateral upper lobe treatment, unilateral lower lobe treatment, or bilateral lower lobe treatment. This strategy applies to a meaningfully higher portion of all patients because a segmental approach for LVR may be appropriate for patients with true inter- or intralobar heterogeneous emphysema.
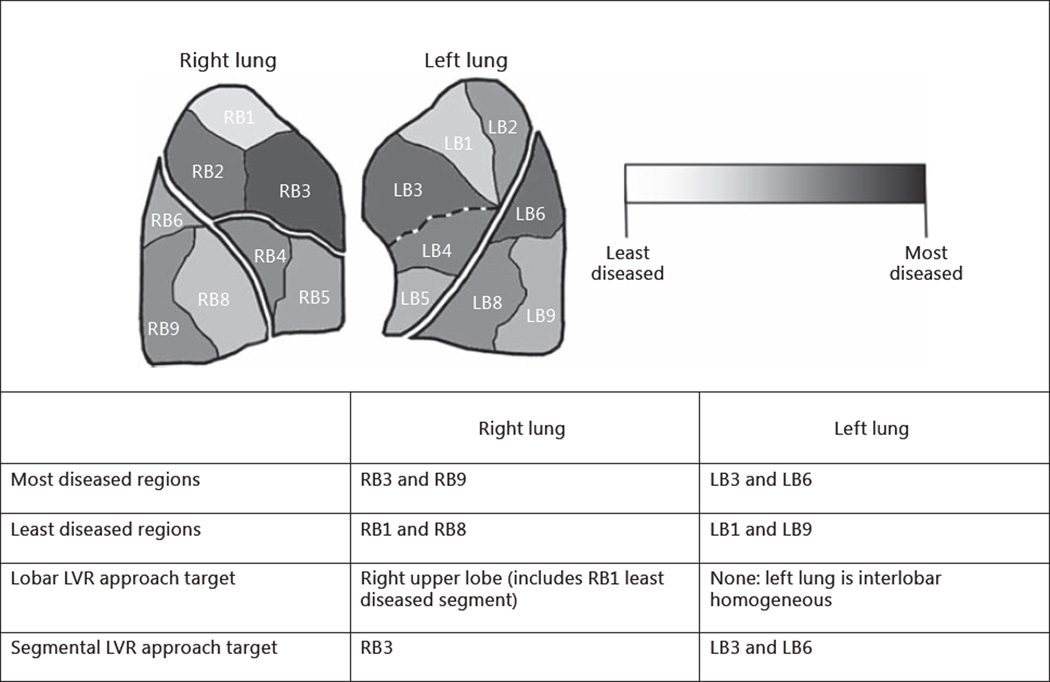
Figure 5 shows tissue destruction at a segmental level as an example emphysema case. The right lung appears to be upper lobe predominant and the left lung appears to be homogeneous based on lobar level tissue destruction. A segmental heterogeneity assessment reveals that the patient has intralobar heterogeneous patterns of emphysema in both lungs. A lobar approach may target the right upper lobe, left upper lobe, or left lower lobe to reduce the most diseased segments of the lung. In doing so, the least diseased segments will also be reduced. An alternate, segmental approach allows for a more personalized therapy of emphysema where segmental heterogeneity can be used to target only the most diseased segments and preserve the less diseased segments. Furthermore, emphysema severity is expected to worsen over time and retreatment may be needed as the disease progresses. Treating only the most diseased portions of the lung without reducing the less diseased portions of the lung will allow for a larger lung reserve for future LVR procedures.
Fig. 5.
Example patient with intralobar heterogeneous emphysema. The left lung is interlobar homogenous but exhibits intralobar heterogeneity. The right upper lobe is perceived to be interlobar heterogeneous but is not considered to be truly interlobar heterogeneous because not all segments are more diseased than the lower lobe.
Specific patterns of emphysema heterogeneity have been attributed to different causes. Previous studies have shown that α1-antitrypsin deficiency is often associated with lower lobe predominant emphysema, and smoking-induced chronic obstructive pulmonary disease (COPD) is often associated with upper lobe predominant emphysema [22]. The frequency of COPD has also been associated with occupational titles and exposure to certain airborne agents [23]. It is possible that these factors could cause a bias in the pattern of emphysema heterogeneity in the current cohort. However, all patients of the cohort were current or former smokers, and 6 patients (4%) were confirmed to have a diagnosis of α1-antitrypsin deficiency. COPD is often attributed to smoking, and about 1–3% of all emphysema patients are estimated to have α1-antitrypsin deficiency [24]. Because emphysema is a sub-set of COPD, an increase in this percentage is expected for COPD patients with α1-antitrypsin deficiency. The study cohort represented a similar population, and we therefore do not expect the results to drastically differ from representative GOLD stage III and IV patients.
Another limitation of the study is the use of low-density regions as a measure of tissue destruction due to emphysema. While this likely has a strong correlation because the cohort is comprised of patients with a >10 pack-year smoking history, there may be other causes of hyperinflation such as asthma and chronic bronchitis. However, because regions of hyperinflation are targeted for LVR in these patients, it was chosen as the best measurement for heterogeneity analysis in this paper [25]. Other measures of emphysema could be used with the same heterogeneity equations.
Conclusion
On detailed CT analysis, the spatial distribution of emphysema is heterogeneous through the whole lung in the majority of severe emphysematous patients. Further studies are needed to understand why some patients develop disease in a particular pattern. Importantly, the pattern of emphysema heterogeneity could be used to indicate which LVR treatment approach and LVR technique will benefit the patient most. LVR techniques that are able to target individual segments provide a broader range of options in terms of determining how much and which portions of a lobe or lung to reduce per procedure. This may prove beneficial especially in patients with intralobar heterogeneity. LVR techniques that are limited to targeting an entire lobe per procedure may unintentionally target and reduce less diseased portions of the lobe during treatment.
Acknowledgments
We would like to acknowledge and thank the COPDGene committee for approval of the study presented (grant Nos. R01 HL089856 and R01 HL089897).
References
- 1.O’Donnell DE, Laveneziana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev. 2006;15:61–67. [Google Scholar]
- 2.Sanchez PG, Kucharczuk JC, Su S, Kaiser LR, Cooper JD. National Emphysema Treatment Trial redux: accentuating the positive. J Thorac Cardiovasc Surg. 2010;140:564–572. doi: 10.1016/j.jtcvs.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 3.Madani A, Keyzer C, Gevenois P. Quantitative computed tomography assessment of lung structure and function in pulmonary emphysema. Eur Respir J. 2001;18:720–730. doi: 10.1183/09031936.01.00255701. [DOI] [PubMed] [Google Scholar]
- 4.Weder W, Thurnheer R, Stammberger U, Bürge M, Russi EW, Bloch KE. Radiologic emphysema morphology is associated with outcome after surgical lung volume reduction. Ann Thorac Surg. 1997;64:313–319. doi: 10.1016/S0003-4975(97)00564-X. discussion 319–320. [DOI] [PubMed] [Google Scholar]
- 5.Valipour A, Herth FJF, Eberhardt R, Shah PL, Gupta A, Barry R, Henne E, Bandyopadhyay S, Snell G. Design of the randomized, controlled sequential staged treatment of emphysema with upper lobe predominance (STEP-UP) study. BMC Pulm Med. 2014;14:190. doi: 10.1186/1471-2466-14-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sciurba FC, Ernst A, Herth FJF, Strange C, Criner GJ, Marquette CH, Kovitz KL, Chiacchierini RP, Goldin J, McLennan G. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–1244. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 7.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, MacNee W, Calverley P, Rennard S, Wouters E, Wedzicha JA. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 8.Agusti A, Calverley PMA, Celli B, Coxson HO, Edwards LD, Lomas DA, MacNee W, Miller BE, Rennard S, Silverman EK, Tal-Singer R, Wouters E, Yates JC, Vestbo J. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. doi: 10.1186/1465-9921-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watz H, Waschiki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33:262–272. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 10.Mahler DA, Ward J, Waterman LA, McCusker C, Zuwallack R, Baird JC. Patient-reported dyspnea in COPD reliability and association with stage of disease. Chest. 2009;136:1473–1479. doi: 10.1378/chest.09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuhmann M, Raffy P, Yin Y, Gompelmann D, Oguz I, Eberhardt R, Hornberg D, Heussel CP, Wood S, Herth FJF. Computed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with Chartis. Am J Respir Crit Care Med. 2015;191:767–774. doi: 10.1164/rccm.201407-1205OC. [DOI] [PubMed] [Google Scholar]
- 13.Heremans A, Verschakelen JA, Demedts M. Measurement of lung density by means of quantitative CT scanning. A study of correlations with pulmonary function tests. Chest. 1992;102:805–811. doi: 10.1378/chest.102.3.805. [DOI] [PubMed] [Google Scholar]
- 14.Bandyopadhyay S, Henne E, Gupta A, Barry R, Snell G, Strange C, Herth FJF. Segmental approach to lung volume reduction therapy for emphysema patients. Respiration. 2015;89:76–81. doi: 10.1159/000369036. [DOI] [PubMed] [Google Scholar]
- 15.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi T, Reed A, Oto T, Holsworth L, Ellis S, Bailey MJ, Williams TJ, Snell G. Relation of interlobar collaterals to radiological heterogeneity in severe emphysema. Thorax. 2006;61:409–413. doi: 10.1136/thx.2005.051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herth FJF, Noppen M, Valipour A, Leroy S, Vergnon J, Ficker JH, Egan JJ, Gasparini S, Agusti C, Holmes-Higgin D, Ernst A. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012;39:1334–1342. doi: 10.1183/09031936.00161611. [DOI] [PubMed] [Google Scholar]
- 18.Herth FJF, Gompelmann D, Ernst A, Eberhardt R. Endoscopic lung volume reduction. Respiration. 2009;79:5–13. doi: 10.1159/000256510. [DOI] [PubMed] [Google Scholar]
- 19.Shah PL, Hopkinson NS. Bronchoscopic lung volume reduction for emphysema: where next? Eur Respir J. 2012;39:1287–1289. doi: 10.1183/09031936.00217411. [DOI] [PubMed] [Google Scholar]
- 20.Gompelmann D, Eberhardt R, Herth F. Endoscopic volume reduction in COPD – a critical review. Dtsch Arztebl Int. 2014;111:827–833. doi: 10.3238/arztebl.2014.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storbeck B, Schröder TH, Oldigs M, Rabe KF, Weber C. Emphysema: imaging for endoscopic lung volume reduction. Rofo. 2015;187:543–554. doi: 10.1055/s-0034-1399424. [DOI] [PubMed] [Google Scholar]
- 22.Dirksen A, Dijkman JH, Madsen F, Stoel B, Hutchison DCS, Ulrik CS, Skovgaard LT, Kok-Jensen A, Rudolphus A, Seersoholdm N, Vrooman HA, Reiber JHC, Hansen NC, Heckscher T, Viskum K, Stolk J. A randomized clinical trial of α1-antitrypsin augmentation therapy. Am J Respir Crit Care Med. 1999;160:1468–1472. doi: 10.1164/ajrccm.160.5.9901055. [DOI] [PubMed] [Google Scholar]
- 23.Bakke PS, Baste V, Hanoa R, Gulsvik A. Prevalence of obstructive lung disease in a general population: relation to occupational title and exposure to some airborne agents. Thorax. 1991;46:863–870. doi: 10.1136/thx.46.12.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 25.Doellinger F, Huebner RH, Kuhnigk JM, Poellinger A. Lung volume reduction in pulmonary emphysema from the radiologist’s perspective. Rofo. 2015;187:662–675. doi: 10.1055/s-0034-1399540. [DOI] [PubMed] [Google Scholar]