Abstract
Introduction
Liver fibrosis develops when hepatic stellate cells (HSC) are activated into collagen-producing myofibroblasts. In non-alcoholic steatohepatitis (NASH), the adipokine leptin is upregulated, and promotes liver fibrosis by directly activating HSC via the hedgehog pathway. We reported that hedgehog-regulated osteopontin (OPN) plays a key role in promoting liver fibrosis. Herein, we evaluated if OPN mediates leptin-profibrogenic effects in NASH.
Methods
Leptin-deficient (ob/ob) and wild-type (WT) mice were fed control or methionine-choline deficient (MCD) diet. Liver tissues were assessed by Sirius-red, OPN and αSMA IHC, and qRT-PCR for fibrogenic genes. In vitro, HSC with stable OPN (or control) knockdown were treated with recombinant (r)leptin and OPN-neutralizing or sham-aptamers. HSC response to OPN loss was assessed by wound healing assay. OPN-aptamers were also added to precision-cut liver slices (PCLS), and administered to MCD-fed WT (leptin-intact) mice to determine if OPN neutralization abrogated fibrogenesis.
Results
MCD-fed WT mice developed NASH-fibrosis, upregulated OPN, and accumulated αSMA+ cells. Conversely, MCD-fed ob/ob mice developed less fibrosis and accumulated fewer αSMA+ and OPN+ cells. In vitro, leptin-treated HSC upregulated OPN, αSMA, collagen 1α1 and TGFβ mRNA by nearly 3-fold, but this effect was blunted by OPN loss. Inhibition of PI3K and transduction of dominant negative-Akt abrogated leptin-mediated OPN induction, while constitutive active-Akt upregulated OPN. Finally, OPN neutralization reduced leptin-mediated fibrogenesis in both PCLS and MCD-fed mice.
Conclusion
OPN overexpression in NASH enhances leptin-mediated fibrogenesis via PI3K/Akt. OPN neutralization significantly reduces NASH fibrosis, reinforcing the potential utility of targeting OPN in the treatment of patients with advanced NASH.
Keywords: Fibrosis, Hepatic, Adipokine Adipokine
1. Introduction
Individuals with nonalcoholic steatohepatitis (NASH), an advanced stage of nonalcoholic fatty liver disease (NAFLD), are at risk of disease progression that leads to liver fibrosis, cirrhosis, and cancer [1–4]. The mechanisms which drive NASH progression are not entirely under-stood, but studies suggest that liver cell injury from lipotoxicity and oxidative stress triggers a fibrogenic repair response. Liver fibrosis develops when this injury-associated repair response becomes excessive or deregulated [5–6].
The hepatic stellate cell (HSC) or liver pericyte is the major source of extracellular matrix, and thus, the key effector of the repair response [7]. When activated by cytokines (transforming growth factor β), growth factors (connective tissue growth factor), or morphogens (hedgehog (Hh), Wnt), quiescent HSC transition into motile, collagen-secreting myofibroblasts [8]. HSCs are also capable of responding to circulating neurotransmitters and hormones [9,10]. Leptin is an adipocyte-derived hormone which, not only controls energy balance and food in-take, but also modulates the liver repair response [11–13]. Binding of leptin to HSC surface receptors for instance, upregulates key fibrogenic genes αSMA and collagen 1α1, while mice deficient in leptin (i.e. ob/ob mice) are protected against liver fibrosis [11,14,15]. In humans, serum and tissue leptin levels are highly elevated in the obese [16–18], helping to explain at least in part, why obese, NAFLD individuals are more at risk of developing liver fibrosis.
Recently, we reported that leptin-induced liver fibrosis occurs via activation of the Hh pathway [19] (a key morphogenic signal that regulates development as well as adult tissue inflammation and fibrosis), and that upregulation of the Hh ligand and downstream target genes requires induction of the PI3K/Akt signaling. Hh pathway activation promotes transition of quiescent HSC into activated myofibroblasts [20], while inhibition of Hh reverses this phenotype. Similarly, the addition of LY294002 (a PI3K inhibitor) or cyclopamine (a specific Hh pathway inhibitor) to leptin-treated HSC blocked all actions of leptin, thus revealing that the PI3K–Hh axis is necessary for leptin to exert its fibrogenic effects.
Osteopontin (OPN) is a pro-inflammatory cytokine and matrix protein that is highly expressed in a variety of inflamed tissues, and plays a critical role in tissue repair [21,22]. OPN levels are upregulated in tissue and blood of patients with chronic liver disease, and have been shown to activate liver progenitors and HSC [23]; functionally resembling Hh. Interestingly, mice with excessive Hh pathway activity express more liver OPN and develop worse liver fibrosis, while inhibition of Hh signaling (with cyclopamine) in HSC represses OPN levels [24]. These results demonstrate that OPN is a downstream effector of Hh, and led us to hypothesize that OPN and leptin may interact in HSC to promote NASH progression.
Using a combination of HSC cultures and diet-induced model of NASH, we confirm that OPN is a downstream effector of leptin, and is required for fibrotic outcomes in NASH. These results are clinically important because humanized antibodies and aptamers which inhibit OPN actions have been developed, and may be used to treat patients with advanced NASH.
2. Methods
2.1. Mice
Lean (WT) and obese (ob/ob) mice (males, C57BL/6 background) were obtained from Jackson Laboratories (Bar Harbor, ME). To induce liver injury and fibrosis, ob/ob and WT mice (n = 5/group) were fed methionine choline-deficient (MCD) diets (MP Biomedicals, Solon, OH) for a total of 8 weeks. In the control arm, ob/ob and WT mice (n = 5/group) were permitted ad libitum consumption of water and control chow (MP Biomedicals, Solon, OH) [23,24].
In a separate study, WT mice were fed the MCD diet for 6 weeks. Sham-aptamers or OPN-specific aptamers (see below) were administered from week 4, via daily tail-vein injection (200 µg/injection/mouse) [25,26].
At the end of treatments, mice were sacrificed and livers harvested for qRTPCR and immunohistochemistry. Animal care and procedures were as per the NIH “Guide for the Care and Use of Laboratory Animals”, and approved by relevant institutions: Duke University Institutional Animal Care and Use Committees, and the University of Calgary Animal Care Committee.
2.2. Immunohistochemistry
Liver tissue was fixed in formalin and embedded in paraffin. To quantify liver fibrosis, five micron sections were stained with picrosirius red (Sigma, St. Louis, MO) and counterstained with fast green (Sigma, St. Louis, MO). Immunohistochemical staining to detect OPN and αSMA was performed using the DAKO Envision System (DAKO Corporation) according to the manufacturer's protocol. Immunostaining was performed as previously described [23]. Antigen retrieval was performed by heating in 10 mM sodium citrate buffer (pH 6.0) or incubating with pepsin (00-3009; Invitrogen). Primary antibodies used were: anti-OPN (R&D; AF808) and anti-αSMA (Abcam; ab32575). Double immunohistochemistry was performed using Vina Green, according to the manufacturer's recommendation (Bio-Care Medical). Negative controls included liver specimens exposed to 1% bovine serum albumin instead of the respective primary antibodies. The proportion of tissue stained with picrosirius red (Sirius-Red) content, αSMA, and OPN were assessed by morphometric analysis with MetaView software (Universal Imaging Corp, Downtownington, PA) as previously described [23]. For morphometric quantification, 50 randomly chosen, 20× fields per section were evaluated for each mouse.
2.3. Rat HSC isolation
Primary HSCs were isolated from Sprague–Dawley rats, assessed for purity and viability, and seeded at a density of 3 × 102 cells/mm2 in DMEM supplemented with 10% fetal bovine serum(FBS) and penicillin (50 U/ml)/streptomycin (50 µg/ml) [20]. For studies that involved treatments with leptin (100 ng/ml; R&D systems, Minneapolis, MN) and/or adenoviral vectors, day 7 HSC cultures were cultured overnight in serum-depleted medium (0.1% FBS) before treatments were initiated.
2.4. Adenoviral transduction of primary rat HSC
Ad5GFP, which contains the GFP gene driven by the cytomegalovirus promoter, was used as a control virus. The Ad5dnAkt and Ad5myrAkt viruses express the dominant negative and activated forms of Akt, respectively [19]. Pilot studies demonstrated that maximally efficient transduction occurred at a multiplicity of infection of 100. Subsequent experiments were carried out with this multiplicity of infection for 24 h; virus-containing medium was then aspirated and replaced with fresh medium.
2.5. HSC line and treatments
The mouse HSC line (GRX) was maintained in DMEM, 5% FBS, and 1% penicillin/streptomycin as described [27]. Cells were treated with recombinant leptin (100 ng/ml; R&D systems, Minneapolis, MN) for 48 h. For neutralization of OPN, 40% confluent cells were treated with OPN-specific aptamers or biologically-inactive mutant sham aptamers (6.66 µg/ml). For PI3K inhibition, LY294002 (25 µM; Cell Signalling Technology, Danvers, MA) was applied to cells 30 min prior to treatment. AG490 (50 µM; Tocris Biosciences, Ellisville, MO) was used to inhibit JAK signaling [19].
2.6. shRNA-mediated OPN knockdown
GRX were seeded in 24-well plates, 5 × 104 per well, serum-starved (0.1% FBS/DMEM), and treated with 5 µg/ml polybrene (Santa Cruz, Dallas TX) in low-serum media (0.1% FBS/DMEM) 24 h prior to infection. Cells were trypsinized and suspended in 250 µl media, and treated with 4 µl lentiviral particles (2 × 104 IFU) containing shRNA constructs specifically targeting OPN (Santa Cruz sc-36130-V) or non-targeting scrambled control shRNA (Santa Cruz sc-108080). The plates containing cell and viral particle suspensions were immediately centrifuged at 750 ×g for 30 min at 25 °C, and placed in the incubator for 48 h. After the infection period, cells were then split, allowed to recover for 24 h, then subjected to 8 µg/ml puromycin selection for 72 h.
2.7. Transmigration and wound healing assays
For transmigration assays, cells were cultured in the upper chamber of a 24-well transwell system (3 µm membrane, Nunc™ Polycarbonate Membrane Inserts, Thermo Fisher Scientific, Loughborough, UK). After 24 h, the upper side of the membrane was gently wiped with a cotton bud to remove non-migrated cells, and the membrane was stained with crystal violet solution (1% crystal violet, 25% methanol) for 10 min, washed in PBS and allowed to air dry. Cells migrated to the bottom side of the membrane were visualized on an inverted micro-scope, and quantified using the average number of migrated cells per 15 randomly-selected fields.
Standard wound healing assays were performed by growing cells to a confluent monolayer, and making a manual scratch using a P200 pipette tip. A reference mark was made on the wound and photo-graphed at time 0, and compared 12 h later. Wound diameters were quantified using NIH Image J version 1.48v (Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, http://imagej.nih.gov/ij/, 1997–2012).
2.8. Semi-quantitative real-time PCR
Total RNA was extracted from cell cultures using TRIzol® (Life Technologies, Carlsbad, CA) according to manufacturer's instructions. RNA (1 µg) was reverse transcribed to cDNA templates using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). For semiquantitative RT-PCR, cDNA (25 ng) was amplified using SYBR® Green PCR Master Mix (Life Technologies) and oligonucleotide primers for specific targets sequences on an Applied Biosystems 7500 Real-Time PCR system. RT-PCR parameters were as follows: denaturating at 95 °C for 10 min, followed by 40 cycles of denaturing at 95 °C for 15 s and annealing/extension at 60 °C for 60 s. Threshold cycles (Ct)were automatically calculated by the system software. Target gene levels were determined relative to the S9 ribosomal protein housekeeping gene using the 2−ΔΔCt method. Primer sequences are listed in (Supplementary Table 1).
2.9. Precision cut liver slice organ culture
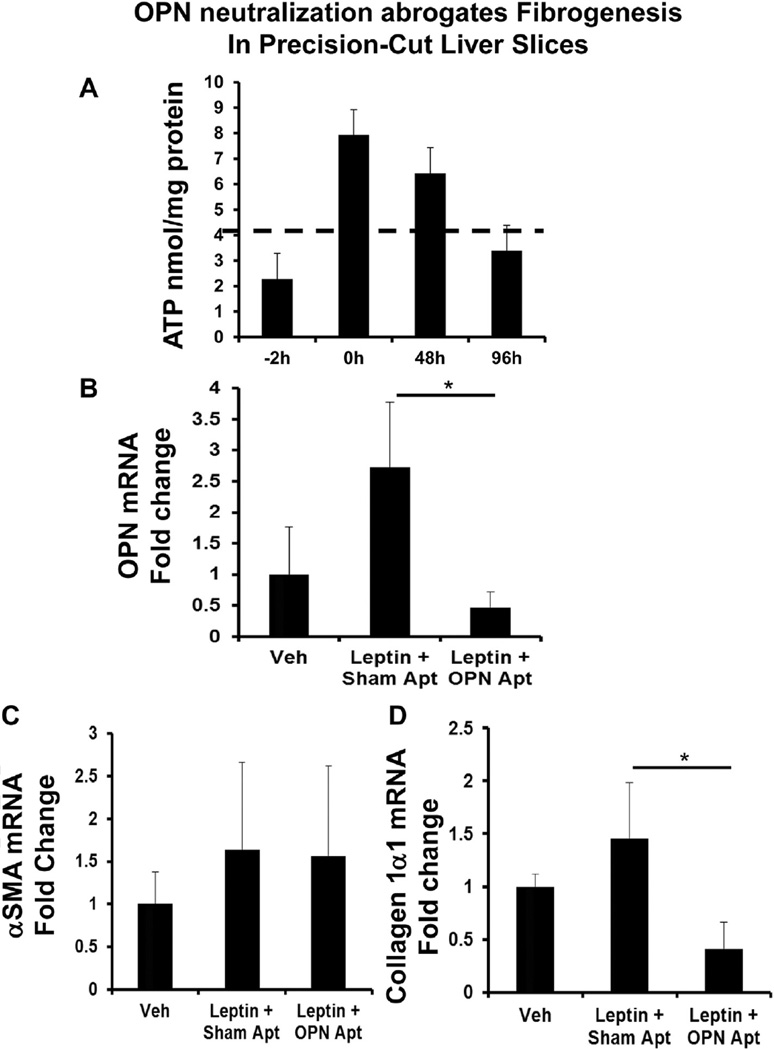
In order to study the impact of OPN neutralization in the intact liver we used a Krumdieck Tissue Slicer (TCS Biologicals) to cut aseptic, 250 µm thick slices of live liver tissue, which could be studied for up to 48 h ex-vivo. Liver tissue was incubated in Williams E media (Sigma) supplemented with 2% FCS, 0.1 µM dexamethasone (Sigma) and 0.5 µM insulin (Novo-Nordisk). Tissues were stimulated with recombinant leptin, in the presence of sham-aptamers or OPN-specific aptamers. At the end of treatment, liver slices were collected for RNA analysis by real-time PCR. Cell viability was evaluated by measuring the content of ATP with the ATP Bioluminescence Assay Kit CLS II (Roche, Basel, Switzerland), normalized against the total amount of proteins quantified by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). The threshold of viability is 4 nmol/mg protein of ATP measured 2 h after recovery (i.e. time 0 h in Fig. 5A).
Fig. 5.
OPN neutralization abrogates fibrogenesis in precision-cut liver slices (PCLS). Precision-cut, viable mouse liver slices (PCLS) (a liver organ culture system) were stimulated with leptin (or vehicle), in the presence of sham- or OPN-specific aptamers for 48 h. At the end of treatment, liver slices were harvested, viability assessed by ATP content, and RNA analyzed by qRTPCR. (A) ATP content (viability) in nmol/mg protein; hashed line denotes the accepted viability threshold. (B) OPN mRNA. (C) αSMA mRNA. (D) Collagen 1α1 mRNA. Results are expressed as fold changes relative to vehicle-treated PCLS, and graphed as mean ± SEM. *p < 0.05 vs. vehicle.
2.10. Statistical analysis
Results are expressed as means ± SEM. Significance was established using the Student's t-test and analysis of variance. Differences were considered significant when p < 0.05.
3. Results
3.1. Leptin-deficient (ob/ob) livers contain less Osteopontin and are less fibrotic than control (WT) livers after MCD treatment
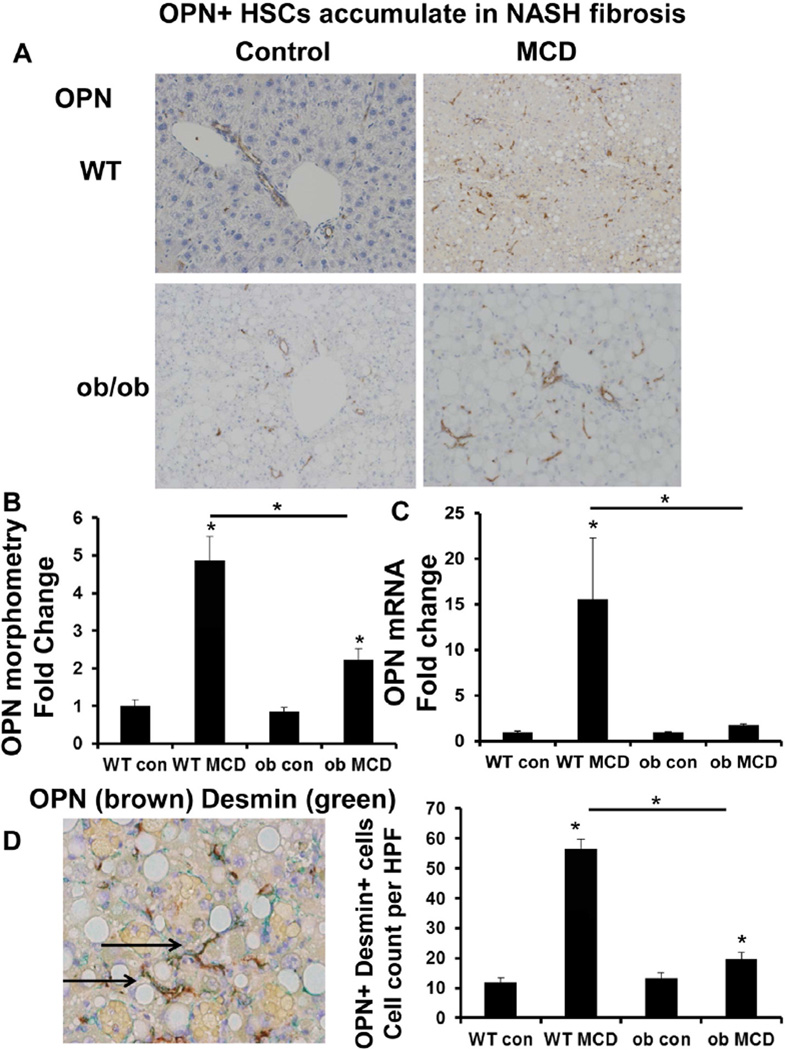
Chronic exposure to the MCD diet induced comparable epithelial cell injury in WT (leptin-intact) and ob/ob (leptin-deficient) mice [28,29]. WT mice developed significant liver fibrosis in response to the MCD diet, as evidenced by ~6-fold accumulation of Sirius red stained collagen fibrils (Suppl Fig. 1A–C), ~2.5-fold increase in hepatic hydroxyproline content (Suppl Fig. 1D), and a 4-fold upregulation in collagen 1α1 mRNA (Suppl Fig. 1E). Collagen deposition was accompanied by the accumulation of αSMA+cells (Suppl Fig. 2A–B), OPN+ cells (Fig. 1A–B), and induction of key fibrogenic genes, αSMA (~4-fold) (Suppl Fig. 2C), TGF-β1 (~3-fold) (Suppl Fig. 2D), and OPN (greater than 10-fold) (Fig. 1C).
Fig. 1.
Osteopontin (OPN)-expressing hepatic stellate cells (HSC) accumulate in non-alcoholic steatohepatitis (NASH) fibrosis. Wild type (WT) and ob/ob (leptin deficient) mice were fed control chow or the methionine choline deficient (MCD) diet for 8 weeks. Livers were harvested for IHC and qRTPCR. Representative staining are shown. (A) OPN staining. (B) OPN morphometry. (C) OPN mRNA; results are expressed as fold change relative to WT control mice. (D) OPN (brown) and Desmin (marker of HSC) (green)—double immunostaining (magnification ×400); graph shows number of OPN/Desmin double-positive cells per HPF (quantified by cell counting); black arrows indicate cells which co-express OPN and Desmin. All results are graphed as mean ± SEM; *p < 0.05 vs. WT control mice or otherwise indicated.
By contrast, fibrosis was significantly less in ob/ob mouse livers after the MCD diet (Suppl Fig. 1): ob/ob livers contained nearly 50% fewer Sirius red stained collagen fibrils (Suppl Fig. 1A–C), ~2.5-fold less liver hydroxyproline (Suppl Fig. 1D), ~50% less TGF-β1 mRNA, and exhibited minimal induction of collagen 1α1, αSMA and OPN mRNA (Suppl Fig. 1E, 2C, 2D, and Fig. 1C). The degree of weight loss however, was similar between (WT and ob/ob) MCD-fed groups. These findings confirm that leptin directly promotes fibrogenesis in MCD-induced NASH [11,19,28].
3.2. OPN is a downstream effector of Leptin in Hepatic Stellate Cells (HSC)
Leptin is a pro-fibrogenic adipokine which directly activates HSC, the liver pericyte responsible for collagen matrix deposition during chronic injury [11,12]. Recently, we reported that OPN, a matrix protein and cytokine, could also promote HSC activation [23,24]. In this study, the loss of circulating leptin was directly associated with repressed liver OPN (Fig. 1), and attenuated liver fibrogenesis (Suppl Fig. 1, 2). These observations led us to hypothesize that leptin could regulate OPN expression in HSC.
Recent studies revealed that HSC isolated from livers subjected to biliary or chemical injury upregulated OPN mRNA (Suppl Fig. 3) [30]. In support, we confirmed by double immunohistochemistry that desmin (+) HSC co-expressed OPN (Fig. 1D). Importantly, the number of desmin/OPN double (+) HSC accumulated by more than 5-fold in MCD-fed mice, but this increase was almost abolished in ob/ob-MCD mice.
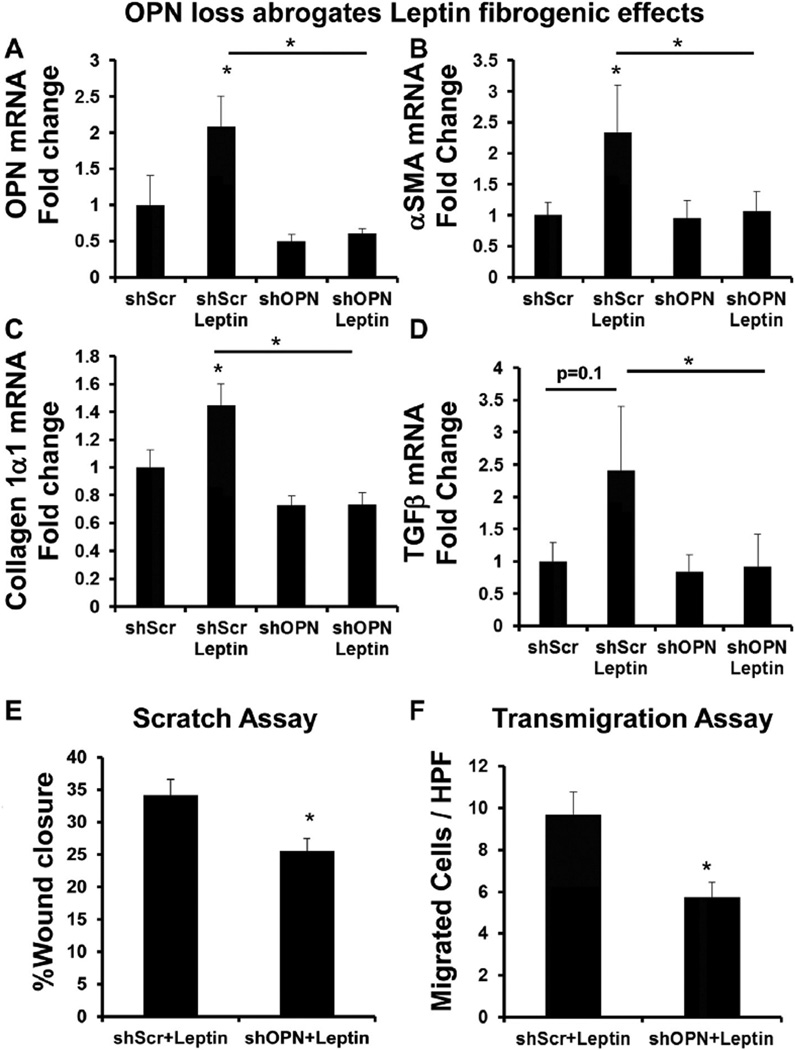
To determine if leptin was directly influencing OPN expression in HSC, we treated the mouse HSC line, GRX, with recombinant leptin (rleptin) for 48 h; cells were then harvested for RNA analysis by qRTPCR. rLeptin activated HSC (Suppl Fig. 4), upregulated OPN, and key fibrogenic markers, αSMA, collagen 1α1, TGFβ (~2-fold) (Fig. 2A–D). We next investigated if loss of OPN would inhibit leptin fibrogenic effects. To this end, GRX were treated with lentiviral particles which contain shRNA constructs specifically targeting OPN (GRX-shOPN). Compared with control (GRX-shScr) (GRX infected with lentiviral particles containing non-targeting scrambled shRNA), OPN knockdown (~80%) (Suppl Fig. 5) significantly abrogated leptin fibrogenic effects by >90% (Fig. 2). As increased cell migration is an important feature of activated HSC, we further examined if OPN knockdown (under leptin-stimulated conditions) would impair HSC migratory properties. Migration was assessed by semi-quantitating the dimensions of a wound dividing the confluent monolayer 12 h after the scratch. OPN knockdown led to ~15% less wound healing compared with controls (p < 0.05) (Fig. 2E). Comparable results observed in the modified cell-invasion assay (Fig. 2F). OPN knockdown led to 50% fewer GRX cells invading across the insert membrane.
Fig. 2.
OPN loss abrogates leptin fibrogenic effects in HSC. HSC (mouse GRX line) with OPN knockdown (shOPN) and control HSC (shScr) were treated with recombinant leptin (or vehicle) for 48 h. Cells were then harvested and RNA analyzed by qRTPCR. (A) OPN mRNA. (B) αSMA mRNA. (C) Collagen 1α1 mRNA. (D) TGFβ mRNA. Results are expressed as fold changes relative to shScr, and graphed as mean ± SEM. Wound-healing and transmigration studies in shOPN or shScr HSC were performed under leptin-treated conditions (E–F). (E) Wound healing; migration was quantified by measuring the distance dividing the two sides of the monolayer. Mean (% wound closure) ± SEM were graphed. (F) Transmigration was evaluated 24 h after seeding of cells, by counting crystal violet-stained cells on the underside membrane in 15 random HPF. Mean cell numbers ± SEM were graphed. *p < 0.05 vs shScr or otherwise indicated.
We verified these results using OPN-specific aptamers which neutralize circulating OPN [25,26], in the presence of rleptin. OPN neutralization blunted αSMA expression (Suppl Fig. 6A), and significantly repressed collagen 1α1 and TGFβ mRNA (~50%) (Suppl Fig. 6B–C). Similar to knockdown experiments, OPN neutralization also inhibited migratory properties of HSC, leading to impaired wound healing (~30%) and transmigration (~50%) across membranes (Suppl Fig. 6D–E). In aggregate, these results demonstrate that OPN is a downstream effector of leptin-induced fibrogenesis, and that OPN is a potential anti-fibrotic target.
3.3. OPN expression is regulated by Leptin-PI3K/Akt signaling in HSC
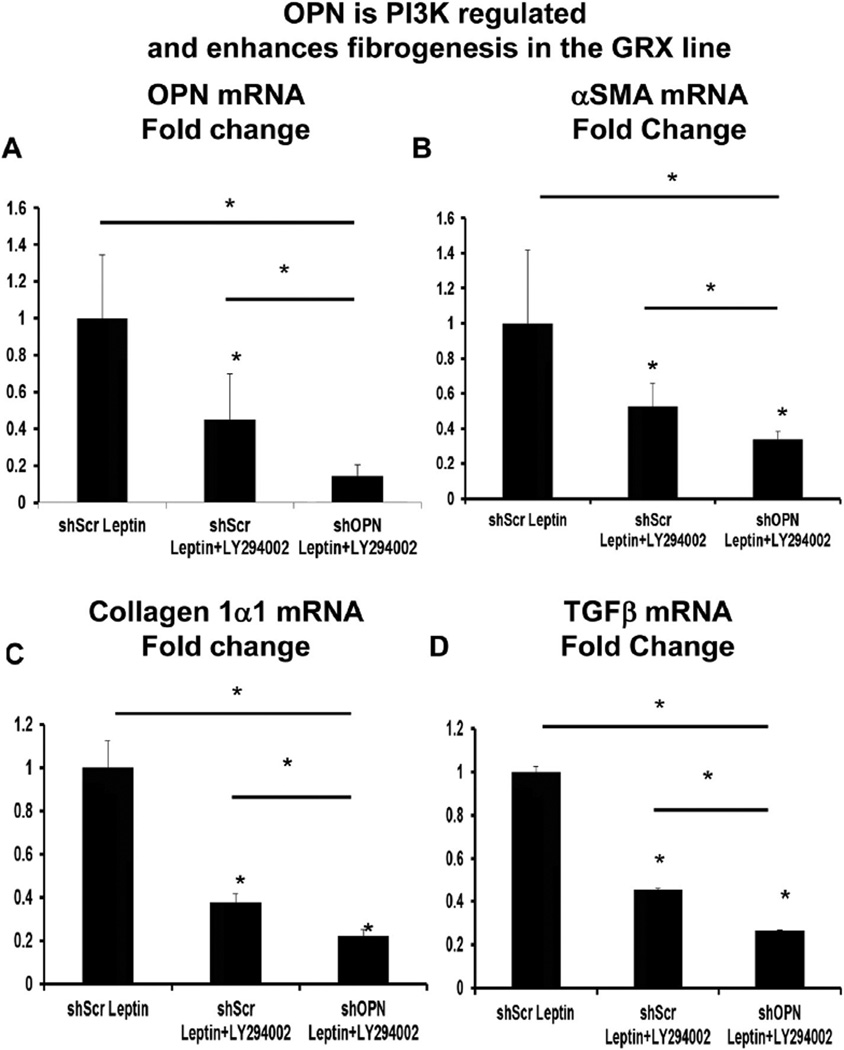
Leptin receptors couple with PI3K and activate Akt, which is required for leptin-associated fibrogenic effects [31,32]. Leptin activates the hedgehog (Hh) pathway (an important morphogenic pathway during development and adult fibrogenesis) in HSC, but this is blocked by LY294002, a PI3K inhibitor [19]. Because OPN is a downstream target of the Hh pathway [24], we investigated if OPN could similarly be regulated by the PI3K/Akt pathway. GRX HSCs were treated with leptin in the presence or absence of LY294002. LY294002 significantly repressed OPN mRNA by >50% (Fig. 3A), and downregulated fibrogenic genes αSMA, collagen 1α1, and TGFβ by up to 60% (Fig. 3B–D). OPN knockdown in combination with LY294002 further enhanced repression of fibrogenic genes by an additional ~20% (Fig. 3).
Fig. 3.
OPN is PI3K regulated and enhances fibrogenesis in GRX HSC line. HSC (mouse GRX line) with OPN knockdown (shOPN) and control HSC (shScr) were treated with recombinant leptin, with or without LY294002 (a PI3K inhibitor). Cells were then harvested and RNA analyzed by qRTPCR. (A) OPN mRNA. (B) αSMA mRNA. (C) Collagen 1α1 mRNA. (D) TGFβ mRNA. Results are expressed as fold changes relative to shScr-Leptin, and graphed as mean ± SEM. *p < 0.05 vs. shScr-Leptin.
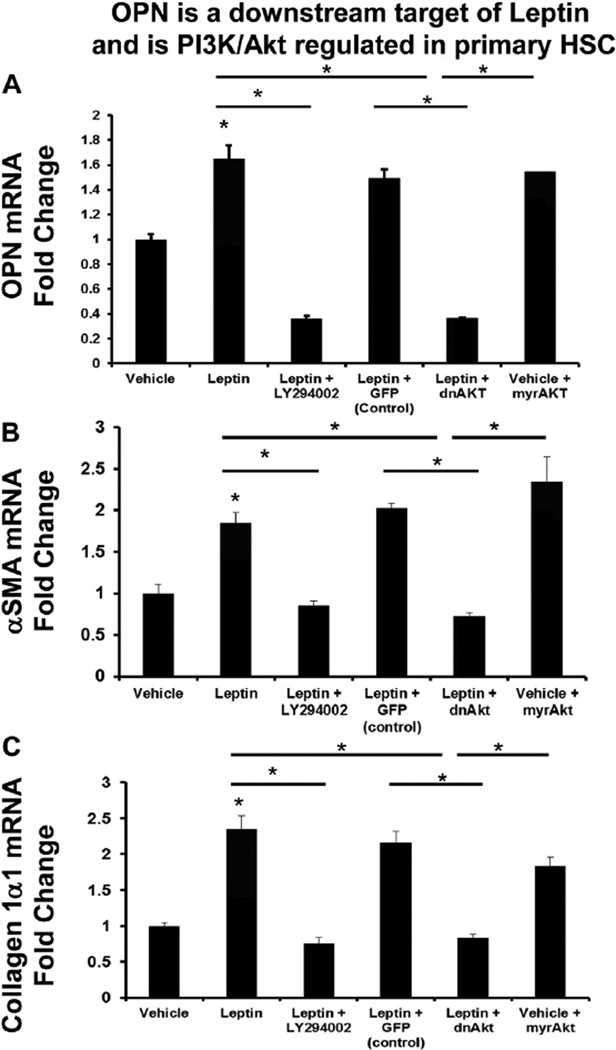
To exclude the possibility that observed responses were GRX-specific, we repeated experiments using freshly isolated rat HSC. Comparable with GRX HSCs, leptin-treated, primary rat HSC upregulated OPN, αSMA, and collagen 1α1 mRNA by ~2-fold (Fig. 4A–C), but this effect was completely blocked in the presence of LY294002. OPN mRNA in particular, was repressed below basal expression. Adenoviral-mediated transfer of dominant negative Akt (dnAkt) similarly inhibited leptin-mediated changes in OPN, αSMA, and collagen 1α1 mRNA (Fig. 4A–C), but infection with mock adenoviral vectors (AdGFP; control) did not. By contrast, infection with the constitutively active Akt vector (Ad5myrAkt) induced OPN, αSMA, and collagen 1α1 mRNA to levels comparable with leptin-treated cells (Fig. 4).
Fig. 4.
OPN is a downstream target of leptin and is PI3K/Akt regulated in primary HSC. Freshly isolated HSC were treated with leptin (or vehicle), with or without LY294002. Additional HSC were also pre-treated with Ad5dnAkt (or Ad5GFP) prior to treatment with leptin (100 ng/ml) or Ad5mryAkt alone. RNA was isolated and changes in gene expression evaluated by qRT-PCR. (A) OPN mRNA. (B) αSMA mRNA. (C) Collagen 1α1 mRNA. Results are expressed as fold changes relative to vehicle-treated HSC, and graphed as mean ± SEM. *p < 0.05 vs. vehicle.
Previous studies suggested that leptin-mediated induction of the JAK/STAT pathway influences HSC fibrogenesis [11,23]. To clarify the role of leptin-mediated JAK/STAT signaling in modulating OPN expression, primary rat HSC were also treated with leptin in the presence or absence of AG490 (an inhibitor of JAK signaling). Compared with DMSO control, pre-treatment with AG490 inhibited OPN mRNA by ~30%. However this effect was significantly less pronounced than LY294002 treatment (Suppl Fig. 7). These data in aggregate confirm that OPN expression in HSC is predominantly leptin-PI3K/Akt-regulated.
3.4. OPN neutralization inhibits leptin-associated fibrogenesis in precision-cut liver slices (PCLS) and in a mouse model of diet-induced NASH
To validate in vitro findings, we used an established liver organ culture system in which precision-cut, viable mouse liver slices (PCLS) were stimulated with leptin, in the presence of sham- or OPN-specific aptamers. PCLS are useful because they can be cultured for several days and better reflect the multicellular in vivo conditions [33,34]. Liver slice viability was maintained as evidenced by the MTT assay (Fig. 5A). Treatment with exogenous leptin upregulated liver slices OPN mRNA by ~2-folds (Fig. 5B), and induced αSMA and collagen 1α1 mRNA by ~20% (Fig. 5C–D). Conversely, neutralizing OPN significantly repressed OPN and collagen 1α1mRNA, even beyond basal levels. No change in total slice αSMA mRNA was detected.
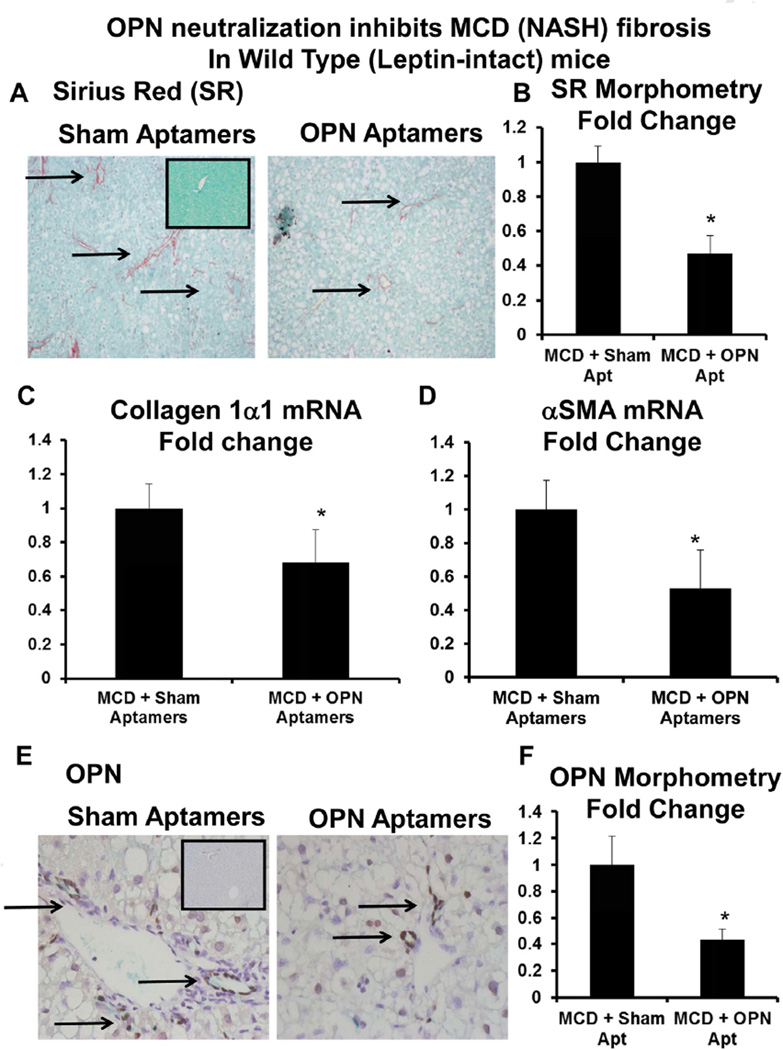
Having demonstrated that OPN is the downstream, fibrogenic effector of leptin, we evaluated the impact of OPN neutralization in MCD-fed, leptin-intact (i.e. WT) mice. MCD-fed mice were administered sham-aptamers or OPN-specific aptamers via tail-vein for 2 weeks. At the end of treatment, livers were harvested and fibrosis was assessed by qRTPCR and IHC. As expected, mice developed NASH-fibrosis after 5 weeks of the MCD diet (Fig. 6 and Suppl Fig. 1A) [23,24]. Consistent with cell culture and PCLS findings, OPN neutralization significantly reduced MCD diet-induced fibrosis, as evidenced by fewer Sirius-Red stained collagen fibrils (Fig. 6A–B), attenuated expression of fibrogenic genes, αSMA, collagen 1α1 (Fig. 6C–D), and reduced liver OPN expression (Fig. 6E–F).
Fig. 6.
OPN neutralization inhibits diet-induced NASH fibrosis in leptin-intact mice. Leptin-intact (WT) mice were fed control chow or the MCD diet for 6 weeks, in the presence of sham or OPN-aptamers. Mice were sacrificed 24 h after final dose of aptamers, and livers analyzed. (A) Representative Sirius-red staining; black arrows indicate Sirius-red stained fibrils. Insert shows Sirius-red staining from a control-fed mouse. (B) Sirius-red morphometry. (C) Collagen 1α1 mRNA. (D) αSMA mRNA. Results are expressed as fold changes relative to sham-aptamer treated mice, and graphed as mean ± SEM. *p < 0.05 vs. sham-aptamer treated mice. (E) Representative OPN staining; black arrows indicated OPN-positive cells. Insert shows OPN staining from control-fed mouse. (F) OPN morphometry.
4. Discussion
Our study confirms that OPN is a key driver of HSC activation, and is an important downstream effector of leptin-induced fibrogenesis. Loss of OPN using OPN-specific aptamers or OPN knockdown abrogates HSC activation despite the presence of exogenous leptin, and these in vitro observations were recapitulated in the ex vivo liver slice model. Importantly, OPN neutralization in a mouse model of diet-induced NASH significantly reduced liver fibrosis, thereby reinforcing the potential utility of targeting OPN in the treatment of patients with advanced stage NASH.
These results are consistent with findings from studies on Hh signaling in liver fibrosis. Both OPN and Hh are leptin-regulated, and are key effectors of leptin-induced liver fibrosis. Although the Hh pathway is recognized for its role in embryonic development, recent studies also show that it regulates adult tissue repair [35]. An over-activation of the Hh pathway leads to tissue fibrosis and cancer, while the loss of Hh signaling impairs wound healing (i.e. less fibrosis) [5,36]. In the liver, we and others recently reported that over-activation of the Hh pathway occurs during human NASH progression [37,38], and that blocking Hh with the commercially available inhibitor, GDC0449 or cyclopamine ameliorates NASH and reduces liver fibrosis in mice [37,38].
The role of OPN in liver inflammation and fibrosis is complex, and has only begun to be unravelled. Outside of the liver, OPN appears to exhibit fairly divergent roles in acute and chronic diseases. In allergic airway disease, OPN is pro-inflammatory during primary systemic sensitization, but anti-inflammatory during secondary antigenic challenge [39]. Similarly, in the lpr model (autoimmune disease that resembles systemic lupus erythematosus), OPN mediates pathology in early disease, but limits exacerbation in late stage disease [40]. In the liver, cumulative evidence suggest that targeting OPN early in disease (i.e. prevention) may result in worsening outcomes [41]. On the other hand, the current data confirms that neutralizing OPN in advanced stage liver disease is beneficial, as demonstrated by the reduced fibrosis in an established model of NASH fibrosis.
The prevalence of NAFLD and NASH is increasing in parallel with the rising tide of obesity and type 2 diabetes mellitus. Studies show that obese individuals and those who harbour additional metabolic risk factors are at a much higher risk of developing NASH and/or NASH-fibrosis [42,43]. Therefore, targeting ‘modifiable’ mediators in these ‘at-risk’ individuals could help alleviate NASH/fibrosis burden. Leptin is an adipose tissue-derived hormone whose physiological role is to induce satiety, but levels of leptin are significantly higher in the obese, and in some with NAFLD [16,17]. To date, studies have evaluated the potential utility of leptin replacement in those with hypoleptinemia-NASH. In one study, leptin administration improved steatosis, but not fibrosis stage [44], and as leptin is directly pro-fibrogenic, it is possible that supplementation of exogenous leptin may inadvertently lead to worsening fibrosis. On the other hand, leptin-neutralization in those with advanced NASH-fibrosis (and excess leptin) may be a useful anti-fibrotic strategy (as observed in our study), although the likely increase in food intake and body weight (and metabolic risk) may well cancel out any potential clinical benefit.
Like leptin, plasma OPN levels are increased in the obese and in patients with type 2 diabetes mellitus, hypertension, and ischemic heart disease [45]. More recently, serum OPN was also found to be associated with the onset of diabetic nephropathy and cardiac events [46]. Thus, high levels of OPN occur in those with the metabolic syndrome, a risk factor for progressive NASH. Importantly, OPN neutralization reduces infiltration of macrophages and immune subsets into adipose tissues, reduces obesity-induced inflammation, and improves systemic glucose tolerance (47–50). In concert with the current findings, these data suggest that OPN could be an important target in the treatment of obese patients with NASH (and the metabolic syndrome), by reducing insulin resistance and limiting NASH-fibrosis (in spite of high prevailing leptin levels).
In summary, our analyses in cell-culture and mice show that OPN is leptin-regulated, and is a key effector of leptin-induced, NASH-fibrosis. Future studies will be necessary to evaluate the utility of OPN neutralization in a different model of obesogenic NASH (such as the high-fat, high fructose/sucrose-fed model), and evaluate if extended periods of treatment could lead to even better anti-fibrotic outcomes.
Supplementary Material
Acknowledgments
The HSC line (GRX) was a kind gift from Professor J Oliveira (Brazil).
Funding
This study was funded predominantly by CORE-UK (WKS), BRET (WKS), EASL (WKS), The Foundation for Liver Research London (WKS), and The Polkemmet Trust (WKS and RW). Additional funding was provided by the National Institute of Health 5K08DK080980 (SSC), R01 DK077794 (AMD), Deutsche Forschungsgemeinschaft (DFG) CA267/8-1 (AC), DFG MA 6864/1-1 (PPM), and the Wilhelm Lapitz Foundation (AC).
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- OPN
osteopontin
- TGF-β
transforming growth factor β
- αSMA
alpha smooth muscle actin
- MCD diet
methionine choline deficient diet
- FFPE
formalin-fixed, paraffin embedded
- HPF
high-powered field
- shScr
short-hairpin RNA against non-target (scrambled) sequence
- shOPN
short-hairpin RNA against OPN sequence
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbadis.2015.10.028.
Disclosures/competing interests
On behalf of authors, I declare no competing interests.
Contributions
JDC, SSC, MSS, PM, EP, RY, MAB, GX, NK, LCC, SB, and LD performed experiments and contributed intellectually to the study; JDC, SSC, MDP, YHO, RW, SC, AMD, ZM, PCK, and SC provided samples and reagents, and contributed to funding. JDC co-wrote the manuscript. WKS is the lead investigator, who designed and supervised the overall project and sub-studies, performed experiments, wrote the manuscript, and is the senior author and guarantor of the manuscript.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- 1.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2015 Apr;13(4):643–654. e1–e9. doi: 10.1016/j.cgh.2014.04.014. quiz e39–40 http://dx.doi.org/10.1016/j.cgh.2014.04.014 (Epub 2014 Apr 24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pais R, Charlotte F, Fedchuk L, et al. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 2013 Sep;59(3):550–556. doi: 10.1016/j.jhep.2013.04.027. http://dx.doi.org/10.1016/j.jhep.2013.04.027 (Epub 2013 May 9). [DOI] [PubMed] [Google Scholar]
- 3.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011 Oct;54(4):1208–1216. doi: 10.1002/hep.24491. http://dx.doi.org/10.1002/hep.24491 (Epub 2011 Aug 9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in US veterans is associated with non-alcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2015 Jul;18 doi: 10.1016/j.cgh.2015.07.019. http://dx.doi.org/10.1016/j.cgh.2015.07.019 (pii: S1542-3565(15)00978–7 [Epub ahead of print]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013 Nov;10(11):656–665. doi: 10.1038/nrgastro.2013.183. http://dx.doi.org/10.1038/nrgastro.2013.183 (Epub 2013 Oct 1). [DOI] [PubMed] [Google Scholar]
- 6.Syn WK, Choi SS, Diehl AM. Apoptosis and cytokines in non-alcoholic steatohepatitis. Clin. Liver Dis. 2009 Nov;13(4):565–580. doi: 10.1016/j.cld.2009.07.003. http://dx.doi.org/10.1016/j.cld.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: concept to treatment. J. Hepatol. 2015 Apr;62(1 Suppl):S15–S24. doi: 10.1016/j.jhep.2015.02.039. http://dx.doi.org/10.1016/j.jhep.2015.02.039 Review. [DOI] [PubMed] [Google Scholar]
- 8.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015 May;64(5):830–841. doi: 10.1136/gutjnl-2014-306842. http://dx.doi.org/10.1136/gutjnl-2014-306842 (Epub 2015 Feb 13. Review. Erratum in: Gut. 2015 Aug;64(8):1337). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena NK, Anania FA. Adipocytokines and hepatic fibrosis. Trends Endocrinol. Metab. 2015 Mar;26(3):153–161. doi: 10.1016/j.tem.2015.01.002. http://dx.doi.org/10.1016/j.tem.2015.01.002 (Epub 2015 Feb 2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oben JA, Roskams T, Yang S, et al. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut. 2004 Mar;53(3):438–445. doi: 10.1136/gut.2003.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean litter-mates of ob/ob mice. Hepatology. 2002 Apr;35(4):762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikejima K, Takei Y, Honda H, et al. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002 May;122(5):1399–1410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- 13.Aleffi S, Petrai I, Bertolani C, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005 Dec;42(6):1339–1348. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- 14.Elinav E, Ali M, Bruck R, et al. Competitive inhibition of leptin signaling results in amelioration of liver fibrosis through modulation of stellate cell function. Hepatology. 2009 Jan;49(1):278–286. doi: 10.1002/hep.22584. http://dx.doi.org/10.1002/hep.22584. [DOI] [PubMed] [Google Scholar]
- 15.Honda H1, Ikejima K, Hirose M, et al. Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology. 2002 Jul;36(1):12–21. doi: 10.1053/jhep.2002.33684. [DOI] [PubMed] [Google Scholar]
- 16.Considine RV, Sinha MK, Heiman M, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996 Feb 1;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 17.Machado MV, Coutinho J, Carepa F, Costa A, Proença H, Cortez-Pinto H. How adiponectin, leptin, and ghrelin orchestrate together and correlate with the severity of nonalcoholic fatty liver Disease. Eur. J. Gastroenterol. Hepatol. 2012 Oct;24(10):1166–1172. doi: 10.1097/MEG.0b013e32835609b0. http://dx.doi.org/10.1097/MEG.0b013e32835609b0. [DOI] [PubMed] [Google Scholar]
- 18.Choi ES, Kim MK, Song MK, et al. Association between serum irisin levels and non-alcoholic fatty liver disease in health screen examinees. PLoS One. 2014 Oct 24;9(10):e110680. doi: 10.1371/journal.pone.0110680. http://dx.doi.org/10.1371/journal.pone.0110680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi SS, Syn WK, Karaca GF, et al. Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J. Biolumin. Chemilumin. 2010 Nov 19;285(47):36551–36560. doi: 10.1074/jbc.M110.168542. http://dx.doi.org/10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi SS, Omenetti A, Witek RP, et al. Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009 Dec;297(6):G1093–G1106. doi: 10.1152/ajpgi.00292.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uede T. Osteopontin, intrinsic tissue regulator of intractable inflammatory diseases. Pathol. Int. 2011 May;61(5):265–280. doi: 10.1111/j.1440-1827.2011.02649.x. http://dx.doi.org/10.1111/j.1440-1827.2011.02649.x (Epub 2011 Mar 8). [DOI] [PubMed] [Google Scholar]
- 22.Kyriakides TR, Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb. Haemost. 2003 Dec;90(6):986–992. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- 23.Coombes JD, Swiderska-Syn M, Dollé L, et al. Osteopontin neutralisation abrogates the liver progenitor cell response and fibrogenesis in mice. Gut. 2015 Jul;64(7):1120–1131. doi: 10.1136/gutjnl-2013-306484. http://dx.doi.org/10.1136/gutjnl-2013-306484 (Epub 2014 Jun 5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syn WK, Choi SS, Liaskou E, et al. Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 2011 Jan;53(1):106–115. doi: 10.1002/hep.23998. http://dx.doi.org/10.1002/hep.23998 (Epub 2010 Oct 21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mi Z, Guo H, Russell MB, Liu Y, Sullenger BA, Kuo PC. RNA aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol. Ther. 2009;17:153–161. doi: 10.1038/mt.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talbot LJ, Mi Z, Bhattacharya SD, Kim V, Guo H, Kuo PC. Pharmacokinetic characterization of an RNA aptamer against osteopontin and demonstration of in vivo efficacy. Surgery. 2011 Aug;150(2):224–230. doi: 10.1016/j.surg.2011.05.015. http://dx.doi.org/10.1016/j.surg.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bitencourt S, Mesquita F, Basso B, et al. Capsaicin modulates proliferation, migration, and activation of hepatic stellate cells. Cell Biochem. Biophys. 2014 Mar;68(2):387–396. doi: 10.1007/s12013-013-9719-0. http://dx.doi.org/10.1007/s12013-013-9719-0. [DOI] [PubMed] [Google Scholar]
- 28.Sahai A, Malladi P, Pan X, et al. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am. J. Physiol. Gastrointest. Liver Physiol. 2004 Nov;287(5):G1035–G1043. doi: 10.1152/ajpgi.00199.2004. (Epub 2004 Jul 15). [DOI] [PubMed] [Google Scholar]
- 29.Machado MV, Michelotti GA, Pereira TA, et al. Accumulation of duct cells with activated YAP parallels fibrosis progression in NonAlcoholic Fatty Liver Disease. J. Hepatol. 2015 Jun;10 doi: 10.1016/j.jhep.2015.05.031. http://dx.doi.org/10.1016/j.jhep.2015.05.031 (pii: S0168-8278(15)00389-X. [Epub ahead of print]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Minicis S, Seki E, Uchinami H, et al. Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology. 2007 May;132(5):1937–1946. doi: 10.1053/j.gastro.2007.02.033. Epub 2007 Feb 21. [DOI] [PubMed] [Google Scholar]
- 31.Saxena NK, Titus MA, Ding X, et al. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J. 2004 Oct;18(13):1612–1614. doi: 10.1096/fj.04-1847fje. (Epub 2004 Aug 19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saxena NK, Sharma D, Ding X, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007 Mar 15;67(6):2497–2507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olinga P, Schuppan D. Precision-cut liver slices: a tool to model the liver ex vivo. J. Hepatol. 2013 Jun;58(6):1252–1253. doi: 10.1016/j.jhep.2013.01.009. http://dx.doi.org/10.1016/j.jhep.2013.01.009 (Epub 2013 Jan 18). [DOI] [PubMed] [Google Scholar]
- 34.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: concept to treatment. J. Hepatol. 2015 Apr;62(1 Suppl):S15–S24. doi: 10.1016/j.jhep.2015.02.039. http://dx.doi.org/10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 35.Petrova R, Joyner AL. Roles for Hedgehog signaling in adult organ homeostasis and repair. Development. 2014 Sep;141(18):3445–3457. doi: 10.1242/dev.083691. http://dx.doi.org/10.1242/dev.083691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu L, Lin X, Lu H, Chen B, Bai Y. An overview of hedgehog signaling in fibrosis. Mol. Pharmacol. 2015 Feb;87(2):174–182. doi: 10.1124/mol.114.095141. http://dx.doi.org/10.1124/mol.114.095141 (Epub 2014 Nov 13). [DOI] [PubMed] [Google Scholar]
- 37.Syn WK, Jung Y, Omenetti A, et al. Hedgehog-mediated epithelial-to- mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterol. 2009 Oct;137(4):1478–1488. doi: 10.1053/j.gastro.2009.06.051. http://dx.doi.org/10.1053/j.gastro.2009.06.051 (e8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirsova P, Ibrahim SH, Bronk SF, Yagita H, Gores GJ. Vismodegib suppresses TRAIL-mediated liver injury in a mouse model of nonalcoholic steatohepatitis. PLoS One. 2013 Jul 22;8(7):e70599. doi: 10.1371/journal.pone.0070599. http://dx.doi.org/10.1371/journal.pone.0070599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xanthou G, Alissafi T, Semitekolou M, et al. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat. Med. 2007 May;13(5):570–578. doi: 10.1038/nm1580. (Epub 2007 Apr 15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber GF, Cantor H. Differential roles of osteopontin/Eta-1 in early and late lpr disease. Clin. Exp. Immunol. 2001 Dec;126(3):578–583. doi: 10.1046/j.1365-2249.2001.01702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorena D, Darby IA, Gadeau AP, et al. Osteopontin expression in normal and fibrotic liver. Altered liver healing in osteopontin-deficient mice. J. Hepatol. 2006 Feb;44(2):383–390. doi: 10.1016/j.jhep.2005.07.024. (Epub 2005 Aug 15). [DOI] [PubMed] [Google Scholar]
- 42.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with non-alcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 43.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J. Clin. Gastroenterol. 2006;40(3 Suppl 1):S5–S10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 44.Safar Zadeh E, Lungu AO, Cochran EK, et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J. Hepatol. 2013 Jul;59(1):131–137. doi: 10.1016/j.jhep.2013.02.007. http://dx.doi.org/10.1016/j.jhep.2013.02.007 (Epub 2013 Feb 21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahles F, Findeisen HM, Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol. Metab. 2014 Mar 22;3(4):384–393. doi: 10.1016/j.molmet.2014.03.004. http://dx.doi.org/10.1016/j.molmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordin D, Forsblom C, Panduru NM, et al. Osteopontin is a strong predictor of incipient diabetic nephropathy, cardiovascular disease, and all-cause mortality in patients with type 1 diabetes. Diabetes Care. 2014 Sep;37(9):2593–2600. doi: 10.2337/dc14-0065. http://dx.doi.org/10.2337/dc14-0065. [DOI] [PubMed] [Google Scholar]
- 47.Nomiyama T, Perez-Tilve D, Ogawa D, et al. Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J. Clin. Invest. 2007 Oct;117(10):2877–2888. doi: 10.1172/JCI31986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiefer FW, Zeyda M, Gollinger K, et al. Neutralization of osteopontin inhibits obesity-induced inflammation and insulin resistance. Diabetes. 2010 Apr;59(4):935–946. doi: 10.2337/db09-0404. http://dx.doi.org/10.2337/db09-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zeyda M, Gollinger K, Todoric J, et al. Osteopontin is an activator of human adipose tissue macrophages and directly affects adipocyte function. Endocrinology. 2011 Jun;152(6):2219–2227. doi: 10.1210/en.2010-1328. http://dx.doi.org/10.1210/en.2010-1328. [DOI] [PubMed] [Google Scholar]
- 50.Kiefer FW, Neschen S, Pfau B, et al. Osteopontin deficiency protects against obesity-induced hepatic steatosis and attenuates glucose production in mice. Diabetologia. 2011 Aug;54(8):2132–2142. doi: 10.1007/s00125-011-2170-0. http://dx.doi.org/10.1007/s00125-011-2170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.