Abstract
Objective:
Combining antidepressants (ADs) for therapy of acute depression is frequently employed, but randomized studies have yielded conflicting results. We conducted a systematic review and meta-analysis aimed at determining efficacy and tolerability of combination therapy.
Methods:
MEDLINE, Embase, PsycINFO, and CENTRAL databases were systematically searched through March 2014 for controlled studies comparing combinations of ADs with AD monotherapy in adult patients suffering from acute depression. The prespecified primary outcome was standardized mean difference (SMD), secondary outcomes were response, remission, and dropouts.
Results:
Among 8688 articles screened, 38 studies were eligible, including 4511 patients. Combination treatment was statistically, significantly superior to monotherapy (SMD 0.29; 95% CI 0.16 to 0.42). During monotherapy, slightly fewer patients dropped out due to adverse events (OR 0.90; 95% CI 0.53 to 1.53). Studies were heterogeneous (I2 = 63%), and there was indication of moderate publication bias (fail-safe N for an effect of 0.1:44), but results remained robust across prespecified secondary outcomes and subgroups, including analyses restricted to randomized controlled trials and low risk of bias studies. Meta-regression revealed an association of SMD with difference in imipramine-equivalent dose. Combining a reuptake inhibitor with an antagonist of presynaptic α2-autoreceptors was superior to other combinations.
Conclusion:
Combining ADs seems to be superior to monotherapy with only slightly more patients dropping out. Combining a reuptake inhibitor with an antagonist of presynaptic α2-autoreceptors seems to be significantly more effective than other combinations. Overall, our search revealed a dearth of well-designed studies.
Keywords: antidepressants, combination therapy, monotherapy, depression
Abstract
Objectif:
Combiner les antidépresseurs pour traiter la dépression aiguë est une méthode fréquemment utilisée, mais les études randomisées ont offert des résultats conflictuels. Nous avons mené une revue systématique et une méta-analyse visant à déterminer l’efficacité et la tolérabilité du traitement combiné ou polythérapie.
Méthodes:
Une recherche des bases de données MEDLINE, Embase, PsycINFO, et CENTRAL a été systématiquement menée jusqu’en mars 2014 pour repérer les études contrôlées comparant les polythérapies d’antidépresseurs avec la monothérapie d’antidépresseur chez des patients adultes souffrant de dépression aiguë. Le résultat principal prédéterminé était la différence des moyennes standardisées (DMS), et les résultats secondaires étaient la réponse, la rémission, et les abandons.
Résultats:
Sur 8688 articles examinés, 38 études étaient admissibles, portant sur 4511 patients. La polythérapie était statistiquement significativement supérieure à la monothérapie (DMS 0,29; IC à 95% 0,16 à 0,42). Durant la monothérapie, un nombre légèrement moindre de patients ont abandonné en raison d’effets indésirables (RC 0,90; 0,53 à 1,53). Les études étaient hétérogènes (I2 = 63%), et il y avait une indication d’un biais de publication modéré (N à sécurité intégrée pour un effet de 0,1: 44), mais les résultats demeuraient solides dans les résultats secondaires prédéterminés et les sous-groupes, y compris les analyses restreintes aux essais randomisés et aux études à faible risque de biais. La méta-régression a révélé une association de la DMS avec une différence d’imipramine pour une dose équivalente. Combiner un inhibiteur du recaptage avec un antagoniste des autorécepteurs α2 présynaptiques était supérieur aux autres combinaisons.
Conclusion:
Combiner des antidépresseurs semble être supérieur à la monothérapie, et un nombre légèrement plus élevé de patients seulement abandonnent le traitement. Combiner un inhibiteur du recaptage avec un antagoniste des autorécepteurs α2 présynaptiques semble être significativement plus efficace que les autres combinaisons. En général, notre recherche a révélé une pénurie d’études bien conçues.
Depressive disorders are major challenges. Twelve-month prevalence estimates differ depending on diagnostic methods but are consistently high ranging between 1% and 10% for major depressive episodes.1 The global burden of disease study ranks major depressive disorder (MDD) as fourth and fifth of the most burdensome disorders in Western Europe and North America, respectively, and globally ranked eleventh, with an increase of illness burden of 37% over the last 2 decades.2
International guidelines by the American Psychiatric Association3 and the Canadian Network for Mood and Anxiety Treatments,4 as well as the German National Clinical Practice Guideline5 recommend use of a single, non-monoamine oxidase inhibitor antidepressant (AD) as initial treatment in severe depression. However, despite the quantity of ADs and despite continuing development of new antidepressive agents, responder rates to initial AD monotherapy remain unsatisfactory, ranging from 40% to 60%,6–8 and remissions occur in only 20% to 30%.9,10
In nonresponders, several second-step treatments are advocated in guidelines,11 especially switching to a different monotherapy, high-dose treatment, augmentation (for example, with lithium), or combining 2 ADs.12 As a consequence, combination treatment is frequent. In a recent naturalistic study13 of nonresponders to initial monotherapy, 19% of patients received combined AD pharmacotherapy. Also, in Veterans Health Administration settings, 11% of all patients with depression were treated with the combination of 2 ADs.14 However, studies have yielded conflicting results regarding the efficacy of combination treatment. For example, Blier et al15 and Maes et al16,17 published results in favour of combining ADs, but studies by Fava et al18 and Leuchter et al19 were negative. Despite a considerable number of trials comparing combination treatment to AD monotherapy, to our knowledge, no comprehensive systematic review and meta-analysis of available controlled trials exists. The authors of 1 earlier meta-analysis of 5 combination studies in treatment-naive patients concluded that there were too few studies to draw definitive conclusions.20 In another systematic review of 5 studies, combination treatment, compared with monotherapy among incomplete responders, was summarized but not quantified, and a lack of evidence was emphasized.21 Both studies applied restrictive selection criteria resulting in the exclusion of many studies carried out on the subject. Moreover, both studies did not contain a meta-analysis of dropouts, a critical aspect of combination treatment. In this vein, Thase,22 in a recent qualitative review, emphasized the lack of adequate research on AD combinations. Accordingly, we carried out a systematic review and meta-analysis of controlled studies comparing combinations of 2 ADs and AD monotherapy in adult patients with acute depression. As data from randomized controlled trials (RCTs) were expected to be sparse, we intended to evaluate all accessible evidence by including controlled trials of different methodological rigour and to present studies both in subgroups according to methodological similarity, as well as in a global assessment across all studies. We hypothesized that combination therapy is superior to monotherapy regarding efficacy but at the price of higher dropout rates.
Methods
This is a systematic literature review and meta-analysis. The protocol has been published on Prospero (Prospero record registration no: CRD42013004407). Methods followed the PRISMA guidelines for systematic reviews and are described in detail in an online supplement (available at cpa.sagepub.com/supplemental). In brief, employing the Cochrane Highly Sensitive Search Strategy we searched PubMed, PsycINFO, Embase, and CENTRAL databases. Trials were included when they met the following criteria: existence of a control group of AD monotherapy (including open label and nonrandomized trials), inclusion of participants aged 18 years or older, of both sexes with depressive disorder, and diagnosis according to standard operationalized criteria. Diagnoses of other psychiatric disorders, as well as comorbid medical conditions, were no exclusion criteria. Studies specifically on bipolar disorder (BD) were excluded. Irrespective of dosage, we included all pharmacological interventions using a combination of 2 ADs, that is, initial combination therapy, as well as adjunctive administration of a second to a first AD. Both trials on first-line treatment and trials among patients with resistance to previous AD treatment(s) were included. We excluded trials on maintenance therapy.
All full texts included were screened independently by 2 reviewers. Study evaluation, data extraction, meta-analyses, tests for heterogeneity, and publication bias, followed the Cochrane Collaboration Handbook.23 Primary outcome criterion was standardized mean difference (SMD) between treatments on an intention-to-treat basis. Secondary outcome criteria were rates of remission, response, and dropouts. Prespecified subgroup analyses included double-blind RCTs, studies in nonresponders to previous treatment trials, and studies with a low risk of bias. Trials were evaluated according to Cochrane’s risk of bias tool23: random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective reporting, sponsorship, and other potential sources of bias. An overall assessment of risk of bias (low, or unknown or high) was added. Post hoc analyses referred to samples restricted to continuation of monotherapy, compared with switch to another monotherapy, to combining monoamine reuptake inhibitors (selective serotonin reuptake inhibitor [SSRI], serotonin-norepinephrine reuptake inhibitor [SNRI], and tricyclic antidepressant [TCA]) with antagonists of presynaptic α2-autoreceptors (mianserin, mirtazapine, and trazodone), to MDD and to unipolar depressive disorder.
Results
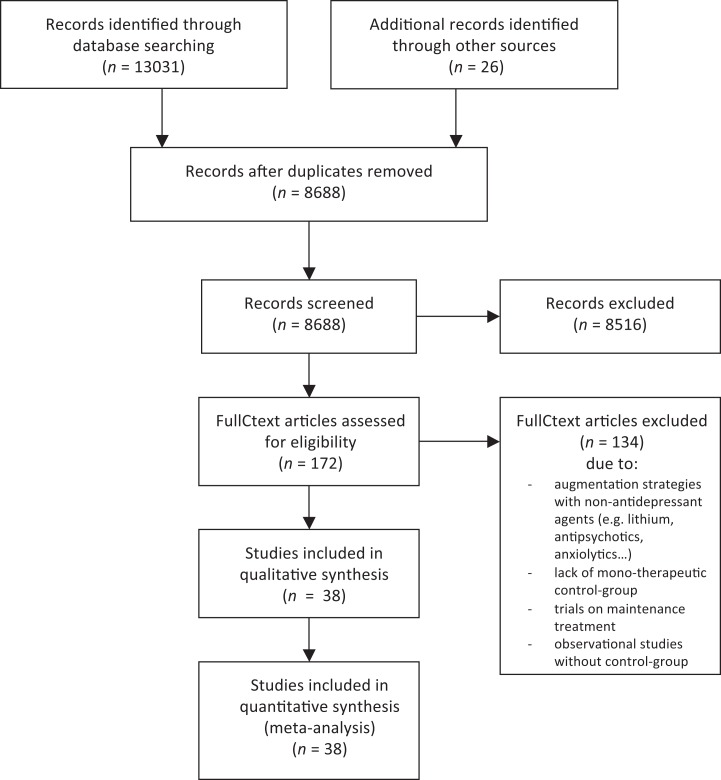
Our literature search retrieved 8688 different articles. After screening titles and abstracts full texts of 172 articles were read, and 38 studies included (Figure 1).
Figure 1.
Flow chart of trials considered, eliminated, and included in study (adapted from PRISMA).
In total, trials included 4511 patients, with 1857 receiving combinations and 2654 receiving monotherapy. Publication dates ranged from 1977 to 2013. Articles were published in English, Chinese (4 articles), and Korean (1 article). Thirty-two studies (84%) were randomized, 27 (71%) used blinding measures, and 20 (53%) were double-blind. Seventeen trials (45%) recruited nonresponders to initial AD treatment only (Table 1).
Table 1.
Characteristics of trials.
| Author | Year | Diagnosis | n | Follow-up, weeks | Combination, n (ITT) | Monotherapy, n (ITT) | Randomized | Blindedness | Non-Responder only | Risk of bias | depression severity at baseline |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bares et al49 | 2013 | MDD, DSM IV, at least Stage I criteria for resistant depression | 61 | 6 | variable, n = 31 | variable, n = 29, | y | open | y | unknown/high | MADRS, C: 28.6+/-3.2, M: 28.4+/-3.2 |
| Blier et al50 | 2009 | MD, DSM-IV | 61 | 6 | Mirtazapine (15 − 45 mg/day) + paroxetine (10 to 30 mg/day), n = 21, | Paroxetine(10-30 mg), N = 19, //Mirtazapin(15-45 mg), N = 21, | y | double | low | MADRS, C: 34.4+/-7.2, M:32.2+/-5.9//32.0+/ − 6.4 | |
| Blier et al15 | 2010 | MDD, DSM-IV | 105 | 6 | Mirtazapine (30 mg/day) + fluoxetine (20 mg/day), n = 25, //mirtazapine (30mg/day) + venlafaxine (75 mg increased to 225 mg/day), n = 26, //mirtazapine + bupropion (150 mg/day), n = 26 | Fluoxetine(20 mg/day), N = 28, | y | double | low | MADRS, C: 32.4+/ − 5//31.7+/ − 4.1//31.0+/ − 4.1, M:31.8+/ − 4.8 HAMD17, C:22.4+/ − 3.5//22.6+/-3.1//21.7+/ − 2.6, M: 22.6+/ − 3.0 | |
| Carpenter et al51 | 2002 | major depressive episode, DSM-IV | 26 | 4 | Mirtazapine (15 to 30 mg/day) + primary antidepressant, n = 11 | Primary antidepressant agents were continued at their pre-study doses throughout the augmentation trial (bupropion 450 mg, citalopram30 to 60 mg, fluoxetine 40 to 50 mg, fluvoxamine 300 mg, paroxetine 30 to 40 mg, sertraline 100 to 200 mg, venlafaxine 200 to 300 mg/day), n = 15 | y | double | y | low | HRSD-17, C:21.9+/ − 3.8, M: 22.5+/ − 5.8 |
| Cha et al52 | 1998 | DSM IV | 36 | 6 | Dothiepin (75 mg/day, dose increase due to clinical state) + sertraline (75 mg/day, dose increase due to clinical state), n = 20 | Dothiepin (150 mg/day, dose increase due to clinical state), n = 16 | y | open | unknown/high | HAM-D-17, C: 22.9+/ − 6.0, M: 21.0 +/ − 4.8 | |
| Dam et al53 | 1998 | depression, ICD-10, moderate-to-severe degree (corresponding to a DSM-Ill-R diagnosis of MDD) | 34 | 6 | Mianserin (30 mg/day) + fluoxetine (20 mg/day), n = 16 | Fluoxetine (20 mg/day), n = 18, | y | double | unknown/high | HAMD-D, median (range), C: 25.0(20 − 34), M: 21.5(17 − 30) | |
| Ebert et al54 | 1995 | dysthymia + superimposed episode of MDD, DSM-III-R, resistant to antidepressant therapy | 36 | 6 | Fluvoxamine (300 mg/day) + moclobemide (600 mg/day), n = 18 | Fluvoxamine (300 mg/day), n = 18 | n | single (raters) | y | unknown/high | HDRS(21items), C: 25.2+/ − 2.7, M: 25.4+/ − 3.7 |
| Fang et al55,56 | 2010 and 2011 | MDD, DSM-IV, stage 2 TRD | 197 | 8 | Paroxetine (20mg/day) + trazodone (100mg/day), n = 47 | Venlafaxine-XR (225 mg/d), n = 50, // mirtazapine (45 mg/d), n = 55, // paroxetine (20 mg/d), n = 45 | y | double | y | unknown/low | HRSD-17, 24.6+/ − 5.8 (only whole − sample data) |
| Fava et al25 | 1994 | MDD, DSM-III-R- Patient Version, refractoriness to initial fluoxetine | 27 | 4 | Fluoxetine (20 mg/day) + desipramine (25 to 50 mg/day), n = 12 | Fluoxetine (40 to 60 mg/day), n = 15 | y | double | y | unknown/high | HAM-D-17, C: 17.5+/-4.7, M: 16.2+/ − 3.9 |
| Fava et al18 | 2002 | MDD, DSM-III-R—Patient Edition | 67 | 4 | Fluoxetine (20 mg/day) + desipramine (25 to 50 mg/day), n = 34 | Fluoxetine (40 to 60 mg/day), n = 33 | y | double | y | unknown/high | HAM-D-17, C: 19.6+/-3.1, M: 17.7+/ − 3.4 |
| Ferreri et al57 | 2001 | MDD, DSM III-R, fluoxetin nonresponders | 103 | 6 | Fluoxetine (20 mg/day) + mianserine (60 mg/day), n = 32 | Mianserine (60 mg/day), n = 33, //fluoxetine (20 mg/day), n = 38 | y | double | y | low | HAMD-17, C: 27.7 + 1.9; M: 27.1 + 2.52//26.9 + 1.9 |
| Gonul et al58 | 2005 | MDD, DSM-IV, resistant to initial SSRI-treatment + consecutive venlafaxine-XR | 39 | 8 | Venlafaxine-XR (225 mg/day) + sertraline (50 to 100 mg/day), n = 19 | Venlafaxine-XR (225 to 375 mg/day), n = 19 | n | open | y | unknown/high | HAMD-D, C: 23.8+/ − 3.1, M: 24.5+/ − 2.3 |
| Gulrez et al59 | 2012 | MDD, DSM-IV-TR, partial responders to SSRI treatment | 60 | 4 | SSRI (escitalopram 10 to 30 mg/day//citalopram 20 to 60 mg/day//paroxetine 25 to 75 mgday//sertraline 50 to 200 mg/day) + bupropion-SR (300 mg/day), n = 30 | SSRI (dosing see left) + placebo, n = 30 | y | single | y | unknown/high | HDRS, C: 17.80 +/ − 0.60, M: 17.57 +/ − 0.48 |
| Lam et al60 | 2004 | MDD, DSM-IV, resistant to minimum 1 antidepressant | 61 | 6 | Citalopram (mean 33.1 +/ − 9.7 mg/day) + bupropion-SR (248.8+/ − 72.4 mg/day), n = 32 | Citalopram (mean 38.8+/ − 13.2 mg/day) or bupropion-SR (283.3+/ − 68.5 mg/day), n = 29 | n | open | y | unknown/high | SIGH-SAD, C: 30.0 +/ − 5.7, M: 31.2 +/ − 7.8 |
| Lauritzen et al61 | 1992 | Hamilton Depression Rating Scale score of >16 and (or) a Melancholia Scale score of >15. Inclusion: patients with depression from 18 to 67 years of age who were either too ill to go through a washout period of 7 days, or who had a depressive episode of more than 1 year (prolonged depression), and patients with depression older than 67 years of age | 40 | 6 | Imipramine (50 to 100 mg/day according to target plasma level 200 nmol/l) + mianserine (30 mg/day), n = 22 | Imipramine (50 to 100 mg/day according to target plasma level 200nmol/l), n = 18 | y | double | low | HAM-D-17, median (25 − 75%), C: 25.2(21.0 − 27.0), M: 22.4(19.0 − 29.0) | |
| Leuchter et al62 | 2009 | MDD, DSM-IV | 220 | 6 | Escitalopram (10 mg/day) + bupropion XL (300 mg/day), n = 74 (PP; ITT not indicated) | Escitalopram(10 mg/day), n = 73 (PP; ITT not indicated), //bupropion XL (300 mg/day), n = 73 (PP; ITT not indicated) | y | open | Unknown/high | HAM-D-17, C: 20.4+/-4.3, M: 20.6+/-4.4, 21.7+/ − 4.0 | |
| Licht and Qvitzau,38 | 2002 | MDD, DSM-IV | 293 | 5 | Sertraline(100 mg/day) + mianserine (30 mg/day), n = 98 | Sertraline (100 mg/day), n = 98, //sertraline (200 mg/day), n = 97 | y | double | y | low | HDRS-17, median(quartiles) C: 23(21 − 26), M: 23(21-26)//23(22 − 26) |
| Liu et al63 | 2000 | Depression, CCMD-2−R | 70 | 6 | Fluoxetine (20 −to 40 mg/day) + amitriptyline (50 to 150 mg/day), n = 35 | Fluoxetine (20 to 40 mg/day), n = 35 | y | single (Pat. only) | unknown/high | HAMD, C: 20.7+/ − 2.5, M: 29.1+/ − 3.1 | |
| Maes et al17 | 1996 | MD, DSM-III-R | 22 | 4 | Fluoxetine (20 mg/day) + trazodone (100 mg/day), n = 12 | Trazodone (100 mg/day), n = 10 | y | double | unknown/high | HAM-D-17, no baseline-data specified | |
| Maes et al16 | 1999 | MDD, DSM-III-R | 23 | 5 | Fluoxetine(20 mg/day)+Mianserine(30 mg/day), N = 11, | Fluoxetine (20 mg/day), n = 12 | y | double | low | HAM-D-17, C: 23.7 +/ − 4.2, M: 21.0 +/ − 4.6 | |
| Matreja et al40 | 2012 | MDD, ICD-10, and DSM-IV | 60 | 6 | SSRI (escitalopram 10 to 30 mg, citalopram 20 to 60 mg, sertraline 50 to 150 mg, fluoxetine 20 to 40 mg, paroxetine 10 to 50 mg/day) + low-dose mirtazapine (7.5 mg/day), n = 30 | SSRI (escitalopram 10 to 30 mg, citalopram 20 to 60 mg, sertraline 50 to 150 mg, fluoxetine 20 to 40 mg, paroxetine 10 to 50 mg/day), n = 30 | y | open | unknown/high | HRDS-17, C: 20.53 ± 0.47, M: 20.76 ± 0.32 | |
| Medhus et al64 | 1994 | Major depressive episode, DSM-I11, after treatment with adequate doses of a TCA | 37 | 3 | TCA (at least 150 mg/day or maximum tolerable dose/range: 100 to 200 mg/day) + mianserin (60 mg/day), n = 18 | TCA (at least 150 mg/day or maximum tolerable dose/range: 75 to 225 mg/day) + placebo, n = 19 | y | double | y | unknown/high | MADRS, C: 32.6 +/ − (4.3), M: 31.7 +/ − (5.6) |
| Murphy39 | 1977 | Depressive illness of sufficient severity to justify treatment with a TCA | 173 | 4 | Clomipramine (10 mg/day) + desipramine (25 mg/day), n = 58 | Clomipramine (10 mg/day), n = 57, //desipramine (25 mg/day), n = 58 | y | double | low | clinical depression rating scale-17 item, mean scores, C: 22.98, M: 24.06//23.71 | |
| Nelson et al65 | 2004 | Yale Depression Inventory, unipolar nonpsychotic MDD | 39 | 6 | Desipramine (target plasma level 160 ng/ml) + fluoxetine (20 mg/day), n = 13 | Fluoxetine (20 mg/day), n = 14, //desipramine (target plasma level 160 ng/ml), n = 12 | y | double | low | MADRS, C: 32 +/ − 6.3, M: 37.2 +/ − 7.1//38.3 +/ − 7.5 | |
| O’Brien et al66 | 1993 | Research diagnostic criteria for MDD | 79 | 6 | AMI (150 mg/day) + TCP (30 mg/day), n = 25 | Amitriptyline (150 mg/day), n = 28, //tranylcypromine (30 mg/day), n = 26 | y | double | unknown/high | HRSD: C: 22.0 +/ − 4.9, M: 24.1 +/ − 6.0// 20.8 +/ − 4.9 | |
| STAR*D (step 2)45–47 | 2006 | nonpsychotic MDD, DSM-IV | 1006 | 12(-14) | Bupropion-SR (200 mg increased to 400 mg/day) + citalopram (mean 54.9 [SD 10.9] mg/day), n = 279 | Bupropion-SR (150 mg increased to 400 mg/day), n=239, //sertraline (50 mg increased to 200 mg/day), n = 238, //venlafaxine-ER (75 mg increased to 375 mg/day), n = 250 | limited | single (remission) /open (response) | (y) | unknown/high | HRSD-17, C: 15.4+/ − 6.8, M: 18.5+/ − 7.7//19.3+/ − 6.9//18.9+/ − 7.3 QIDS − SR − 16, C: 11.2 +/ − 4.7, M: 13.3+/ − 5.1//13.3+/ − 4.7//13.1+/ − 5 |
| STAR*D (step 4)45,46,48 | 2006 | nonpsychotic MDD, DSM-IV | 109 | 12 (–14) | Venlafaxine-ER (37.5 mg increased to 300 mg/day) + mirtazapine (15 mg increased to 45 mg/day), n = 51 | Tranylcypromine (10 mg increased to 60 mg/day), n = 58 | limited | single (remission) /open (response) | y | unknown/high | HDRS, C: 19.7+/ − 5.5, M: 19.6+/ − 7.6 QIDS-SR − 16, C: 14.9 +/ − 4.1, M: 13.6 +/ − 5.1 |
| Stewart et al67 | 2014 | MDD, DSM-IV-TR | 245 | 12 | Escitalopram (40 mg/d) + bupropion (450 mg/d), n = 78 | Escitalopram (40 mg/d), N = 84, // Bupropion (450mg/d), N = 83, | y | double | low | HAM-D-17, C: 20+/-4, M: 20+/-5 | |
| Raisi et al68 | 2007 | MDD, DSM-IV | 45 | 8 | Nortriptyline (50 mg/day) + citalopram (40 mg/day), n = 23 | Citalopram (40 mg/day), n = 22 | y | double | unknown/high | HAM-D-17, C: 30.80+/-4.16, M: 31.2+/-5.07 | |
| Rush et al69 | 2011 | DSM-IV-TR, recurrent or chronic (current episode lasting at least 2 years), MDD | 665 | 12 | Bupropion-SR (150 to 400 mg/day) + escitalopram (10 to 20 mg/day), n = 221, //venlafaxine-ER (150 to 300 mg/day) + mirtazapine (15 to 45 mg/day), n = 220 | Escitalopram (10 to 20 mg/day), n = 224 | y | single | low | HAM-D, C: 23.8+/-4.6//24.3+/-5.0, M: 23.4+/-4.9 | |
| Tanghe et al70 | 1997 | DSM-III R, major depressive episode, resistant to minimum 2 antidepressants | 39 | 4 | Moclobemide (200600 mg/day) + amitriptyline (increased to 280 mg/day), n = 20 | Moclobemide (200 to 600 mg/day), n = 19, //amitriptyline (increased to 280 mg/day), n = 19 | y | double | y | unknown/high | MADRS, C: 41.8+/-6.08, M: 41.26+/-8.22//39.16+/-5.1 |
| Tong and Lan71 | 2005 | Comorbid depression and anxiety disorder, DSM-IV | 63 | 6 | Fluoxetine (20 +/- 4 mg/day) + maprotiline (139 +/- 20 mg/day), n = 31 | Venlafaxine (204 +/- 44 mg/day), n = 32 | n | open | unknown/high | HAM-D, data not available | |
| Vezmar et al72 | 2009 | MD, DSM-IV | 22 | 2 (response) – 4/6 (remission) | Amitriptyline (75 mg/day) + fluvoxamine (100 mg/day), n = 7 | Amitriptyline (75 mg/day), n = 9, //fluvoxamine (100 mg/day), n = 6 | y | open | unknown/high | HAM-D, C: 28.0+/-5.5, M: 29.3+/-5.8//29.2+/-8.1 | |
| White et al73 | 1980 | minor or MDD | 30 | 4 | AMI (50 to 150 mg/day) + TCP (10 to 15 mg/day), n = 10, | Amitriptyline (75 to 300 mg/day), n = 9, //tranylcypromine(10 to 20 mg/day), n = 11 | y | open | unknown/high | HAM-D, no baseline-data specified | |
| Xu et al24 | 2004 | comorbid depression and anxiety disorder, DSM-IV | 84 | 8 | Fluoxetine (20 mg/day) + amitriptyline (112.5 mg/day), n = 28 | Fluoxetine (20 mg/day), n = 28, //amitriptyline (75 to 150 mg/day), n = 28 | y | single (Pat. only) | unknown/high | SDS, data not available | |
| Yang et al74 | 2005 | refractory depression | 36 | 6 | Citalopram (20 mg increased to 40 mg/day) + amitriptylin (50 mg/day), n = 20 | Citalopram (20 mg increased to 40 mg/day), n = 16 | y | single | y | unknown/high | HAM-D, C: 28.9 + /-6.3, M: 28.5 + /-6.8 |
| Yazicioglu et al75 | 2006 | MDD, DSM-IV | 43 | 10 | Reboxetine (4 mg increased to 8 mg/day) + sertraline (50 mg/day), n = 21 | Venlafaxine-XR(75 mg increased to 150 mg/day), N = 22, | y | open | unknown/high | HDRS-17, C: 18.1 + /-1.7, M: 18.3 + /-1.6 | |
| Young et al76 | 1979 | mild or moderate depression not requiring ECT or inpatient admission | 135 | 6 | Trimipramine (mean 102 mg/day) + phenelzine (mean 44 mg/day) // trimipramine (mean 96 mg/day) + isocarboxazide (mean 30 mg/day), n = 51 | Trimipramine (mean 106 mg/day) + placebo//phenelzine (mean 45 mg/day) + placebo//isocarboxazide (mean 32 mg/day) + placebo, n = 84 | y | double | unknown/high | HDRS, mean score, C: 22.9//26.6, M: 22.5//24.8//24.8 | |
| HAM-D range: 15.4 − 31.2, MADRS range: 28.4 − 41.8 |
AMI = amitriptyline; CCMD-2-R = Chinese Classification of Mental Disorders, Second Edition, Revised; DSM = Diagnostic and Statistical Manual of Mental Disorders;
ECT = electroconvulsive therapy; ICD = International Classification of Diseases; ITT = intention-to-treat population; MDD = major depressive disorder;
PP = per-protocol population; SSRI = selective serotonin reuptake inhibitor; TCA = tricyclic antidepressant; TCP = tranylcypromine;
TRD = treatment-resistant depression
Efficacy
Primary Outcome
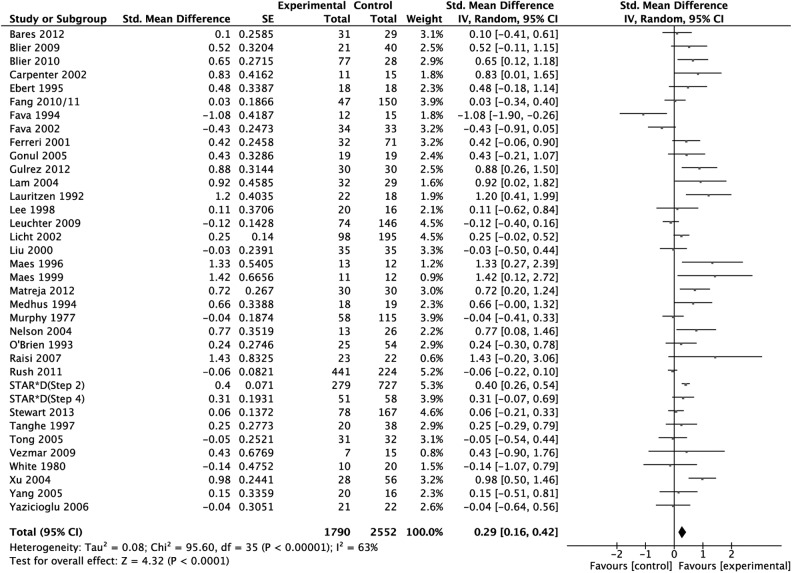
The analysis sample regarding our primary outcome criterion—efficacy as measured in SMD—consisted of 36 studies with 4342 patients. The SMD was 0.29 (95% CI 0.16 to 0.42) in favour of combination treatment (P < 0.001; random effects model) (Figure 2). Between-study heterogeneity was substantial (I2 = 63%, τ2 = 0.08).
Figure 2.
Primary outcome: treatment effect as measured in standardized mean difference weighted according to random effects analysis.
To avoid undue reliance on single studies, we removed studies one by one from the analysis. None of the 36 rounds resulted in a substantial change of point estimate or significance. Effect sizes varied between 0.26 (after elimination of Xu et al24) and 0.31 (when Fava et al18,25 were removed).
Sensitivity and Subgroup Analyses
The effect remained robust across subgroup analyses restricted to randomized, double-blind trials (SMD 0.33; 95% CI 0.11 to 0.54, P = 0.003) to low risk of bias-trials (SMD 0.36; 95% CI 0.13 to 0.59, P = 0.002), to trials excluding patients with BD (SMD 0.30; 95% CI 0.07 to 0.53, P = 0.01), and to RCTs of MDD patients treated with standard doses (SMD 0.25; 95% CI 0.04 to 0.46, P = 0.02). In trials limited to nonresponders, the direction of effect remained but effect sizes were lower (SMD 0.13; 95% CI –0.18 to 0.44, P = 0.42).
Secondary Outcomes
Secondary outcome analyses based on remission and response supported the superiority of combination treatment over monotherapy: odds ratios of 1.63 (95% CI 1.25 to 2.12, P < 0.001), and of 1.68 (95% CI 1.32 to 2.14, P < 0.001), respectively. In a meta-analysis confined to continuous data (rating scale differences) a similar effect emerged (SMD –0.20; 95% CI –0.37 to –0.03, P = 0.02). Primary and secondary outcomes, as well as subgroup analyses, are presented in Table 2.
Table 2.
Results and outcomes across subgroup analyses
| Results | Primary outcome | Secondary outcomes | ||||
|---|---|---|---|---|---|---|
| SMD | Remission | Response | SMD at study endpoint | Dropouts | Dropouts due to AE | |
| SMD (95% CI) | OR (95% CI) | OR (95% CI) | SMD (95% CI) | OR (95% CI) | OR (95% CI) | |
| Whole sample (38 studies) | 0.29 (0.16 to 0.42); n = 4342 (36 studies) | 1.63 (1.24 to 2.08); n = 3884 (27 studies) | 1.68 (1.32 to 2.14); n = 3769 (29 studies) | –0.20 (–0.37 to –0.03); n = 2989 (20 studies) | 0.82 (0.62 to 1.08]; n = 2044 (29 studies) | 0.90 (0.53 to 1.53); n = 2857 (17 studies) |
| rand.db. (20 studies) | 0.33 (0.11 to 0.54); n = 1643 (18 studies) | 1.63 (1.07 to 2.48); n = 1287 (12 studies) | 2.38 (1.50 to 3.77); n = 1130 (12 studies) | –0.13 (–0.61 to 0.36); n = 441 (6 studies) | 0.79 (0.59 to 1.07); n = 1405 (16 studies) | 0.87 (0.51 to 1.48); n = 883 (10 studies) |
| Non(rand.db.) (18 studies) | 0.27 (0.09 to 0.44); n = 2699 (18 studies) | 1.65 (1.15 to 2.37); n = 2597 (15 studies) | 1.35 (1.04 to 1.75); n = 2639 (17 studies) | –0.23 (–0.41 to –0.05); n = 2548 (14 studies) | 1.03 (0.49 to 2.19); n = 639 (13 studies) | 0.89 (0.34 to 2.34); n = 1974 (7 studies) |
| rand.db. nonresponder (8 studies) | 0.13 (–0.18 to 0.44); n = 808 (8 studies) | 1.22 (0.71 to 2.07); n = 713 (6 studies) | 1.53 (0.87 to 2.68); n = 619 (4 studies) | 0.01 (–0.81 to 0.82); n = 157 (4 studies) | 0.65 (0.37 to 1.14); n = 450 (5 studies) | 0.94 (0.23 to 3.83); n = 156 (3 studies) |
| Low risk of bias (11 studies) | 0.30 (0.09 to 0.50); n = 1773 (11 studies) | 1.84 (1.18 to 2.89); n = 1537 (8 studies) | 2.00 (1.27 to 3.14); n = 1528 (10 studies) | –0.21 (–0.54 to 0.11); n = 975 (4 studies) | 0.73 (0.52 to 1.04); n = 956 (8 studies) | 0.72 (0.39 to 1.32); n = 1408 (8 studies) |
| High /unknown risk of bias (27 studies) | 0.25 (0.09 to 0.42); n = 2569 (25 studies) | 1.54 (1.08 to 2.18); n = 2347 (19 studies) | 1.57 (1.16 to 2.12); n = 2241 (19 studies) | –0.19 (–0.39 to 0.01); n = 2014 (16 studies) | 0.99 (0.63 to 1.54); n = 1088 (21 studies) | 1.25 (0.67 to 2.34); n = 1449 (9 studies) |
| Exclusion of patients with bipolar disorder (12 studies) | 0.30 (0.07 to 0.53); n = 1661 (12 studies) | 2.13 (1.28 to 3.55); n = 1661 (12 studies) | 1.59 (1.00 to 2.53); n = 1210 (8 studies) | –0.17 (–0.47 to 0.12); n = 1114 (6 studies) | 0.84 (0.56 to 1.27); n = 700 (8 studies) | 0.61 (0.29 to 1.27); n = 1253 (7 studies) |
| Patients with MDD rand.db. (15 studies) | 0.25 (0.04 to 0.46); n = 1382 (14 studies) | 1.63 (1.07 to 2.48); n = 1287 (12 studies) | 1.94 (1.27 to 2.97); n = 869 (8 studies) | –0.14 (–0.55 to 0.26); n = 499 (7 studies) | 0.78 (0.55 to 1.09); n = 1019 (11 studies) | 0.96 (0.50 to 1.85); n = 680 (8 studies) |
Primary outcome: SMD > 0 in favour of combination. Secondary outcomes: OR > 1 designates superiority of combination treatment; SMD < 0 designates superiority of combination treatment. Study number in the first column refers to the number of studies in a subgroup: for example, there are 20 randomized double-blind studies. Note, depending on design specifics, studies from column 1 may not be included in all outcome analyses (for example, only 10 randomized double-blind studies reported data on the number of dropouts due to adverse effects).
AE = adverse events; MDD = major depressive disorder; rand.db. = randomized, double-blind trials; SMD = standardized mean difference
Tolerability
Regarding both dropouts, due to any reason and to dropouts due to adverse effects, fewer patients dropped out in monotherapy arms (OR 0.82; 95% CI 0.62 to 1.08 and OR 0.90; 95% CI 0.53 to 1.53, respectively). Results were not significant and high P values indicate a possible role of chance (0.16 and 0.70, respectively).
Heterogeneity
Between-study heterogeneity was substantial in the primary outcome meta-analysis (I2 = 63%, τ2 = 0.08, Figure 2) and in most of the subgroup analyses. In some subgroup analyses and secondary outcomes I2 indicated lower heterogeneity between studies, especially in dropout analyses (I2 = 0%) and analyses restricted to studies of patients with MDD only (I2 = 49%). Heterogeneity as measured by tau-squared varied from 0.07 to 0.12 in primary outcome and primary outcome subgroup analyses. Accordingly, Tau ranged from 0.26 to 0.35, indicating that the standard deviation of the weighted SMD estimate was about equal to the effect size.
Publication Bias
The funnel plot of studies included in the primary outcome analysis did not rule out publication bias. Eggers test was borderline negative ( df = 34, P = 0.07), a trim and fill procedure (Duval and Tweedie) with 9 studies trimmed to the left of the mean resulted in a reduced effect size (0.16; 95% CI 0.02 to 0.29). Forty-four studies with an effect size of 0 would be necessary to reduce the overall effect to 0.1 (Orwin’s fail-safe N).
Trials selected had an average power of 26.4% (9.3% to 98.6%) to detect an effect size of 0.29 (SMD)–the primary outcome effect estimate.
Post Hoc Analyses
Two different modalities of monotherapy were compared with add-on combination treatment: continuation of monotherapy (10 studies) and switch to another monotherapy (2 studies). Both favoured add-on combination SMD (0.39; 95% CI 0.25 to 0.52, compared with 0.35; 95% CI 0.01 to 0.69).
Another post hoc analysis revealed similar effects in samples of only patients with MDD (SMD 0.30; 95% CI 0.15 to 0.44) (23 studies). The difference between combination and monotherapy was more pronounced in treatment-naive patients than in patients with treatment-resistant depression (TRD) (SMD 0.41; 95% CI 0.10 to 0.72) [6 studies], compared with 0.13; 95% CI –0.18 to 0.44 [8 studies]; randomized, double-blind studies, patients with MDD only).
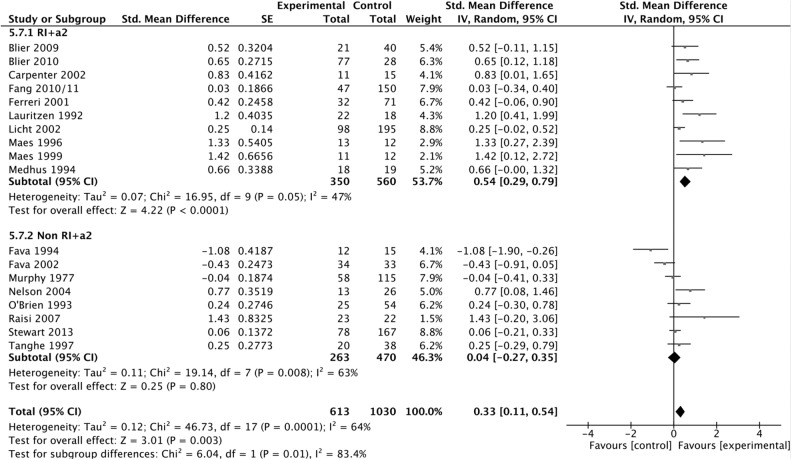
Combination of a monoamine reuptake inhibitor (SSRI, SNRI, and TCA) with an antagonist of the presynaptic α2-autoreceptor (mianserin, mirtazapine, and trazodone) (13 studies) resulted in a statistically, significantly higher effect than other combinations (24 studies) (SMD 0.47; 95% CI 0.24 to 0.71, compared with SMD 0.17; 95% CI 0.02 to 0.32, P = 0.03). Restriction of this analysis to randomized, double-blind studies only consolidated the finding (SMD 0.54; 95% CI 0.29 to 0.79 [10 studies], compared with SMD 0.04; 95% CI –0.27 to 0.35 [8 studies]; P = 0.01); I2 = 47%) (Figure 3).
Figure 3.
Primary outcome: treatment effect as measured in standardized mean difference (SMD) subgroup analysis: RI+α2 versus other pharmacologic combinations. Randomized, double-blind trials only weighted according to random effects analysis.
The fourth post hoc analysis confirmed an advantage of combination over monotherapy in patients with BD as well (SMD 0.30; 95% CI 0.07 to 0.53).
Meta-Regressions and Moderator Analyses
Average imipramine-equivalent doses were 269.8 mg (SD 134.1 mg) in combination arms and 170.1 mg (SD 72.9 mg) in monotherapy arms, a ratio of 1.6:1 (n = 35 studies). In meta-regression, dosage difference was substantially associated with SMD (slope 0.0014; 95% CI 0.00003 to 0.0028, P = 0.045, n = 34). A similar result emerged for dosage ratio (slope 0.286; 95% CI 0.0285 to 0.543, P = 0.029, n = 35).
Meta-regression revealed no association of duration of follow-up in weeks with SMD (slope –0.01; 95% CI –0.06 to 0.04, P = 0.60, n = 36).
Moderator analyses of dichotomized variables did not result in strong signals: randomized, compared with nonrandomized trials (P = 0.66); low, compared with non-low risk of bias studies (P = 0.46); and treatment resistant, compared with nontreatment-resistant patient samples (P = 0.78) (Table 2).
Discussion
Our study yielded 3 main results. First, combination treatment seems to be superior to monotherapy. Second, combination treatment is not associated with substantially more dropouts. Third, the combination of monoamine reuptake inhibitors and α2-adrenergic receptor antagonists seems to be superior to other AD combinations.
In this area of research an SMD of about 0.3, as estimated in this meta-analysis, is substantial, particularly because it emerged from comparisons between active treatments. Even when compared with placebo, AD monotherapy has effect sizes of no more than about 0.3.26 Thus, even the lower end of the effect size confidence interval of the primary outcome, 0.16, indicates a non-negligible effect in AD pharmacotherapy. In the same vein, the subgroup analysis regarding remission lead to number needed to treat estimate of 9 (6 to 21) for combination treatment in comparison with monotherapy. In contrast, regarding dropouts, the point estimate of number needed to harm amounts to 50 (not significant).
Some, but not all, previous reviews judged combination therapy to be superior.20,27–31 Nevertheless, owing to small numbers of studies, or in the absence of meta-analyses, differences were not quantified or the role of chance was not estimated. Unfortunately, previous reviews did not quantify adverse effects but some, largely on theoretical grounds, highlighted increased risks of adverse events during combination therapy.20,28,29 Thus, our tolerability analyses are of clinical importance: while monotherapy resulted in fewer dropouts due to any reason to adverse events, both effects were small and P values were high. The results refute our hypothesis of lower tolerability during combination treatment.
Regarding other treatment strategies in TRD, studies support lithium augmentation and augmentation with atypical antipsychotics.32,33 However, switching to another AD cannot be considered evidence based. Bschor and Baethge,34 in a systematic review and meta-analysis found switching not to be superior to continuation of initial ADs. Similarly, our subgroup analyses showed the advantage of add-on combination treatment to be more pronounced when compared with switching, than to continuation of monotherapy. As a result, it seems justified to particularly prefer combining ADs, compared with switching to another AD. It is important to note that, in our analysis, combination treatment in TRD was only marginally and not statistically, significantly effective in comparison with monotherapy. The finding may prove clinically useful anyway for 2 reasons. First, patients with TRD are a difficult-to-treat subgroup—and one that is probably frequently subjected to combination treatment—and any approach that shows some potential may have clinical merit. Second, the effect estimated in our study could be real even in the absence of statistical significance, mainly because the positive results in our various comparisons between combination and monotherapy increase the a priori probability of superiority in patients with TRD as well. In any event, additional methodologically rigorous research on this topic is essential.
Combinations of monoamine reuptake inhibitors and α2-adrenergic receptor antagonists turned out to be particularly effective. On pharmacodynamic grounds, monoamine reuptake inhibitors may act synergistically with α2-adrenergic receptor antagonists. Reuptake inhibitors are thought to be limited in effect, because increased levels of monoamines in the synaptic cleft result in increased activation of inverse feedback by stimulation of presynaptic α2-adrenergic receptors.35,36 However, this finding has to be viewed with caution as it resulted from a post hoc analysis.
In our regression model, dosage difference accounted for about one-half of the total difference in treatment effect. Still, it has to be borne in mind that several studies did not provide detailed data on dosages. However, if the dose difference is correct it is all the more remarkable that tolerability, as measured in dropouts, seems to be sufficient when ADs are combined. Additionally, in a systematic review, a dose–effect relation could be demonstrated for some, but not for all ADs.37 Licht and Qvitzau,38 comparing combination therapy to standard- and high-dose sertraline, found the latter to be the least effective regimen. However, more 3-armed studies including a standard-dose monotherapy arm, a high-dose monotherapy arm, and a combination arm are needed to shed light on the significance of dosage.
Murphy et al39 examined application of ADs at subtherapeutic doses. Matreja et al40 observed application of low-dose mirtazapine in combination treatment. Both were not part of the MDD subgroup analyses. These may simultaneously represent subgroup analyses of therapeutic AD dosing.
Limitations
First, this meta-analysis included studies covering different patient populations, ADs, dosages, or durations of treatment, as well as studies of different methodological rigour. However, as earlier studies were contradictory and many underpowered, we aimed at including as many publications as possible. Ioannidis et al41 argue that in most cases, even in the presence of heterogeneity, quantitative synthesis may be preferable to approaches frequently found in narrative reviews, as long as limitations of meta-analyses are acknowledged. In this vein, had we limited our calculations to meta-analyses restricted to a small group of studies we would not have found preliminary evidence for the superiority of combining reuptake inhibitors with α2-autoreceptor antagonists.
I2 values indicated substantial heterogeneity of effects. However, they are known to increase with accumulating N, additional tau-squared statistics were calculated, indicating a spread of data not unfamiliar in medical studies. The standard deviation had the same order of magnitude as the effect size. Nevertheless, heterogeneity was carefully considered in various sensitivity and subgroup analyses of more homogeneous study samples, in using random effects models, in testing whether results remained robust after each study was left out, and in considering possible effects of publication bias. Finally, 2 meta-regressions and 3 additional moderator analyses addressed the role of possible confounders. Therefore, our study does not represent an analysis of broad, instead of narrow, inclusion criteria but rather several analyses of different grades of homogeneity regarding trials selected, narrow and broad. Although we acknowledge that different confounding factors are interconnected, unfortunately, we could not calculate a single meta-analysis adjusting for all covariates simultaneously.
The meta-analyses of double-blind randomized trials and double-blind randomized trials among patients with MDD supported our findings. In fact, the advantage of combination treatment became stronger. Similar results apply to the more homogeneous subgroup populations. Some factors causing variability in the group of studies included likely have made the sample as a whole more conservative (for example, selection of studies employing low dose combinations, or including a subgroup of patients with BD). Still, rather than to find an exact point estimate for the efficacy of combined ADs relative to monotherapy it was the primary aim of our study to determine whether there is a difference at all between these 2 treatment principles and whether such a difference would be outweighed by an increase in dropouts. Note, secondary outcome analyses using different effect estimates aimed at confirming or falsifying primary results and not at finding significant results. Every effect estimate has to be viewed as what it is—an estimate that makes sense only with its confidence interval.
Some of the trials (N = 10) required nonresponse to an initial period of AD monotherapy. Trials consisted of AD monotherapy of this agent (in continuation), compared with add-on therapy with an additional AD. Therefore, dropouts due to adverse effects may have been decreased in the monotherapy group, as patients may have dropped out during the pretrial period. In these studies, dropout rates may be biased against combination treatment—a methodological problem that strengthens our findings.
Further, it has been shown that the results of some meta-analyses are inflated. While Pereira and Ioannidis42 confirmed that results of most meta-analyses represent true effects, they calculated that there may occur effect inflation, particularly in meta-analyses with sparse data. However, our study benefitted from many studies and events. With a sample size of 4300 patients for our primary outcome, there seems reason to believe that the results of our meta-analysis will be stable over time.43
It is possible that we did not include all relevant studies, but with MEDLINE, Embase, PsycINFO, and CENTRAL, 4 different large international databases were searched. It is doubtful that, in the field of combination treatment, publication bias is as important as it is in placebo studies of AD monotherapies. Nevertheless, adjusting for possible publication bias resulted in a weakened but still positive effect (SMD 0.16).
Finally, the limitations of our study will always be those of the included trials. For example, the trials that were published as blinded studies did not report on measures to ensure blinding. Therefore, it is difficult to judge the quality of blinding—a problem that has been shown to be prevalent in psychiatric research.44 In addition, some of the study arms (step 2 in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study45–47) were not meant to be directly compared, and step 2 and step 4 of the STAR*D trial45–48 were sequential treatments among the same study population. It is, therefore, reassuring that results were supported by the analysis of trials with low risk of bias.
Conclusions
AD combination seems to be superior to monotherapy and this advantage does not come at the cost of higher rates of severe adverse events. Possible areas of application might be in particularly severe cases of depression and treatment-resistant populations (although in this population the effect estimate was low). Our results point to a possibly important role of higher dosage in combination therapy and to promising combinations of reuptake inhibitors with α2-autoreceptor antagonists. Still, even with our systematic search of ample and broad inclusion criteria we find a dearth of evidence in this field of research. Future research should focus on the clinically important field of TRD.
Supplementary Material
Supplementary Material
Acknowledgements
We gratefully acknowledge the generous help by Ming Deng, Dr Sun-Hee Lee, He-Loung Jeong, Stanislav Bleer, and Ulla Beck in translating articles in Chinese, Korean, Russian, and Danish.
Dr Baethge and Dr Bschor had the idea for the study and its design. Dr Henssler conducted the literature search and screened the articles. All authors reviewed all full texts for inclusion and collected the data independently. Dr Baethge and Dr Henssler analyzed the data. All authors drafted and revised the paper, and approved the final version.
Both Dr Henssler and Dr Bschor contributed equally to this work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and (or) publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and (or) publication of this article: Until June 2014, Dr Bschor has received honoraria for speaking from Lilly, AstraZeneca, Esparma, Bristol-Myers Squibb, Sanofi-Aventis, Servier, and Lundbeck, and has accepted reimbursements of travelling expenses to congresses from AstraZeneca and Lilly. Since then, Dr Bschor has discontinued all financial relations with pharmaceutical companies..
Supplemental Material: The online supplements are available at http://cpa.sagepub.com/supplemental.
References
- 1. Wittchen HU, Jacobi F, Rehm J, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–679. [DOI] [PubMed] [Google Scholar]
- 2. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. [DOI] [PubMed] [Google Scholar]
- 3. American Psychiatric Association (APA). Practice guideline for the treatment of patients with major depressive disorder. 3rd ed Arlington (VA): APA; 2010. [Google Scholar]
- 4. Lam RW, Kennedy SH, Grigoriadis S, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. III. Pharmacotherapy. J Affect Disord. 2009;117(Suppl1):S26–S43. [DOI] [PubMed] [Google Scholar]
- 5. Härter M, Klesse C, Bermejo I, et al. Unipolar depression: diagnostic and therapeutic recommendations from the current S3/National Clinical Practice Guideline. Dtsch Arztebl International. 2010;107(40):700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schulberg HC, Katon W, Simon GE, et al. Treating major depression in primary care practice: an update of the Agency for Health Care Policy and Research Practice Guidelines. Arch Gen Psychiatry. 1998;55(12):1121–1127. [DOI] [PubMed] [Google Scholar]
- 7. Williams JW, Jr, Mulrow CD, Chiquette E, et al. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med. 2000;132(9):743–756. [DOI] [PubMed] [Google Scholar]
- 8. Bschor T, Adli M. Treatment of depressive disorders. Dtsch Arztebl Int. 2008;105(45):782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19(2):179–200. [DOI] [PubMed] [Google Scholar]
- 10. Fava M, Rush AJ, Trivedi MH, et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am. 2003;26(2): 457–494. [DOI] [PubMed] [Google Scholar]
- 11. National Institute for Health and Care Excellence (NICE). Depression in adults: the treatment and management of depression in adults (CG90). London (GB): NICE; 2009. [PubMed] [Google Scholar]
- 12. Bschor T, Bauer M, Adli M. Chronic and treatment resistant depression: diagnosis and stepwise therapy. Dtsch Arztebl Int. 2014;111(45):766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohler S, Unger T, Hoffmann S, et al. Comparing augmentation with non-antidepressants over sticking to antidepressants after treatment failure in depression: a naturalistic study. Pharmacopsychiatry. 2013;46(2):69–76. [DOI] [PubMed] [Google Scholar]
- 14. Valenstein M, McCarthy JF, Austin KL, et al. What happened to lithium? Antidepressant augmentation in clinical settings. Am J Psychiatry. 2006;163(7):1219–1225. [DOI] [PubMed] [Google Scholar]
- 15. Blier P, Ward HE, Tremblay P, et al. Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry. 2010;167(3):281–288. [DOI] [PubMed] [Google Scholar]
- 16. Maes M, Libbrecht I, Hunsel F, et al. Pindolol and mianserin augment the antidepressant activity of fluoxetine in hospitalized major depressed patients, including those with treatment resistance. J Clin Psychopharmacol. 1999;(2):177–182. [DOI] [PubMed] [Google Scholar]
- 17. Maes M, Vandoolaeghe E, Desnyder R. Efficacy of treatment with trazodone in combination with pindolol or fluoxetine in major depression. J Affect Disord. 1996;41(3):201–210. [DOI] [PubMed] [Google Scholar]
- 18. Fava M, Alpert J, Nierenberg A, et al. Double-blind study of high-dose fluoxetine versus lithium or desipramine augmentation of fluoxetine in partial responders and nonresponders to fluoxetine. J Clin Psychopharmacol. 2002;22(4):379–387. [DOI] [PubMed] [Google Scholar]
- 19. Leuchter AF, Cook IA, Marangell LB, et al. Comparative effectiveness of biomarkers and clinical indicators for predicting outcomes of SSRI treatment in major depressive disorder: results of the BRITE-MD study. Psychiatry Res. 2009;169(2):124–131. [DOI] [PubMed] [Google Scholar]
- 20. Rocha FbL, Fuzikawa Cn, Riera R, et al. Combination of antidepressants in the treatment of major depressive disorder: a systematic review and meta-analysis. J Clin Psychopharmacol. 2012;32(2):278–281. [DOI] [PubMed] [Google Scholar]
- 21. Lopes Rocha F, Fuzikawa C, Riera R, et al. Antidepressant combination for major depression in incomplete responders—a systematic review. J Affect Disord. 2013;144(1-2):1–6. [DOI] [PubMed] [Google Scholar]
- 22. Thase ME. Antidepressant combinations: cutting edge psychopharmacology or passing fad? Topical collection on mood disorders. Curr Psychiatry Rep. 2013;15(10):403. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. The Cochrane Collaboration; [updated 2011 Mar; cited 2014 Jun]. Available from www.cochrane-handbook.org.
- 24. Xu JX, Tan JW, Ou LM. [Comparison of the effect of fluoxetine and amitriptyline in treating comorbid depression disorder and anxiety disorder]. Chinese Journal of Clinical Rehabilitation. 2004; (15):2812–4 [Google Scholar]
- 25. Fava M, Rosenbaum JF, McGrath PJ, et al. Lithium and tricyclic augmentation of fluoxetine treatment for resistant major depression: a double-blind, controlled study. Am J Psychiatry. 1994;151(9):1372–1374. [DOI] [PubMed] [Google Scholar]
- 26. Turner EH, Matthews AM, Linardatos E, et al. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358(3):252–260. [DOI] [PubMed] [Google Scholar]
- 27. Lam RW, Wan DD, Cohen NL, et al. Combining antidepressants for treatment-resistant depression: a review. J Clin Psychiatry. 2002;63(8):685–693. [DOI] [PubMed] [Google Scholar]
- 28. Lopes Rocha Fb, Fuzikawa Cn, Riera R, et al. Antidepressant combination formajor depression in incomplete responders, a systematic review. J Affect Disord. 2013;144(1-2):1–6. [DOI] [PubMed] [Google Scholar]
- 29. Dodd S, Horgan D, Malhi GS, et al. To combine or not to combine: a literature review of antidepressant combination therapy. J Affect Disord. 2005;89(1-3):1–11. [DOI] [PubMed] [Google Scholar]
- 30. Feetam C. Are two antidepressants always better than one? Prog. Neurol. Psychiatry. 2012;16(3):5–8. [Google Scholar]
- 31. Keks NA, Burrows GD, Copolov DL, et al. Beyond the evidence: Is there a place for antidepressant combinations in the pharmacotherapy of depression? Med J Aust. 2007;186(3):142–4. [DOI] [PubMed] [Google Scholar]
- 32. Bschor T, Bauer M. Efficacy and mechanisms of action of lithium augmentation in refractory major depression. Curr Pharm Des. 2006;12(23):2985–2992. [DOI] [PubMed] [Google Scholar]
- 33. Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. 2009;166(9):980–991. [DOI] [PubMed] [Google Scholar]
- 34. Bschor T, Baethge C. No evidence for switching the antidepressant: systematic review and meta-analysis of RCTs of a common therapeutic strategy. Acta Psychiatr Scand. 2010;121(3):174–179. [DOI] [PubMed] [Google Scholar]
- 35. Bschor T, Bauer M, Adli M. Chronic and treatment resistant depression: diagnosis and stepwise therapy. Dtsch Arztebl Int. 2014;111(45):766–775; quiz 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bschor T. Therapy-resistant depression. Expert Rev Neurother. 2010;10(1):77–86. [DOI] [PubMed] [Google Scholar]
- 37. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387–400. [DOI] [PubMed] [Google Scholar]
- 38. Licht RW, Qvitzau S. Treatment strategies in patients with major depression not responding to first-line sertraline treatment. A randomised study of extended duration of treatment, dose increase or mianserin augmentation. Psychopharmacology (Berl). 2002;161(2):143–151. [DOI] [PubMed] [Google Scholar]
- 39. Murphy JE. A comparative trial of Anafranil, Pertofran and an Anafranil/Pertofran combination. J Int Med Res. 1977;5(1Suppl):16–23. [PubMed] [Google Scholar]
- 40. Matreja PS, Badyal DK, Deswal RS, et al. Efficacy and safety of add on low-dose mirtazapine in depression. Indian J Pharmacol. 2012;44(2):173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ioannidis JP, Patsopoulos NA, Rothstein HR. Reasons or excuses for avoiding meta-analysis in forest plots. BMJ. 2008;336(7658):1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pereira TV, Ioannidis JP. Statistically significant metaanalyses of clinical trials have modest credibility and inflated effects. J Clin Epidemiol. 2011;64(10):1060–1069. [DOI] [PubMed] [Google Scholar]
- 43. Trikalinos TA, Churchill R, Ferri M, et al. Effect sizes in cumulative meta-analyses of mental health randomized trials evolved over time. J Clin Epidemiol. 2004;57(11):1124–1130. [DOI] [PubMed] [Google Scholar]
- 44. Baethge C, Assall OP, Baldessarini RJ. Systematic review of blinding assessment in randomized controlled trials in schizophrenia and affective disorders 2000-2010. Psychother Psychosom. 2013;82(3):152–160. [DOI] [PubMed] [Google Scholar]
- 45. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. [DOI] [PubMed] [Google Scholar]
- 46. Rush AJ, Warden D, Wisniewski SR, et al. STAR*D: revising conventional wisdom. CNS Drugs. 2009;23(8):627–647. [DOI] [PubMed] [Google Scholar]
- 47. Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164(5):739–752. [DOI] [PubMed] [Google Scholar]
- 48. McGrath PJ, Stewart JW, Fava M, et al. Tranylcypromine versus venlafaxine plus mirtazapine following three failed antidepressant medication trials for depression: a STAR*D report. Am J Psychiatry. 2006;163(9):1531–1541; quiz 666. [DOI] [PubMed] [Google Scholar]
- 49. Bares M, Novak T, Kopecek M, et al. Antidepressant monotherapy compared with combinations of antidepressants in the treatment of resistant depressive patients: a randomized, openlabel study. Int J Psychiatry Clin Pract. 2013;17(1):35–43. [DOI] [PubMed] [Google Scholar]
- 50. Blier P, Gobbi G, Turcotte JE, et al. Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. European Neuropsychopharmacology. 2009;19(7):457–465. [DOI] [PubMed] [Google Scholar]
- 51. Carpenter LL, Yasmin S, Price LH. A double-blind, placebocontrolled study of antidepressant augmentation with mirtazapine. Biol Psychiatry. 2002;51(2):183–188. [DOI] [PubMed] [Google Scholar]
- 52. Cha JH, Jung IK, Lee MS. Comparison between dothiepin-sertraline combination and dothiepin alone therapy in the treatment of depressive disorder. J Korean Soc Biol Psychiatry. 1997;4(2):251–8. [Google Scholar]
- 53. Dam J, Ryde L, Svejsø J, et al. Morning fluoxetine plus evening mianserin versus morning fluoxetine plus evening placebo in the acute treatment of major depression. Pharmacopsychiatry. 1998;(2):48–54. [DOI] [PubMed] [Google Scholar]
- 54. Ebert D, Albert R, May A, et al. Combined SSRI-RIMA treatment in refractory depression. Safety data and efficacy. Psychopharmacology (Berl). 1995;119(3):342–344. [DOI] [PubMed] [Google Scholar]
- 55. Fang Y, Yuan C, Xu Y, et al. A pilot study of the efficacy and safety of paroxetine augmented with risperidone, valproate, buspirone, trazodone, or thyroid hormone in adult Chinese patients with treatment-resistant major depression. J Clin Psychopharmacol. 2011;31(5):638–642. [DOI] [PubMed] [Google Scholar]
- 56. Fang Y, Yuan C, Xu Y, et al. Comparisons of the efficacy and tolerability of extended-release venlafaxine, mirtazapine, and paroxetine in treatment-resistant depression: a double-blind, randomized pilot study in a Chinese population. J Clin Psychopharmacol. 2010;30(4):357–364. [DOI] [PubMed] [Google Scholar]
- 57. Ferreri M, Lavergne F, Berlin I, et al. Benefits from mianserin augmentation of fluoxetine in patients with major depression non-responders to fluoxetine alone. Acta Psychiatr Scand. 2001;103(1):66–72. [DOI] [PubMed] [Google Scholar]
- 58. Gonul AS, Doksat K, Eker OD, et al. Managing treatment-resistant depression: focusing on increasing serotonergic transmission In:Shirley AC, editor. Trends in serotonin uptake inhibitor research. Hauppauge (NY): Nova Biomedical Books; 2005. p 1–27. [Google Scholar]
- 59. Gulrez G, Badyal DK, Deswal RS, et al. Bupropion as an augmenting agent in patients of depression with partial response. Basic Clin Pharmacol Toxicol. 2012;110(3):227–230. [DOI] [PubMed] [Google Scholar]
- 60. Lam RW, Hossie H, Solomons K, et al. Citalopram and bupropion-SR: combining versus switching in patients with treatment-resistant depression. J Clin Psychiatry. 2004;65(3):337–340. [PubMed] [Google Scholar]
- 61. Lauritzen L, Clemmesen L, Klysner R, et al. Combined treatment with imipramine and mianserin: a controlled pilot study. Pharmacopsychiatry. 1992;25(4):182–186. [DOI] [PubMed] [Google Scholar]
- 62. Leuchter AF, Cook IA, Gilmer WS, et al. Effectiveness of a quantitative electroencephalographic biomarker for predicting differential response or remission with escitalopram and bupropion in major depressive disorder. Psychiatry Res. 2009;169(2):132–138. [DOI] [PubMed] [Google Scholar]
- 63. Liu HP, Zhang HK, Liu ZZ. A controlled study of fluoxetine combining with amitriptyline in treatment of depression. West China Medical Journal. 2000;(3):354–355. [Google Scholar]
- 64. Medhus A, Heskestad S, Tjemsland L. Mianserin added to tricyclic antidepressants in depressed patients not responding to a tricyclic antidepressant alone: a randomized, placebo-controlled, double-blind study. Nord J Psychiatry. 1994;48(5):355–358. [Google Scholar]
- 65. Nelson JC, Mazure CM, Jatlow PI, et al. Combining norepinephrine and serotonin reuptake inhibition mechanisms for treatment of depression: a double-blind, randomized study. Biol Psychiatry. 2004;55(3):296–300. [DOI] [PubMed] [Google Scholar]
- 66. O’Brien S, McKeon P, O’Regan M. The efficacy and tolerability of combined antidepressant treatment in different depressive subgroups. Br J Psychiatry. 1993;162:363–368. [DOI] [PubMed] [Google Scholar]
- 67. Stewart JW, McGrath PJ, Blondeau C, et al. Combination antidepressant therapy for major depressive disorder: speed and probability of remission. J Psychiatr Res. 2014;52:7–14. [DOI] [PubMed] [Google Scholar]
- 68. Raisi F, Habibi N, Nasehi AA, Akhondzadeh S. Combination of citalopram and nortriptyline in the treatment of severe major depression: a double-blind, placebo-controlled trial. Therapy. 2007;4(2):187–192. [Google Scholar]
- 69. Rush AJ, Trivedi MH, Stewart JW, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. Am J Psychiatry. 2011;168(7):689–701. [DOI] [PubMed] [Google Scholar]
- 70. Tanghe A, Steeman J, Bollen E, et al. Moclobeminde and amitriptyline, alone or in combination, in therapy resistant depression. Hum Psychopharmacol. 1997;12(5):509–510. [Google Scholar]
- 71. Tong JM, Lan JF. Fluoxetine combined with maprotiline in treating depressive disorder associated with anxiety. Chinese Journal of New Drugs and Clinical Remedies. 2005; (1):57–60. [Google Scholar]
- 72. Vezmar S, Miljkovic B, Vucicevic K, et al. Pharmacokinetics and efficacy of fluvoxamine and amitriptyline in depression. J Pharmacol Sci. 2009;110(1):98–104. [DOI] [PubMed] [Google Scholar]
- 73. White K, Pistole T, Boyd JL. Combined monoamine oxidase inhibitor-tricyclic antidepressant treatment: a pilot study. Am J Psychiatry. 1980;137(11):1422–1425. [DOI] [PubMed] [Google Scholar]
- 74. Yang C, Wen Q, Wang X, et al. Comparative study of citalopram combined with amitriptyline for treatment of refractory depression. International Medicine and Health Guidance News. 2005;11(4):69–70. [Google Scholar]
- 75. Yazicioglu B, Akkaya C, Sarandol A, et al. A comparison of the efficacy and tolerability of reboxetine and sertraline versus venlafaxine in major depressive disorder: a randomized, open-labeled clinical trial. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1271–1276. [DOI] [PubMed] [Google Scholar]
- 76. Young JP, Lader MH, Hughes WC. Controlled trial of trimipramine, monoamine oxidase inhibitors, and combined treatment in depressed outpatients. Br Med J. 1979;2(6201):1315–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.