Abstract
Background: Increasing skeletal muscle carnitine content represents an appealing intervention in conditions of perturbed lipid metabolism such as obesity and type 2 diabetes but requires chronic l-carnitine feeding on a daily basis in a high-carbohydrate beverage.
Objective: We investigated whether whey protein ingestion could reduce the carbohydrate load required to stimulate insulin-mediated muscle carnitine accretion.
Design: Seven healthy men [mean ± SD age: 24 ± 5 y; body mass index (in kg/m2): 23 ± 3] ingested 80 g carbohydrate, 40 g carbohydrate + 40 g protein, or control (flavored water) beverages 60 min after the ingestion of 4.5 g l-carnitine tartrate (3 g l-carnitine; 0.1% 2[H]3-l-carnitine). Serum insulin concentration, net forearm carnitine balance (NCB; arterialized-venous and venous plasma carnitine difference × brachial artery flow), and carnitine disappearance (Rd) and appearance (Ra) rates were determined at 20-min intervals for 180 min.
Results: Serum insulin and plasma flow areas under the curve (AUCs) were similarly elevated by carbohydrate [4.5 ± 0.8 U/L · min (P < 0.01) and 0.5 ± 0.6 L (P < 0.05), respectively] and carbohydrate+protein [3.8 ± 0.6 U/L · min (P < 0.01) and 0.4 ± 0.6 L (P = 0.05), respectively] consumption, respectively, compared with the control visit (0.04 ± 0.1 U/L · min and −0.5 ± 0.2 L). Plasma carnitine AUC was greater after carbohydrate+protein consumption (3.5 ± 0.5 mmol/L · min) than after control and carbohydrate visits [2.1 ± 0.2 mmol/L · min (P < 0.05) and 1.9 ± 0.3 mmol/L · min (P < 0.01), respectively]. NCB AUC with carbohydrate (4.1 ± 3.1 μmol) was greater than during control and carbohydrate-protein visits (−8.6 ± 3.0 and −14.6 ± 6.4 μmol, respectively; P < 0.05), as was Rd AUC after carbohydrate (35.7 ± 25.2 μmol) compared with control and carbohydrate consumption [19.7 ± 15.5 μmol (P = 0.07) and 14.8 ± 9.6 μmol (P < 0.05), respectively].
Conclusions: The insulin-mediated increase in forearm carnitine balance with carbohydrate consumption was acutely blunted by a carbohydrate+protein beverage, which suggests that carbohydrate+protein could inhibit chronic muscle carnitine accumulation.
Keywords: doubly labeled water, energy requirement, resting energy expenditure, short bowel syndrome, total energy expenditure
INTRODUCTION
There is growing interest in the role of skeletal muscle carnitine and its associated enzymes in the perturbation of muscle lipid metabolism and the etiology of obesity and type 2 diabetes. Flux through the carnitine palmitoyltransferase 1 (CPT1)2 reaction is considered rate-limiting to fatty acid oxidation, and incomplete or insufficient β-oxidation of fatty acids has been implicated in the development of skeletal muscle insulin resistance (1, 2). In line with the proposal that availability of skeletal muscle carnitine is limiting to CPT1 flux and fatty acid oxidation (3), the elevation of muscle carnitine content via acute l-carnitine infusion (4) or chronic feeding (5) promotes physiologic and gene expression adaptations that are consistent with enhanced fat oxidation at rest and during moderate-intensity exercise. Conversely, during high-intensity exercise, when mitochondrial acetyl-group provision is limited (at least partially) by the activation kinetics of the pyruvate dehydrogenase complex (PDC) enzyme (6), increasing skeletal muscle carnitine availability and acetylation improves the matching of glycolytic, PDC, and mitochondrial substrate fluxes (7). The latter observation has implications for the enhancement of mitochondrial ATP delivery, a pertinent consideration in disease states that feature impaired or inadequate skeletal muscle perfusion (8, 9).
Increasing the availability of carnitine within skeletal muscle is therefore a useful research tool to investigate the importance of these processes in humans in vivo as well as to manipulate muscle metabolism for health benefits. However, muscle carnitine accretion, facilitated by the sodium-dependent novel organic cation transporter 2 (OCTN2) (10), occurs against a 100-fold concentration gradient and hence l-carnitine feeding (11, 12) or intravenous infusion (13, 14) alone has no impact on muscle carnitine content. The simultaneous elevation of plasma carnitine and serum insulin concentrations has proven effective in stimulating muscle carnitine uptake in healthy subjects (4, 14), and as such, a 20% increase in muscle carnitine can be achieved through twice-daily consumption of l-carnitine in a beverage containing 80 g carbohydrate over a 12- to 24-wk period (5, 7). However, such a large carbohydrate load per se (160 g/d) will likely affect metabolism and alter body composition (5), and so investigation into alternative oral insulinogenic formulations that can stimulate muscle carnitine accumulation with the use of lower carbohydrate loads is warranted. For example, whey protein has previously been given with carbohydrate to promote insulin-mediated muscle creatine retention (15), and unlike carbohydrate, prolonged protein supplementation is unlikely to influence body fat content (16). Therefore, the aim of the present study was to acutely assess forearm net carnitine balance (NCB) and uptake after oral l-carnitine ingestion in combination with 80 g carbohydrate alone compared with a protein-carbohydrate blend that would produce a similar serum insulin response.
METHODS
Volunteers
Seven healthy, nonvegetarian men [aged 24.2 ± 5.0 y; BMI (in kg/m2) 23.3 ± 3.1] provided written informed consent and attended a routine medical screening before starting the study, which was approved by the University of Nottingham Medical School Ethics Committee.
Experimental protocol
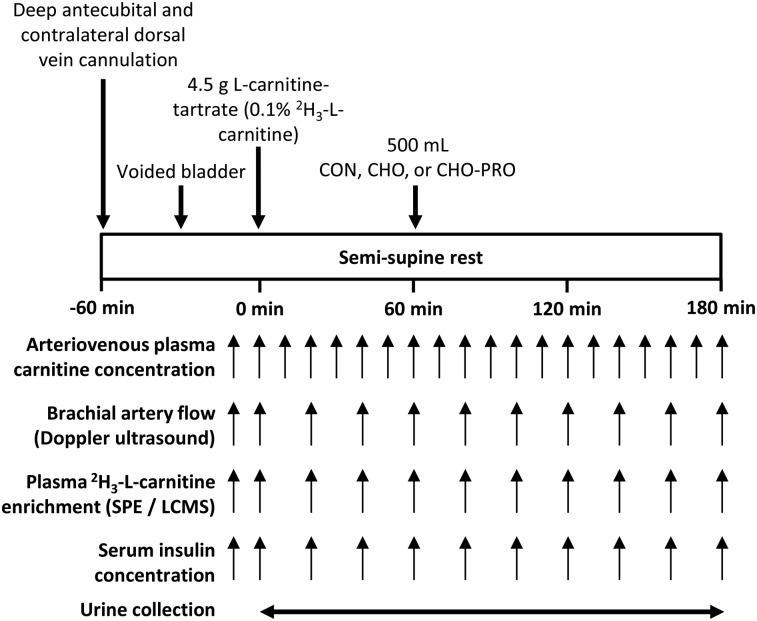
Subjects completed 3 single-blind, randomized (randomization.com) visits (Figure 1) after a 10-h fast. On arrival at the University of Nottingham David Greenfield Physiology Unit, volunteers voided their bladder and laid semi-supine with one hand heated to ∼55°C in an air-warming unit. This method typically achieves arterialized venous oxygen saturation that is within 3% of arterial blood (17) and consistently obtained >95% in the one volunteer for whom it was determined in the current study. A cannula was placed retrograde into a dorsal vein of the heated hand for arterialized venous blood sampling and into a deep-lying antecubital vein of the contralateral arm to sample venous blood draining the forearm muscle bed (18, 19). At t = 0 min, subjects ingested 4.5 g l-carnitine tartrate (Lonza) with 30 mg [methyl-2H3]-l-carnitine (Cambridge Isotopes) in 200 mL water. At t = 60 min, subjects were given a 500-mL beverage of either 80 g carbohydrate (CHO visit), 40 g carbohydrate mixed with 40 g pure whey protein isolate (CHO-PRO visit), or flavored water (CON visit). The type (Vitargo orange; Swecarb) and amount of carbohydrate chosen were used previously to increase muscle carnitine content (5, 7). Whey protein was chosen for its insulinotropic potency (20), with the isocaloric composition of carbohydrate+protein intended to elicit a comparable serum insulin response to carbohydrate (15). The whey protein isolate (PRO10.com) contained no detectable amounts of l-carnitine as assessed by radioisotopic assay. All drinks were identically colored and flavored.
FIGURE 1.
Experimental protocol for study visits. CHO, carbohydrate; CHO-PRO, carbohydrate+protein; CON, control; LCMS, liquid chromatography–mass spectrometry; SPE, solid phase extraction.
Sampling and analysis
Blood was sampled at 10-min intervals for the determination of arterialized venous serum insulin (Coat-A-Count Insulin; Siemens Health Care) and plasma acylcarnitine as well as arterialized and deep-venous whole-blood glucose (Yellow Springs Instruments) and plasma free carnitine (FC) (21) concentrations and 2[H]3-carnitine enrichment. For the latter, plasma was purified by strong cation exchange solid phase extraction (30 mg Oasis MCX 33 μm, 80Å Waters), dried to a residue, and resuspended in 0.1% formic acid for analysis by liquid chromatography–tandem mass spectrometry. Chromatography was performed in isocratic mode by using 5% acetonitrile, 0.1% formic acid in water with a C18 Brownlee column (2.1 × 300 mm, 5 μm; PerkinElmer). The peak area ratio of carnitine (m/z 162 → 60) to 2H3-carnitine (m/z 165 → 63) was subsequently determined in positive electrospray ionization mode (Quattro Ultima triple quad; Micromass Ltd.). Brachial artery blood flow of the nonheated arm was determined by ultrasound imaging (Aplio SSA-770A; Toshiba Medical Systems) with a 12-MHz transducer synchronized to a 3-lead electrocardiogram. Luminal diameter was imaged 10 cm proximal to the antecubital fossa and measured with the use of online video calipers. Mean blood velocity was determined at the same anatomic location by integration of the pulsed-wave Doppler signal (22). Blood-flow measurements were made by a single experienced operator (intrarater CV = 14%; calculated from 21 pairs of repeated baseline measurements), and values were converted to plasma flow by using individual hematocrit fractional concentrations. Urine was collected from t = 0 until t = 180 min for the determination of urinary total carnitine excretion (21).
Calculations
Plasma flow (F) was calculated as blood flow × (1 – hematocrit fraction). NCB was calculated by the Fick principle, as follows: NCB = F · (Ca − Cv′), where Ca is the arterialized venous and Cv′ is the deep-venous FC concentration adjusted for non–steady state conditions (23, 24). Fractional carnitine and glucose extraction were calculated to provide a flow-independent marker of forearm balance: Extraction = (Ca − Cv′)/Ca · 100.
The rate of carnitine disappearance (Rd) across the forearm was calculated from the steady state 2[H]3-carnitine molar percent excess (MPE) by using arterialized venous plasma as the precursor pool (25): Rd = (MPEa · Ca − MPEv · Cv) · F ÷ MPEa, where MPE is the tracer-to-tracee ratio (TTR) expressed as a percentage enrichment: MPE = TTR ÷ (1 + TTR) × 100. The rate of carnitine appearance (Ra) was also calculated: Ra = Rd – NCB.
Consistent with small-molecule pharmacokinetics, the calculations of Rd and Ra assume rapid carnitine equilibration between plasma and interstitial fluid compartments. All of the values were averaged over 20 min. Area under the (variable) × time curve (AUC) above baseline was integrated over 0–180 min.
Statistical analysis
On the basis of our previous carnitine feeding and infusion studies (26, 27), this study was powered at 80% to detect a 10% difference in NCB in 7 subjects with an α level of 0.05. Time-dependent variables (serum insulin and plasma free concentrations, plasma flow, NCB, carnitine extraction, MPE, Rd, and Ra) were analyzed by using 2-factor repeated-measures ANOVA with Bonferroni-corrected paired t test to isolate main effects post hoc. Urinary carnitine excretion and AUC were compared by using 1-factor ANOVA with Tukey’s post hoc test. All statistical analyses were performed with GraphPad Prism 6 (GraphPad Software). Data presented are means ± SEMs (SEs) for 7 subjects.
RESULTS
Serum insulin and forearm glucose balance
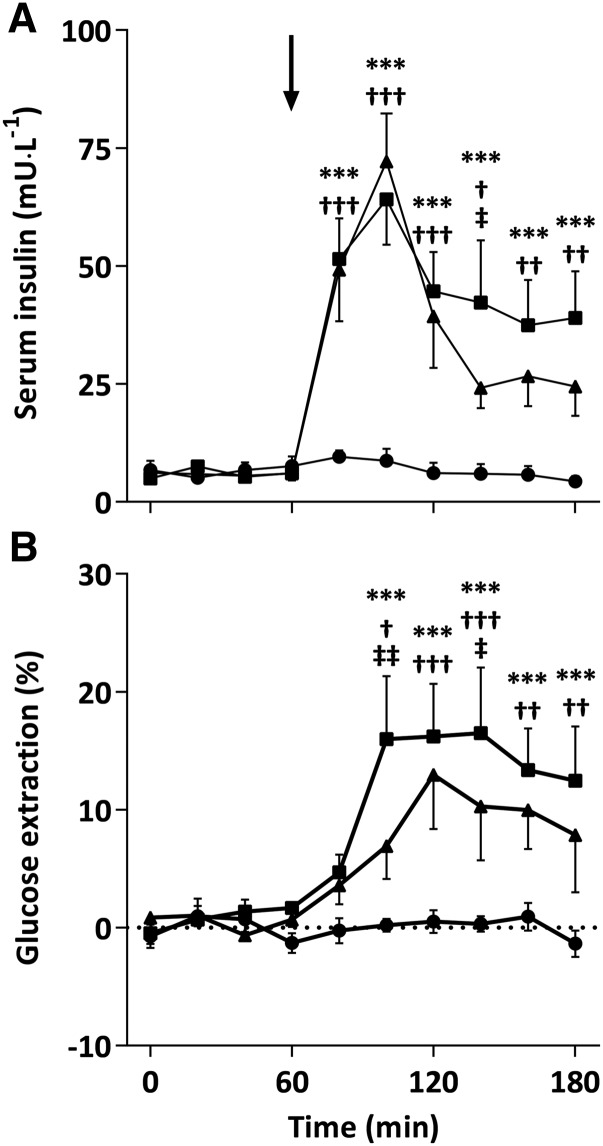
Serum insulin concentrations were similar between CON, CHO, and CHO-PRO groups at 0 min (7 ± 2, 5 ± 1, and 6 ± 1 mU/L, respectively) and did not change during the CON condition (Figure 2A). Insulin concentration increased rapidly after carbohydrate and carbohydrate+protein ingestion, peaking at 100 min (64 ± 10 and 72 ± 10 mU/L, respectively), and remained elevated above the CON condition thereafter. AUCs during the CHO (4.5 ± 0.8 U/L · min) and CHO-PRO (3.8 ± 0.6 U/L · min) visits were 102- and 85-fold greater, respectively, than in the CON visit (0.04 ± 0.1 U/L · min; P < 0.01) but were no different between CHO and CHO-PRO visits. Forearm glucose balance AUC was higher during the CHO visit (9.5 ± 3.9; P < 0.05) but not in the CHO-PRO visit (4.3 ± 1.0; P = 0.55) compared with the CON visit (1.2 ± 0.3 mmol). An interaction effect (P < 0.001) for glucose extraction was observed such that glucose extraction was higher during the CHO and CHO-PRO visit than during the CON visit from 100 to 180 min and also higher during the CHO than during the CHO-PRO visit at 100 and 140 min (Figure 2B).
FIGURE 2.
Arterialized venous serum insulin (A) and whole-blood glucose extraction (B) after ingestion of 4.5 g l-carnitine tartrate (t = 0 min) and a 500-mL drink (arrow) containing flavored water (CON; circles), 80 g carbohydrate (squares), or 40 g carbohydrate + 40 g whey protein (triangles) at t = 60 min. Values are means ± SEs (n = 7) and were compared by using 2-factor (drink × time) ANOVA with Tukey’s or Bonferroni tests used for post hoc subgroup comparisons after the observation of a significant main or interaction effect, respectively. A significant interaction effect was found for serum insulin (P < 0.001) and glucose extraction (P < 0.001) with post hoc subgroup comparisons denoted as follows: ***P < 0.001 (CHO compared with CON); †P < 0.05, ††P < 0.01, and †††P < 0.001 (CHO-PRO compared with CON); ‡P < 0.05 and ‡‡P < 0.01 (CHO compared with CHO-PRO). CHO, carbohydrate; CHO-PRO, carbohydrate+protein; CON, control.
Plasma free, acyl, and urinary total carnitine
Baseline plasma arterialized venous FC concentrations were no different (42 ± 3, 44 ± 2, and 42 ± 3 μmol/L) for CON, CHO, and CHO-PRO visits, respectively, and increased equivalently over the first hour after l-carnitine ingestion. After the ingestion of the treatment drink, FC increased sharply during the CHO-PRO visit and remained elevated above CHO and CON conditions, which continued to increase steadily, for the remainder of the visit (P < 0.001; Figure 3A). FC AUCs during the CHO-PRO condition (3.5 ± 0.5 mmol/L·min) were 67% and 84% greater than during the CON (2.1 ± 0.2 mmol/L · min; P < 0.05) and CHO (1.9 ± 0.3 mmol/L · min; P < 0.01) conditions, respectively. Plasma acylcarnitine was unchanged during the CON and CHO visits (P > 0.05 compared with baseline) but increased after carbohydrate+protein ingestion (P < 0.05 compared with baseline), such that the AUC in the CHO-PRO visit (0.56 ± 0.24 mmol/L · min) was greater (P < 0.05) than in both CON (0.34 ± 0.15 mmol/L · min) and CHO (0.34 ± 0.19 mmol/L · min) visits. Urinary total carnitine excretion was similar during CON (46 ± 14 mg) and CHO (45 ± 34 mg) conditions but was 98% and 106% greater in the CHO-PRO visit (92 ± 18 mg) than in the CON and CHO visits, respectively (both P < 0.05).
FIGURE 3.
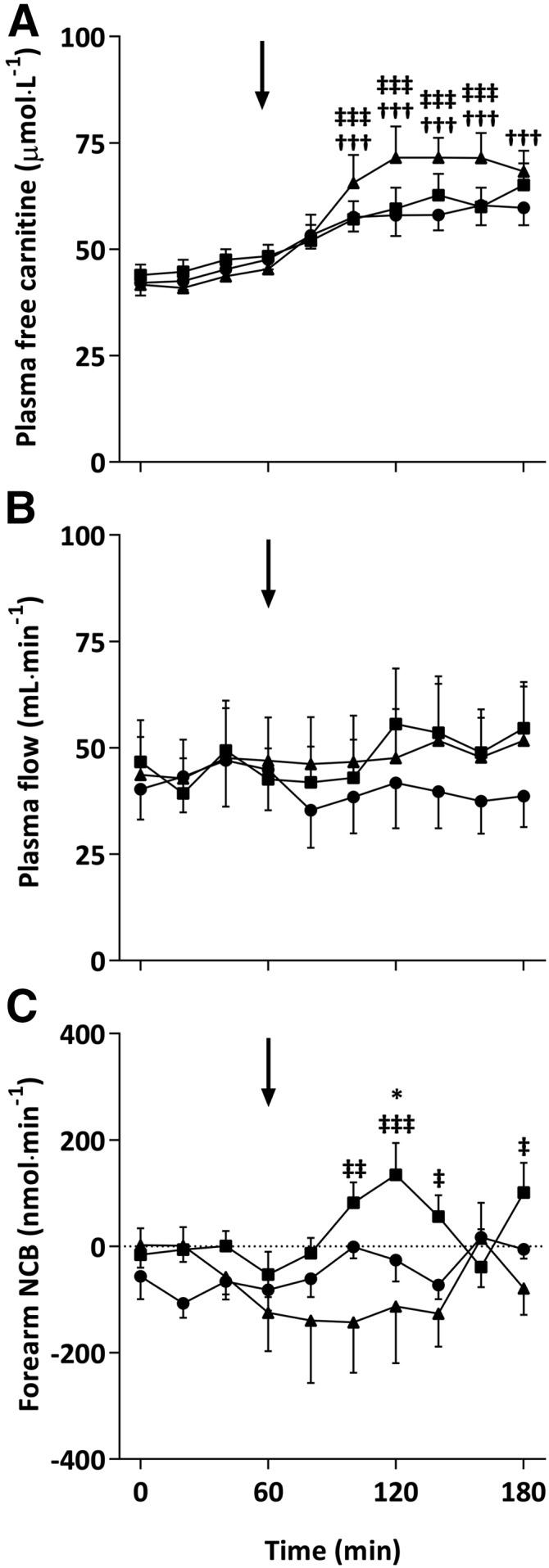
Plasma arterialized venous free carnitine concentration (A), brachial artery plasma flow (B), and forearm NCB (C) after ingestion of 4.5 g l-carnitine tartrate (t = 0 min) and a 500-mL drink (arrows) containing flavored water (CON; circles), 80 g carbohydrate (squares), or 40 g carbohydrate + 40 g whey protein (triangles) at t = 60 min. Values are means ± SEs (n = 7) and were compared by using 2-factor (drink × time) ANOVA with Tukey’s or Bonferroni tests used for post hoc subgroup comparisons after the observation of a significant main or interaction effect, respectively. Plasma flow was greater in both CHO and CHO-PRO groups compared with the CON group (main effect of drink, P < 0.05). An interaction effect (P < 0.05) was found for plasma free carnitine and NCB with post hoc subgroup comparisons denoted as follows: *P < 0.05 (CHO compared with CON); †††P < 0.001 (CHO-PRO compared with CON); ‡P < 0.05, ‡‡P < 0.01, and ‡‡‡P < 0.001 (CHO compared with CHO-PRO). CHO, carbohydrate; CHO-PRO, carbohydrate+protein; CON, control; NCB, net carnitine balance.
Plasma flow
Plasma flow was similar at baseline for the CON, CHO, and CHO-PRO trials (40 ± 7, 47 ± 10, and 44 ± 9 mL/min, respectively) and did not change over the first hour. A main effect of drink (P < 0.05) was observed such that plasma flow was greater in CHO and CHO-PRO visits than in the CON visit (Figure 3B). Plasma flow AUC above baseline (0–60 min) was greater in the CHO visit (0.5 ± 0.6 L; P < 0.05) and tended to be greater in the CHO-PRO visit (0.4 ± 0.6 L; P = 0.05) than in the CON visit (−0.5 ± 0.2 L).
Net forearm carnitine balance and extraction
NCB across the forearm is shown in Figure 3C and was unchanged over the 1 h after l-carnitine ingestion. After consumption of the treatment drink, NCB increased in the CHO visit only (interaction effect, P < 0.05), peaking at 120 min (135 ± 60 nmol/min) above CON (−26 ± 40 nmol/min; P < 0.05) and CHO-PRO (−113 ± 107 nmol/min; P < 0.05) conditions. This resulted in a greater AUC in the CHO visit (4.1 ± 3.1 μmol) than in the CON (−8.6 ± 3.0 μmol) and CHO-PRO (−14.6 ± 6.4 μmol) visits (P < 0.05). Carnitine extraction was also higher (P < 0.05) in the CHO trial (3.1% ± 1.2%, 3.2% ± 1.7%, and 3.0% ± 0.9%) than in the CHO-PRO trial (−2.9% ± 2.5%, −2.4% ± 2.9%, and −2.9% ± 2.3%) at t = 100, 120, and 180 min, respectively, and tended to be higher (P = 0.09) in the CHO trial than in the CON trial (−1.2% ± 1.5%) at t = 120 min.
Plasma [2H3]-carnitine enrichment, Rd, and Ra
Plasma [2H3]-carnitine enrichment reached a steady state after t = 80 min in all trials, with a similar MPE attained in CON and CHO visits (∼0.14%) but with a slightly higher MPE of ∼0.2% in the CHO-PRO visit (Figure 4A). The plasma carnitine Rd was unchanged throughout in the CON and CHO-PRO visits but tended to increase in the CHO visit (Figure 4B), resulting in a 1.8- and 2.4-fold greater AUC above zero in the CHO visit (35.7 ± 25.2 μmol) than in the CON (19.7 ± 15.5 μmol; P = 0.07) and CHO-PRO (14.8 ± 9.6 μmol; P < 0.05) visits, respectively. Carnitine Ra was similar and no different from zero in the CON, CHO, and CHO-PRO visits (Figure 4C).
FIGURE 4.
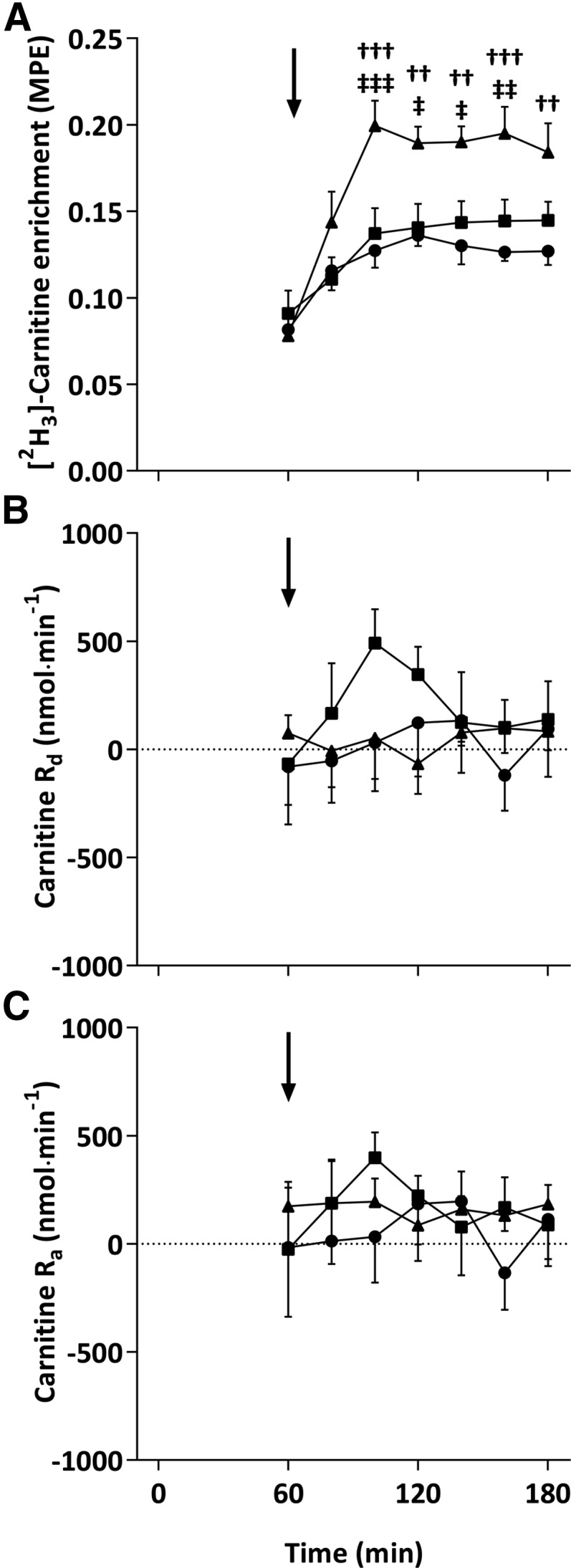
Arterialized venous plasma [3H2]-carnitine enrichment (MPE; A), Rd (B), and Ra (C) after ingestion of 4.5 g l-carnitine tartrate (t = 0 min) and a 500-mL drink (arrows) containing flavored water (CON; circles), 80 g carbohydrate (squares), or 40 g carbohydrate + 40 g whey protein (triangles) at t = 60 min. Values are means ± SEs (n = 7) and were compared by using 2-factor (drink × time) ANOVA with Tukey’s or Bonferroni tests used for post hoc subgroup comparisons after the observation of a significant main or interaction effect, respectively. An interaction effect (P < 0.001) was found for plasma [3H2]-carnitine enrichment with post hoc subgroup comparisons denoted as follows: ††P < 0.01 and †††P < 0.001 (CHO-PRO compared with CON); ‡P < 0.05, ‡‡P < 0.01, and ‡‡‡P < 0.001 (CHO compared with CHO-PRO). CHO, carbohydrate; CHO-PRO, carbohydrate+protein; CON, control; MPE, molar percent excess; Ra, rate of carnitine appearance; Rd, rate of carnitine disappearance.
DISCUSSION
To our knowledge, these novel data provide the most direct measurement to date of acute muscle carnitine uptake in vivo in humans and show that the ingestion of a carbohydrate beverage is able to promote a positive NCB across the forearm, indicative of accelerated muscle carnitine accretion. Moreover, the replacement of some of this carbohydrate with whey protein prohibited any increase in NCB, despite inducing a similar serum insulin and plasma flow response. Thus, it would appear that the mechanism by which insulin stimulates muscle carnitine transport is antagonized by a large bolus of whey protein.
Studies from this laboratory previously showed that the elevation of plasma carnitine concentration via intravenous infusion alone has no impact on muscle carnitine content (14), whereas acute (28) or chronic (11) oral dosing of l-carnitine does not affect net leg carnitine balance or muscle carnitine content, respectively. A recent study of porcine arteriovenous carnitine fluxes confirmed that net muscle carnitine uptake/efflux is negligible under normal conditions, with systemic concentrations of carnitine and acylcarnitines largely governed by gut absorption, hepatic release, and renal filtration (29). The latter is consistent with the preservation of total muscle carnitine under conditions of increased metabolic flux, such as during hyperinsulinemic-euglycemic insulin clamp (4), or exercise (7). These data refute the speculated role of skeletal muscle acetylcarnitine influx/efflux in metabolic health (30, 31) and question the validity of the physiologic inferences that can be made from in vitro studies of carnitine metabolism (31, 32). In agreement with previous in vivo data and consistent with the high concentration gradient between plasma and tissues, carnitine ingestion in the current study had no detectable impact on NCB during the CON condition. In contrast, carbohydrate facilitated a positive NCB across the forearm, further validating the ingestion of l-carnitine in a carbohydrate beverage as a means to augment muscle carnitine content (7). The majority of the measured plasma carnitine extraction across the forearm likely occurred into skeletal muscle (33), and thus it is possible to estimate whole-body rates of insulin-stimulated muscle carnitine accretion. Insulin was elevated from 80 to 180 min during the CHO condition, over which period the NCB AUC was 7.9 μmol greater than with the CON condition. Assuming an average forearm muscle mass of 0.6 kg (18, 34) and a whole-body muscle mass of 30 kg, this equates to a whole-body muscle carnitine uptake of 390 μmol above the CON condition. This aligns well with the 370 μmol (60 mg) of carnitine retention predicted from differences in urinary carnitine excretion in a previous study (27). Extended to a chronic feeding scenario, this would equate to a daily increase in muscle carnitine content of 13 μmol · kg wet weight−1, which would augment muscle total carnitine content stores (∼5 mmol · kg wet weight−1) by 22% over 12 wk. Again, this extrapolation is in good agreement with the 21% increase in muscle carnitine content reported by Stephens et al. (5) and provides indirect validation for our values of NCB.
Limb/organ balance models normally preclude definitive conclusions on whether substrate uptake, efflux, or a combination of the 2 has occurred (29). Here, the use of 2H3-carnitine tracer enabled a more direct interrogation of muscle carnitine uptake. The average rate of forearm carnitine disappearance throughout the CON trial (∼9 μmol · kg · h−1) is very similar to estimated rates of basal muscle carnitine uptake (11.6 μmol · kg · h−1) from compartmental modeling of intravenously administered [3H]-carnitine kinetics (35). Peak Rd during carbohydrate ingestion was numerically 15-fold higher than the equivalent time point in the CON condition and tended to be greater when compared over the entire treatment period (AUC). It could be argued that increases in NCB and Rd during carbohydrate ingestion were related to greater plasma flow. However, carnitine fractional extraction, which does not depend on flow, was also elevated after carbohydrate ingestion but not with the CON condition, whereas plasma flow was similarly elevated above the CON condition in the CHO-PRO visit (in which extraction was unchanged). Moreover, peak carnitine Rd during the CHO condition coincided with peak serum insulin concentration, suggesting that the positive NCB during carbohydrate ingestion is more likely attributable to an upregulation of muscle carnitine transport, rather than plasma flow.
Serum insulin concentrations were elevated above the 50-mU/L threshold for stimulating muscle carnitine uptake (26) for a similar duration in the CHO and CHO-PRO visits, coinciding with the elevation of NCB in the CHO visits. It was thus surprising that NCB was not similarly increased during the CHO-PRO condition, a finding supported by the observations that Rd was suppressed, whereas plasma carnitine concentration and urinary carnitine excretion were augmented in the CHO-PRO relative to CHO conditions. One possible explanation for this is an antagonism of muscle carnitine transport by acylcarnitines, which were elevated in the plasma throughout the period of hyperinsulinemia in the CHO-PRO visit and are known to inhibit the activity of OCTN2 (36), the obligate transporter protein in muscle carnitine accumulation (10, 37). Indeed, plasma acylcarnitines have been shown to accumulate in response to excessive amino acid oxidation or incomplete β-oxidation (38) and are characteristically elevated in the insulin-resistant state (39), a scenario in which muscle carnitine accumulation is purportedly compromised.
Whey protein ingestion (∼30-g bolus) was recently shown to impair insulin-stimulated glucose disposal (40), which is consistent with the lower forearm glucose extraction in CHO-PRO compared with CHO conditions in the current study. A similar effect of increased amino acid availability on insulin-mediated muscle carnitine uptake cannot be ruled out, because high rates of insulin-responsive, sodium-dependent amino acid flux could plausibly restrict cationic muscle carnitine transport (41, 42), although this requires further study. It should also be noted that the increased glucose extraction in the CHO condition aligns with previous reports of increased glucose disposal after an acute increase in skeletal muscle carnitine (4, 43). Why the uptake of carnitine by skeletal muscle would be inhibited by protein, given that the predominant dietary source of carnitine is meat (44), is somewhat perplexing, although it should be noted that the large bolus of protein ingested in this study is excessive compared with the protein content of a normal mixed meal. Nevertheless, a slow postprandial transport of carnitine into muscle, together with a negligible Ra of carnitine from muscle, is entirely consistent with the stability and slow turnover of the muscle carnitine pool (35).
It was previously suggested that amino acids could inhibit l-carnitine intestinal absorption (45). However, the greater urinary and plasma carnitine in the CHO-PRO visit during the current study may provide evidence to the contrary. When compared with the CHO visit, the elevated plasma and urinary carnitine in the CHO-PRO visit can reasonably be accounted for by the estimated difference in muscle carnitine uptake. When comparing CON and CHO-PRO trials, however, during which NCB was similarly negligible, the differences in plasma and urinary carnitine would suggest that carnitine absorption was not equal across all trials. Considering the low rate of endogenous carnitine synthesis, ∼25 nmol/min for a 75-kg nonvegetarian man (46) and assuming a negligible intracellular release of carnitine during the CON and CHO-PRO conditions (Ra was not different from zero), the greater plasma MPE during the CHO-PRO condition likely reflects a greater absorption of exogenous carnitine into the endogenous carnitine pool. On the basis of previous studies (47–49), it was expected that a 3-g dose of l-carnitine would saturate intestinal active carnitine transport and thus facilitate equivalent carnitine absorption across all trials. However, and in contrast to the suggestion that amino acids may inhibit intestinal carnitine absorption, these findings infer that protein ingestion may increase coingested carnitine absorption. This reconciles with the predominant dietary sources of carnitine and, given the blunting of forearm NCB in the CHO-PRO condition, also implies that the mechanism and regulation of intestinal carnitine absorption are perhaps different from those of skeletal muscle. It is important to acknowledge that the use of arterialized venous sampling may lead to a systematic underestimation of arterial carnitine extraction, but assuming a similar arterialization was obtained across all visits, the implications of these data still remain valid.
In conclusion, the novel use of an acute arteriovenous forearm balance model with 2H3-l-carnitine tracer methodology affirms the absence of appreciable muscle carnitine uptake (or efflux) after l-carnitine ingestion alone and confirms the efficacy of a carbohydrate beverage in promoting muscle carnitine accretion. Conversely, a carbohydrate+protein blend entirely blunted this stimulation of muscle carnitine uptake, despite comparable serum insulin concentration, plasma flow responses, and apparent increased intestinal carnitine absorption.
Acknowledgments
The authors’ responsibilities were as follows—CES, PLG, and FBS: designed the research; CES and AVN: conducted the research and analyzed the data; CES: wrote the manuscript; PLG and FBS: critically revised the manuscript; FBS: had primary responsibility for the final content; and all authors: read and approved the final manuscript. The authors had no conflicts of interest to declare.
Footnotes
Abbreviations used: CHO, carbohydrate; CHO-PRO, carbohydrate+whey protein; CON, control; CPT1, carnitine palmitoyltransferase 1; FC, free carnitine; MPE, molar percent excess; NCB, net carnitine balance; OCTN2, novel organic cation transporter 2; PDC, pyruvate dehydrogenase complex; Ra, rate of appearance; Rd, rate of disappearance; TTR, tracer-to-tracee ratio.
REFERENCES
- 1.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, et al. . Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 2008;7:45–56. [DOI] [PubMed] [Google Scholar]
- 2.Keung W, Ussher JR, Jaswal JS, Raubenheimer M, Lam VH, Wagg CS, Lopaschuk GD. Inhibition of carnitine palmitoyltransferase-1 activity alleviates insulin resistance in diet-induced obese mice. Diabetes 2013;62:711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Loon LJC, Greenhaff PL, Constantin-Teodosiu D, Saris WHM, Wagenmakers AJM. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol 2001;536:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. An acute increase in skeletal muscle carnitine content alters fuel metabolism in resting human skeletal muscle. J Clin Endocrinol Metab 2006;91:5013–8. [DOI] [PubMed] [Google Scholar]
- 5.Stephens FB, Wall BT, Marimuthu K, Shannon CE, Constantin-Teodosiu D, Macdonald IA, Greenhaff PL. Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans. J Physiol 2013;591:4655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenhaff PL, Campbell-O’Sullivan SP, Constantin-Teodosiu D, Poucher SM, Roberts PA, Timmons JA. An acetyl-group deficit limits mitochondrial ATP production at the onset of exercise. Biochem Soc Trans 2002;30. [DOI] [PubMed] [Google Scholar]
- 7.Wall BT, Stephens FB, Constantin-Teodosiu D, Marimuthu K, Macdonald IA, Greenhaff PL. Chronic oral ingestion of l-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. J Physiol 2011;589:963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvert LD, Shelley R, Singh SJ, Greenhaff PL, Bankart J, Morgan MD, Steiner MC. Dichloroacetate enhances performance and reduces blood lactate during maximal cycle exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:1090–4. [DOI] [PubMed] [Google Scholar]
- 9.Bauer TA, Regensteiner JG, Brass EP, Hiatt WR. Oxygen uptake kinetics during exercise are slowed in patients with peripheral arterial disease. J Appl Physiol 1999;87:809–16. [DOI] [PubMed] [Google Scholar]
- 10.Tamai I, Ohashi R, Nezu J, Yabuuchi H, Oku A, Shimane M, Sai Y, Tsuji A. Molecular and functional identification of sodium ion-dependent, high affinity human carnitine transporter OCTN2. J Biol Chem 1998;273:20378–82. [DOI] [PubMed] [Google Scholar]
- 11.Wachter S, Vogt M, Kreis R, Boesch C, Bigler P, Hoppeler H, Krahenbuhl S.. Long-term administration of l-carnitine to humans: effect on skeletal muscle carnitine content and physical performance. Clin Chim Acta 2002;318(1–2):51–61. [DOI] [PubMed] [Google Scholar]
- 12.Vukovich MD, Costill DL, Fink WJ. Carnitine supplementation: effect on muscle carnitine and glycogen content during exercise. Med Sci Sports Exerc 1994;26:1122–9. [PubMed] [Google Scholar]
- 13.Brass EP, Hoppel CL, Hiatt WR. Effect of intravenous l-carnitine on carnitine homeostasis and fuel metabolism during exercise in humans. Clin Pharmacol Ther 1994;55:681–92. [DOI] [PubMed] [Google Scholar]
- 14.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. Insulin stimulates l-carnitine accumulation in human skeletal muscle. FASEB J 2006;20(2):377–9. [DOI] [PubMed] [Google Scholar]
- 15.Steenge GR, Simpson EJ, Greenhaff PL. Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. J Appl Physiol 2000;89:1165–71. [DOI] [PubMed] [Google Scholar]
- 16.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64. [DOI] [PubMed] [Google Scholar]
- 17.Liu D, Moberg E, Kollind M, Lins PE, Adamson U, Macdonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia 1992;35:287–90. [DOI] [PubMed] [Google Scholar]
- 18.Andres R, Zierler KL, Anderson HM, Stainsby WN, Cader G, Ghrayyib AS, Lilienthal JL Jr. Measurement of blood flow and volume in the forearm of man; with notes on the theory of indicator-dilution and on production of turbulence, hemolysis, and vasodilatation by intra-vascular injection. J Clin Invest 1954;33:482–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallen IW, Macdonald IA. Effect of two methods of hand heating on body temperature, forearm blood flow, and deep venous oxygen saturation. Am J Physiol 1990;259:E639–43. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson M, Holst JJ, Bjorck IM. Metabolic effects of amino acid mixtures and whey protein in healthy subjects: studies using glucose-equivalent drinks. Am J Clin Nutr 2007;85:996–1004. [DOI] [PubMed] [Google Scholar]
- 21.Cederblad G, Lindstedt S.. A method for the determination of carnitine in the picomole range. Clin Chim Acta 1972;37:235–43. [DOI] [PubMed] [Google Scholar]
- 22.Hoskins PR. Measurement of arterial blood flow by Doppler ultrasound. Clin Phys Physiol Meas 1990;11:1–26. [DOI] [PubMed] [Google Scholar]
- 23.Zierler KL. Theory of the use of arteriovenous concentration differences for measuring metabolism in steady and non-steady states. J Clin Invest 1961;40:2111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tessari P, Inchiostro S, Biolo G, Vincenti E, Sabadin L. Effects of acute systemic hyperinsulinemia on forearm muscle proteolysis in healthy man. J Clin Invest 1991;88:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and proctice of kinetic analysis. 2nd ed. Hoboken (NJ): John Wiley & Sons; 2005. [Google Scholar]
- 26.Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. A threshold exists for the stimulatory effect of insulin on plasma l-carnitine clearance in humans. Am J Physiol Endocrinol Metab 2007;292:E637–41. [DOI] [PubMed] [Google Scholar]
- 27.Stephens FB, Evans CE, Constantin-Teodosiu D, Greenhaff PL. Carbohydrate ingestion augments l-carnitine retention in humans. J Appl Physiol 2007;102:1065–70. [DOI] [PubMed] [Google Scholar]
- 28.Soop M, Bjorkman O, Cederblad G, Hagenfeldt L, Wahren J. Influence of carnitine supplementation on muscle substrate and carnitine metabolism during exercise. J Appl Physiol 1988;64:2394–9. [DOI] [PubMed] [Google Scholar]
- 29.Schooneman MG, Ten Have GA, Van Vlies N, Houten SM, Deutz NE, Soeters MR. Transorgan fluxes in a porcine model reveal a central role for liver in acylcarnitine metabolism. Am J Physiol Endocrinol Metab 2015;309:E256–64. [DOI] [PubMed] [Google Scholar]
- 30.Muoio DM, Noland RC, Kovalik JP, Seiler SE, Davies MN, DeBalsi KL, Ilkayeva OR, Stevens RD, Kheterpal I, Zhang J, et al. . Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab 2012;15:764–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seiler SE, Martin OJ, Noland RC, Slentz DH, DeBalsi KL, Ilkayeva OR, An J, Newgard CB, Koves TR, Muoio DM. Obesity and lipid stress inhibit carnitine acetyltransferase activity. J Lipid Res 2014;55:635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seiler SE, Koves TR, Gooding JR, Wong KE, Stevens RD, Ilkayeva OR, Wittmann AH, DeBalsi KL, Davies MN, Lindeboom L, et al. . Carnitine acetyltransferase mitigates metabolic inertia and muscle fatigue during exercise. Cell Metab 2015;22:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brass EP. Pharmacokinetic considerations for the therapeutic use of carnitine in hemodialysis patients. Clin Ther 1995;17:176–85; discussion: 5. [DOI] [PubMed] [Google Scholar]
- 34.Allwood MJ, Hensel H, Papenberg J. Muscle and skin blood flow in the human forearm during insulin hypoglycaemia. J Physiol 1959;147:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rebouche CJ, Engel AG. Kinetic compartmental analysis of carnitine metabolism in the human carnitine deficiency syndromes: evidence for alterations in tissue carnitine transport. J Clin Invest 1984;73:857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohashi R, Tamai I, Yabuuchi H, Nezu J-I, Oku A, Sai Y, Shimane M, Tsuji A. Na+-dependent carnitine transport by organic cation transporter (OCTN2): its pharmacological and toxicological relevance. J Pharmacol Exp Ther 1999;291:778–84. [PubMed] [Google Scholar]
- 37.Treem WR, Stanley CA, Finegold DN, Hale DE, Coates PM. Primary carnitine deficiency due to a failure of carnitine transport in kidney, muscle, and fibroblasts. N Engl J Med 1988;319:1331–6. [DOI] [PubMed] [Google Scholar]
- 38.Stephens FB, Mendis B, Shannon CE, Cooper S, Ortori CA, Barrett DA, Mansell P, Tsintzas K. Fish oil omega-3 fatty acids partially prevent lipid-induced insulin resistance in human skeletal muscle without limiting acylcarnitine accumulation. Clin Sci (Lond) 2014;127(5):315–22. [DOI] [PubMed] [Google Scholar]
- 39.Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, Hwang DH, Newman JW, Garvey WT. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 2009;139:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith GI, Yoshino J, Stromsdorfer KL, Klein SJ, Magkos F, Reeds DN, Klein S, Mittendorfer B. Protein ingestion induces muscle insulin resistance independent of leucine-mediated mTOR activation. Diabetes 2015;64:1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen GJ, Russell JB. Sodium-dependent transport of branched-chain amino acids by a monensin-sensitive ruminal peptostreptococcus. Appl Environ Microbiol 1989;55:2658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White MF, Gazzola GC, Christensen HN. Cationic amino acid transport into cultured animal cells. I. Influx into cultured human fibroblasts. J Biol Chem 1982;257:4443–9. [PubMed] [Google Scholar]
- 43.De Gaetano A, Mingrone G, Castagneto M, Calvani M. Carnitine increases glucose disposal in humans. J Am Coll Nutr 1999;18:289–95. [DOI] [PubMed] [Google Scholar]
- 44.Durán JM, Peral MJ, Calonge ML, Ilundain AA. Functional characterization of intestinal l-carnitine transport. J Membr Biol 2002;185:65–74. [DOI] [PubMed] [Google Scholar]
- 45.Taylor PM. Absorbing competition for carnitine. J Physiol 2001;532(2):283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rebouche CJ, Chenard CA. Metabolic fate of dietary carnitine in human adults: identification and quantification of urinary and fecal metabolites. J Nutr 1991;121:539–46. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton JW, Li BU, Shug AL, Olsen WA. Carnitine transport in human intestinal biopsy specimens: demonstration of an active transport system. Gastroenterology 1986;91:10–6. [DOI] [PubMed] [Google Scholar]
- 48.Li B, Lloyd ML, Gudjonsson H, Shug AL, Olsen WA. The effect of enteral carnitine administration in humans. Am J Clin Nutr 1992;55:838–45. [DOI] [PubMed] [Google Scholar]
- 49.Harper P, Elwin CE, Cederblad G. Pharmacokinetics of bolus intravenous and oral doses of l-carnitine in healthy subjects. Eur J Clin Pharmacol 1988;35:69–75. [DOI] [PubMed] [Google Scholar]