 A concise synthesis of (–)-chloramphenicol, based on the catalytic asymmetric aldol reaction between 4-nitrobenzaldehyde and benzhydryl isocyanoacetate, is reported.
A concise synthesis of (–)-chloramphenicol, based on the catalytic asymmetric aldol reaction between 4-nitrobenzaldehyde and benzhydryl isocyanoacetate, is reported.
Abstract
The highly enantio- and diastereoselective aldol reaction of isocyanoacetates catalysed by Ag2O and cinchona-derived amino phosphines applied to the synthesis of (–)- and (+)-chloramphenicol is described. The concise synthesis showcases the utility of this catalytic asymmetric methodology for the preparation of bioactive compounds possessing α-amino-β-hydroxy motifs.
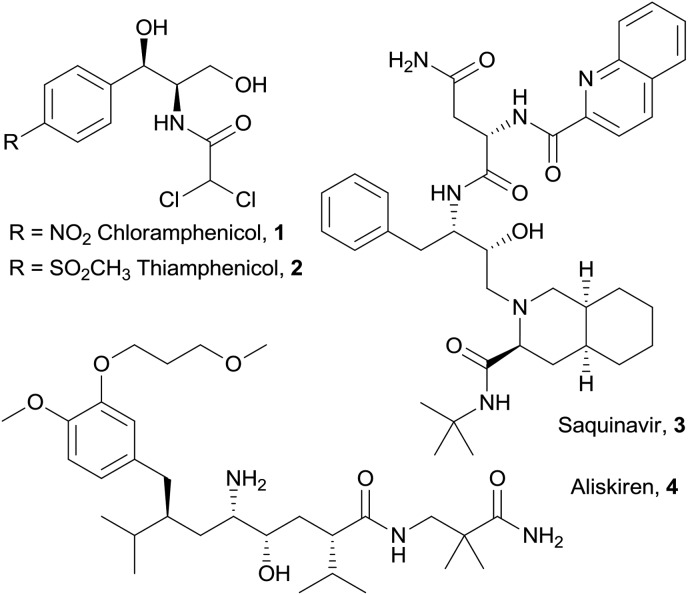
Chiral vicinal amino alcohols represent a very important class of compounds, which are of interest to synthetic chemists not only as valuable building blocks and chiral auxiliaries, but also by virtue of their pharmacological properties.1 Bioactive vicinal amino alcohols of differing complexity include the broad spectrum antibiotics chloramphenicol2 (1) and thiamphenicol3 (2), the protease inhibitor for HIV treatment saquinavir4 (3) and the antihypertensive drug aliskiren5 (4, Fig. 1). Among others,1,6 a privileged access to these structures is offered by the aldol reaction of glycine equivalents,7 including isocyanoacetates,8 followed by reduction of the carboxylic group. Recently, our group developed a cooperative catalytic system consisting of a Lewis acid (Ag+) and a cinchona-derived amino phosphine ligand, bearing both Brønsted and Lewis basic sites, for the activation of isocyanoacetate pronucleophiles towards electrophiles, such as aldehydes,9 ketimines10 and ketones.11
Fig. 1. Selected pharmaceuticals containing vicinal amino alcohol fragments.
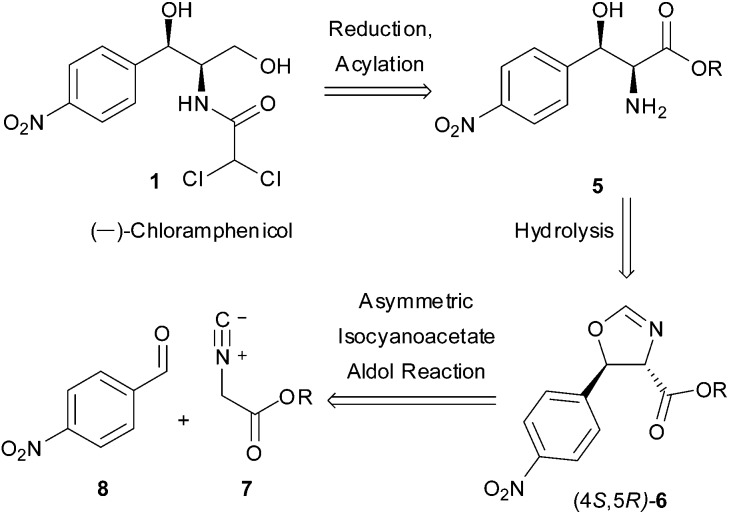
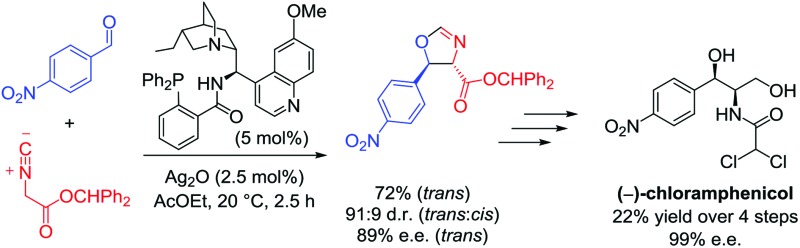
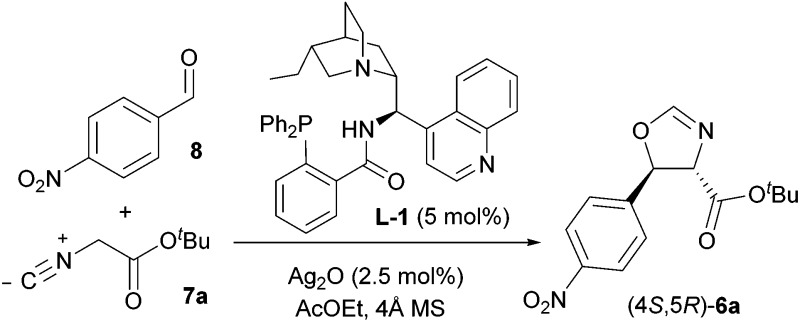
As a direct demonstration of the utility of this asymmetric methodology, herein we report a short asymmetric synthesis of (–)-chloramphenicol,12 which is the first one relying on a catalytic enantio- and diastereoselective aldol reaction.13 According to our retrosynthetic plan, outlined in Scheme 1, (–)-chloramphenicol would be derived through standard chemical manipulations from the trans oxazoline (4S,5R)-6.14 It was envisioned that the latter could be obtained via the Ag-catalysed asymmetric isocyanoacetate aldol reaction (IAR) between a suitable isocyanoacetate ester15 7 and 4-nitrobenzaldehyde (8). Specifically, on the basis of our previous work9 it was anticipated that the use of cinchonine-derived amino phosphine L-1 as chiral ligand in the IAR would provide oxazoline 6 with the desired absolute configuration for the preparation of (–)-chloramphenicol (Table 1).
Scheme 1. Retrosynthetic approach to (–)-chloramphenicol.
Table 1. Temperature and concentration screening in the isocyanoacetate aldol reaction between 8 and 7a a .
| ||||||
| Entry | T (°C) | [7a] (M) | Time (h) | Yield b (%) | d.r. c (trans : cis) | e.e. d (%) |
| 1 e | –20 | 0.3 | 21 f | 45 | 91 : 9 | 44 |
| 2 e | 20 | 0.3 | 0.25 | 59 | 91 : 9 | 53 |
| 3 | 20 | 0.05 | 0.5 | 73 | 91 : 9 | 69 |
| 4 | 20 | 0.01 | 0.5 | 70 | 91 : 9 | 78 |
| 5 | 20 | 0.0025 | 17 g | 70 | 90 : 10 | 80 |
| 6 | 0 | 0.01 | 16 f | 72 | 90 : 10 | 72 |
| 7 | 50 | 0.01 | 2 | 61 | 88 : 12 | 65 |
aReaction performed on 0.25 mmol of 7a using 1.1 eq. of 8. Configuration of 6a assigned by analogy with previous work.9
bIsolated yield of trans diastereomer after FCC.
cd.r. determined by 1H NMR analysis of the crude reaction mixture.
de.e. of trans diastereomer determined by HPLC on chiral stationary phase.
e0.50 mmol of 7a.
fStirred overnight, as TLC control after 3 hours indicated that the reaction was progressing.
gStirred overnight, as TLC control after 6 hours indicated that the reaction was progressing.
Our investigation thus began by performing the reaction between 8 and tert-butyl isocyanoacetate 7a under the conditions that had already been optimised for a range of aldehydes, namely in AcOEt at –20 °C and 0.3 M concentration, employing 5 mol% L-1 and 2.5 mol% Ag2O. To our surprise, the desired trans oxazoline 6a was obtained with modest yield (45%) and low enantioselectivity (44% e.e., Table 1, entry 1), pointing out the need for optimisation of the IAR. Screening of several reaction parameters (concentration, temperature, solvent, nature of the isocyanoacetate ester group, structure of the ligand and Ag/ligand ratio) was therefore undertaken, and the main findings are reported below.
Adjustment of the temperature to 20 °C was beneficial both for yield (59%) and enantiocontrol (53% e.e., entry 2). At this temperature, dilution of the reaction mixture to 0.01 M isocyanoacetate concentration afforded the desired product 6a in 30 minutes with good yield and stereoselectivity (70% yield, 91 : 9 d.r., 78% e.e., entry 4). These conditions improved solubility, diminishing a competitive non-asymmetric background reaction catalysed by Ag2O only, which we hypothesized was responsible for the poor enantioselectivity observed at lower temperature and higher concentration. At the same time, dilution of the reaction mixture increased the yield of the product by reducing the amount of undesired double aldol side product. Further dilution to 0.0025 M resulted in slightly improved enantiocontrol (80% e.e., entry 5) over longer reaction time, but it was discarded for practical scale-up reasons. A temperature screen at 0.01 M (entries 6 and 7) revealed that 20 °C was the optimal temperature. A quick solvent survey confirmed AcOEt to be optimal (see ESI, Table S1†).
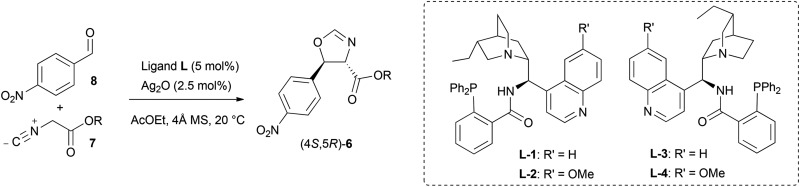
With the optimised reaction conditions established, the performance of isocyanoacetates with different ester groups was then investigated (Table 2). Methyl isocyanoacetate 7b (entry 2) performed better than its tert-butyl analogue 7a (entry 1), suggesting that excessive steric bulk hampered the transmission of stereochemical information. However, the presence of a benzyl or benzhydryl group was well-tolerated: from isocyanoacetates 7c and 7f the desired oxazolines could be obtained with the highest enantioselectivity (87% e.e., entries 3 and 7). Isocyanoacetates 7d and 7e, possessing 4-methoxybenzyl and 3,5-bis(trifluoromethyl)benzyl groups respectively, provided enantioenriched oxazolines with similar e.e. (86% and 84% e.e. respectively, entries 5 and 6), suggesting that electronic factors didn't play a major role in enantiocontrol.
Table 2. Pronucleophile and ligand screening in the isocyanoacetate aldol reaction between 8 and 7 a .
| |||||||
| Entry | R | 7 | Ligand | Time (min) | Yield b (%) | d.r. c (trans : cis) | e.e. d (%) |
| 1 | (CH3)3C | 7a | L-1 | 30 | 70 | 91 : 9 | 78 |
| 2 | CH3 | 7b | L-1 | 100 | 80 | 88 : 12 | 82 |
| 3 | PhCH2 | 7c | L-1 | 180 | 61 | 90 : 10 | 87 |
| 4 | PhCH2 | 7c | L-2 | 80 | 64 | 90 : 10 | 87 |
| 5 | 4-(OCH3) C6H4CH2 | 7d | L-2 | 60 | 63 | 89 : 11 | 86 |
| 6 | 3,5-(CF3)2 C6H3CH2 | 7e | L-2 | 60 | 56 | 90 : 10 | 84 |
| 7 | Ph2CH | 7f | L-1 | 100 | 81 | 93 : 7 | 87 |
| 8 | Ph2CH | 7f | L-2 | 45 | 78 | 91 : 9 | 89 |
| 9 | Ph2CH | 7f | L-3 | 200 | 82 | 93 : 7 | 88 e |
| 10 | Ph2CH | 7f | L-4 | 60 | 68 | 92 : 8 | 93 e |
aReaction performed on 0.25 mmol of 7 (0.01 M in AcOEt) using 1.1 eq. of 8. Configuration of 6 assigned by analogy with previous work.9
bIsolated yield of trans diastereomer after FCC.
cd.r. determined by 1H NMR analysis of the crude reaction mixture.
de.e. of trans diastereomer determined by HPLC on chiral stationary phase.
eOpposite enantiomer obtained.
Next the effect of fine tuning of the ligand was taken into account by testing four amino phosphines prepared from 9-amino(9-deoxy) epicinchona alkaloids (Table 2). The use of quinidine-derived L-2 in the reaction between the benzhydryl isocyanoacetate 7f and 8 resulted in further improved enantiocontrol (89% e.e., entry 8), whereas no boost in enantioselectivity was observed starting from benzyl isocyanoacetate 7c (87% e.e., entry 4). The pseudoenantiomeric catalytic system comprising quinine-derived L-4 afforded the enantiomeric oxazoline (4R,5S)-6f in slightly lower yield (68%) and better stereocontrol (92 : 8 d.r., 93% e.e., entry 10).
Finally catalyst loading studies confirmed the ideal Ag/ligand ratio to be 1 : 1, specifically with 2.5 mol% Ag2O and 5 mol% L-2 (see ESI, Table S2†).
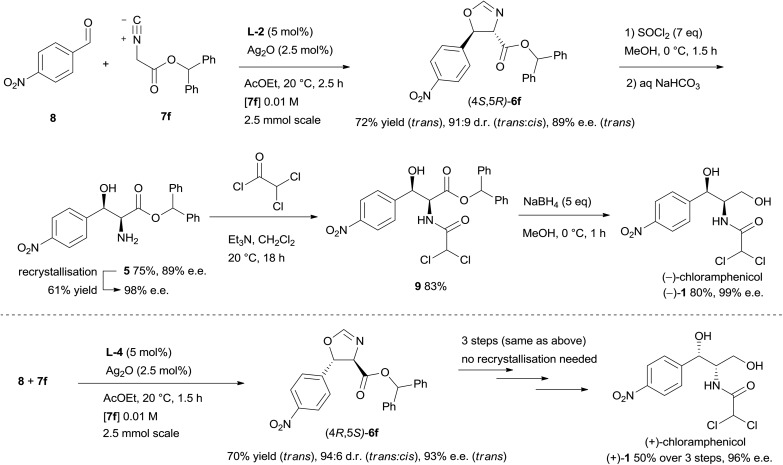
After having successfully improved yield and stereocontrol for the isocyanoacetate aldol reaction, oxazoline (4S,5R)-6f was prepared on 2.5 mmol scale with 72% yield and 89% e.e.,16 and then was readily elaborated to the target molecule (Scheme 2). Ring opening of 6f using thionyl chloride in methanol proceeded with 75% yield to afford amino alcohol 5, whose enantiomeric purity could be improved to 98% e.e. by a single recrystallisation from toluene (61% yield, first crop). The amino alcohol was then acylated with dichloroacetyl chloride to provide dichloroacetamide 9 in 83% yield. Finally, chemoselective reduction of the ester group with excess sodium borohydride delivered (–)-chloramphenicol in 80% yield and 99% e.e.17 (+)-Chloramphenicol was prepared in an analogous manner from oxazoline (4R,5S)-6f.17
Scheme 2. Synthesis of (–)- and (+)-chloramphenicol.
In summary, a catalytic asymmetric synthesis of (–)-chloramphenicol has been accomplished, delivering the target molecule in 4 steps and 22% yield calculated from 4-nitrobenzaldehyde. The concise synthetic route relies on the enantio- and diastereoselective aldol reaction of isocyanoacetates catalysed by Ag2O and cinchona-derived amino phosphine ligands. Extensive screening of the reaction parameters has been undertaken to optimise the key step, eventually achieving the formation of the two contiguous stereocentres of the target molecule with good enantiocontrol. The present work demonstrates the utility of this asymmetric methodology for the preparation of bioactive molecules bearing an α-amino-β-hydroxy motif.
The authors gratefully acknowledge the EPSRC (leadership fellowship to D.J.D. and postdoctoral fellowship to P.J.) and the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (A.F., FP7/2007-2013, REA grant agreement no. 316955).
Footnotes
References
- Bergmeier S. C. Tetrahedron. 2000;56:2561. [Google Scholar]
- (a) Ehrlich J., Bartz Q. R., Smith R. M., Joslyn D. A., Burkholder P. R. Science. 1947;106:417. doi: 10.1126/science.106.2757.417. [DOI] [PubMed] [Google Scholar]; (b) Rebstock M. C., Crooks H. M., Controulis J., Bartz Q. R. J. Am. Chem. Soc. 1949;71:2458. [Google Scholar]
- Cutler R. A., Stenger R. J., Suter C. M. J. Am. Chem. Soc. 1952;74:5475. [Google Scholar]
- de Clercq E. J. Clin. Virol. 2004;30:115. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Wood J. M., Maibaum J., Rahuel J., Grütter M. G., Cohen N.-C., Rasetti V., Rüger H., Göschke R., Stutz S., Fuhrer W., Schilling W., Rigollier P., Yamaguchi Y., Cumin F., Baum H.-P., Schnell C. R., Herold P., Mah R., Jensen C., O'Brien E., Stanton A., Bedigian M. P. Biochem. Biophys. Res. Commun. 2003;308:698. doi: 10.1016/s0006-291x(03)01451-7. [DOI] [PubMed] [Google Scholar]
- Karjalainen O. K., Koskinen A. M. P. Org. Biomol. Chem. 2012;10:4311. doi: 10.1039/c2ob25357g. [DOI] [PubMed] [Google Scholar]
- and references therein; ; (a) Patel J., Clavé G., Renard P.-Y., Franck X. Angew. Chem., Int. Ed. 2008;47:4224. doi: 10.1002/anie.200800860. [DOI] [PubMed] [Google Scholar]; (b) Jakubowska A., Kulig K. Curr. Org. Synth. 2013;10:547. [Google Scholar]
- (a) Kohta S., Halder S. Synlett. 2010:337. [Google Scholar]; (b) Gulevich A. V., Zhdanko A. G., Orru R. V. A., Nenajdenko V. G. Chem. Rev. 2010;110:5235. doi: 10.1021/cr900411f. [DOI] [PubMed] [Google Scholar]; (c) Chakrabarty S., Choudhari S., Doshi A., Liu F.-Q., Mohan R., Ravindra M. P., Shah D., Yang X., Fleming F. F. Adv. Synth. Catal. 2014;356:2135. doi: 10.1002/adsc.201400017. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Boyarskiy V. P., Bokach N. A., Luzyanin K. V., Kukushkin V. Y. Chem. Rev. 2015;115:2698. doi: 10.1021/cr500380d. [DOI] [PubMed] [Google Scholar]
- Sladojevich F., Trabocchi A., Guarna A., Dixon D. J. J. Am. Chem. Soc. 2011;133:1710. doi: 10.1021/ja110534g. [DOI] [PubMed] [Google Scholar]
- Ortín I., Dixon D. J. Angew. Chem., Int. Ed. 2014;53:3462. doi: 10.1002/anie.201309719. [DOI] [PubMed] [Google Scholar]
- de la Campa R., Ortín I., Dixon D. J. Angew. Chem., Int. Ed. 2015;54:4895. doi: 10.1002/anie.201411852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For racemic syntheses of chloramphenicol see: ; (a) Controulis J., Rebstock M. C., Crooks H. M. J. Am. Chem. Soc. 1949;71:2463. [Google Scholar]; (b) Long L. M., Troutman H. D. J. Am. Chem. Soc. 1949;71:2469. [Google Scholar]; (c) Long L. M., Troutman H. D. J. Am. Chem. Soc. 1949;71:2473. [Google Scholar]; (d) Ehrhart G., Siedel W., Nahm H. Chem. Ber. 1957;90:2088. [Google Scholar]; (e) Hazra B. G., Pore V. S., Maybhate S. P. Synth. Commun. 1997;27:1857. [Google Scholar]; (f) Schöllkopf U., Beulshausen T. Liebigs Ann. Chem. 1989:223. [Google Scholar]; (g) Hajra S., Karmakar A., Maji T., Medda A. K. Tetrahedron. 2006;62:8959. [Google Scholar]; (h) Li Q., Zhang H., Li C., Xu P. Chin. J. Chem. 2013;31:149. [Google Scholar]; (i) Easton C. J., Hutton C. A., Merrett M. C., Tiekink E. R. T. Tetrahedron. 1996;52:7025. [Google Scholar]; (j) Veeresa G., Datta A. Tetrahedron Lett. 1998;39:8503. [Google Scholar]; (k) Rao A. V. R., Rao S. P., Bhanu M. N. J. Chem. Soc., Chem. Commun. 1992:859. [Google Scholar]; (l) Lou B.-L., Zhang Y.-Z., Dai L.-X. Chem. Ind. 1993;7:249. [Google Scholar]; (m) Bhaskar G., Kumar V. S., Rao B. V. Tetrahedron: Asymmetry. 2004;15:1279. [Google Scholar]; (n) George S., Narina S. V., Sudalai A. Tetrahedron. 2006;62:10202. [Google Scholar]; (o) Park J. N., Ko S. Y., Koh H. Y. Tetrahedron Lett. 2000;41:5553. [Google Scholar]; (p) Boruwa J., Borah J. C., Gogoi S., Barua N. C. Tetrahedron Lett. 2005;46:1743. [Google Scholar]; (q) Chênevert R., Thiboutot S. Synthesis. 1989:444. [Google Scholar]; (r) Loncaric C., Wulff W. D., Org. Lett., 2001, 3 , 3675 , (catalytic enantioselective aziridination) . [DOI] [PubMed] [Google Scholar]
- For an asymmetric synthesis of chloramphenicol relying on a diazaborolidine-mediated aldol reaction, proceeding via a chiral boron enolate, see: Corey E. J., Choi S., Tetrahedron Lett., 2000, 41 , 2765 . [Google Scholar]
- For previous work on the synthesis of oxazolines and their hydrolysis to vicinal amino alcohols, see: ; (a) Ito Y., Sawamura M., Hayashi T. J. Am. Chem. Soc. 1986;108:6405. [Google Scholar]; (b) Hoppe D., Schöllkopf U. Liebigs Ann. Chem. 1972;763:1. doi: 10.1002/jlac.19727630102. [DOI] [PubMed] [Google Scholar]; (c) Schöllkopf U., Scheunemann K.-H. Liebigs Ann. Chem. 1980:1348. [Google Scholar]
- The preparation of isocyanoacetates 7a–f from commercial reagents over three steps is detailed in the ESI.
- Removal of 4 Å molecular sieves had no detrimental effect on the reaction
- The observed slight upgrade in e.e. between 9 and 1 is linked to the chromatographic purification process
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.