Abstract
Background
Due to the clinical challenges of treatment-resistant depression (TRD), we evaluated the efficacy of Mindfulness-Based Cognitive Therapy (MBCT) relative to a structurally equivalent active comparison condition as adjuncts to treatment-as-usual (TAU) pharmacotherapy in TRD.
Methods
This single site, randomized controlled trial compared 8-week courses of MBCT and the Health-Enhancement Program (HEP), comprising physical fitness, music therapy and nutritional education, as adjuncts to TAU pharmacotherapy for outpatient adults with TRD.
The primary outcome was change in depression severity, measured by percent reduction in total score on the 17-item Hamilton Depression Rating Scale (HAM-D17), with secondary depression indicators of treatment response and remission.
Results
We enrolled 173 adults, mean length of current depressive episode was 6.8 years (sd = 8.9). At the end of 8-week treatment, a multivariate analysis showed that relative to the HEP condition, the MBCT condition was associated with a significantly greater mean percent reduction on the HAM-D17 (36.6% versus 25.3%; p=.01) and a significantly higher rate of treatment responders (30.3% versus 15.3%; p=.03). Although numerically superior for MBCT than for HEP, the rates of remission did not significantly differ between treatments (22.4% versus 13.9%; p=.15). In these models, state anxiety, perceived stress, and the presence of personality disorder had adverse effects on outcomes.
Conclusions
MBCT significantly decreased depression severity and improved treatment response rates at 8 weeks, but not remission rates. MBCT appears to be a viable adjunct in the management of TRD.
Keywords: treatment-resistant, depression, mindfulness-based, cognitive, therapy, psychotherapy, major depressive disorder, treatment, mindfulness meditation
Introduction
Major depressive disorder (MDD) is a debilitating disease estimated to affect up to 14 million adults annually in the United States [1]. Despite many pharmacologic treatment options for depression, as many as 60% of patients treated with treatment-as-usual (TAU) pharmacotherapy fail to fully remit after one year [2]. Clinical consensus regarding the definition of TRD is wide-ranging following in the footsteps of Thase and Rush who developed the first staging protocol for treatment resistance [3]. Currently many experts define TRD as the failure to remit after at least two antidepressant trials of adequate dose and duration [4]. Current TRD definitions are somewhat limited since they generally do not include psychotherapy trials in their classification systems. Many individuals with TRD will suffer chronic depression, typically diagnosed when depressive symptoms have been present for two or more years. Although in DSM IV-TR chronic major depression and less symptomatic dysthymia were differentiated, in DSM V, they have been combined into persistent depressive disorder [5]. We should note that TRD is sometimes incorrectly identified (i.e. pseudo-treatment-resistance), when there has been inadequate diagnosis or treatment [6]. Even with multiple medication trials, however, treatment resistance is quite common [7].
Although most studies of TRD have focused on medication strategies, recent approaches have utilized evidence-based psychotherapeutic interventions. In the STAR*D study, patients at level 2 who had not responded adequately to an initial trial with citalopram were randomized to CBT or a change in medication [8]. CBT was offered either alone or as augmentation to continued citalopram. Medication changes were either switches or augmentations. All of the arms had similar remission rates of approximately 25%, although the medication augmentation strategies achieved significantly faster remission compared to CBT augmentation [9].
In an earlier study by Paykel et al. [10], CBT was compared to medication management in patients with incomplete remission. Both groups showed similar reductions in depression ratings although CBT appeared to offer protection against relapse. The cognitive behavioral analysis system of psychotherapy (CBASP) was investigated in comparison to nefazodone in patients with chronic depression, although only a minority were treatment-resistant. Both medication and CBASP produced individual remission rates of approximately 30% and when combined had a rate of 48% [11]. Kocsis et al. [12] investigated individuals with chronic depression, including 491 patients with TRD. The study compared CBASP or supportive psychotherapy augmentation of medication treatment with a flexible, optimized course of pharmacotherapy alone. In this study, neither form of psychotherapy was superior to the pharmacotherapy alone. Brakemeier et al. 2015 investigated the application of CBASP in an inpatient setting with 70 patients with chronic depression and treatment-resistance. In this uncontrolled pre-post design, 40% of patients remitted after the 12-week intervention. At 52 weeks, 48% of patients had a sustained response. In uncontrolled studies of MBCT for TRD, both Kenny and Williams in a study of 79 patients and Eisendrath et al. study of 55 patients demonstrated large effects sizes (>1.0) in reducing depression severity.
TRD is associated with poorer clinical outcome and adherence,[13] as well as higher rates of disability, mortality, morbidity and lower overall quality of life compared to those with non-resistant unipolar depression [14, 15]. Given these factors, there is an urgent need for innovative and effective treatment strategies for TRD.
Because of this need and our pilot data [16], we examined the possibility that MBCT might be effective as an adjunctive treatment for TRD. Studies have suggested that Mindfulness-based Cognitive Therapy (MBCT) strengthens cognitive control of emotion regulation [17]. TRD is believed to be related to decreased dorsal executive control system's regulation of the ventral affective processing system [18-20]. Strengthening the dorsal system (e.g. dorsolateral prefrontal cortex or dorsal anterior cingulate) through meditation effects such as enhanced selective attention, executive functioning, and emotion regulation helps restore cognitive control processes to down-regulate the ventral system (such as amygdala and ventrolateral prefrontal cortex) and depression-related processes such as rumination [21, 22]. As Barnhofer and colleagues have pointed out [23, 24], meditation, by strengthening cognitive control mechanisms, may lessen ruminative processes and thereby diminish depression. This theoretical background led us to hypothesize that MBCT could prove effective in diminishing TRD. We specifically hypothesized that MBCT would be more effective than another credible intervention that controlled for non-specific effects.
MBCT is an 8-week group program originally developed as an intervention to protect against relapse for patients with unipolar depression who had achieved remission after initial treatment [17]. Multiple randomized controlled trials (RCTs) have since confirmed its effectiveness in preventing relapse when compared to routine clinical management or maintenance antidepressant treatment [17, 25-27]. Because most of the RCTs evaluating MBCT for relapse prevention used treatment as usual as the control condition, it was difficult to ascertain whether sustained treatment effects were related to specific components of MBCT or non-specific effects.
With this limitation in mind, we designed this study to evaluate the effects of MBCT as an adjunct to TAU pharmacotherapy for adults with TRD compared to a structurally equivalent comparator condition plus TAU. The comparator, the Health-Enhancement Program (HEP), controlled for the following non-specific factors: group support and morale; reduction of stigma, facilitator attention; treatment duration; and time spent on at-home practice. Because of these features of HEP, we believed the two arms of the study offered clinical equipoise deserving investigation, especially since MBCT had never been studied in an RCT in the TRD population when we commenced our study. We named the study the “Practicing Alternatives Techniques to Heal Depression Study” to avoid biasing participants with investigators' hypotheses. Secondary aims included exploration of potential mediators of MBCT effects, including mindfulness, self-compassion, rumination, and experiential avoidance. We predicted that MBCT would be superior to HEP primarily in reducing depression severity while also enhancing response and remission rates.
Methods
Study Design
We conducted the study at the University of California, San Francisco with a four-year period of active recruitment from September 2009 to September 2013. The study design and rationale have previously been published [28]. The primary outcome analysis evaluated percent change in depression severity after 8-week acute treatment, the study's primary endpoint. Clinical evaluations were conducted in person by research assistants (RAs), who held advanced degrees in psychology and were blind to treatment assignment. To assure blinding, participants were instructed not to discuss treatment assignment with RAs prior to assessments and both intervention groups were held on the same day and time to minimize raters having cues to participant assignment. Lastly, treatment groups were held in the evenings, outside of traditional work hours, when the RAs had already left the research offices. We obtained approval from our local institutional review board that operates in accordance with the Declaration of Helsinki. All participants signed informed consent forms. The trial was registered at clinicaltrials.gov (identifier: NCT01021254). The research was regularly reviewed by a data safety monitoring board.
Participants
Participants were recruited from outpatient psychiatry and general medicine clinics at UCSF and the outpatient psychiatry clinic at Kaiser Permanente in San Francisco and from the community. Clinics were located in large medical centers that serve an ethnically diverse population of privately and publicly insured adults in the San Francisco Bay Area. In addition to direct enrollment from these clinics, recruitment strategies included clinician referrals, flyers in public places, online advertisements, and local print advertisements in newspapers and on municipal buses and trains.
To be eligible for the study, participants had to be age 18 and older, fluent in English, meet DSM-IV diagnostic criteria for unipolar MDD based on evaluation with the Structured Clinical Interview for DSM-IV Diagnosis (SCID),[29] and have a score of 14 or greater on the 17-item Hamilton Depression Rating Scale (HAM-D17).[30] Additionally, participants were required to be taking antidepressant medications with evidence of two or more adequate trials prescribed during the current episode assessed with the Antidepressant Treatment History Form (ATHF) (described below) [31]. Participants were excluded for any of the following reasons: they (1) had a lifetime history of bipolar illness, schizophrenia or any psychotic disorder; (2) met DSM-IV criteria for substance abuse or dependence within 3 months prior to study entry; (3) were imminently suicidal, a danger to others, or were currently exhibiting self-injurious behavior; (4) were in concurrent individual or group psychotherapy and not willing to discontinue these during 8-week acute treatment duration of the study; (5) or had cognitive impairment, as defined by a score of <25 on the Mini Mental Status Exam (MMSE) [32]. Anxiety related disorders and eating disorders were acceptable for enrollment if these co-occurring disorders were stable and not the primary disorder.
Randomization
Randomization was stratified by gender using computer-generated numbers. Participants were randomized by person after baseline assessment. These assessments occurred within 2 weeks of treatment initiation to ensure participants continued to meet inclusion criteria for depression severity listed above. Participants and facilitators were blind to hypotheses and were informed of treatment assignments by the study coordinator who was not blinded or involved in outcome evaluation.
Study Treatment Interventions
Both treatments followed detailed manuals and were administered to small groups (6-12 participants) once per week for eight consecutive weeks. Groups lasted 2 hours and 15 minutes, and in addition to weekly attendance, participants were assigned 45-minutes of homework 6 days per week. Participants in both conditions were encouraged to continue their antidepressant treatment, as prescribed by their outside provider and to report any medication changes weekly throughout the study.
Although treatment conditions were led by two different sets of facilitators, both sets were extensively trained in their respective techniques, having at least 3 years of experience in their respective components. All passed trial runs of their interventions, and had strong beliefs in the value of their approach. All group sessions were audio recorded and three recordings per group were randomly selected and reviewed by external and internal evaluators. Internally, HEP facilitator fidelity was evaluated at UCSF by a trained reviewer (EG) and externally HEP facilitator fidelity was evaluated by an investigator at the University of Wisconsin, Madison where HEP was developed [33]. Similarly, MBCT fidelity was assessed through an internal UCSF reviewer (SE) and externally by an unaffiliated psychiatrist trained in MBCT. Based on reviews and results of adherence and competency measures,[34] (MBCT Competency, HEP Adherence and HEP Competency Scales are unpublished and available from the first author) corrective feedback was provided when needed to group leaders by the PI during weekly supervision meetings with each team of interventionists.
Mean adherence ratings per item were 1.79 (sd=.06) out of 2 points maximum for MBCT using the MBCT Adherence Scale [34] and 1.82 (sd=.04 out of 2 points maximum for the HEP Adherence Scale. The mean competency ratings per item were 4.95 (sd=.02) out of 5 points on the MBCT Competency Scale and 4.90 (sd=.04) out of 5 points on the HEP Competency Scale. All scores were in the highly acceptable range.
Mindfulness-Based Cognitive Therapy (MBCT) was adapted from the original manual developed by Segal et al.[35], with modifications for TRD for the purpose of this study (published elsewhere) [36]. Using guided meditations and exercises from CBT, participants were taught skills to identify cognitive distortions and to disengage from depression-focused ruminative thinking patterns through the use of non-judgmental and present-focused awareness. MBCT utilizes body scans, sitting meditations, three-minute breathing spaces, and mindful movement as core techniques. Group discussion of how individuals are developing their meditation practice occurs in each session. Psycho-education regarding depression is also an important component. Home practice consisted of mindfulness meditation, mindfulness of everyday life, and mindful movement. With our TRD population we modified the original protocol several ways [28, 37], such as shortening the length of meditations to a maximum of 30 minutes, emphasizing mindful movement, exploring barriers to practice, and incorporating a focus on acceptance of emotional events.
The original HEP manual, had been developed for use in a non-clinical sample as a control condition for Mindfulness-Based Stress Reduction, the forerunner of MBCT [33]. The HEP manual was adapted for the TRD population by linking the components to having potential beneficial effects on mood in class discussions. Course content focused on aerobic exercise, functional movement, music therapy, and dietary education. Participants were asked to do specific exercises (e.g.. walking/stretching/agility training), receive nutritional education, and participate in musical activities such as drumming or song writing. They were told that these activities could reduce stress and promote overall health. Home practice consisted of activities such as exercise, stretching, monitoring food intake with a daily journal, and musical activities. For both MBCT and HEP, daily diaries of home practice were completed online.
Medication Treatments
During the trial, patients continued to be seen for medication visits an average of once monthly. No prohibitions were made for medication changes during the trial. We encouraged reporting of any changes in dose or medications and recorded theses.
Assessment of Outcomes and Measures
Depression
Patients were evaluated with the HAM-D17 at baseline (week 0), midway through treatment (week 4), and at post-treatment (week 8) as our main focus. We examined the primary outcome of change in depression severity (overall percentage change in the total score from baseline), as well as secondary outcomes of treatment response (scores ≥ 50% decrease from baseline) and remission (post-treatment scores ≤ 7 on the HAM-D17). As a more global measure of change in depression severity we utilized the Clinical Global Impression (CGI) instrument [38] to assess improvement and severity changes at similar time points as the HAM-D17
Mediators of Treatment Efficacy
The theoretical basis for MBCT's mechanisms of change has been posited to include improved emotional regulation [39] related to enhanced mindfulness,[40] increased self-compassion[26], reduced rumination [41], and decreased experiential avoidance of dysphoric feelings [42].
We explored these potential mediators of treatment efficacy at weeks 4 and 8 using four instruments. Mindfulness was evaluated using the 39-item Five Facet Mindfulness Questionnaire (FFMQ) [43], a self-report measure that assesses mindfulness across 5 domains: observing, describing, acting with awareness, accepting without judgment, and non-reactivity to internal experience. We used the total score from the 26-item Self-Compassion Scale (SCS) [44] to evaluate the level of self-compassion across 6 subscales including: self-kindness, self-judgment, common humanity, isolation, mindfulness, and over-identification. We measured rumination with the Ruminative Response Scale (RRS) [45] a 22-item self-report scale. The reliability of the scale has been repeatedly confirmed for patients with depression and high scores appear to be associated with a vulnerability factor to depression and predict depression severity in clinically depressed populations. Finally, experiential avoidance, which has been associated with increased levels of depression [46] and is defined by attempts to avoid thoughts, feelings, memories, physical sensations or other internal experiences – was assessed using the 9-item Acceptance and Action Questionnaire (AAQ) [47]. Increased acceptance and decreased avoidance have been associated with decreased depression.[42]
Moderators of Treatment Efficacy
Psychiatric factors that may impact depression severity and treatment resistance were analyzed as potential moderators of treatment. These included demographic factors such as ethnicity, minority and socio-economic status as well as psychiatric history factors such as age of depression onset, number of lifetime depressive episodes, and co-morbid psychiatric illness (e.g. personality disorders[48]). We also examined other potential moderators of outcome such as anxiety utilizing the State and Trait Anxiety Inventory,[49] current stress with the Perceived Stress Scale (PSS) [50], and early childhood stress with the Childhood Trauma Questionnaire (CTQ) [51]. The CTQ assesses physical, sexual or emotional abuse and trauma or neglect, all of which are widely reported to influence depression [52]. We also assessed the presence of personality disorders using the SCID-II [53]. Finally, since patients' expectations and beliefs about treatment may affect outcome [54], we included a measure of treatment expectancy and credibility, the Treatment Rationale Scale [55], which participants completed after session 1. The scale includes expectancy of treatment success and how confident the individual would be in recommending the treatment to a friend.
Statistical Analysis
Standard summary statistics were used to describe the study sample. Treatment conditions were compared on the primary outcome measure (percent change in depression severity from baseline on the HAM-D17). We regarded this continuous variable as the most sensitive indicator of change in this population. We also assessed the dichotomous variables of percent responding, and percent achieving remission at the end of treatment (Week 8). We used generalized logistic and linear regression models and generalized estimating equations to account for non-independence due to experiencing a group-based intervention. Covariate terms were added to the models testing primary efficacy after screening for their relationship to the outcomes. Mediational effects were tested using the methods outline by MacKinnon[56] and implemented in Mplus v7.2. The model of mediation of treatment condition on percent reduction in severity was estimated and tested using the potential mediators (FFMQ scales, SCS, RRS and AAQ) measured at week 4. As 4 weeks may not have been sufficient for more thorough skill acquisition, the model was re-estimated using those measures when taken at the end of treatment. Moderation was tested by including interaction terms in the statistical models. Model estimation and testing was conducted under the intent-to-treat principle and all available data was used in analyses. Power was estimated for each hypothesis and the methods of estimation were aligned with the methods of analysis. Estimates of effect size were derived from pilot work and the extant literature. Primary analyses were conducted using SAS v9.4.
Results
A total of 229 adults provided informed consent and were screened for eligibility (see Consort Table, Figure 1 for details). Of the total screened, 173 subjects were eligible for randomization to either MBCT or HEP. Summary demographic statistics and clinical characteristics of the sample of 173 participants by treatment condition at the baseline assessment are shown in Table 1. There were no statistically significant differences between the conditions on any demographic or clinical variables. Table 1 shows that the sources of participant recruitment were distributed evenly across the three sites and the treatment conditions did not differ by recruitment site (X2 (2) = 0.33, p = .84). In the MBCT cohort, there were 4 changes in medication during the eight weeks, while in the HEP cohort there were 5 changes.
Figure 1. Consort Diagram: Enrollment and Study flow in Randomized Controlled Trial of Mindfulness-Based Cognitive Therapy (MBCT) versus Health Enhancement Program (HEP) for treatment-resistant depression.
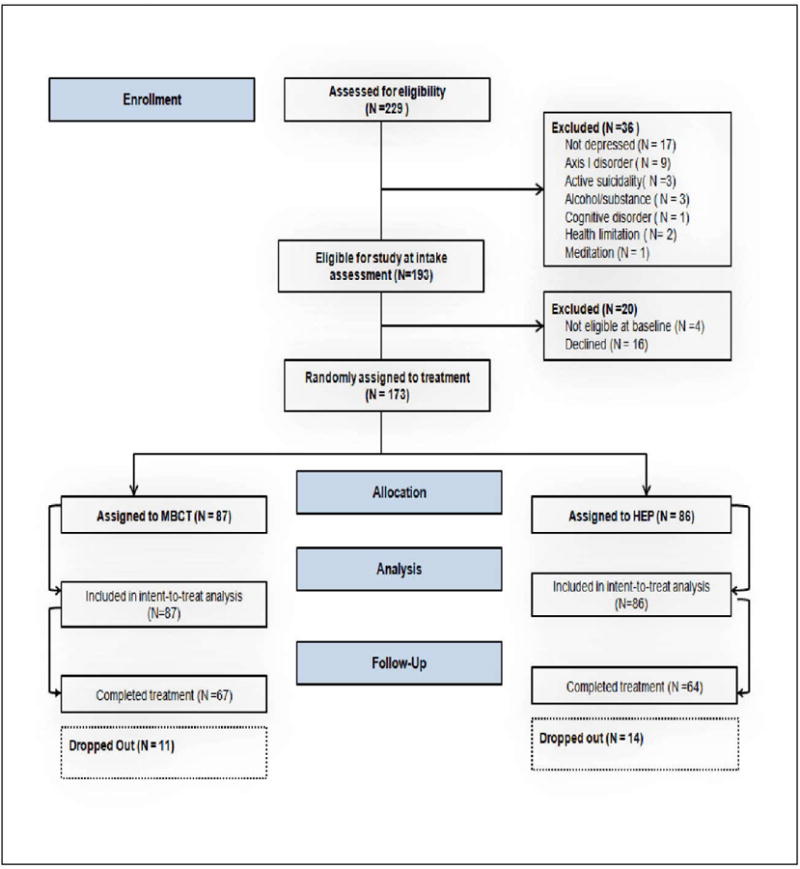
Table 1. Demographic and clinical characteristics between treatment conditions of study participants with treatment-resistant depression at baseline (N=173).
| Demographic Characteristics | MBCT* | HEP* | ||
|---|---|---|---|---|
|
| ||||
| Variable | Mean | SD | Mean | SD |
| Age years (range) | 47.1(21-69) | 13.46 | 45.2(16-85) | 11.19 |
| Education (years) | 15.6 | 2.01 | 15.9 | 2.15 |
| Female (%) | 75.9 | 76.7 | ||
| Ethnicity (%) | ||||
| Hispanic | 8.3 | 11.6 | ||
| Race (%) | ||||
| White | 82.4 | 82.1 | ||
| Asian | 4.7 | 5.6 | ||
| African American | 8.2 | 8.3 | ||
| Other | 4.7 | 2.4 | ||
| Employment (%) | ||||
| Full-time | 28.7 | 39.5 | ||
| Part-time | 18.4 | 22.1 | ||
| Unemployed/student/homemaker | 36.8 | 31.4 | ||
| Retired | 11.5 | 7.0 | ||
| Disability (%) | ||||
| Mental Health | 19.5 | 15.1 | ||
| Physical | 8.1 | 7.0 | ||
| Marital Status (%) | ||||
| Single | 37.9 | 47.7 | ||
| Married/cohabitating | 25.3 | 24.4 | ||
| Divorced/separated/widowed | 21.8 | 12.8 | ||
|
| ||||
| Clinical Characteristics | MBCT* | HEP* | ||
|
| ||||
| Variable | Mean | SD | Mean | SD |
|
| ||||
| Age at depression onset | 18.8 | 10.86 | 21.7 | 13.24 |
| Total number depressive episodes | 3.8 | 2.61 | 3.5 | 2.37 |
| Length of current depressive episode (months) | 84.4 1 | 19.51 | 78.5 | 93.5 |
| HAM-D17 score | 18.3 | 3.36 | 17.4 | 3.48 |
| Chronic depression (> 2 years duration) | 54.0 | 64.0 | ||
| <3 episodes (%) | 35.2 | 40.3 | ||
| ≥ 3 Lifetime episodes (%) | 64.8 | 59.7 | ||
| Previous Number of depressive episodes | 3.80 | 3.52 | ||
| Number of adequate ATHF trials in episode | 2.9 | 3.06 | ||
| Previous Psychiatric Hospitalizations (%) | 16.1 | 18.6 | ||
| History of Suicide Attempt (%) | 21.8 | 22.1 | ||
| Comorbid Anxiety Disorder | 56.3 | 63.9 | ||
| Eating Disorder | 13.8 | 11.6 | ||
| Personality Disorder | 11.49 | 20.9 | ||
| Recruitment Source (%) | ||||
| General Internal Medicine | 34.5 | 36.1 | ||
| Psychiatry Clinic | 43.7 | 39.5 | ||
| Community | 21.8 | 24.4 | ||
MBCT= Mindfulness-Based Cognitive Therapy; HEP = Health Enhancement Program
There were no significant differences between conditions on any demographic or clinical variable.
In another secondary analysis, eight measures considered potential confounders in the preliminary plan were screened. Of the eight, four were significantly correlated with outcomes: PSS total score, presence of a personality disorder, and state and trait anxiety on the STAI. The number of treatment sessions attended were inversely correlated with percent change in depression severity. These five measures were included as covariates in the main models testing treatment effect.
By the end of treatment, Week 8 data were available on 83.7% of the HEP participants and 87.3% of the MBCT participants, indicating a dropout rate of 16.3% (HEP) and 12.7% (MBCT). The most common dropout reasons in both conditions, in descending frequency, were the following: 1) lost to contact; 2) worsened medical condition; 3) time burden; 4) scheduling conflict; 5) change in family circumstance.
A multivariate analysis showed that relative to the HEP condition, the MBCT condition produced a significantly greater mean percent reduction in depression severity (36.6% versus 25.3%; p=.01) at endpoint. MBCT also produced a significantly greater number of treatment responders (30.3% versus 15.3%; p=.03) based on the HAM-D17 at endpoint (Table 2). Although also favoring MBCT, no statistically significant difference was found for rates of remission (22.4% versus 13.9%; p = .15). Additionally, no differences based on gender were found.
Table 2.
Percentage Reduction, Response Rate, and Remission Rate in Hamilton Depression Rating Scale (HAM-D17) by mid-treatment and end-of-treatment assessment points.
| Percentage Reduction | Response (%) | Remission (%) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Week 4 | Week 8* | Week 4 | Week 8* | Week 4 | Week 8 | |
| HEP | 23 | 25.3 | 10.1 | 15.3 | 9.3 | 13.9 |
| MBCT | 24 | 36.6 | 10.1 | 30.7 | 10.1 | 22.4 |
p < .05; MBCT= Mindfulness-Based Cognitive Therapy; HEP= Health Enhancement Program
In these models, state anxiety was related to diminished HAM-D17 per cent reduction of symptom severity and lower response rate, but did not interact with condition. Stress (PSS score) and the presence of a personality disorder were related to diminished percent reduction, but did not interact with condition.
Similar modeling was used to estimate and test for effects of childhood trauma on the outcomes—both as predictors of outcome and moderators of the treatment effect. Only the Emotional Abuse subscale reached statistical significance in relationship to both response (p = .05) and remission (p = .03) with participants who responded and those who remitted having lower mean values. No evidence of moderation, as evidenced by a significant interaction term, was seen. Similar modeling was used to test for effects of ≥3 episodes of depression but we did not find evidence of a relationship of this characteristic to outcome.
Treatment expectancy did not differ statistically between conditions after one session. The MBCT group and HEP group did not differ on the two treatment expectancy items: 1) how likely the treatment would be successful (p=.62), and 2) how confident the individual would be in recommending the treatment to a friend (p=.23).
In terms of other factors that could have affected outcomes, we found no statistically significant differences in medication changes (e.g. number of increased or decreased dosages or medication switches or augmentations) between conditions or evidence that such changes were related to clinical outcomes. Moreover, the number of ATHF adequate prior treatment trials did not significantly differ between the two conditions (MBCT mean 2.90 [sd 1.31], HEP mean 3.08 [sd 1.37]) indicating similar levels of treatment resistance. Finally, although all of the putative mediators (Table 3) changed in the direction anticipated (i.e. increased mindfulness, reduced rumination, enhanced self-compassion, and decreased experiential avoidance), only the FFMQ observing subscale showed a greater change with MBCT than HEP (p=.019). Moreover, the mediation models did not indicate any of these variables drove the observed clinical change in either arm of the study.
Table 3. Putative mediators over time.
| Putative Mediators |
Baseline |

|
4 Weeks |

|
8 Weeks |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MBCT | SD | HEP | SD | MBCT | SD | HEP | SD | MBCT | SD | HEP | SD | |
| AAQ Total score | 42.5 | 6.4 | 42.5 | 7.3 | 41.7 | 6.4 | 39.3 | 6.8 | 37.8 | 7.1 | 37.8 | 6.5 |
| SCS Total mean | 2.3 | 0.6 | 2.3 | 0.6 | 2.4 | 0.6 | 2.5 | 0.6 | 2.8 | 0.7 | 2.7 | 0.7 |
| RRS Rumination Total | 59.0 | 11.1 | 58.9 | 11.1 | 55.9 | 11.5 | 55.9 | 12.4 | 49.3 | 11.5 | 49.9 | 11.5 |
| FFMQ Observing subscale | 23.3 | 6.4 | 24.1 | 6.0 | 25.5 | 5.5 | 25.2 | 6.4 | 27.1 | 6.2 | 24.7 | 5.9 |
| FFMQ Describing subscale | 24.0 | 7.4 | 24.3 | 7.1 | 25.1 | 7.1 | 25.4 | 6.7 | 27.3 | 7.4 | 26.8 | 6.7 |
| FFMQ Acting subscale | 20.2 | 6.4 | 20.2 | 5.9 | 20.2 | 5.5 | 22.0 | 6.7 | 23.8 | 6.4 | 23.8 | 7.1 |
| FFMQ Nonjudging subscale | 22.1 | 6.8 | 21.9 | 7.6 | 23.2 | 6.3 | 23.6 | 7.8 | 27.0 | 5.9 | 26.6 | 6.8 |
| FFMQ Nonreactive subscale | 17.6 | 4.6 | 18.1 | 5.2 | 19.0 | 4.6 | 20.3 | 4.4 | 20.9 | 5.1 | 19.8 | 4.8 |
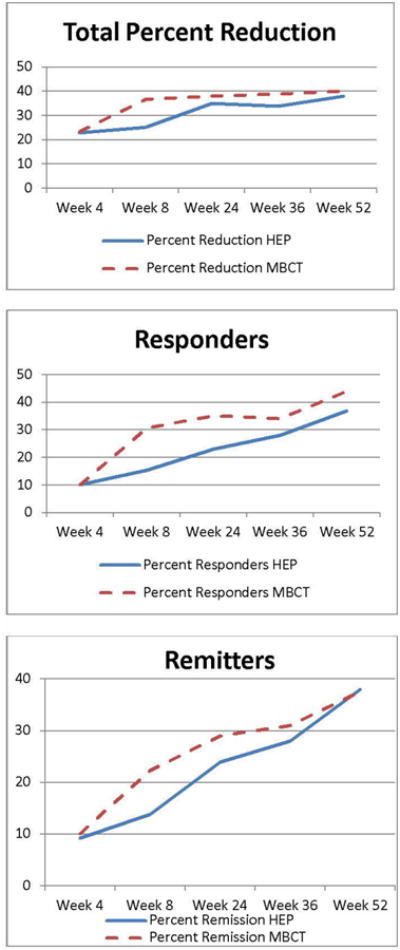
We also assessed a number of secondary variables over a 52-week follow-up interval. The Clinical Global Impression (CGI) demonstrated beneficial effects for both conditions but MBCT had more favorable changes for improvement (p=.001) and decreased severity (p=.038) at 8 weeks. By 52 weeks, there was no significant difference between conditions, although both continued to be significantly improved from baseline. The presence of increased perceived stress, personality disorder, and short-term or trait anxiety predicted decreased response for both conditions. There were no significant differences between conditions at 24, 36, and 52 weeks for the putative mediators of avoidance, rumination, mindfulness, or self-compassion, although all continued to be significantly improved from baseline.
Clinical outcomes at 52 weeks using the HAMD-17 indicated that MBCT was significantly greater for percent responding but not for percent reduction or remitting. These findings are illustrated in Figure 2.
Figure 2. HAMD17 outcomes over 52-week follow-up.
Discussion
This study is the first full scale RCT to investigate MBCT as an adjunctive treatment with medications for individuals with TRD who are currently depressed. In the current study, using a manualized and previously published control condition, the Health-Enhancement Program [33, 57], both arms demonstrated high rates of compliance and low dropout rates for a TRD population [58]. MBCT produced a significantly greater percent decrease in depression severity and response rate than HEP at 8-week endpoint. Although the remission rate was numerically greater for MBCT than for HEP, the difference was not statistically significant. In this treatment-resistant population comprised of patients who had failed to remit despite an average of 3 medication trials prior to study entry, low remission rates would be expected and differences between low rates would be difficult to detect in a sample of this size. In addition, although our entry criteria was a HAMD17 score of ≥ 14 (the same cutoff used in the STAR*D study), both MBCT and HEP mean HAMD17 entry scores were greater than 17, indicating the population was significantly depressed upon study initiation.
To place our results in context we can compare our findings to other psychotherapeutic efforts in depression and TRD. For example, our dropout rates of 16.3% (HEP) and 12.7% (MBCT) compared favorably to the rate of 24.3% for outpatient group CBT for unipolar depressed patients in a recent meta-analysis [59]. Brakemeier et al.[60] completed an uncontrolled study of CBASP with inpatients suffering chronic depression and TRD. Our results were modest in comparison. Our 8-week response/remission rates of 30.7/22.4% compared to rates of 75.7/40.0% of Brakemeier et al.[60] at 12-weeks. However, their inpatient treatment offered a longer and more intense milieu treatment. Our 52-week responder rate (43.7%) was similar to their 48% rate. Perhaps more comparably, our results can be assessed in relation to other randomized outpatient trials of TRD management. Our 8-week results demonstrate outcomes that were superior to the 12-week medication steps 3 or 4 of the STAR*D study. That study found response/remission rates of 24.0%/14.1% at step 3 and 23.2%/13.1% at step 4 [61]. In a trial of CBASP with patients having chronic depression and TRD, rates for response/remission were 22.5%/15.0% [12] whereas in our study MBCT produced rates that were again superior at 8 weeks. Taken as a whole, these studies suggest that expectations must be tempered by what is feasible, yet there is room for cautious optimism in undertaking treatment in TRD. Moreover, recently, Kuyken et al. [62] demonstrated that MBCT with discontinuation of antidepressants was not inferior to maintenance medications in terms of preventing relapse. Our study raises the possibility that MBCT offers improvement similar to additional medication trials for those with TRD with a potential for relapse prevention as well.
This study strengthens the finding of a previous study by Chiesa et al.[63] that had similar main effects of MBCT, but was based on a smaller, preliminary sample with less stringent requirement for TRD that utilized a psychoeducational control. In a review of treatment approaches for TRD, Carvalho et al. [64] noted that cognitive therapy appears to be an effective adjunctive treatment to medication for TRD. Our report broadens consideration of psychotherapeutic approaches for this challenging condition to include MBCT.
Identifying appropriate controls for mindfulness interventions has been challenging and researchers have often utilized relatively weak controls such as waitlists. We chose HEP as the comparator condition for several reasons. First, the University of Wisconsin and NCCAM specifically developed it to be a credible, structurally equivalent control for mindfulness intervention such as Mindfulness-Based Stress Reduction (MBSR) and its derivative, MBCT. Second, it provides the non-specific elements of therapy including group support, attention (time spent with therapist), home practice, diminished stigma, and provision of hope. Third, exercise, the central component of the HEP, was initially conceived as a control for the movement component of mindfulness interventions (e.g. mindful walking and yoga) and as a way of building a credible alternative condition. Since exercise has been shown to have significant antidepressant effects [65], this may help to explain why the HEP group improved. The fact that HEP demonstrated some change in depression levels after 8 weeks could have been expected due to its nonspecific effects. In addition, HEP has been shown to be as efficacious as MBSR for reducing perceived stress, a contributing factor of depression, in two previous studies [33, 57]. In our pre-trial evaluations, we observed that HEP has a behavioral activation element that was not described in its initial research. Encouraging participants to engage in activities such as exercise, listening to music, or eating nutritionally could all have helped make HEP a credible intervention as well as potentially having beneficial mood effects. Finally, structural elements of HEP's design also made it a strong comparator condition. For example, HEP was equivalent to MBCT in number of sessions and home practice; it also was led by two highly motivated facilitators (> 3 years teaching meditation or the components of HEP) for each group. Although the same interventionists did not deliver both conditions, both MBCT and HEP interventionists believed their procedures could help alleviate depression.
How MBCT may achieve its effects needs further elucidation [66]. While the putative mediators we examined changed in the expected direction with MBCT, none of them met the statistical test of mediation. Although it is possible that we lacked sufficient power to detect a mediator in a secondary analysis, the lack of a significant effect of our hypothesized mediators raises the possibility that another factor may be a key ingredient. There could be another variable like attentional control, autobiographical memory or self-discrepancy that may have played a role, but we faced a limit in both respondent burden and the ability to usefully analyze a larger number of variables.
Without being clear on what the actual mediator was, we cannot be sure why we did not detect it. We did examine the most commonly reported mediators of mindfulness, self-compassion, rumination and experiential avoidance and failed to identify any as passing the test of mediation [56]. Moreover, it is possible that the current measures of mindfulness may not accurately capture what actually changes with treatment. For example, there is evidence that exercise, such as included in HEP, may have an impact on what we measure as mindfulness. Mothes et al. [67] demonstrated that regular aerobic exercise can increase measures of dispositional mindfulness, using the Mindfulness Attention Awareness Scale [68]. Such findings point to the importance of refining our definitions of mindfulness and being aware of how well they match the sample being investigated [69] ; i.e. are measures equally valid in community, clinical, and meditator samples.
In addition to the above factors, the long-term antidepressant treatment that our TRD population underwent may have washed out a clearer picture of the effects of our putative mediators. As Fava points out [70], certain features of long-term antidepressant treatment may play a role in contributing to treatment resistance that may interfere with psychotherapeutic responsivity. These features could potentially influence the effects of mediators making it more difficult to identify which ones may be playing a significant role.
We examined a number of potential predictors of the outcomes and found that the following factors did not have a significant effect on outcomes: duration of depressive episode, number of episodes, presence of disability, early onset of illness, socio-demographic group, education level, medical illness, depression severity, amount of homework completed, or recruitment site. Of particular note, some studies of MBCT for relapse prevention [17, 27] have found efficacy only in individuals with 3 or more episodes of depression. We did not find such a relationship, perhaps because the duration of episodes in our sample produced a picture of chronicity that shared features of those with 3 or more episodes.
While the above factors did not appear to have a significant impact on outcomes, several other factors were predictors of outcome independent of treatment. Data showed that a greater number of sessions attended were positively correlated with a greater treatment effect. We also found that the presence of a personality disorder or anxiety on both a state and trait level influenced outcomes adversely. This is consistent with other studies showing personality disorders and anxiety's impact on depression treatment [71]. Perceived stress also had a significant adverse effect. Stress has been regarded as a key contributor to depression, so this effect is not unanticipated. None of the above factors showed an interaction with treatment condition. Finally, one subscale of the CTQ, emotional abuse, also showed a relationship to adverse outcomes but not differentially by treatment condition. This was in contrast to one study suggesting MBCT was more efficacious than a psychoeducational control condition when childhood trauma was present [72].
The long-term outcomes indicate that the beneficial effects of MBCT occurred early and were maintained over the 52 weeks, while HEP effects appeared to have a later onset. In both conditions, people got better after 8 weeks with sustained improvement over 1-year, although the improvements were more substantial in MBCT at the 8-week time point, the primary focus of this study. While MBCT remained numerically superior for percentage reduction in HAMD17 and percentage of responders, there was no statistical difference at 52 weeks. The decrease in condition separation over 52 weeks is not atypical for psychotherapy studies which often report that benefits are maintained over time but that condition differences do not continue after intervention cessation [73, 74]. Although powered for 8 week differences in clinical outcomes (i.e. percent reduction in HAMD17), there is a possibility that with time, attrition effects occurred with participants who were not doing as well and may have dropped out so that only individuals who were doing better agreed to complete long-term follow-up assessments in both conditions; such a process may have decreased the separation. Additionally, although HEP was not chosen as a therapeutic control, it has components that could have produced beneficial antidepressant effects; e.g. exercise has been identified as improving depressive disorders, particularly as an augmentation intervention [75] and these effects may have played also played a role in reducing the separation of the conditions by 52 weeks.
The study had a number of strengths. It utilized two manualized interventions, MBCT and HEP, that offered structural equivalence. HEP provided a credible, active comparator for MBCT as evidenced by the week one Therapeutic Rationale Scale scores for each condition. We carefully monitored facilitator fidelity to each model and competency. We note, however, that only the MBCT Adherence Scale [34] was published and the measures of MBCT competency and HEP adherence and competency have not been validated or published thus far. However, on most of the items rated by 2 people there was perfect agreement on a 5-point scale and when they disagreed, they differed by only one point. We should also comment that the PI, trained in both MBCT and HEP, delivered feedback to both MBCT and HEP interventionists. Since he knew the hypothesis, this introduced a potential for bias. Another limitation of the study was that the two interventions were delivered by two different sets of individuals. However, it would be difficult to find one set of individuals who would be skilled across both domains.
Another limitation relates to the 8-week length of treatment. It is possible with a longer intervention, or mandated monthly alumni meetings, that long term results may have improved. Longer treatment lengths may have been particularly useful for individuals with chronic depression.
We screened participants to meet rigorous criteria for TRD, and they represented a seriously ill population. The participants, although blind to study hypotheses, were not masked to intervention assignment that could have introduced some bias. The study's title, “Practicing Alternative Techniques to Heal Depression,” was chosen to prevent introducing a perceived an investigator bias. Another factor that may have played a role in outcomes was our decision not to limit medication changes during the treatment period. Medication changes were tracked on a weekly basis and we found no differences between conditions on the number of medication changes that occurred. Furthermore, there was no evidence of a relationship between medication changes and treatment outcomes.
Patient preference for treatment assignment was not measured before randomization, and this may have played a role in outcomes. Some studies, however, suggest that treatment preferences in RCTs may not significantly affect outcomes [76]. In addition, Steidtman et al. [77] indicates that patient preference may act as moderators, but in unexpected ways. For example, in their study, individuals expressing a preference for medication treatment of depression who then received such treatment, had higher attrition rates than those who had not expressed such a preference receiving that treatment. Individuals' past psychotherapy and medication treatment histories and illness belief models are additional factors that affect patient preference. Hence, although the relationship between patient preference and outcome is complex, it would have been useful to have collected initial preference prior to randomization in order to assess potential effects.
In summary, MBCT appears to have specific effects that make it a useful treatment for patients with TRD. Many depressed individuals are interested in learning non-pharmacological and empowering approaches to control their illness. Moreover, in contrast to the suggestion that actively depressed patients could not concentrate well enough to utilize MBCT,[35] our findings did not support this belief. Subsequent papers will describe the changes in brain function associated with MBCT and HEP. The suffering related to TRD is pervasive, long lasting, and challenging. New approaches such as MBCT for augmentation of medication management may play an important role in the therapeutic armamentarium.
Acknowledgments
The National Institutes of Health/National Center for Complementary and Alternative Medicine (NCCAM) funded this study with grant number R01AT004572. The authors would like to thank the research staff of Lauren Erickson, Christa Hogan, Natalie Holbrook, and George Dugan, the research participants, and consultant Dr. Ian Gotlib.
Abbreviations
- MBCT
Mindfulness-Based Cognitive Therapy
- HEP
Health-Enhancement Program
- MDD
major depressive disorder
- TRD
treatment-resistant depression
- RCT
randomized controlled trial
- RA
research assistant
Footnotes
All authors report no conflict of interest.
References
- 1.Trevino K, McClintock SM, McDonald Fischer N, Vora A, Husain MM. Defining treatment-resistant depression: A comprehensive review of the literature. Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 2014;26:222–232. [PubMed] [Google Scholar]
- 2.Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: A review of current concepts and methods. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2007;52:46–54. doi: 10.1177/070674370705200108. [DOI] [PubMed] [Google Scholar]
- 3.Thase ME, Rush AJ. Treatment resistant depression. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The fourth generation of progress. New York, NY: Raven Press; 1995. pp. 1081–1097. [Google Scholar]
- 4.Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 5.Psychiatric Association A. Dsm-5: Diagnostic and statistical manual of mental disorders. Fifth. Arlington, Virgina: American Psychiatric Association; 2013. [Google Scholar]
- 6.Bschor T, Bauer M, Adli M. Chronic and treatment resistant depression: Diagnosis and stepwise therapy. Dtsch Arztebl Int. 2014;111:766–775. doi: 10.3238/arztebl.2014.0766. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janicak PG, Dowd SM. Treatment-resistant depression: An update on diagnosis and management. Psychopharm Review. 2009;44:41–43. [Google Scholar]
- 8.Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, Fava M, Nierenberg AA, McGrath PJ, Warden D, Niederehe G, Hollon SD, Rush AJ. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: A star*d report. Am J Psychiatry. 2007;164:739–752. doi: 10.1176/ajp.2007.164.5.739. [DOI] [PubMed] [Google Scholar]
- 9.Warden D, Trivedi MH, Wisniewski SR, Davis L, Nierenberg AA, Gaynes BN, Zisook S, Hollon SD, Balasubramani GK, Howland R, Fava M, Stewart JW, Rush AJ. Predictors of attrition during initial (citalopram) treatment for depression: A star*d report. Am J Psychiatry. 2007;164:1189–1197. doi: 10.1176/appi.ajp.2007.06071225. [DOI] [PubMed] [Google Scholar]
- 10.Paykel ES, Scott J, Teasdale JD, Johnson AL, Garland A, Moore R, Jenaway A, Cornwall PL, Hayhurst H, Abbott R, Pope M. Prevention of relapse in residual depression by cognitive therapy: A controlled trial. Arch Gen Psychiatry. 1999;56:829–835. doi: 10.1001/archpsyc.56.9.829. [DOI] [PubMed] [Google Scholar]
- 11.Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, Markowitz JC, Nemeroff CB, Russell JM, Thase ME, Trivedi MH, Zajecka J. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med. 2000;342:1462–1470. doi: 10.1056/NEJM200005183422001. [DOI] [PubMed] [Google Scholar]
- 12.Kocsis JH, Gelenberg AJ, Rothbaum BO, Klein DN, Trivedi MH, Manber R, Keller MB, Leon AC, Wisniewski SR, Arnow BA, Markowitz JC, Thase ME. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: The revamp trial. Arch Gen Psychiatry. 2009;66:1178–1188. doi: 10.1001/archgenpsychiatry.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fekadu A, Wooderson SC, Markopoulo K, Donaldson C, Papadopoulos A, Cleare AJ. What happens to patients with treatment-resistant depression? A systematic review of medium to long term outcome studies. J Affect Disord. 2009;116:4–11. doi: 10.1016/j.jad.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Russell JM, Hawkins K, Ozminkowski RJ, Orsini L, Crown WH, Kennedy S, Finkelstein S, Berndt E, Rush AJ. The cost consequences of treatment-resistant depression. The Journal of clinical psychiatry. 2004;65:341–347. doi: 10.4088/jcp.v65n0309. [DOI] [PubMed] [Google Scholar]
- 15.Greden JF. The burden of disease for treatment-resistant depression. The Journal of clinical psychiatry. 2001;62(Suppl 16):26–31. [PubMed] [Google Scholar]
- 16.Eisendrath SJ, Delucchi K, Bitner R, Fenimore P, Smit M, McLane M. Mindfulness-based cognitive therapy for treatment-resistant depression: A pilot study. Psychotherapy and psychosomatics. 2008;77:319–320. doi: 10.1159/000142525. [DOI] [PubMed] [Google Scholar]
- 17.Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of consulting and clinical psychology. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, LaBar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, Krishnan RR, McCarthy G. Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res. 2008;163:143–155. doi: 10.1016/j.pscychresns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozano AM, Giacobbe P, Hamani C, Rizvi SJ, Kennedy SH, Kolivakis TT, Debonnel G, Sadikot AF, Lam RW, Howard AK, Ilcewicz-Klimek M, Honey CR, Mayberg HS. A multicenter pilot study of subcallosal cingulate area deep brain stimulation for treatment-resistant depression. J Neurosurg. 2012;116:315–322. doi: 10.3171/2011.10.JNS102122. [DOI] [PubMed] [Google Scholar]
- 20.Mayberg HS. Defining the neural circuitry of depression: Toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61:729–730. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12:163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz A, Slagter HA, Rawlings NB, Francis AD, Greischar LL, Davidson RJ. Mental training enhances attentional stability: Neural and behavioral evidence. J Neurosci. 2009;29:13418–13427. doi: 10.1523/JNEUROSCI.1614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnhofer T, Crane C, Hargus E, Amarasinghe M, Winder R, Williams JM. Mindfulness-based cognitive therapy as a treatment for chronic depression: A preliminary study. Behav Res Ther. 2009;47:366–373. doi: 10.1016/j.brat.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnhofer T, Crane C, Hargus E, Amarasinghe M, Winder R, Williams JM. The effects of mindulness meditation on cognitive processes and affect in patients with past depression. Behav Res Ther. 2009;47:366–373. doi: 10.1016/j.brat.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, Bloch R, Levitan RD. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67:1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuyken W, Byford S, Byng R, Dalgleish T, Lewis G, Taylor R, Watkins ER, Hayes R, Lanham P, Kessler D, Morant N, Evans A. Study protocol for a randomized controlled trial comparing mindfulness-based cognitive therapy with maintenance anti-depressant treatment in the prevention of depressive relapse/recurrence: The prevent trial. Trials. 2010;11:99. doi: 10.1186/1745-6215-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: Replication and exploration of differential relapse prevention effects. Journal of consulting and clinical psychology. 2004;72:31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- 28.Eisendrath SJ, Gillung EP, Delucchi KL, Chartier M, Mathalon DH, Sullivan JC, Segal ZV, Feldman MD. Mindfulness-based cognitive therapy (mbct) versus the health-enhancement program (hep) for adults with treatment-resistant depression: A randomized control trial study protocol. BMC Complement Altern Med. 2014;14:95. doi: 10.1186/1472-6882-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.First MB, Pincus HA. The dsm-iv text revision: Rationale and potential impact on clinical practice. Psychiatric services. 2002;53:288–292. doi: 10.1176/appi.ps.53.3.288. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton M. Development of a rating scale for primary depressive illness. The British journal of social and clinical psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 31.Prudic J, Haskett RF, Mulsant B, Malone KM, Pettinati HM, Stephens S, Greenberg R, Rifas SL, Sackeim HA. Resistance to antidepressant medications and short-term clinical response to ect. Am J Psychiatry. 1996;153:985–992. doi: 10.1176/ajp.153.8.985. [DOI] [PubMed] [Google Scholar]
- 32.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 33.MacCoon DG, Imel ZE, Rosenkranz MA, Sheftel JG, Weng HY, Sullivan JC, Bonus KA, Stoney CM, Salomons TV, Davidson RJ, Lutz A. The validation of an active control intervention for mindfulness based stress reduction (mbsr) Behav Res Ther. 2012;50:3–12. doi: 10.1016/j.brat.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Segal Z, Teasdale JD, Williams MJ, Gemar MC. The mindfulness-based cognitive therapy adherence scale: Inter-rater reliability, adherence to protocol and treatment distinctiveness. Clinical Psychology and Psychotherapy. 2002;9:131–138. [Google Scholar]
- 35.Segal Z, Williams JM, Teasdale J. Mindfulness-based cognitive therapy for depression. New York: The Guilford Press; 2002. [Google Scholar]
- 36.Chartier M, Bitner R, Peng T, Coffelt N, McLane M, Eisendrath S. Adapting ancient wisdom for the treatment of depression: Mindfulness-based cognitive therapy group training. Group. 2010;34:319–327. [PMC free article] [PubMed] [Google Scholar]
- 37.Eisendrath S, Chartier M, McLane M. Adapting mindfulness-based cognitive therapy for treatment-resistant depression: A clinical case study. Cogn Behav Pract. 2011;18:362–370. doi: 10.1016/j.cbpra.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guy W. Ecdeu assessment manual for psychopharmacology revised (dhew publ no adm 76-338) Rockville, MD: U.S Dept Health, Education and Welfare, Public Health Service, Alcohol, Drug Abuse and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 39.Arch JJ, Craske MG. Mechanisms of mindfulness: Emotion regulation following a focused breathing induction. Behav Res Ther. 2006;44:1849–1858. doi: 10.1016/j.brat.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 40.Batink T, Peeters F, Geschwind N, van Os J, Wichers M. How does mbct for depression work? Studying cognitive and affective mediation pathways. Plos One. 2013;8 doi: 10.1371/journal.pone.0072778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu J, Strauss C, Bond R, Cavanagh K. How do mindfulness-based cognitive therapy and mindfulness-based stress reduction improve mental health and wellbeing? A systematic review and meta-analysis of mediation studies. Clinical psychology review. 2015;37:1–12. doi: 10.1016/j.cpr.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Zettle RD, Rains JC, Hayes SC. Processes of change in acceptance and commitment therapy and cognitive therapy for depression: A mediation reanalysis of zettle and rains. Behavior modification. 2011;35:265–283. doi: 10.1177/0145445511398344. [DOI] [PubMed] [Google Scholar]
- 43.Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- 44.Neff KD. The development and validation of a scale to measure self-compassion. Self and Identity. 2003;2:223–250. [Google Scholar]
- 45.Nolen-Hoeksema S, Parker LE, Larson J. Ruminative coping with depressed mood following loss. Journal of personality and social psychology. 1994;67:92–104. doi: 10.1037//0022-3514.67.1.92. [DOI] [PubMed] [Google Scholar]
- 46.Hayes AM, Beevers CG, Feldman GC, Laurenceau JP, Perlman C. Avoidance and processing as predictors of symptom change and positive growth in an integrative therapy for depression. International journal of behavioral medicine. 2005;12:111–122. doi: 10.1207/s15327558ijbm1202_9. [DOI] [PubMed] [Google Scholar]
- 47.Hayes SC, Strosahl K, Wilson KG, Bissett RT, Pistorello J, Toarmino D, Polusny MA, Dykstra TA, Batten SV, Bergan J, Stewart SH, Zvolensky MJ, Eifert GH, Bond FW, Forsyth JP, Karekla M, McCurry SM. Measuring experiential avoidance: A preliminary test of a working model. Psychol Rec. 2004;54:553–578. [Google Scholar]
- 48.Souery D, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, Kasper S, Lecrubier Y, Montgomery S, Serretti A, Zohar J, Mendlewicz J. Clinical factors associated with treatment resistance in major depressive disorder: Results from a european multicenter study. The Journal of clinical psychiatry. 2007;68:1062–1070. doi: 10.4088/jcp.v68n0713. [DOI] [PubMed] [Google Scholar]
- 49.Spielberger C, Gorush RR, Luchene RE. State-trait anxiety inventory. Palo Alto Consulting Psychologists Press; 1970. [Google Scholar]
- 50.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of health and social behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- 51.Bernstein DP, F L. A retrospective self-report Manual. San Antonio, TX: The Psychological Corporation; 1998. Ctq childhood trauma questionnaire; p. 1998. [Google Scholar]
- 52.Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF, Ninan PT, McCullough JP, Jr, Weiss PM, Dunner DL, Rothbaum BO, Kornstein S, Keitner G, Keller MB. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14293–14296. doi: 10.1073/pnas.2336126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.First M, Spitzer R, Gibbon M, Williams J, Benjamin L. Structured clinical interview for dsm-iv axis ii personality disorders (scid ii) New York, NY, New York: Biometric Research Department; 1994. [Google Scholar]
- 54.Greenberg RP, Constantino MJ, Bruce N. Are patient expectations still relevant for psychotherapy process and outcome? Clinical psychology review. 2006;26:657–678. doi: 10.1016/j.cpr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psy. 1972;3:257–260. [Google Scholar]
- 56.MacKinnon DP. Introduction to statistical mediation analysis. New York: Taylor & Francis Group; 2008. [Google Scholar]
- 57.MacCoon DG, MacLean KA, Davidson RJ, Saron CD, Lutz A. No sustained attention differences in a longitudinal randomized trial comparing mindfulness based stress reduction versus active control. Plos One. 2014;9:e97551. doi: 10.1371/journal.pone.0097551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, Ritz L, Nierenberg AA, Lebowitz BD, Biggs MM, Luther JF, Shores-Wilson K, Rush AJ, Team SDS. Medication augmentation after the failure of ssris for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 59.Hans E, Hiller W. A meta-analysis of nonrandomized effectiveness studies on outpatient cognitive behavioral therapy for adult anxiety disorders. Clinical psychology review. 2013;33:954–964. doi: 10.1016/j.cpr.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Brakemeier EL, Radtke M, Engel V, Zimmermann J, Tuschen-Caffier B, Hautzinger M, Schramm E, Berger M, Normann C. Overcoming treatment resistance in chronic depression: A pilot study on outcome and feasibility of the cognitive behavioral analysis system of psychotherapy as an inpatient treatment program. Psychotherapy and psychosomatics. 2015;84:51–56. doi: 10.1159/000369586. [DOI] [PubMed] [Google Scholar]
- 61.Warden D, Rush AJ, Trivedi MH, Fava M, Wisniewski SR. The star*d project results: A comprehensive review of findings. Curr Psychiatry Rep. 2007;9:449–459. doi: 10.1007/s11920-007-0061-3. [DOI] [PubMed] [Google Scholar]
- 62.Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, Lewis G, Watkins E, Brejcha C, Cardy J, Causley A, Cowderoy S, Evans A, Gradinger F, Kaur S, Lanham P, Morant N, Richards J, Shah P, Sutton H, Vicary R, Weaver A, Wilks J, Williams M, Taylor RS, Byford S. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (prevent): A randomised controlled trial. Lancet. 2015;386:63–73. doi: 10.1016/S0140-6736(14)62222-4. [DOI] [PubMed] [Google Scholar]
- 63.Chiesa A, Castagner V, Andrisano C, Serretti A, Mandelli L, Porcelli S, Giommi F. Mindfulness-based cognitive therapy vs. Psycho-education for patients with major depression who did not achieve remission following antidepressant treatment. Psychiatry Res. 2015;226:474–483. doi: 10.1016/j.psychres.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Carvalho AF, Berk M, Hyphantis TN, McIntyre RS. The integrative management of treatment-resistant depression: A comprehensive review and perspectives. Psychotherapy and psychosomatics. 2014;83:70–88. doi: 10.1159/000357500. [DOI] [PubMed] [Google Scholar]
- 65.McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, Barakat M, Miguelez M. Treatment-resistant depression: Definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1–7. doi: 10.1016/j.jad.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 66.Bieling PJ, Hawley LL, Bloch RT, Corcoran KM, Levitan RD, Young LT, Macqueen GM, Segal ZV. Treatment-specific changes in decentering following mindfulness-based cognitive therapy versus antidepressant medication or placebo for prevention of depressive relapse. Journal of consulting and clinical psychology. 2012;80:365–372. doi: 10.1037/a0027483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mothes H, Klapersi S, Seelig H, Schmidt S, Fuchs R. Regular aerobic exercise increases dispositional mindfulness in men: A randomized controlled trial. Mental Health and Physical Activity. 2014;7:111–119. [Google Scholar]
- 68.Brown KW, Ryan RM. The benefits of being present: Mindfulness and its role in psychological well-being. Journal of personality and social psychology. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- 69.Williams MJ, Dalgleish T, Karl A, Kuyken W. Examining the factor structures of the five facet mindfulness questionnaire and the self-compassion scale. Psychol Assess. 2014;26:407–418. doi: 10.1037/a0035566. [DOI] [PubMed] [Google Scholar]
- 70.Fava GA. Rational use of antidepressant drugs. Psychotherapy and psychosomatics. 2014;83:197–204. doi: 10.1159/000362803. [DOI] [PubMed] [Google Scholar]
- 71.Ionescu DF, Niciu MJ, Richards EM, Zarate CA., Jr Pharmacologic treatment of dimensional anxious depression: A review. The primary care companion to CNS disorders. 2014;16 doi: 10.4088/PCC.13r01621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams JM, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJ, Hackmann A, Krusche A, Muse K, Von Rohr IR, Shah D, Crane RS, Eames C, Jones M, Radford S, Silverton S, Sun Y, Weatherley-Jones E, Whitaker CJ, Russell D, Russell IT. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: A randomized dismantling trial. Journal of consulting and clinical psychology. 2014;82:275–286. doi: 10.1037/a0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohr DC, Hart SL, Julian L, Catledge C, Honos-Webb L, Vella L, Tasch ET. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry. 2005;62:1007–1014. doi: 10.1001/archpsyc.62.9.1007. [DOI] [PubMed] [Google Scholar]
- 74.Beutler LECJ. Systematic treatment selection: Toward targeted therapeutic interventions. New York, NY: Brunner/Mazel; 1990. [Google Scholar]
- 75.Trivedi MH, Greer TL, Church TS, Carmody TJ, Grannemann BD, Galper DI, Dunn AL, Earnest CP, Sunderajan P, Henley SS, Blair SN. Exercise as an augmentation treatment for nonremitted major depressive disorder: A randomized, parallel dose comparison. The Journal of clinical psychiatry. 2011;72:677–684. doi: 10.4088/JCP.10m06743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dunlop BW, Kelley ME, Mletzko TC, Velasquez CM, Craighead WE, Mayberg HS. Depression beliefs, treatment preference, and outcomes in a randomized trial for major depressive disorder. Journal of psychiatric research. 2012;46:375–381. doi: 10.1016/j.jpsychires.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steidtmann D, Manber R, Arnow BA, Klein DN, Markowitz JC, Rothbaum BO, Thase ME, Kocsis JH. Patient treatment preference as a predictor of response and attrition in treatment for chronic depression. Depress Anxiety. 2012;29:896–905. doi: 10.1002/da.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]


