Abstract
A tumor originates from a normal cell that has undergone tumorigenic transformation as a result of genetic mutations. This transformed cell is the cell-of-origin for the tumor. In contrast, an established clinical tumor is sustained by subpopulations of self-renewing cancer cells operationally called cancer stem cells (CSCs) that can generate, intraclonally, both tumorigenic and non-tumorigenic cells. Identifying and characterizing tumor cell-of-origin and CSCs should help elucidate tumor cell heterogeneity, which, in turn, should help understand tumor cell responses to clinical treatments, drug resistance, tumor relapse, and metastatic spread. Both tumor transplantation and lineage-tracing assays have been helpful in characterizing these cancer cell populations, although each system has its strengths and caveats. In this essay, we briefly review and summarize advantages and limitations of both assays in support of a combinatorial approach in order to accurately define the roles of both cancer-initiating and cancer-propagating cells. As an aside, we also wish to clarify the definitions of cancer cell-of-origin and CSCs, which are often interchangeably used by mistake.
Keywords: cell-of-origin, cancer stem cells, transplantation assay, lineage-tracing assay
Epithelial cancers are complex and exhibit inter-tumoral and intra-tumoral heterogeneity. Identifying specific cell types that initiate and sustain tumorigenesis is key to addressing tumor cell heterogeneity and other outstanding tumor biology questions. Cancer-initiating cell, or the cell-of-origin of cancer, is the normal cell that receives the first cancer-causing mutations. In other words, the cancer-initiating cell founds a future clinical tumor. Cancer stem cells (CSCs), on the other hand, are the cells that maintain tumor propagation (1-3). Aptly referred to as cancer-propagating cells, CSCs are defined by two attributes, self-renewal and multipotency. The phenotypes between cancer-initiating cells and cancer-propagating cells may differ and dynamically change and, in most cases, the relationship between the two is not well understood. Two assays have been helpful in characterizing these two cell types: transplantation assays and lineage tracing assays (Figure 1). The serial tumor transplantation assay is the current “gold standard” for identifying CSCs because it can assess both self-renewal and multipotency. Transplantation assays can also be utilized to determine the cell-of-origin of cancers. Lineage tracing is the current gold standard for defining the cell-of-origin of transformation in mouse models. Lineage tracing is also being used to provide insight into the proliferative potential and fate of stem cells during tumor formation as evidenced by recent progress in identifying CSCs in solid tumors.
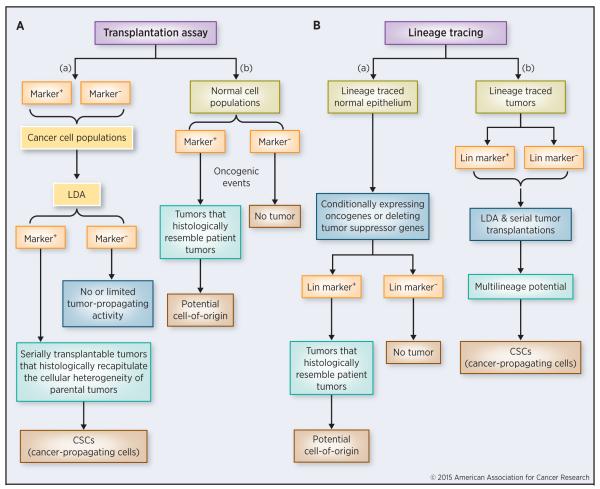
Figure 1. A comparison of transplantation and lineage tracing assays.
(A) Tumor transplantation assay. LDA can be used in combination with serial transplantations to assess CSC abundance properties in a candidate marker-positive tumor cell population (a). The transplantation assay can also be utilized to determine cell-of-origin in cancers (b).
(B) Lineage-tracing assay. This is most commonly utilized to determine the potential cell-of-origin for a cancer. In a specific normal cell population, transformation events can be introduced via activation of oncogenes or inactivation of tumor-suppressor genes. Labeled tumors can then be traced back to a specific cell-of-origin (a). Lineage tracing can also be used to determine and/or authenticate the CSC properties of the marked cell population in the established, traced tumor (b).
The transplantation assay
In the transplantation assay, tumor cell populations are fractionated and xenografted into immunocompromised mice. When identifying CSCs, cancer cell subpopulations are sorted using FACS based on relatively specific or presumed CSC markers followed by limiting dilution assay (LDA) and serial tumor transplantations in order to determine the CSC frequency and multi-lineage potential of a given marker phenotype (Figure 1A, a). Populations of CSC marker-positive cells that give rise to serially transplantable tumors that histologically recapitulate the cellular heterogeneity of the parental tumors can therefore be classified as CSCs whereas populations of CSC marker-negative cells with no or limited tumor-propagating activity can be excluded from the CSC candidates (Figure 1A, a). These assays have demonstrated the existence of CSCs in human cancers including acute myeloid leukemia (AML), chronic myeloid leukemia (CML), breast cancer, glioblastoma, colorectal cancer, and others (1-3)
Historically, in the early 1950’s, it was shown that a small fraction of cells in murine tumors could withstand freeze-thawing as indicated by proliferation in vitro, providing early evidence for functional heterogeneity in sarcoma cell subpopulations (4). Subsequent quantitative transplantation assays determined that 1 out of 27 viable cells was capable of giving rise to a tumor when cells from a murine sarcoma tumor, S37, were xenotransplanted into 4-6 week old albino mice (5). Direct proof for the existence of leukemic stem cells (LSCs) was provided in the mid-1990’s (reviewed in 3), and in the 2000’s, evidence for CSCs was extended to human solid tumors. It was first shown that in human breast cancer as few as 100 cells bearing the CD44+CD24−/lowLin− cell surface marker profile could regenerate serially transplantable tumors in mice (6). Shortly thereafter, xenotransplantation assays evinced CSCs in human brain tumors, with the CD133+ tumor cell fraction containing cells capable of tumor regeneration in non-obese diabetic-severe combined immunodeficiency (NOD-SCID) mouse brains (7).
The key elements in properly using the xenotransplantation assay to identify and characterize CSCs in human tumors are to perform rigorous LDA and serial tumor transplantations (Figure 1A, a). In the LDA, CSC frequency is measured by transplanting increasingly diluted singe-cell preparations. After tumor regeneration is evaluated for each cell dose, the frequency of cancer cells present in a given cell population that can regenerate a xenograft tumor can be approximated. For this reason, CSCs are also frequently called tumor-initiating cells, which is actually not very accurate and should rather be termed tumor-regenerating cells. In subsequent serial transplantations, true CSCs or CSC-enriched population should be able to perpetuate the xenograft tumors for multiple generations, thus attesting that these particular cells have an inherently unlimited life span when propagated in vivo (Figure 1A, a). Important, LDA combined with serial tumor transplantations help assess one of the most important biological traits of CSCs, i.e., self-renewal in vivo. As an example, in a recent study, prostate specific antigen (PSA) positive (PSA+) and PSA−/lo human prostate cancer (PCa) cells were separated and used in serial tumor transplantations (8). The study revealed that the PSA−/lo population could regenerate and propagate xenograft tumors virtually indefinitely whereas the PSA+ PCa cell population could only propagate xenograft tumors for ~3 generations (8). This study illustrates that the serial tumor transplantation assay has the ability to compare isogenic subpopulations under identical experimental conditions in order to determine differences in tumor regeneration and long-term tumor-propagating capacities. Similar serial transplantation studies have demonstrated that human breast (6) and colon (9) CSCs can initiate serially transplantable tumors and thus can self-renew in immunodeficient mice.
Transplantation assays can be utilized to probe the potential cell-of-origin of cancer as well (Figure 1A, b). In this scenario, normal cell subpopulations are sorted via FACS based on specific markers followed by introduction of oncogenic events (overexpressing oncogenes and/or knocking out tumor suppressor genes) and subsequent survey of differential tumor formation in xenotransplantation assays. When a marker-positive population gives rise to tumors that histologically resemble parental or patient tumors, cells within this population can then be considered as a potential cell-of-origin for that specific type of cancer (Figure 1A, b). One example comes from a recent study that demonstrates that the basal epithelial cells from primary benign human prostate tissue, upon tumorigenic transformation, can initiate PCa in immunodeficient mice (10). The authors developed a system whereby naïve adult human prostate epithelium is directly transformed with genetic alterations commonly found in human PCa. When primary human prostate basal and luminal cells transduced with lentivirus carrying red fluorescent protein were combined with murine urogenital sinus mesenchyme cells in Matrigel and injected subcutaneously into NOD-SCID-IL-2Rγnull (NSG) mice, outgrowths were observed only from basal cells (10). Important, when the lentivirus cocktail included both activated (myristoylated) AKT and ERG, basal cell but not luminal cell-derived lesions fulfilled the histological criteria for the diagnosis of high-grade precursor lesion (10). With the addition of AR (androgen receptor) to the mix, adenocarcinomas developed from transformed basal cells but not luminal cells (10). This study (10) thus indicates that the human basal prostate epithelial cells can function as a potential cell-of-origin for PCa. Using similar transplantation assays, Taylor et al also demonstrated that basal epithelial cells could act as cells-of-origin for PCa (11). One word of caution when using transplantation assays to study cancer cell-of-origin is that a positive outcome only indicates that a specific cell population CAN function as the target of tumorigenic transformation but may not necessarily BE the actual cell-of-origin for cancer in vivo.
For obvious reasons, human tumor cells can only be xenotransplanted to immunodeficient mice to assess their inherent CSC properties. As a result, a major disadvantage of cell transplantation assays is that dissociated single cells may not behave the same way as they do in their natural tissue microenvironment (i.e., niche), thereby misrepresenting the existence or abundance of CSCs (see below). By teasing apart the intact tissue to resolve subpopulations, we inevitably change the cells’ metabolism, their apparent role in the tissue hierarchy, and potentially their developmental trajectory. Therefore it may not be certain whether transplantation assays demonstrate selection of phenotypically plastic cells that survive and proliferate in the new environment, or whether they are actually assaying the implicit CSC traits. Additionally, solid tumor cells exist in complex microenvironments that are not readily modeled by transplantation because xenotransplants differ in architecture and stroma compared to their native environment. Another caveat associated with xenotransplantations lies in the lack of an immune-competent microenvironment such that many have argued that the transplantation-based CSC assays may not assess the intrinsic properties of stem cells but may instead be assessing the ability of transplanted human cancer cells in evading immune surveillance. However, this may be a circular argument - it is precisely because CSCs lack the expression of differentiation markers such as MHC molecules that they can better escape host immune-mediated attack, take root, and initiate and propagate human tumors in mice (12).
Understandably, the outcome of xenotransplantation experiments can be influenced by many variables including the level of malignancy (or differentiation) of donor human tumors and the level of immunodeficiency of recipient mice (2). For instance, the frequency of human melanoma CSCs was found to be as high as 15-25% when assayed in NSG mice compared to 1 in 105 cells in NOD/SCID mice (13). The high frequency of melanoma CSCs in NSG mice has been interpreted by many as evidence for lack of tumorigenic hierarchy in melanoma. However, in that study (13) most melanoma samples used were very advanced high-grade tumors and it is well known that advanced, undifferentiated human cancers are highly enriched in CSCs (1-3, 8). Indeed, when early stages of melanoma specimens were later used in CSC studies, it was found that CD271+ melanoma cells identify rare melanoma CSCs (14). Along the same line, in a syngeneic transplantation study of pre-B/B lymphoma cells from Eμ-myc transgenic mice, a very high frequency of the tumor-initiating cells was observed (15), which again was construed by many as evidence to refute the CSC concept. However, the Eμ-myc lymphoma cells are known to be extremely aggressive, resembling undifferentiated human tumors in which CSCs are greatly enriched (2,3). Recent lineage-tracing studies in mouse models of tumors have also provided direct evidence for CSCs (see below).
The lineage-tracing assay
The lineage-tracing assay is mostly commonly used to determine the potential cell-of-origin of tumors (Figure 1B, a) although it can also be employed to study CSCs (Figure 1B, b). In the lineage-tracing assay, use of different cell-specific promoters allows distinct cell subpopulations to be labeled, allowing tracking of a single cell-derived clone in animals (see below). The ability to resolve individual cell fate is the greatest advantage of this assay. In order to determine cell-of-origin, normal (epithelial) cells are genetically labeled followed by introduction of activating and inactivating mutations in various oncogenes and tumor suppressors in the same cell type. The fully transformed cell that forms a tumor can then be traced and identified as the cellular source of the tumor (Figure 1B, c). On the other hand, in the established, traced tumors, single marked tumor cells can be purified out and used in the LDA and serial tumor transplantations to determine whether the lineage-traced tumor cells have true CSC properties, i.e., self-renewing and long-term tumor-propagating activity (Figure 1B, d). This latter tracing strategy can also be adapted to dissect tumor cell heterogeneity in cultured cancer cells and human xenograft tumors. For example, a PSA promoter was used to drive reporters (GFP and RFP) in a lentiviral vector, which was used to infect cultured as well as xenograft PCa cells and to separate the PSA−/lo and PSA+ PCa cells (8). When traced in vitro and in vivo, the PSA−/lo PCa cells were found to be able to undergo asymmetric cell division generating PSA+ cells under time-lapse microscopy as well as in serially transplanted tumors (8). Similarly, a Wnt reporter was employed to trace colorectal CSCs and to demonstrate that the secreted soluble molecules from the neighboring myofibroblasts activate the Wnt signaling in these CSCs (16).
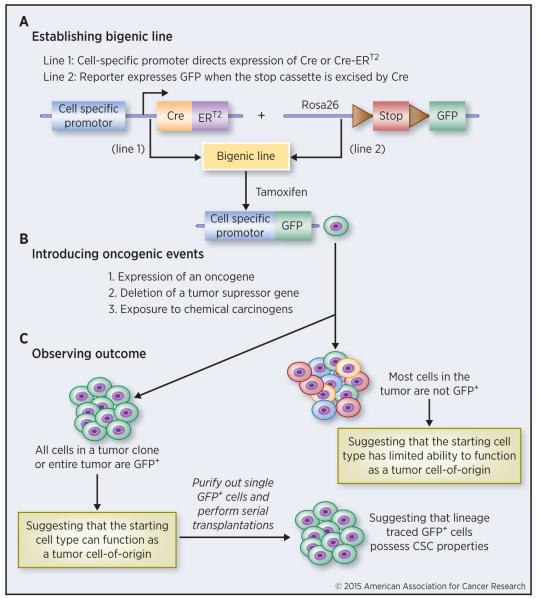
Figure 2 depicts a basic scheme for performing a lineage tracing study. The first step is to establish a bigenic mouse line by crossing an inducible Cre line, which expresses the Cre recombinase, with a ‘generic’ reporter line to achieve cell-specific labeling (Figure 2A). For the inducible Cre transgenic (Tg) line, a cell type specific gene promoter is used to drive the expression of modified, Tamoxifen-inducible Cre (CreER or CreERT2). The gene promoters can be stem cell specific (e.g., p63, Lrig1, Lgr5, Sox9, Nkx3.1, etc) or the ones active in differentiated lineages (e.g., K8 and K18). Promoter can be endogenous, in which CreER or CreERT2 expression cassette is knocked into the endogenous promoter locus. Alternatively, an exogenous promoter can be used to drive Cre expression in a conventional Tg line. An example is the use of human PSA gene promoter to drive Cre expression in a Tg model to show that regenerated prostatic epithelial cells upon castration – androgen supplementation were derived from the pre-existent luminal cells (17). In the second mouse line, a reporter (either fluorogenic such as GFP and RFP or colorigenic such as β-galactosidase) is flanked by a loxP-STOP-loxP sequence (Figure 2A). In the bigenic mice, tamoxifen activates Cre expression via excising loxP-STOP-loxP, which in turn activates the reporter in cells that express the promoter activity (Figure 2A). Doxycycline-inducible TetO-Cre system or orthotopic adenoviral delivery of lineage-specific Cre-recombinases (AdCre) can also be used to express Cre in specific cell types.
Figure 2. Schematic for a lineage tracing study.
Three main step are outlined. See text for detailed descriptions.
To study tumor development, the second step is to introduce oncogenic events, which can be either genetic or chemical, to the specifically labeled cell types (Figure 2B). For genetic approaches, this is accomplished by crossing the above bigenic line with the third Tg line that overexpresses certain oncogenes (Myc, Ras, Tcf, etc), or has some tumor suppressors (e.g., Pten, Rb, p53) deleted. For chemical carcinogenesis, the bigenic animals are challenged by chemical carcinogens (e.g., DMBA) known to cause cancer in the specific tissues/cells. The molecular basis of chemical carcinogenesis is still genetic – for instance, DMBA mainly causes mutations in K-ras. In the final step of lineage tracing (Figure 2C), tumor development is observed by monitoring the expression pattern of the trace label. For instance, if all cells in a tumor clone or the entire tumor is label (e.g., GFP) positive, it suggests that the specific cell type marked by GFP CAN function as a cancer cell-of-origin in that specific context. These GFP+ tumor cells can be purified out in serial tumor transplantations to further demonstrate their CSC or tumor-propagating activities (Figure 2C). On the other hand, if the majority of tumor cells are GFP−, it would suggest that the initially traced cell type does not function as the cell-of-origin for the tumor development (Figure 2C).
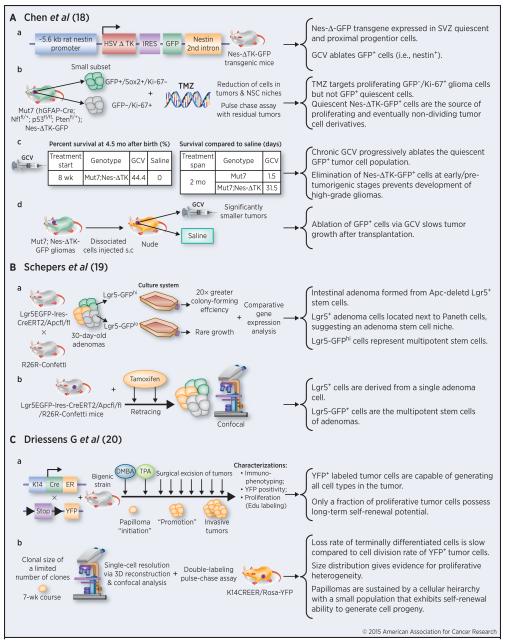
Recently, three lineage-tracing studies (18-20) provided support for the CSC model across three different types of solid tumors—skin, intestinal, and brain (Figure 3). In one study (18), lineage tracing was used to pinpoint a putative endogenous glioma stem cell population that plays a pivotal role in tumor maintenance and recurrence after chemotherapy (Figure 3A). A nestin-ΔTK-IRES-GFP transgene labeled quiescent subventricular zone (SVZ) adult neural stem cells and the labeled GFP+ cells could be ablated by ganciclovir (GCV) through the action of the truncated thymidine kinase (TK) (Figure 3A, a). When this Tg line was crossed with glioma-prone mouse line called Mut7 (induced by concerted deletion of 3 tumor suppressors, i.e., Nf1, p53, and Pten in GFAP-expressing cellular compartment), a subset of endogenous glioma tumor cells was also labeled by GFP and these GFP+ cells could also be ablated by GCV (Figure 3A, b). Interestingly, most GFP+ glioma cells were Sox2+ and not dividing (i.e., Ki67−) whereas most Ki67+ glioma cells were GFP− (Figure 3A, b). Temozolomide (TMZ), a drug used to treat glioma patients, eliminated most GFP−/Ki67+ tumor cells leaving behind a significant fraction of GFP+/Ki67− quiescent mouse glioma cells, which mediated recurrent tumor formation (Figure 3A, b). Remarkably, ablation of the GFP+ cells with chronic GCV administration significantly retarded tumor growth leading to extended animal survival, and combined TMZ and ganciclovir treatment impeded tumor development (Figure 3A, c) (13). GCV administration also reduced tumor growth in secondary tumor transplants (Figure 3A, d). This lineage tracing study demonstrates that a relatively quiescent subset of endogenous glioma cells, with properties similar to those proposed for CSCs, is responsible for sustaining long-term tumor growth.
Figure 3. Summary of three recent lineage tracing studies providing support for the CSC model.
Details for each study are discussed in the text. The lineage tracing tumor models are presented on the left and main outcomes and conclusions summarized on the right.
In the second study (19), use of a multicolor Cre-reporter R26R-Confetti and the β-naphtoflavone–inducible Ah-Cre mouse strain demonstrated that the crypt stem cell marker Lgr5 also marked a subpopulation of adenoma cells induced by loss of Apc (Figure 3B, a). When R26R-Confetti was crossed into the Lgr5 knock-in Cre-expressing mouse strain, tamoxifen injection allowed single Lgr5+ stem cells to randomly adopt one of the four fluorescent colors encoded in the R26R-Confetti allele. The formation of adenomas was derived from individual Apc-mutant stem cells. Additionally, the location of the labeled Lgr5+ cells was near the base of the wedge-shaped adenoma segments, concurrent with the crypt stem cell niche (Figure 3B, a). Transcriptional profiles and clonogenic potential of Lgr5-GFPhi cells suggested that this population constituted multipotent stem cells. The fate of Lgr5+ adenoma cells in individual clones was back-traced in vivo, and it was concluded that the cells were derived from a single adenoma stem cell (Figure 3B, b).
The third lineage tracing study employed a classical chemical two-stage carcinogenesis model (20), in which skin papillomas were initiated by the carcinogen DMBA and then propagated by tumor promoter TPA (Figure 3C, a). The bigenic line, K14CreER/Rosa-YFP, was created by crossing K14-driven CreER line with the Rosa26-YFP reporter line (Figure 3C, a). In the presence of tamoxifen, all K14-expressing basal keratinocytes would be labeled as YFP+. Upon DMBA/TPA treatment and tamoxifen application, cells within the papillomas were labeledand the YFP+ tumor cells were capable of generating all cell types that comprised the tumor (Figure 3C, a). Interestingly, the majority of labeled tumor cells in benign papillomas had limited proliferative potential, whereas a particular fraction had the capacity to persist long term (20). Specifically, the more persistent population displayed stem cell-like characteristics and cycled twice per day, whereas a slower cycling transient population gave rise to terminally differentiated tumor cells. Data from 3 dimensional (3D) reconstruction using confocal analyses of clones indicated that papillomas were sustained by a cellular hierarchy in which a minor population of tumor cells with stem-cell-like properties gave rise to a more transient progenitor cell pool (Figure 3C, c).
Lineage tracing has now been utilized to identify probable cells-of-origin for many mouse models of cancers including intestinal, prostate and basal cell carcinomas, brain and breast tumors, as well as pancreatic ductal adenocarcinoma. In PCa, for example, the lineage-tracing assay was used to identify a rare luminal epithelial population, termed CARNs (castration-resistant Nkx3.1-expressing cells) with stem cell properties during prostate regeneration in mice (21). These cells were marked using a genetically engineered mouse line in which an inducible CreERT2 recombinase was put under the control of the endogenous promoter for Nkx3.1, a putative prostate tumor suppressor. After Cre activation by tamoxifen treatment in castrated Nkx3.1CreERT2/+; R26R-YFP/+ male mice, YFP expression was observed in luminal epithelial cells, corresponding to lineage-marked CARNs. All CARNS in the regressed prostate were strictly luminal and growth-quiescent. However, after regeneration upon testosterone treatment, the percentage of lineage-marked cells increased nine-fold, indicating their proliferative potential, and, important, basal cells appeared, indicating that CARNS contained bipotential progenitors. Subsequent single-cell transplantation of lineage-marked CARNs further indicated the multipotency of these cells. The authors further demonstrated that, by crossing the compound reporter mice with Pten mutant mice, the CARNs could function as cells-of-origin for PCa (21). The same group later showed that basal cells, upon loss of Pten, gave rise to tumors with luminal phenotypes, but they noted that these basal cells displayed substantial phenotypic plasticity when removed from their endogenous tissue microenvironment (22). Hence, the authors stressed that transplantation-based assays have a tendency to over-estimate the frequency of putative stem cells and that genetic lineage tracing in vivo should be preferably employed for identification of potential tumor cell-of-origin (22).
Another group genetically marked mouse prostate basal cells and luminal cells in adult mice using K14-CreER and K8-CreERT2, respectively, in order to further interrogate the cellular origin of PCa (23). It was found that prostate basal cells only generated basal cells whereas luminal cells only generated luminal cells suggesting that adult prostate epithelial lineages are maintained by unipotent progenitors or self-duplication of epithelial cells. Pten was then knocked out in both cell types and interestingly, prostatic intraepithelial neoplasia (PIN), a precursor lesion to PCa, appeared in both mice but only after a long latency in the K14-CreER mice, suggesting that basal cells had to convert into luminal cells in the context of tumor development by Pten deletion. This study demonstrates that both prostate basal and luminal cells can serve as the cellular origin for PCa.
As in all other techniques, there are caveats and problems associated with lineage tracing. First of all, lineage tracing can only be conducted in mice and there are fundamental differences between human and rodent organs and cells. Take prostate again, mouse prostate has 4 distinct lobes that do not exist in the human counterpart. Mouse prostate does not even express PSA, the most ‘important’ molecule in defining the prostate as a male glandular organ and in defining fully differentiated luminal epithelial cells. Also, somatic mouse cells possess high telomerase activity and express significantly longer telomeres than the human cells, suggesting that mouse cells in most lineages may never undergo true terminal differentiation. These latter molecular features underlie the reasons why rodent cells are highly susceptible to spontaneous immortalization as well as experimental tumorigenic transformation. Consequently, results with mouse studies should never be directly equated to human systems and always be put in appropriate context.
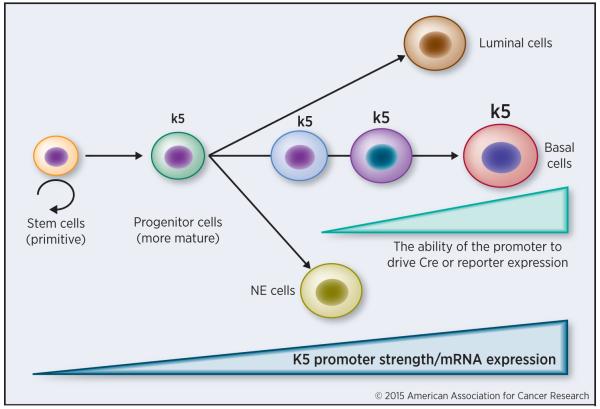
Secondly, the labeling efficiency in lineage tracing studies is highly variable depending on the Cre- or reporter-driving promoters and generally low and the results may oftentimes be subject to alternative interpretations. When endogenous promoters are used, the promoter activity in less differentiated cells may not be strong enough to turn on the transgene leading to low efficiency and spurious interpretations. In the example presented in Figure 4, when CreER is driven by the endogenous k5 promoter, lineage-tracing studies in adult mice may lead to the conclusion that k5-expressing (basal) cells can only regenerate more k5-positive basal cells. However, in multi-potent progenitor cells that have the ability to differentiate into not just k5 cells but also luminal or even neuroendocrine (NE) cells, because they have just started expressing k5 mRNA and the k5 promoter activity in these progenitor cells is too weak to activate the Cre, these cells will not be labeled (Figure 4). In other words, only more differentiated cells that have already undergone lineage specification will be tagged by the reporter (Figure 4), leading to inaccurate or even erroneous conclusions. Along this line, when exogenous or heterologous promoters are used in lineage tracing, they will randomly integrate into the genome resulting in patterns of expression different from the endogenous gene. Furthermore, such promoters are likely regulated by quite different mechanisms than the endogenous promoters. In some cases, promoters exhibit some expression in tissues outside of those predicted, due to regulatory elements or read-through transcripts at the site of insertion.
Figure 4. Potential problems associated with lineage tracing.
In this example, primitive stem cells with self-renewing activity do not express k5 whereas more mature progenitor cells start expressing low levels of k5 mRNA but the promoter activity is not strong enough to drive Cre or reporter gee expression. Hence, the promoter strength in less differentiated cells may not be strong enough to reach the labeling threshold, thereby failing to label the true primitive stem cells that possess the ability to undergo multi-lineage differentiation. NE, neuroendocrine.
Thirdly, when lineage tracing is combined with mouse tumor models to elucidate cell-of-origin, there exists another problem. Most human epithelial cancers develop through decades of clonal evolution and accumulation of genetic mutations and epigenetic alterations. However, in mouse models of human cancer, the promoter is instantly turned on leading to all-at-once genetic defects in an entire population of cells, a phenomenon fundamentally unlike the sequential acquisition of mutations found in most human cancers. Ideally, a cancer model should recapitulate the natural history of the disease by introducing a low frequency of sporadic mutations at a defined time.
Finally, the remaining construct and its insertional effects must be optimized. In most inducible lines in which there is a single element to control Cre, the system frequently becomes leaky, having minor but detectable Cre activity in the absence of the inducer, resulting in spontaneous background recombination. In some cases, an alternative transgenic line, AhcreERT, can be used to eliminate background recombination via controlling Cre activity both transcriptionally by the Ah promoter and by Tamoxifen binding (24). In addition, transgene insertion of Cre recombinase under the control of a specific promoter may alter the function of the endogenous locus via activation or silencing. Incomplete incorporation of regulatory elements into the driver construct or alternatively unexpected excision can also occur (25). LoxP-flanked target genes can differ dramatically with respect to their sensitivity to Cre-mediated recombination (26). Finally, Cre activity can be modified by strain genetic background and variable maternal/paternal germline expression can occur, highlighting the need for animal model optimization.
Concluding remarks and perspectives
The preceding discussions highlight the varying conclusions that may reflect differences and limitations in each assay utilized. A combinatorial approach of the two assays has the potential to lead to a better understanding of the cellular origins of cancer and CSCs and the development of more effective cancer therapies. A major consideration in performing both CSC and tumor cells-of-origin studies is the emergence of CSCs via cellular dedifferentiation (reviewed in detail in 2). Several recent studies have demonstrated that non-CSCs can acquire CSC-like activity under certain conditions (27-30). For instance, many aggressive CSCs within individual tumors can be newly derived from their non-CSC counterparts, and this dedifferentiation may occur continually during the development of the tumor. In melanoma, differentiated cancer cells can dedifferentiate into cells resembling embryonic stem cells that organize into vessel-like structures (30). In breast cancer, CSCs exist in distinct mesenchymal-like and epithelial-like states (27). Remarkably, these two populations of CSCs manifest different locations in the tumors and functional activities: while mesenchymal-like CSCs (CD24−CD44+) are mainly localized at the invasive tumor front and are largely quiescent, the epithelial-like CSCs (ALDH+) are located more centrally and are highly proliferative (30). Understanding the plasticity of CSCs and identifying the subpopulations of non-CSCs that are poised to convert to CSCs (2) should greatly facilitate the efforts in dissecting tumor cell heterogeneity and developing CSC-specific therapeutics. It has become clear that to eradicate cancer and prevent relapse both CSCs and their less tumorigenic progeny must be targeted.
Acknowledgements
We thank colleagues in the Tang lab for helpful discussions and advice and Ms. Joi Holcomb for graphical assistance. Work in the authors’ lab was supported, in part, by grants from NIH (R01-CA155693), Department of Defense (W81XWH-13-1-0352 and W81XWH-14-1-0575), CPRIT (RP120380), and MDACC Center for Cancer Epigenetics (all to DGT). KR was supported in part by an NIH postdoctoral fellowship. We apologize to authors whose work could not be cited due to space constraint.
Footnotes
Disclosure of Potential Conflict of Interest
The authors disclose no potential conflict of interest.
References
- 1.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–22. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 2.Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell research. 2012;22:457–72. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–91. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Passey RD, Dmochowski L, Lasnitzki I, Millard A. Cultivation in vitro of frozen and desiccated mouse tumour tissues. Br Med J. 1950;2:1134–6. doi: 10.1136/bmj.2.4689.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hewitt HB. Studies of the quantitative transplantation of mouse sarcoma. Br J Cancer. 1953;7:367–83. doi: 10.1038/bjc.1953.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 8.Qin J, Liu X, Laffin B, Chen X, Choy G, Jeter CR, et al. The PSA(−/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration. Cell Stem Cell. 2012;10:556–69. doi: 10.1016/j.stem.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumor growth in immunodeficient mice. Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–71. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor RA, Toivanen R, Frydenberg M, Pedersen J, Harewood L, Australian Prostate Cancer B et al. Human epithelial basal cells are cells of origin of prostate cancer, independent of CD133 status. Stem Cells. 2012;30:1087–96. doi: 10.1002/stem.1094. [DOI] [PubMed] [Google Scholar]
- 12.Domingo-Domenech J, Vidal SJ, Rodriguez-Bravo V, Castillo-Martin M, Quinn SA, Rodriguez-Barrueco R, et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 2012;22:373–88. doi: 10.1016/j.ccr.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–8. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boiko AD, Razorenova OV, van de Rijn M, Swetter SM, Johnson DL, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466:133–7. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 16.Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Pascal LE, Isharwal S, Metzger D, Ramos Garcia R, Pilch J, et al. Regenerated luminal epithelial cells are derived from preexisting luminal epithelial cells in adult mouse prostate. Mol Endocrinol. 2011;25:1849–57. doi: 10.1210/me.2011-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–6. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schepers AG, Snippert HJ, Stange DE, van den Born M, van Es JH, van de Wetering M, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–5. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 20.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–30. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang ZA, Mitrofanova A, Bergren SK, Abate-Shen C, Cardiff RD, Califano A, et al. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nat Cell Biol. 2013;15:274–83. doi: 10.1038/ncb2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi N, Zhang B, Zhang L, Ittmann M, Xin L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell. 2012;21:253–65. doi: 10.1016/j.ccr.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemp R, Ireland H, Clayton E, Houghton C, Howard L, Winton DJ. Elimination of background recombination: somatic induction of Cre by combined transcriptional regulation and hormone binding affinity. Nucleic Acids Res. 2004;32:e92. doi: 10.1093/nar/gnh090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heffner CS, Herbert Pratt C, Babiuk RP, Sharma Y, Rockwood SF, Donahue LR, et al. Supporting conditional mouse mutagenesis with a comprehensive cre characterization resource. Nat Commun. 2012;3:1218. doi: 10.1038/ncomms2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–8. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports. 2013;2:78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, et al. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146:633–44. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 29.Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141:583–94. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Molecular plasticity of human melanoma cells. Oncogene. 2003;22:3070–5. doi: 10.1038/sj.onc.1206447. [DOI] [PubMed] [Google Scholar]