Abstract
Background/Aims
Primary hyperoxaluria results from an alteration in enzymes that metabolize glyoxylate. The metabolism that leads to glyoxylate synthesis is not well defined. The aim of this study was to investigate the production of glyoxylate in liver mitochondria when they metabolize hydroxyproline.
Methods
Mitochondria were isolated from mouse liver using Percoll gradient centrifugation. The metabolism of hydroxyproline was examined by a combination of HPLC and ion chromatography/mass spectrometry techniques.
Results
Glyoxylate production was substantially greater when mitochondria were incubated with hydroxyproline in comparison with proline. Inclusion of malate and glutamate with hydroxyproline resulted in a drop in glyoxylate and an increase in glycolate in the incubation mixture. This suggests an increased NAD(P)+ reduction which occurred with the inclusion of glutamate/malate and that the NAD(P)H production was required to stimulate the glyoxylate reductase-catalyzed conversion of glyoxylate to glycolate. The presence of glyoxylate reductase in these mitochondria was confirmed by measuring enzymatic activity and by Western blotting.
Conclusion
These results indicate that studies on isolated mitochondria have the potential to help unravel the metabolism associated with glyoxylate and oxalate production and understand the metabolic function of glyoxylate reductase.
Keywords: Glyoxylate, Mitochondria, Primary hyperoxaluria
Introduction
Primary hyperoxaluria type 1 (PH1) results from a deficiency in peroxisomal alanine:glyoxylate aminotransferase (AGT1) activity [1]. In peroxisomes, AGT converts the glyoxylate produced from glycolate oxidation to glycine. The source of the glycolate has not yet been identified. PH2 results from a deficiency in glyoxylate reductase (GR) activity [1]. This enzyme converts glyoxylate to glycolate and limits the conversion of glyoxylate to oxalate. Liver and kidney are two tissues that are enriched in GR activity [2]. These two tissues are also involved in hydroxyproline metabolism, which results in the formation of glyoxylate, an oxalate precursor [3]. The breakdown of hydroxyproline occurs in mitochondria, raising the issue of how mitochondria handle the glyoxylate produced. For many years, GR was thought to be solely a cytoplasmic enzyme. However, our recent investigations in cultured hepatocytes (Hep G2 cells) revealed that GR was also localized in mitochondria, seemingly with similar concentrations in both the cytoplasm and mitochondria [4]. Proteonomic analyses have indicated that this enzyme is also present in heart mitochondria [5]. The functional role for GR in mitochondria may relate to the metabolism of hydroxyproline that occurs in this organelle [3]. To examine whether GR plays a role in converting the glyoxylate formed from hydroxyproline to glycolate, we have investigated hydroxyproline metabolism in isolated mouse liver mitochondria.
Materials and Methods
Materials
All chemicals were of reagent grade and were primarily purchased from Sigma (St. Louis, Mo., USA).
Preparation of Mitochondria
Mitochondria were purified from the livers of male BALB/c wild-type mice as described previously, with modifications [6]. Liver (approximately 1 g) was minced in isolation buffer (1:10 w/v; 0.25 M sucrose, 1 mM EDTA and 5 mM Tris-HCl, pH 7.5) and gently homogenized with a Potter-Elvehjem tissue homogenizer with a Teflon pestle. The homogenate was spun at 1,500 g (10 min) to remove nuclei, unbroken cells and cellular debris. The postnuclear supernatant was subsequently centrifuged at 16,000 g (10 min) to obtain a crude mitochondrial pellet. The pellet was re-suspended in 2 ml isolation buffer containing 15% Percoll, which was layered over a 40%/23% Percoll discontinuous gradient. Gradients were centrifuged at 30, 700 g for 10 min, and the mitochondria were collected at the interface between the 23 and 40% Percoll layers. Purified mitochondria were incubated at room temperature for 1 h, with gentle stirring, in a mixture containing 20 mM Hepes, pH 7.1, 100 mM KCl, 1 mM MgCl2 and either no amino acids, 1 mM proline, 1 mM hydroxyproline or 1mM hydroxyproline with 5 mM glutamate and 5 mM malate.
Mitochondrial Anion Analyses
To determine anion levels, mitochondrial incubation mixtures were extracted with ice-cold 10% trichloroacetic acid for 30 min. The trichloroacetic acid was removed from extracts by vigorously vortexing with an equal volume of 1,1,2-trichlorotrifluoroethane (Freon)-trioctylamine (3:1 v/v; Aldrich, Milwaukee, Wisc., USA), centrifuging at 4°C to promote phase separation and collecting the upper aqueous layer for analysis [7]. The phenylhydrazone derivatives of glyoxylate were measured by reverse-phase HPLC, as previously described [8]. Other anions were determined in extracts after treatment with Ag-treated resin, as described by Hagen et al. [9], by ion chromatography coupled with electrospray mass spectrometry (IC/MS; Dionex Corporation, Sunnyvale, Calif., USA). The ion chromatography equipment consisted of a Dionex ED50 conductivity detector, Dionex AS11_HC 2 × 250 mm ion exchange column with guard column at a controlled temperature of 30°C, and a Dionex ASRS-ULTRA 2-mm suppressor. A sodium hydroxide gradient 1–35 mM over 40 min at a flow rate of 0.4 ml/min was used.
Amino Acid Analysis
Amino acid analysis was performed by the AccQ Tag method (Waters Corp., Milford, Mass., USA), as previously described [10].
GR Assay
Crude liver homogenate and purified mitochondria were first sonicated in hypotonic lysis buffer (25 mM Hepes, pH 7.1, 0.1% Triton X-100). GR activity was measured spectrophotometrically at 37°C in an assay mixture containing 50 mM Hepes, pH 7.1, 0.2 mM NAD(P)H, 100 mM KCl and 6 mM glyoxylate, as previously described [2].
Western Blot Analysis
For Western blot analysis, samples were electroblotted from 12% (w/v) acrylamide gels onto Hybond-C nitrocellulose membranes (Amersham Biosciences, Piscataway, N.J., USA). Membranes were incubated for 1 h in 5% (w/v) nonfat dried milk dissolved in PBS, pH 7.4, containing 1% (v/v) Tween-20, followed by incubation for 1.5 h at 25°C with rabbit antirecombinant human GR (hGR) antibody (1 μg/ml). The membrane was then washed and incubated for 1 h at room temperature with peroxidase-conjugated goat antirabbit immunoglobulin G (0.2 μg/ml; Dako Corporation, Carpenteria, Calif., USA). GR was visualized by using enhanced chemiluminescence (Amersham Biosciences).
Protein Analysis
Protein content was measured after dissolution of the lysates with 0.1 M NaOH. A Coomassie Blue dye binding method (Pierce, Rockford, Ill., USA) with bovine serum albumin as the standard was used. The absorbance at 595 nm of samples was compared with a standard curve to determine protein concentration.
Data Analysis
The results are presented as means ± SE. The data shown are representative of four independent experiments that showed similar results.
Results
Oxidation of Hydroxyproline to Glyoxylate and Glycolate
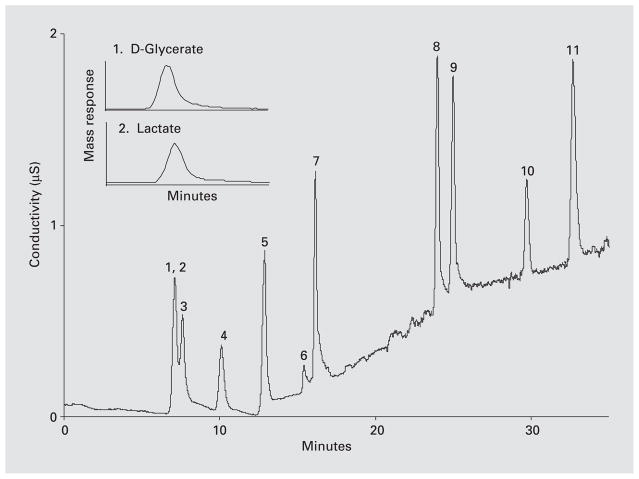
Glyoxylate levels in mitochondrial incubation mixtures were readily measured by HPLC after derivatization with phenylhydrazine, but glycolate could only be measured by time-consuming procedures with a low sensitivity. However, IC/MS was able to readily measure glycolate with high sensitivity. The separation of a standard range of anions by IC/MS is shown in figure 1. Thus, D-glycerate and lactate coelute from the column and cannot be distinguished by conductivity measurements. However, mass spectral analysis allows these two anions to be measured, as shown in the insert in figure 1.
Fig. 1.
Separation of anions by ion chromatography coupled with IC/MS using a sodium hydroxide gradient. The insert in the figure illustrates how D-glycerate (MW 106) and lactate (MW 90), which coelute from the column, can be measured by mass spectrometry. 1, 2 = D-Glycerate and lactate, respectively; 3 = glycolate; 4 = formate; 5 = pyruvate; 6 = glyoxylate; 7 = chloride; 8 = sulphate; 9 = oxalate; 10 = phosphate; 11 = citrate.
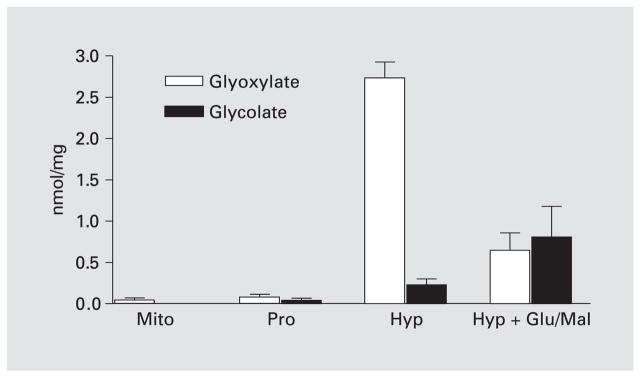
Mitochondria incubated in 20 mM Hepes, pH 7.1, 100 mM KCl, 1 mM MgCl2 contained very low levels of glyoxylate, 0.04 ± 0.027 nmol/mg protein, and no detectable glycolate (fig. 2). Following incubation with hydroxyproline, mean glyoxylate and glycolate levels were 45- and 5-fold higher than that seen after incubation with proline. Incubation with hydroxyproline and a mixture of malate and glutamate resulted in a 77% drop in glyoxylate levels and a 72% increase in glycolate levels compared with hydroxyproline alone. This change suggested that an increased NAD(P)+ reduction occurred with the inclusion of glutamate/malate and that NAD(P)H production was required to stimulate the GR-catalyzed conversion of glyoxylate to glycolate. Amino acid analysis of all mitochondrial incubation mixtures showed low levels of glycine and L-alanine, 0.58 ± 0.31 and 0.15 ± 0.07 pmol/mg protein, respectively.
Fig. 2.
Glyoxylate and glycolate levels following the incubation of mitochondria (Mito) with 1 mM proline (Pro), 1 mM hydroxyproline (Hyp) and a mixture of 1 mM hydroxyproline and 5 mM glutamate/malate (Glu/Mal).
Glyoxylate Reductase
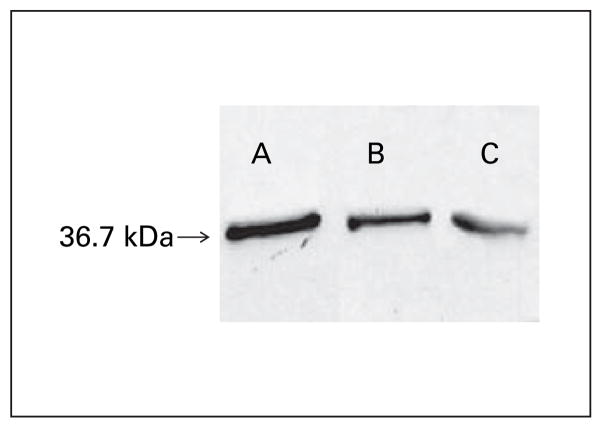
Purified liver mitochondria and crude liver homogenates were sonicated in hypotonic lysis buffer containing 0.1% Triton and assayed for GR activity. Mean GR activity of purified mitochondria and crude liver homogenate was 15.25 ± 1.7 nmol/min/mg (range 9.88–35.98) and 48.13 ± 5.7 nmol/min/mg (range 34.3–61.4), respectively. Western Blot analysis confirmed the presence of GR in both crude homogenate and purified mitochondria (fig. 3).
Fig. 3.
Western blot analysis of recombinant hGR (0.1 ng) (A), GR in crude mouse liver homogenate (3 μg) (B) and purified mouse liver mitochondria (26 μg) (C). Samples were electroblotted from 12% acrylamide gels onto nitrocellulose membranes and probed with rabbit antirecombinant hGR antibody. Secondary peroxidase-antibody conjugate was detected by enhanced chemiluminescence.
Discussion
We recently reported that mitochondria isolated from a line of human liver cells contain GR activity at a concentration that is similar to that in the cytoplasm [4]. Our examination of mouse liver mitochondria has revealed that they also contain GR, but at a concentration that appears to be lower than in the cytoplasm (fig. 3). Mitochondria also contain AGT2 activity, but the kinetic properties of the enzyme suggest that this activity is not a favored reaction of the enzyme [4]. This enzyme also has D-3-aminobutyrate:pyruvate aminotransferase, β-alanine:pyruvate aminotransferase and dimethylarginine: pyruvate aminotransferase activities [11, 12]. Rodent liver mitochondria are distinct from human liver mitochondria in containing AGT1 activity; however, the AGT1 concentration in rodent liver is only a small fraction of that found in human liver [13]. Whether the AGT1 activity in mouse liver mitochondria plays a role in metabolizing glyoxylate derived from hydroxyproline oxidation is unclear from our studies. Its low concentration, the low concentration of alanine and the failure to detect an increase in mitochondrial glycine content with hydroxyproline oxidation suggests that its contribution is minor under the experimental conditions we used.
The ability of isolated mitochondria to metabolize hydroxyproline is clearly evident in their production of glyoxylate from added hydroxyproline but not proline. The conversion of this glyoxylate to glycolate was only evident when 5 mM glutamate/malate was added with hydroxyproline to the incubation mixture to stimulate the reduction of NAD(P)+. It appears that the levels produced with 1 mM hydroxyproline oxidation were insufficient to produce significant GR activity. This sensitivity to the NAD(P)H concentration is in keeping with the apparent low affinity of GR for pyridine nucleotide cofactors that has been reported [2]. The interaction of GR with pyridine nucleotide cofactors needs closer scrutiny to be able to fully understand the role of GR in disposing of intra-mitochondrial-generated glyoxylate.
The amount of hydroxyproline degraded each day in humans as a result of collagen turnover is 300–450 mg, with the formation of 150–250 mg of glyoxylate. Further hydroxyproline is derived from the diet with the ingestion of meat and gelatin-containing foods. GR may play an important physiological role by converting this glyoxylate to glycolate, thereby limiting glyoxylate conversion to oxalate. Such a role would make mitochondria an important source of glycolate. In individuals with PH2 who lack GR activity, mitochondria may be an important source of the glyoxylate that fuels the excessive oxalate synthesis in this condition. In individuals with PH1, the combined urinary excretion of oxalate and glycolate, 200–400 mg/day, matches the estimated amount of hydroxyproline metabolized reasonably well, suggesting that mitochondrial glycolate production could be the principal source of this substrate for oxalate synthesis in the disease. Thus, hydroxyproline metabolism could play an important role in both PH1 and PH2, and this metabolism could be an important target for limiting oxalate production. As the targeting of GR to mitochondria will most likely depend on unique binding sites or structural motifs in GR that are separate from regions of the protein associated with its enzymatic activity, it would seem possible that a mutation that interfered with mitochondrial targeting could produce a cellular phenotype with normal cytoplasmic GR but lacking mitochondrial GR. Whether such a phenotype would result in excessive oxalate synthesis deserves consideration as an explanation for the phenotype observed in individuals with unclassified PH [14].
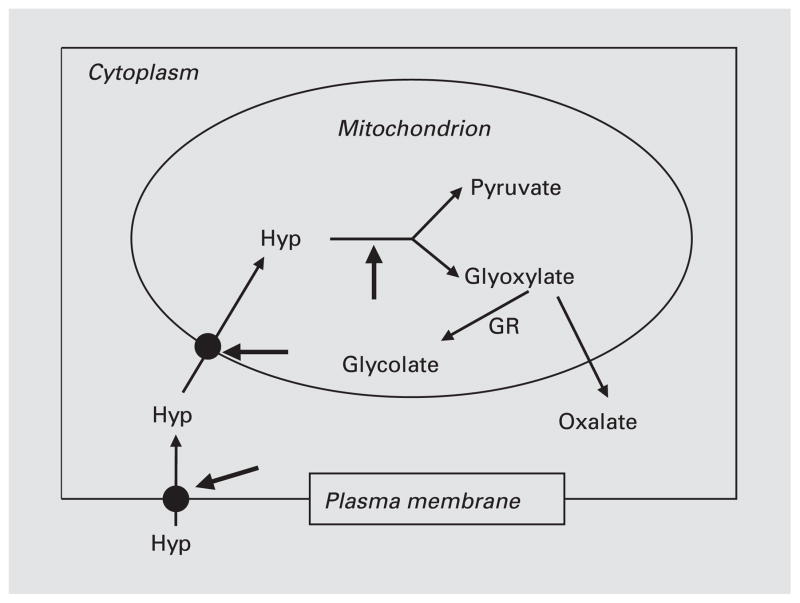
If hydroxyproline metabolism plays a major role in generating glyoxylate and glycolate that cannot be effectively metabolized in the primary hyperoxalurias, there are several potential target sites as shown in figure 4. They include the transport of hydroxyproline across the plasma membrane and mitochondrial membranes and the oxidation of hydroxyproline in mitochondria by hydroxyproline oxidase. Hydroxyproline analogues are ideally poised to act at these sites because of their presumed low toxicity and structural similarity to trans-4-hydroxy-L-proline. The use of isolated liver mitochondria has the potential to elucidate the functional role of GR in hydroxyproline metabolism and to evaluate the efficacy of hydroxyproline analogues in modifying it.
Fig. 4.
Hydroxyproline transport and metabolism in cells. The heavy arrows identify potential target sites for blocking hydroxyproline metabolism. Hyp = Trans-4-hydroxy-L-proline.
Acknowledgments
The expertise of Martha Kennedy, Persida Tahiri and Ying Yang in assisting with these experiments was greatly appreciated. This research was funded by NIH grant DK54468.
References
- 1.Danpure CJ. Primary hyperoxaluria. In: Scriver CR, Beaudet AL, Sly WS, Vallee D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. New York: McGraw-Hill; 2001. pp. 3323–3367. [Google Scholar]
- 2.Giafi CF, Rumsby G. Kinetic analysis and tissue distribution of human D-glycerate dehydrogenase/glyoxylate reductase and its relevance to the diagnosis of primary hyperoxaluria type 2. Ann Clin Biochem. 1998;35:104–109. doi: 10.1177/000456329803500114. [DOI] [PubMed] [Google Scholar]
- 3.Phang JM, Hu CA, Valle D. Disorders of proline and hydroxyproline metabolism. In: Scriver CR, Beaudet AL, Sly WS, Vallee D, Childs B, Kinzler KW, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. 8. New York: McGraw-Hill; 2001. pp. 1821–1838. [Google Scholar]
- 4.Baker PR, Cramer SD, Kennedy M, Assimos DG, Holmes RP. Glycolate and glyoxylate metabolism in HepG2 cells. Am J Physiol Cell Physiol. 2004;287:C1359–C1365. doi: 10.1152/ajpcell.00238.2004. [DOI] [PubMed] [Google Scholar]
- 5.Taylor SW, Fahy E, Zhang B, Glenn GM, Warnock DE, Wiley S, Murphy AN, Gaucher SP, Capaldi RA, Gibson BW, Ghosh SS. Characterization of the human heart mitochondrial proteome. Nat Biotechnol. 2003;21:281–286. doi: 10.1038/nbt793. [DOI] [PubMed] [Google Scholar]
- 6.Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- 7.Palmero S, de Marchis M, Prati M, Fugassa E. HPLC analysis of free aminoacids and aminoacids of total proteins in cultured cells – An application to the study of rat Sertoli cell protein metabolism. Anal Biochem. 1992;202:152–158. doi: 10.1016/0003-2697(92)90220-2. [DOI] [PubMed] [Google Scholar]
- 8.Poore RE, Hurst CH, Assimos DG, Holmes RP. Pathways of hepatic oxalate synthesis and their regulation. Am J Physiol. 1997;272:C289–C294. doi: 10.1152/ajpcell.1997.272.1.C289. [DOI] [PubMed] [Google Scholar]
- 9.Hagen L, Walker VR, Sutton RA. Plasma and urinary oxalate and glycolate in healthy subjects. Clin Chem. 1993;39:134–138. [PubMed] [Google Scholar]
- 10.Reverter M, Lundh T, Lindberg JE. Determination of free amino acids in pig plasma by precolumn derivatization with 6-N-aminoquinolyl-N-hydroxysuccinimidyl carbamate and high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1997;696:1–8. doi: 10.1016/s0378-4347(97)00217-x. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa T, Kimoto M, Sasaoka K. Dimethylarginine:pyruvate aminotransferase in rats. Purification, properties, and identity with alanine:glyoxylate aminotransferase 2. J Biol Chem. 1990;265:20938–20945. [PubMed] [Google Scholar]
- 12.Kontani Y, Kaneko M, Kikugawa M, Fujimoto S, Tamaki N. Identity of D-3-aminoisobutyrate-pyruvate aminotransferase with alanine-glyoxylate aminotransferase 2. Biochim Biophys Acta. 1993;1156:161–166. doi: 10.1016/0304-4165(93)90131-q. [DOI] [PubMed] [Google Scholar]
- 13.Danpure CJ, Guttridge KM, Fryer P, Jennings PR, Allsop J, Purdue PE. Subcellular distribution of hepatic alanine:glyoxylate aminotransferase in various mammalian species. J Cell Soc. 1990;97:669–678. doi: 10.1242/jcs.97.4.669. [DOI] [PubMed] [Google Scholar]
- 14.Monico CG, Persson M, Ford GC, Rumsby G, Milliner DS. Potential mechanisms of marked hyperoxaluria not due to primary hyperoxaluria I or II. Kidney Int. 2002;62:392–400. doi: 10.1046/j.1523-1755.2002.00468.x. [DOI] [PubMed] [Google Scholar]