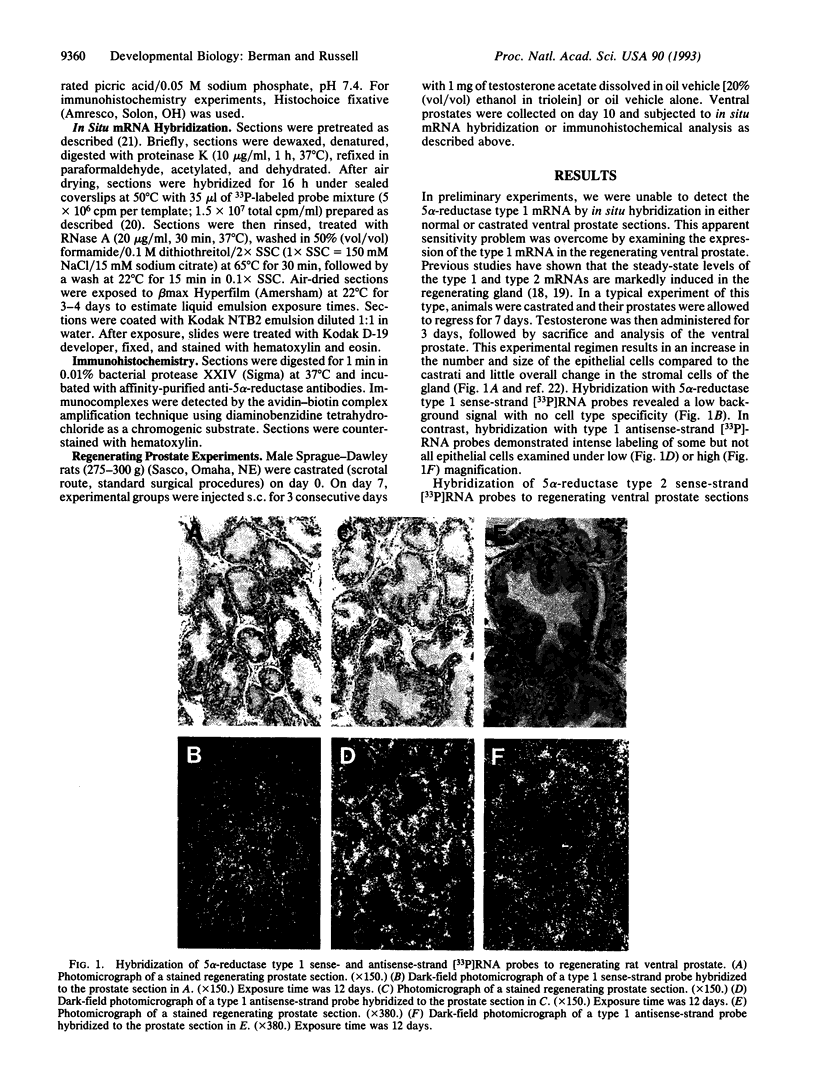
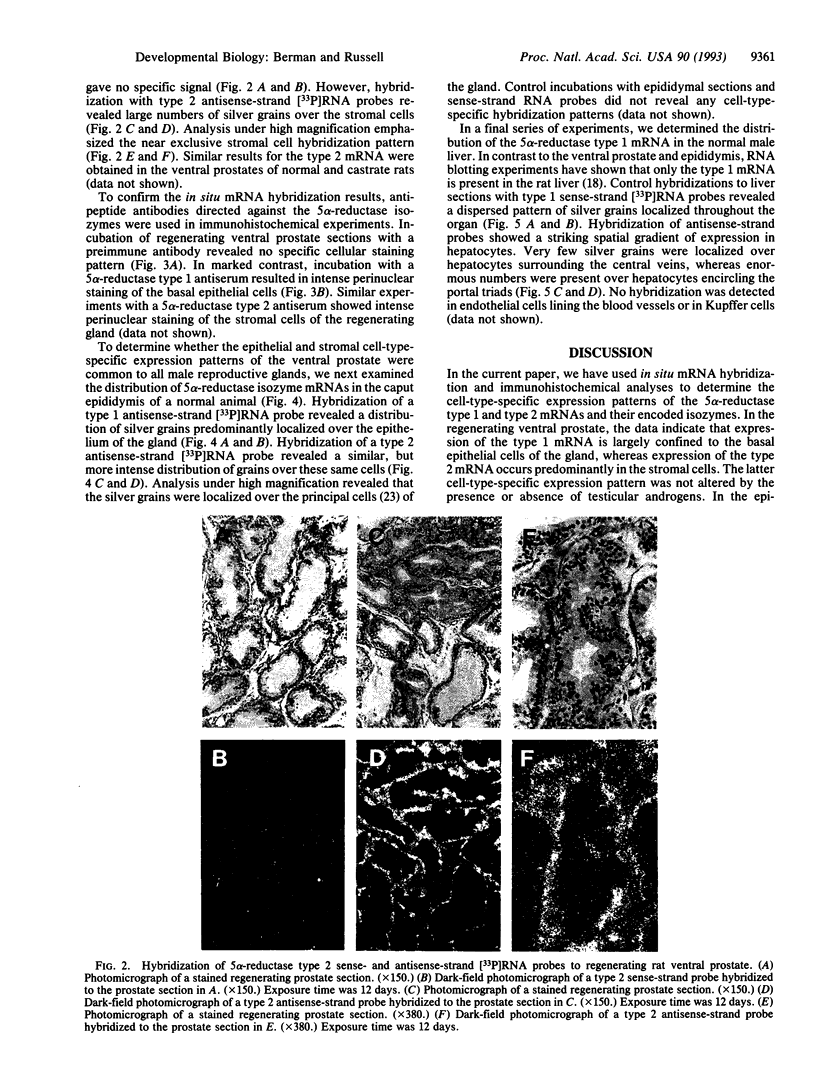
Abstract
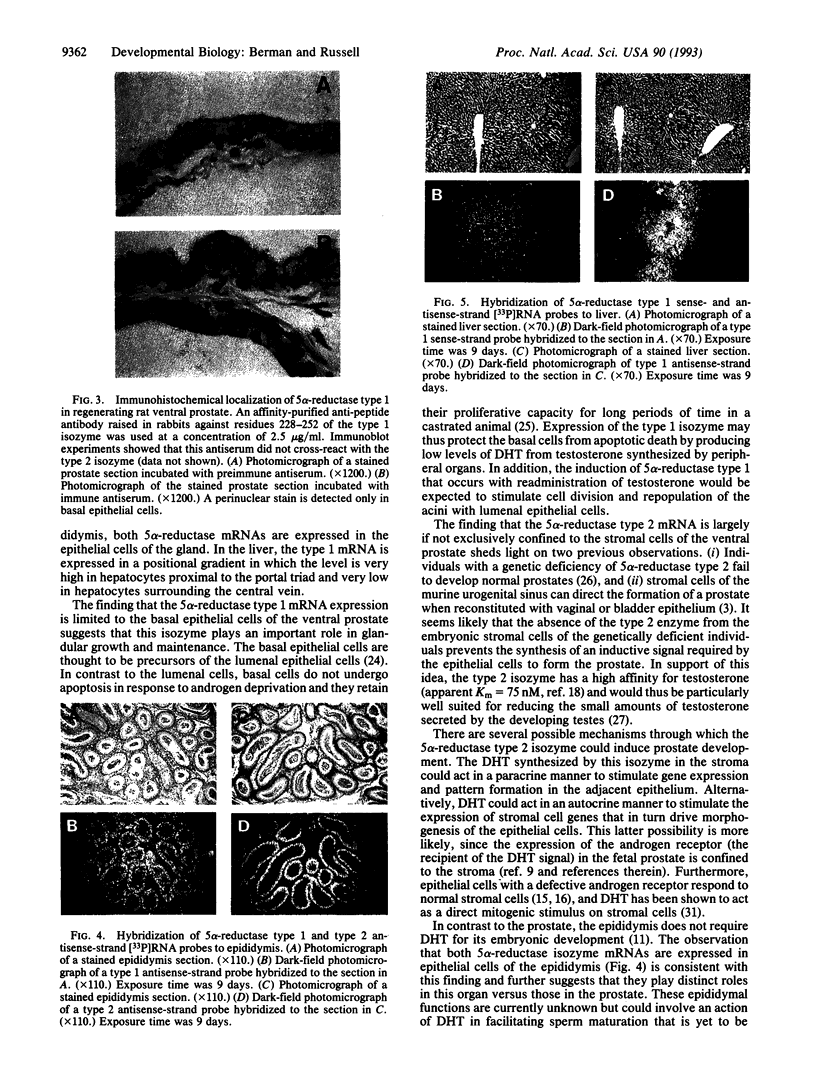
The enzyme steroid 5 alpha-reductase (EC 1.3.99.5) is a component of an intercellular signaling pathway that determines cell fate in the primordium of the mammalian reproductive tract. During male phenotypic sexual differentiation, the dihydrotestosterone product of this enzyme binds to the androgen receptor and initiates development of the external genitalia and prostate. Genes encoding two isozymes of steroid 5 alpha-reductase with different biochemical properties and tissue distributions have recently been isolated. In the current study, we utilize in situ hybridization analysis to determine cell-type-specific expression patterns of the 5 alpha-reductase isozyme mRNAs in two androgen target tissues (regenerating ventral prostate and epididymis) and a peripheral tissue (liver). In regenerating ventral prostate, the type 1 mRNA is expressed in basal epithelial cells whereas expression of the type 2 mRNA is largely confined to stromal cells. These results were confirmed by immunohistochemical analysis and are consistent with distinct roles played by the isozymes in the prostate. In the epididymis, both 5 alpha-reductase isozyme mRNAs are expressed in epithelial cells. Only the type 1 mRNA is present in the liver. This mRNA is distributed in a striking spatial gradient extending from hepatocytes surrounding the portal triad (high expression) to those surrounding the central vein (low to absent expression). These findings demonstrate cell-type-specific expression of the steroid 5 alpha-reductase isozymes and underscore their distinct and overlapping functions in androgen physiology.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Bishop R. W., Russell D. W. Expression cloning and regulation of steroid 5 alpha-reductase, an enzyme essential for male sexual differentiation. J Biol Chem. 1989 Sep 25;264(27):16249–16255. [PMC free article] [PubMed] [Google Scholar]
- Cate R. L., Mattaliano R. J., Hession C., Tizard R., Farber N. M., Cheung A., Ninfa E. G., Frey A. Z., Gash D. J., Chow E. P. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986 Jun 6;45(5):685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- Chang S. M., Chung L. W. Interaction between prostatic fibroblast and epithelial cells in culture: role of androgen. Endocrinology. 1989 Nov;125(5):2719–2727. doi: 10.1210/endo-125-5-2719. [DOI] [PubMed] [Google Scholar]
- Cunha G. R. Age-dependent loss of sensitivity of female urogenital sinus to androgenic conditions as a function of the epithelia-stromal interaction in mice. Endocrinology. 1975 Sep;97(3):665–673. doi: 10.1210/endo-97-3-665. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Chung L. W., Shannon J. M., Reese B. A. Stromal-epithelial interactions in sex differentiation. Biol Reprod. 1980 Feb;22(1):19–42. doi: 10.1095/biolreprod22.1.19. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Donjacour A. A., Cooke P. S., Mee S., Bigsby R. M., Higgins S. J., Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987 Aug;8(3):338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- Cunha G. R. Epithelio-mesenchymal interactions in primordial gland structures which become responsive to androgenic stimulation. Anat Rec. 1972 Feb;172(2):179–195. doi: 10.1002/ar.1091720206. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Lung B. The importance of stroma in morphogenesis and functional activity of urogenital epithelium. In Vitro. 1979 Jan;15(1):50–71. doi: 10.1007/BF02627079. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978 Aug;205(2):181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- DeKlerk D. P., Coffey D. S. Quantitative determination of prostatic epithelial and stromal hyperplasia by a new technique. Biomorphometrics. Invest Urol. 1978 Nov;16(3):240–245. [PubMed] [Google Scholar]
- George F. W., Russell D. W., Wilson J. D. Feed-forward control of prostate growth: dihydrotestosterone induces expression of its own biosynthetic enzyme, steroid 5 alpha-reductase. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8044–8047. doi: 10.1073/pnas.88.18.8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B. M. Epithelial morphogenesis. Cell. 1992 May 1;69(3):385–387. doi: 10.1016/0092-8674(92)90440-n. [DOI] [PubMed] [Google Scholar]
- Husmann D. A., McPhaul M. J., Wilson J. D. Androgen receptor expression in the developing rat prostate is not altered by castration, flutamide, or suppression of the adrenal axis. Endocrinology. 1991 Apr;128(4):1902–1906. doi: 10.1210/endo-128-4-1902. [DOI] [PubMed] [Google Scholar]
- Imperato-McGinley J., Gautier T., Zirinsky K., Hom T., Palomo O., Stein E., Vaughan E. D., Markisz J. A., Ramirez de Arellano E., Kazam E. Prostate visualization studies in males homozygous and heterozygous for 5 alpha-reductase deficiency. J Clin Endocrinol Metab. 1992 Oct;75(4):1022–1026. doi: 10.1210/jcem.75.4.1400866. [DOI] [PubMed] [Google Scholar]
- Montesano R., Matsumoto K., Nakamura T., Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991 Nov 29;67(5):901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Normington K., Russell D. W. Tissue distribution and kinetic characteristics of rat steroid 5 alpha-reductase isozymes. Evidence for distinct physiological functions. J Biol Chem. 1992 Sep 25;267(27):19548–19554. [PubMed] [Google Scholar]
- Ratanasavanh D., Beaune P., Morel F., Flinois J. P., Guengerich F. P., Guillouzo A. Intralobular distribution and quantitation of cytochrome P-450 enzymes in human liver as a function of age. Hepatology. 1991 Jun;13(6):1142–1151. [PubMed] [Google Scholar]
- Reid L. M. Stem cell biology, hormone/matrix synergies and liver differentiation. Curr Opin Cell Biol. 1990 Feb;2(1):121–130. doi: 10.1016/s0955-0674(05)80042-0. [DOI] [PubMed] [Google Scholar]
- Sariola H., Saarma M., Sainio K., Arumäe U., Palgi J., Vaahtokari A., Thesleff I., Karavanov A. Dependence of kidney morphogenesis on the expression of nerve growth factor receptor. Science. 1991 Oct 25;254(5031):571–573. doi: 10.1126/science.1658930. [DOI] [PubMed] [Google Scholar]
- Siiteri P. K., Wilson J. D. Testosterone formation and metabolism during male sexual differentiation in the human embryo. J Clin Endocrinol Metab. 1974 Jan;38(1):113–125. doi: 10.1210/jcem-38-1-113. [DOI] [PubMed] [Google Scholar]
- Thigpen A. E., Davis D. L., Milatovich A., Mendonca B. B., Imperato-McGinley J., Griffin J. E., Francke U., Wilson J. D., Russell D. W. Molecular genetics of steroid 5 alpha-reductase 2 deficiency. J Clin Invest. 1992 Sep;90(3):799–809. doi: 10.1172/JCI115954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarfaty I., Resau J. H., Rulong S., Keydar I., Faletto D. L., Vande Woude G. F. The met proto-oncogene receptor and lumen formation. Science. 1992 Aug 28;257(5074):1258–1261. doi: 10.1126/science.1387731. [DOI] [PubMed] [Google Scholar]
- Verhagen A. P., Aalders T. W., Ramaekers F. C., Debruyne F. M., Schalken J. A. Differential expression of keratins in the basal and luminal compartments of rat prostatic epithelium during degeneration and regeneration. Prostate. 1988;13(1):25–38. doi: 10.1002/pros.2990130104. [DOI] [PubMed] [Google Scholar]
- Viger R. S., Robaire B. Differential regulation of steady state 4-ene steroid 5 alpha-reductase messenger ribonucleic acid levels along the rat epididymis. Endocrinology. 1991 May;128(5):2407–2414. doi: 10.1210/endo-128-5-2407. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. The endocrine control of sexual differentiation. Harvey Lect. 1983 1984;79:145–172. [PubMed] [Google Scholar]
- Wylie C. Embryology. Activins and induction. Nature. 1990 Sep 27;347(6291):337–338. doi: 10.1038/347337a0. [DOI] [PubMed] [Google Scholar]